Abstract
The prevalence and frequency of the dihydrofolate reductase (dhfr) and dihydropteroate synthetase (dhps) mutations associated with sulfadoxine–pyrimethamine (SP) resistance at 13 sentinel surveillance sites in southern Mozambique were examined regularly between 1999 and 2004. Frequency of the dhfr triple mutation increased from 0.26 in 1999 to 0.96 in 2003, remaining high in 2004. The dhps double mutation frequency peaked in 2001 (0.22) but declined to baseline levels (0.07) by 2004. Similarly, parasites with both dhfr triple and dhps double mutations had increased in 2001 (0.18) but decreased by 2004 (0.05). The peaking of SP resistance markers in 2001 coincided with a SP–resistant malaria epidemic in neighboring KwaZulu-Natal, South Africa. The decline in dhps (but not dhfr) mutations corresponded with replacement of SP with artemether–lumefantrine as malaria treatment policy in KwaZulu-Natal. Our results show that drug pressure can exert its influence at a regional level rather than merely at a national level.
INTRODUCTION
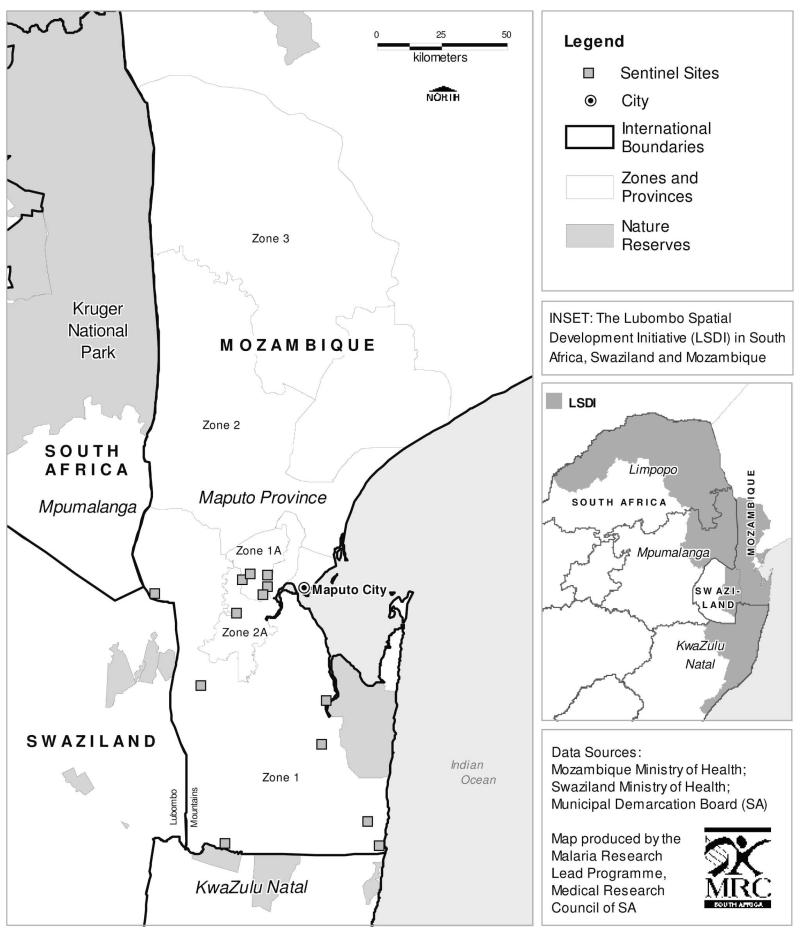
Malaria-related morbidity and mortality has risen in Africa, principally because of increasing resistance to chloroquine and sulfadoxine–pyrimethamine (SP) in Plasmodium falciparum.1,2 The economic impact of malaria is massive, costing Africa an estimated US$ 12 billion per annum.3 Malaria control programs that effectively control both the mosquito vector and the falciparum parasite can reduce the malaria burden substantially.4–6 The Lubombo Spatial Development Initiative (LSDI) initiated in 1999, is a cross-border collaboration between the governments of Mozambique, Swaziland, and South Africa, to develop the Lubombo region into a globally competitive economic zone (Figure 1). As malaria control is a necessary precursor to economic development, this is a core component of the initiative and comprises two arms: vector control through widespread community-based, indoor residual insecticide spraying (IRS), and parasite control through the phased implementation of effective artemisinin-based combination treatment of definitively diagnosed malaria cases.7
Figure 1.
Zones and sentinel sites of the Lubombo Spatial Development Initiative malaria control programme.
As the emergence of drug resistance is a potential threat to the success of the LSDI, population-based antimalarial resistance surveillance was conducted from the start of the intervention. First line malaria treatment policy varied across the LSDI region (Figure 1). In KwaZulu-Natal (KZN), a province in South Africa, SP replaced the chloroquine treatment policy in 1988, following a high prevalence of treatment failures associated with the emergence of chloroquine resistant parasites.8,9 An SP in-vivo therapeutic efficacy study with a 42-day follow-up period conducted in KZN 12 years after SP introduction found adequate clinical and parsitological response in only 11% of patients with uncomplicated falciparum malaria.5,10 This prompted a treatment policy change to the artemisinin-based combination therapy (ACT), artemether–lumefantrine, in January 2001.5 In Mozambique, chloroquine was the first-line treatment and SP the second-line treatment until 2004, when the LSDI started phased implementation of the ACT, artesunate plus SP, as the first-line treatment in Maputo Province. This ACT has now become Mozambique’s national malaria treatment policy.
Accumulation of point mutations in the 2 enzyme systems targeted by SP, namely, dihydrofolate reductase (dhfr) and dihydropteroate synthetase (dhps), confer resistance to pyrimethamine and sulfadoxine, respectively,11,12 with higher levels of treatment failure observed with multiple mutations.13,14 The combination of 3 dhfr point mutations at codons 108, 51, and 59 and 2 dhps mutations at codons 437 and 540 is strongly correlated with SP treatment failure.15 Emergence of SP resistance in southern and east Africa is a result of a combination of increased drug pressure, applied through SP use for malaria treatment, and migration of resistant genes from areas of established resistance.16 In the case of dhfr, the highly resistant triple-mutant allele has been shown to have originated in southeast Asia.17 The dhps double-mutant allele found throughout the southeast African region is descended from a common ancestral lineage,16 although its site of origin remains to be identified.
To explore the effect of gene flow on the development of resistance in neighboring countries with different malaria treatment policies, we analyzed the prevalence and frequency of dhfr and dhps alleles between 1999 and 2004 in the LSDI areas in southern Mozambique closest to KZN in South Africa (Figure 1).
MATERIALS AND METHODS
Study population and blood sample collection
Finger-prick blood samples were collected during systematic community-based sampling of 120 children (of age 2–15 years) at each of the 13 sentinel sites in LSDI zones 1, 1A, and 2A in Maputo Province, southern Mozambique (Figure 1). These surveys commenced in the 1999–2000 malaria-transmission season and were repeated regularly.7 Baseline P. falciparum prevalence was 63.5% in Zone 1, 88% in Zone 1A, and 76% in Zone 2A.7 Capillary blood samples were blotted on filter paper [Whatman filter paper No. 1; Merck Laboratory Supplies (Pty) Ltd., Durban, South Africa] and individually stored at room temperature in zip-lock packets containing desiccant. Ethical approval for this study was obtained from South African Medical Research Council and the Maputo Province Directorate of Health, Mozambique. Blood samples were only taken after full consent from a parent/guardian had been obtained.
Sample preparation and analysis
Parasite DNA was extracted using the Chelex method18 from the blood spots of participants found to be rapid-test positive (ICT, Global Diagnostics, Cape Town, South Africa, and Kat Quick, Kat Medical, Johannesburg, South Africa, which detect P. falciparum histidine-rich protein 2). Once a sample was confirmed as P. falciparum positive by nested PCR,19 polymorphism analyses of dhfr and dhps genes were conducted. Primers, PCR amplification conditions, and restriction endonucleases used to detect polymorphisms in the dhfr (codons 51, 59, 108, and 164) and dhps (codons 436, 437, 540, and 581) genes have been described previously.20,21 Digestion products separated on a 2% agarose gel using electrophoresis were visualized and photographed using a MiniBIS documentation system (Bio-Systematica, Ceredigion, Wales, UK). Codons were classified as either pure sensitive, pure mutant, or mixed (both mutant and sensitive genotypes present in an individual sample). Genotyping was run in duplicate, with a third assay being performed on any discordant results.
Prevalence of mutant genotypes
When overall prevalence of infections with mutant genotypes was calculated, codons with mixed genotypes were grouped with pure mutant codons.
Frequency of mutant genotypes
For mutant allele frequency calculations, the mixed codons were reclassified as either sensitive or mutant, depending on which allele was more dominant (visually darker band on the gel). When the two alleles were equally dominant (similar visual intensities on the gel), the sample was excluded from the frequency (but not prevalence) analyses as the actual haplotype could not be determined.
Statistical analysis
Statistical analysis was preformed using Stata 9.0 (Stata Corp., College Station, TX). Univariate analysis and multiple variable logistic regression were carried out to determine whether any of the prospectively defined factors (namely, age, gender, fever, asexual parasite prevalence at the given sentinel site, zone, year) were significantly associated with mutation frequency and prevalence. Statistical inference took account of within-sentinel site correlations of mutational markers. Confidence limits were set at 95%.
RESULTS
A total of 6,179 blood samples were collected over the study period, 2,175 (35%) of which were rapid-test positive for P. falciparum. DNA was extracted from 1,215 randomly selected rapid-test positive samples collected in 1999, 2001, 2003, and 2004, 1,114 (92%) of which were confirmed to be P. falciparum positive by PCR. The falciparum-positive samples were taken from children with a median age of 7 (IQR 4–10) years, 48.4% were female and 4.9% were febrile (axillary temperature ≥ 37.5°C). Mixed genotypes were detected in 243 of 1,114 (21.8%) samples. Of the samples with mixed genotypes, 194 (79.8%) could not be haplotyped.
The dhfr 164 mutation was not found in any of the samples analyzed. The dhps 436 and 581 mutations were rare, with prevalence of 0.01% and < 0.001%, respectively.
Baseline prevalence of the dhfr triple mutation differed markedly across the 3 zones (47.5% in Zone 1, 9% in Zone 1A, and 18.4% in Zone 2A). The greatest variation in sentinel site dhfr triple prevalence was detected in Zone 1, where the prevalence ranged from 5% to 65%. The prevalence of the dhfr triple mutation across all 3 zones increased from 26% in 1999 to 96% in 2003 (OR: 77.3; 95% CI: 29.4–202.9; P < 0.0001), with prevalences remaining essentially unchanged thereafter (Figure 2A).
Figure 2.
Prevalence of dhfr triple (A), dhps double (B) and quintuple (C) mutations by study zone and year.
Baseline prevalence of the dhps double mutant was relatively low across all 3 zones (11% in Zone 1, 5% in Zone 1A, and 11% in Zone 2A), with little variation in prevalence between sentinel sites. Prevalence of the dhps double haplotype increased significantly between 1999 and 2001, from 9.1% to 36.3% (OR: 5.70; 95% CI: 2.84–11.43; P < 0.0001; Figure 2B). Similarly, the prevalence of parasites with both the dhfr triple and dhps double mutation, sometimes referred to as the “quintuple” genotype, increased from 4% in 1999 to 28% in 2001 (OR: 5.73; 95% CI: 2.12–15.50; P = 0.001; Figure 2C). However, unlike the triple dhfr mutation, there had been a decline in the prevalence of the dhps double (OR: 0.22; 95% CI: 0.08–0.59; P = 0.004) and the quintuple mutants (OR: 0.33; 95% CI: 0.13–0.83; P = 0.021) between 2001 and 2004 (Figures 2B and 2C, respectively).
Allelic frequencies of the dhfr triple and dhps double haplotypes and quintuple genotype mirrored the trends observed in the prevalence analyses. These frequencies are reported by zone and year in Table 1.
Table 1.
dhfr triple and dhps double and quintuple mutation frequency, by zone and year
| Mutant type | Year | Total | Zone 1 | Zone 1A | Zone 2A | OR | 95% CI | P value |
|---|---|---|---|---|---|---|---|---|
| 1999 | 0.26 (61/238) | 0.48 (40/84) | 0.09 (7/78) | 0.18 (14/76) | 1 | |||
| 2001 | 0.62 (197/320) | 0.59 (102/174) | 0.69 (79/115) | 0.52 (16/31) | 4.65 | 1.69–12.81 | 0.005 | |
| 2003 | 0.96 (213/221) | 0.96 (49/51) | 0.97 (105/108) | 0.95 (59/62) | 77.36 | 29.42–202.89 | 0.000 | |
| dhfr triple | 2004 | 0.85 (175/206) | 0.89 (47/53) | 0.80 (87/109) | 0.93 (41/44) | 16.38 | 7.04–38.11 | 0.000 |
|
| ||||||||
| 1999 | 0.07 (16/237) | 0.06 (5/83) | 0.05 (4/78) | 0.09 (7/76) | 1 | |||
| 2001 | 0.22 (80/369) | 0.19 (35/186) | 0.25 (38/151) | 0.22 (7/32) | 3.82 | 2.19–6.670 | 0.000 | |
| 2003 | 0.06 (13/212) | 0.04 (2/50) | 0.08 (8/105) | 0.05 (3/57) | 0.90 | 0.42–191 | 0.780 | |
| dhps double | 2004 | 0.07 (14/217) | 0.05 (3/56) | 0.09 (10/117) | 0.02 (1/44) | 0.95 | 0.33–2.71 | 0.925 |
|
| ||||||||
| 1999 | 0.04 (9/237) | 0.06 (5/83) | 0.03 (2/78) | 0.03 (2/76) | 1 | |||
| 2001 | 0.18 (47/256) | 0.14 (19/137) | 0.26 (25/96) | 0.13 (3/24) | 5.69 | 2.41–13.42 | 0.000 | |
| 2003 | 0.06 (13/212) | 0.04 (2/50) | 0.08 (8/105) | 0.05 (3/57) | 1.66 | 0.68–4.03 | 0.254 | |
| SP quintuple | 2004 | 0.05 (13/201) | 0.06 (3/53) | 0.09 (9/106) | 0.02 (1/42) | 1.75 | 0.71–4.34 | 0.216 |
Univariate analysis showed that the occurrence of the dhfr triple mutant allele was negatively associated with site specific asexual parasite prevalence (OR: 0.96 per % change in site asexual parasite prevalence; 95% CI: 0.93–0.98; P = 0.001) and age (OR: 0.92 per year of age; 95% CI: 0.88–0.97; P = 0.004). After adjusting for survey year, multiple logistic regression analysis was able to confirm that the dhfr triple mutation prevalence was independently negatively associated with age (OR: 0.92; 95% CI: 0.88–0.96; P = 0.001) but not with the asexual-stage parasite prevalence of a sentinel site.
The prevalence of the quintuple genotype was higher in children who were febrile (temperature ≥ 37.5°C; OR: 1.32; 95% CI: 1.03–1.68; P = 0.03) and decreased with increasing age (OR: 0.94 per year of age; 95% CI: 0.88–1.0; P = 0.048). None of the other predefined explanatory variables was found to be associated with the prevalence of the double dhps or quintuple mutations. Results were similar when mixed genotype infections were excluded and allelic frequencies rather than prevalence were analyzed.
DISCUSSION
Despite high levels of therapeutic failure associated with chloroquine resistance, Mozambique continued to use chloroquine as first-line treatment and SP as the second-line treatment until 2004.22 Given this limited SP use within southern Mozambique, it is not surprising that the prevalence of dhfr and dhps mutations was relatively low in 1999. However, by 2001 all markers associated with SP resistance had increased markedly in southern Mozambique. This is most likely a consequence of increased SP drug pressure within the region. Neighboring KZN experienced a severe SP-resistant malaria epidemic peaking in 2000, when 89% of patients with uncomplicated malaria failed SP treatment within 42 days.5,10 In 1999, the population-wide frequency of the dhfr triple and dhps double mutants in northern KZN was 0.38 and 0.15, respectively.16 However, among symptomatic malaria cases during the same year, frequencies were considerably higher; dhfr triple mutant allele was 0.62, and dhps double mutant was 0.47.16
This reduced SP efficacy resulted in the KZN Ministry of Health becoming the first in Africa to implement an artemisinin-based combination therapy (ACT) drug policy when artemether–lumefantrine replaced SP as the first-line antimalarial in January 2001.5 After this policy change in KZN, prevalences of both the dhps double and quintuple mutations in southern Mozambique began to decline, reaching baseline values by 2004. An in vivo SP therapeutic efficacy study with a 42-day follow-up period conducted in Zone 1 in 2003 found adequate clinical and parasitological response in 87.7% of the patients with uncomplicated malaria (K. I. Barnes, unpublished data). However the prevalence of the dhps double in symptomatic children in a neighboring district, in 200423 was higher than the levels observed in this study. Other than the change in malaria treatment policy in KZN, there were no major changes in SP drug use in the region that could explain the significant increase and then decrease in the dhps double and quintuple genotypes that we observed.
It seems probable that once the high SP drug pressure was removed, sulfadoxine-sensitive parasites began to re-emerge in southern Mozambique. This observation supports the view that the mutant allele carries some fitness cost which is disadvantageous in the absence of widespread drug use.24 Although this has not been observed before in the context of the dhps double haplotype with decreased sulfadoxine use, it appears to be the case for the Pfcrt mutation when chloroquine use is diminished.25
In contrast, the dhfr triple mutation prevalence did not decline once SP drug pressure from KZN was markedly reduced. Instead the mutation continued to increase almost to fixation within the population, a finding seen previously in Mozambique.23 There are 3 possible explanations for this. Firstly, dhfr triple mutants may have undergone compensatory mutations which minimized the reduced fitness effect of the dhfr mutations,26 thereby allowing the mutants to thrive in the absence of SP drug pressure, a trend observed in South East Asia.27 Secondly, there may have been insufficient sensitive parasites remaining in the population for a recovery once the drug pressure is removed. Our data show that sensitive parasites were uncommon in the study area after 2001 (prevalence of 3% in 2003 and 8% in 2004). They were predominantly found in older age groups, who may be less likely to receive treatment once partial immunity is acquired. Thirdly, the use of antifolate–sulfonamide combinations, like Co-trimoxazole, as prophylaxis against opportunistic infections in HIV/AIDS patients,15,28 may have contributed to the selection for the dhfr triple mutant. In such a scenario, we would expect to see a similar increase in dhps double mutants, a trend not observed in this study.
The presence of the dhfr triple haplotype results in more than a 1,000-fold increase in the pyrimethamine IC50 in vitro.29 Given the high frequency of the dhfr triple mutant in southern Mozambique, it would seem that SP therapeutic efficacy is primarily being provided by sulfadoxine, although pyrimethamine may still be exerting some synergistic benefit. The therapeutic efficacy of sulfonamide monotherapy has previously been shown.30
A policy shift to ACTs is widely advocated as it is expected that 2 drugs with differing modes of action would increase therapeutic efficacy, and the artemisinins specifically would decrease malaria transmission, thus reducing the risk of resistance spreading.31 Phased implementation of the artemisinin-based combination, artesunate plus SP, began in LSDI districts in southern Mozambique in 2004. This ACT has since become the Mozambican national malaria treatment policy.
Unfortunately, the benefits of combination therapy are negated by inclusion of an ineffective partner drug.32 At present, there is no direct evidence that the addition of artesunate to SP, even when SP remains reasonably effective, could avert the spread of SP resistance. Resistance to SP spreads particularly rapidly, mainly due to 2 factors. Firstly, the selection pressure exerted by SP’s relatively long elimination half-life33 and secondly, patients carrying resistance parasites tend to have enhanced gametocyte carriage and are more infectious to mosquitoes than patients with wild type parasites.34,35 Close surveillance of sulfadoxine resistance markers and the highly pyrimethamine (and dapsone)-resistant 164 dhfr mutation is essential now that artesunate plus SP is being widely deployed in the region, to ensure that artesunate does not in effect become a monotherapy.
Results from this study highlight the importance of considering the regional impact of neighboring countries’ drug policies. Although never first-line treatment in Mozambique, SP use in KZN has strongly influenced SP-resistance marker prevalence in southern Mozambique, with most P. falciparum isolates now carrying the dhfr triple mutation. Although dhps mutations are currently uncommon, increased SP drug pressure is likely to rapidly select for sulfadoxine-resistant parasites. This has far-reaching consequences when considering new drug policies involving SP and related antifolates such as chlorproguanil–dapsone. Ongoing monitoring of dhfr and dhps mutation prevalence and the therapeutic efficacy of the SP-containing combination would be imperative to ensure effective treatment policies in the region.
Acknowledgments
The authors thank the Global Fund for AIDS, Tuberculosis and Malaria, The South African Business Trust, and South African Department of Health for financial support without which this project would not have been possible, Ms. Natashia Morris for GIS support, members of the Database section of the Malaria Lead Programme, particularly Mr. Dayalan Govender, for assistance with database management, and 2 anonymous reviewers for their constructive comments.
Financial support: The Global Fund for Aids, Tuberculosis and Malaria (grants MAF-202-GO1-M-00 and MAF-202-GO2-M-00); The South African Business Trust; and The South African Department of Health.
Contributor Information
Jaishree Raman, Malaria Lead Programme, Medical Research Council, 491 Ridge Road, Durban, 4067, KwaZulu-Natal, South Africa, Telephone: +27 31 203 4782, Fax: +27 31 203 4704, jaishree.raman@mrc.ac.za..
Immo Kleinschmidt, London School of Hygiene and Tropical Medicine, Keppel Street, London, WC1E 7HT, England, Telephone: +44 207 927 2103, Fax: +44 207 636 8739, immo.kleinschmidt@lshtm.ac.uk..
Cally Roper, Department of Infectious and Tropical Diseases, London School of Hygiene and Tropical Medicine, Keppel Street, London, WC1E 7HT, England, Telephone: +44 207 927 2331, Fax: +44 207 636 8739; cally.roper@lshtm.ac.uk..
Elizabeth Streat, Maputo Province Directorate of Health, Av. Gov. Raimundo Bila 438, Matola City, Maputo Province, Mozambique, Telephone: +258 2172 2929, Fax: +258 2172 2929, lsdi_coord@tvcabo.co.mz..
Val Kelly, Malaria Lead Programme, Medical Research Council, 491 Ridge Road, Durban, 4067, KwaZulu-Natal, South Africa, Telephone: +27 31 203 4782, Fax: +27 31 203 4704, val.kelly@mrc.ac.za.
Karen I. Barnes, Division of Clinical Pharmacology, Department of Medicine, University of Cape Town, Groote Schuur Hospital, Old Main Road, Cape Town, 7707, Western Cape, South Africa, Telephone: +27 21 406 6294, Fax: +27 21 406 6759, karen.barnes@uct.ac.za.
REFERENCES
- 1.Trape JF, Pison G, Preziosi MP, Enel C, Desgrees du Lou A, Delaunay V, Samb B, Lagarde E, Molez JF, Simondon F. Impact of chloroquine resistance on malaria mortality. CR Acad Sci III. 1998;321:689–697. doi: 10.1016/s0764-4469(98)80009-7. [DOI] [PubMed] [Google Scholar]
- 2.Snow RW, Trape JF, Marsh K. The past, present and future of childhood malaria mortality in Africa. Trends Parasitol. 2001;17:593–597. doi: 10.1016/s1471-4922(01)02031-1. [DOI] [PubMed] [Google Scholar]
- 3.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415:680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 4.Barat LM. Four malaria success stories: how malaria burden was successfully reduced in Brazil, Eritrea, India and Vietnam. Am J Trop Med Hyg. 2006;74:12–16. [PubMed] [Google Scholar]
- 5.Barnes KI, Durrheim DN, Little F, Jackson A, Mehta U, Dlamini SS, Tsoka J, Bredenkamp B, Mthembu DJ, White NJ, Sharp BL. Effect of artemether–lumefantrine policy and improved vector control on malaria burden in Kwazulu-Natal, South Africa. PloS Med. 2005;2:e330. doi: 10.1371/journal.pmed.0020330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mabaso MLH, Sharp B, Lengeler C. Historical review of malarial control in southern Africa with emphasis on the use of indoor residual house-spraying. Trop Med Int Health. 2004;9:846–856. doi: 10.1111/j.1365-3156.2004.01263.x. [DOI] [PubMed] [Google Scholar]
- 7.Sharp BL, Kleinschmidt I, Streat E, Maharaj R, Barnes KI, Durrheim DN, Ridl FC, Morris N, Seocharan I, Kunene S, La Grange JJ, Mtembu JD, Maartens F, Martin CL, Barreto A. Seven years of regional malaria control collaboration—Mozambique, South Africa and Swaziland. Am J Trop Med Hyg. 2007;76:42–47. [PMC free article] [PubMed] [Google Scholar]
- 8.Hansford CF. Chloroquine resistance in Plasmodium falciparum in KwaZulu, 1983–1988. S Afr Med J. 1989;76:546–547. [PubMed] [Google Scholar]
- 9.Freese JA, Sharp BL, Ngxongo SM, Markus MB. In vitro confirmation of chloroquine-resistant Plasmodium falciparum malaria in KwaZulu. S Afr Med J. 1988;74:576–578. [PubMed] [Google Scholar]
- 10.Bredenkamp B, Sharp B, Mthembu SD, Durrheim DN, Barnes KI. Failure of sulfadoxine–pyrimethamine in treating Plasmodium falciparum malaria in KwaZulu-Natal. S Afr Med J. 2001;91:970–971. [PubMed] [Google Scholar]
- 11.Peterson DS, Milhous WK, Wellems TE. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci USA. 1990;87:3018–3022. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Triglia T, Menting JG, Wilson C, Cowman AF. Mutations in dihydropteroate synthase are responsible for sulfone and sulfonamide resistance in Plasmodium falciparum. Proc Natl Acad Sci USA. 1997;94:13944–13949. doi: 10.1073/pnas.94.25.13944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorsey G, Njama D, Kamya MR, Cattamanchi A, Kyabayinze D, Staedke SG, Gasasira A, Rosenthal PJ. Sulfadoxine/pyrimethamine alone or with amodiaquine or artesunate for treatment of uncomplicated malaria: a longitudinal randomised trial. Lancet. 2002;360:2031–2038. doi: 10.1016/S0140-6736(02)12021-6. [DOI] [PubMed] [Google Scholar]
- 14.Mendez F, Munoz A, Carrasquilla G, Jurado D, Arevalo-Herrera M, Cortese JF, Plowe CV. Determinants of treatment response to sulfadoxine–pyrimethamine and subsequent transmission potential in falciparum malaria. Am J Epidemiol. 2002;156:230–238. doi: 10.1093/aje/kwf030. [DOI] [PubMed] [Google Scholar]
- 15.Happi CT, Gbotosho GO, Folarin OA, Akinboye DO, Yusuf BO, Ebong OO, Sowunmi A, Kyle DE, Milhous WK, Wirth DF, Oduola AMJ. Polymorphisms in Plasmodium falciparum dhfr and dhps genes and age related in vivo sulfadoxine–pyrimethamine resistance in malaria-infected patients from Nigeria. Acta Trop. 2005;95:183–193. doi: 10.1016/j.actatropica.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Roper C, Pearce R, Bredenkamp B, Gumede J, Drakeley C, Mosha F, Chandramohan D, Sharp B. Antifolate antimalarial resistance in southeast Africa: a population-based analysis. Lancet. 2003;361:1174–1181. doi: 10.1016/S0140-6736(03)12951-0. [DOI] [PubMed] [Google Scholar]
- 17.Roper C, Pearce R, Nair S, Sharp B, Nosten F, Anderson TJC. Intercontinental spread of pyrimethamine-resistant malaria. Science. 2004;305:1124. doi: 10.1126/science.1098876. [DOI] [PubMed] [Google Scholar]
- 18.Wooden J, Kyes S, Sibley CH. PCR and strain identification in Plasmodium falciparum. Parasitol Today. 1993;9:303–305. doi: 10.1016/0169-4758(93)90131-x. [DOI] [PubMed] [Google Scholar]
- 19.Snounou G, Viriyakosol S, Zhu XP, Jarra W, Pinheiro L, do Rosario VE, Thaithong S, Brown KN. High sensitivity to detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–320. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 20.Plowe CV, Cortese JF, Djimde A, Nwanyanwu OC, Watkins WM, Winstanley PA, Estrada-France JG, Mollinedo R, Avila JC, Cespedes JL, Carter D, Doumbo OK. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine–sulfadoxine use and resistance. J Infect Dis. 1997;176:1590–1596. doi: 10.1086/514159. [DOI] [PubMed] [Google Scholar]
- 21.Duraisingh MT, Curtis J, Warhurst DC. Plasmodium falciparum: detection of polymorphisms in the dihydrofolate reductase and dihydropteroate synthase genes by PCR and restriction digestion. Exp Parasitol. 1998;89:1–8. doi: 10.1006/expr.1998.4274. [DOI] [PubMed] [Google Scholar]
- 22.Abacassamo F, Enosse S, Aponte JJ, Gomez-Olive FX, Quinte L, Mabunda S, Barreto A, Magnussen P, Ronn AM, Thompson R, Alonso PL. Efficacy of chloroquine and combination therapy with artesunate in Mozambican children with non-complicated malaria. Trop Med Int Health. 2004;9:200–208. doi: 10.1046/j.1365-3156.2003.01182.x. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes N, Figueiredo P, do Rosario VE, Cravo P. Analysis of sulphadoxine–pyrimethamine resistance-conferring mutations of Plasmodium falciparum from Mozambique reveals the absence of the dihydrofolate reductase 164L mutant. Malaria J. 2007;6:1–4. doi: 10.1186/1475-2875-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walliker D, Hunt P, Babiker H. Fitness of drug-resistant malaria parasites. Acta Trop. 2005;94:251–259. doi: 10.1016/j.actatropica.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, Takala SL, Taylor TE, Plowe CV. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355:1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 26.Nair S, Williams JT, Brockman A, Paiphun L, Mayxay M, Newton PN, Guthman J-P, Smithuis FM, Hien TT, White NJ, Nosten F, Anderson TJC. A selective sweep driven by pyrimethamine treatment in SE Asian malaria parasites. Mol Biol Evol. 2003;20:1526–1536. doi: 10.1093/molbev/msg162. [DOI] [PubMed] [Google Scholar]
- 27.Nair S, Brockman A, Paiphum L, Nosten F, Anderson TJC. Rapid genotyping of loci involved in antifolate drug resistance in Plasmodium falciparum by primer extension. Int J Parasitol. 2002;32:852–858. doi: 10.1016/s0020-7519(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 28.White NJ. Antimalarial drug resistance. J Clin Invest. 2004;113:1084–1092. doi: 10.1172/JCI21682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins WM, Mberu EK, Winstanley PA, Plowe CV. The efficacy of antifolate antimalarial combinations in Africa: a predictive model based on pharmacodynamic and pharmacokinetic analyses. Parasitol Today. 1997;13:459–464. doi: 10.1016/s0169-4758(97)01124-1. [DOI] [PubMed] [Google Scholar]
- 30.Clyde DF. Antimalarial effect of diaphenylsulfone and three sulfonamides among semi-immune Africans. Am J Trop Med Hyg. 1968;16:7–10. doi: 10.4269/ajtmh.1967.16.7. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organisation Guidelines for the Treatment of Malaria. 2006. WHO/HTM/MAL/2006.1108.
- 32.Yeka A, Banek K, Bakyaita N, Staedke SG, Kamya MR, Talisuna A, Kironde F, Nsobya SL, Kilian A, Slater M, Reingold A, Rosenthal PJ, Wabwire-Mangen F, Dorsey G. Artemisinins versus nonartemisinin combination therapy for uncomplicated malaria: randomised clinical trials from four sites in Uganda. PloS Med. 2005;2:e190. doi: 10.1371/journal.pmed.0020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Watkins WM, Mosobo M. Treatment of Plasmodium falciparum malaria with pyrimethamine–sulfadoxine: selective pressure for resistance is a function of long elimination halflife. Trans R Soc Trop Med Hyg. 1993;87:75–78. doi: 10.1016/0035-9203(93)90431-o. [DOI] [PubMed] [Google Scholar]
- 34.Mabuza A, Govere J, La Grange K, Mngomezulu N, Allen E, Zitha A, Mbokazi F, Durrheim D, Barnes K. Therapeutic efficacy of sulfadoxine–pyrimethamine for Plasmodium falciparum malaria. S Afr Med J. 2005;95:346–349. [PubMed] [Google Scholar]
- 35.Barnes KI, White NJ. Population biology and antimalarial resistance: the transmission of antimalarial drug resistance in Plasmodium falciparum. Acta Trop. 2005;94:230–240. doi: 10.1016/j.actatropica.2005.04.014. [DOI] [PubMed] [Google Scholar]