Abstract
Objective
Examine efficacy of U.S. primary care pediatric obesity treatment recommendations, within two randomized trials.
Methods
Between November 2005 - September 2007, 182 families (children aged 4–9 years, body mass index (BMI) ≥ 85th percentile) were recruited for two separate trials and randomized within trial to a 6-month intervention. Each trial had one intervention that increased child growth monitoring frequency and feedback to families (GROWTH MONITORING). Each trial also had two interventions, combining GROWTH MONITORING with an 8-session, behavioral, parent-only intervention targeting two energy-balance behaviors (Trial 1: reducing snack foods and sugar sweetened beverages [DECREASE] and increasing fruits, vegetables, and low-fat dairy [INCREASE]; Trial 2: decreasing sugar sweetened beverages and increasing physical activity [TRADITIONAL] and increasing low-fat milk consumption and reducing TV watching [SUBSTITUTES]). Child ZBMI and energy intake were assessed at 0, 6, and 12 months.
Results
In both trials, main effects of time were found for ZBMI, which decreased at 6 and 12 months (p < 0.01). In Trial 1, ZBMI reduced from 0–6 months, which was maintained from 6–12 months (ΔZBMI 0–12 months = −0.12 ± 0.22). In Trial 2, ZBMI reduced from 0–6 and from 6–12 months (ΔZBMI 0–12 months = −0.16 ± 0.31). For energy intake, main effects of time were found in both trials and intake reduced from 0–6 months (p < 0.05), with Trial 1 reducing intake from 0–12 months (p < 0.05).
Conclusions
All interventions improved weight status. Future research should examine effectiveness and translatability of these approaches into primary care settings.
Keywords: Intervention, Growth Monitoring, Parent-only, Pediatric Obesity, Primary Care, Efficacy
Treatment for childhood obesity in a research setting has progressed in the past 25 years (1), with family-based behavioral treatment being the most consistently investigated intervention (1). A recent review of eight, family-based, pediatric obesity intervention trials conducted over the past 25 years in research settings describes an intervention that has predominantly targeted children aged 8 to 12 years, and has provided 6 months (16 to 20 sessions) of intensive behavioral treatment to parents and children, using a combined group and individual format for each session (1). Children were asked to reduce energy intake to 800 to 1200 kcals/day, and increase physical activity and/or decrease sedentary behaviors. This type of intervention has significantly improved weight status in participating children (1). Unfortunately, these highly effective, intensive, research interventions are likely not feasible for primary care settings (2).
In 1997, a U.S. Expert Committee developed recommendations for pediatric obesity treatment in primary care settings (3). The Committee acknowledged there was no intervention designed for a non-research setting that was known to be efficacious. General guidelines were provided, which included: 1) starting with children as young as 3 years of age; 2) monitoring growth outcomes regularly (i.e., monthly); 3) applying a family-based model; and 4) using behavior modification techniques to change the child’s eating and activity behaviors (3). The Committee also emphasized that two or three energy-balance behaviors should be targeted during treatment (3). Although these recommendations suggest using components from empirically supported treatment, it is unclear whether a less intensive treatment approach will be effective in enhancing weight control. These recommendations were updated in 2007 and were very similar to the previous general guidelines, particularly in regards to the use of a family-based model and behavior modification techniques (4). Determining the efficacy of such an approach, including which energy balance behaviors to target, is imperative.
Thus, this investigation examined the efficacy of the 1997 U.S. pediatric obesity treatment recommendations for primary care with two separate randomized trials (RTs) in children aged 4 to 9 years. Since the focus was on efficacy, not effectiveness, these interventions were delivered primarily in research settings to maximize treatment integrity. Both RTs had three interventions: one intervention focused on increasing child growth monitoring and providing feedback to families (GROWTH MONITORING); and two interventions that combined GROWTH MONITORING with a 6-month, behavioral, parent-only intervention that focused on two energy-balance behaviors ( decreasing sugar sweetened beverage and sweet and salty snack food intake [DECREASE] vs. increasing fruit, vegetable, and low-fat dairy intake [INCREASE]) (Trial 1) and decreasing sugar sweetened beverage intake and increasing physical activity [TRADITIONAL] vs. increasing low-fat milk intake and decreasing TV watching [SUBSTITUTE]) (Trial 2). It was hypothesized that the combined interventions would show greater improvements in child weight status.
Methods
Participants
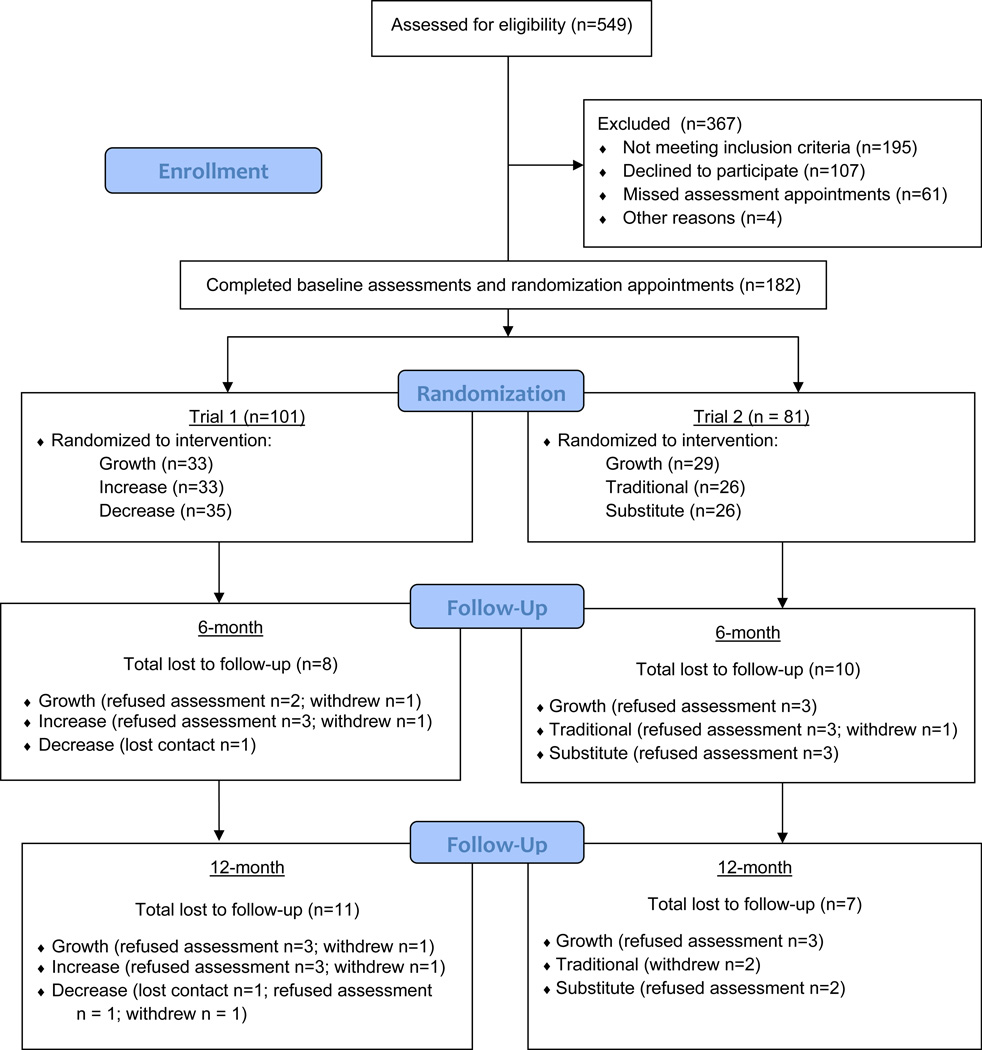
Between November 2005 and September 2007 continuous general recruitment for both trials occurred, with 549 families phone-screened. Families were referred by pediatrician/family physician or self-initiated contact and became aware of the trials through mailings, advertisements, community fairs, and posters and flyers at community centers and schools. Eligible families were assigned to the trial that was actively enrolling participants at the time the family made contact. Child eligibility criteria included: aged 4 to 9 years, ≥ 85th percentile for body mass index (BMI) as determined by the CDC growth charts (5), and having no dietary or physical activity restrictions. Families were ineligible if the participating parent could not read English, had a psychological disorder that would impair ability to participate, or if the family was planning to move out of the area during the program. Families chose which parent would participate in the trial. Of the 549 families phone-screened, 243 families consented/assented to participate and enrolled in the trials, with 182 families starting intervention (n = 101 in Trial 1; n = 81 in Trial 2) (see Figure 1 for participant flow). Participants were enrolled into the trials by research staff engaged in assessments.
Figure 1.
Participant Flow.
All participating families lived in Rhode Island, Connecticut, and Massachusetts at the time of enrollment. Both trials were approved by the Institutional Review Board at Rhode Island Hospital (Providence, RI).
Sample size calculations presumed 2-sided hypothesis testing at 6-month assessment, with type one error rate (alpha) equal to 0.05. To reject with 80% power the null hypothesis of no pre- to post-treatment difference between intervention conditions versus the alternative that the pre-to post-treatment difference is 0.6 or greater (effect size)(6), 24 participants per group were needed.
Study Design
Participants in each trial were randomly assigned to one of three interventions in a 1:1:1 allocation ratio. Using random permuted blocks within strata (gender), cards with intervention assignment were sealed in an envelope by research staff not engaged in intervention or assessments and provided to families at a randomization visit, following completion of baseline assessments.
Both trials had one intervention that focused on increasing child growth monitoring and providing feedback to families (GROWTH MONITORING). In this intervention, families received a monthly newsletter with information about healthy eating and leisure-time behaviors, and growth was assessed at 0, 3, and 6 months. Letters providing changes in height, weight, body mass index (BMI), BMI percentile (BMI-for-age percentile chart was also provided), and percent overweight along with interpretation of these changes were mailed to families and the child’s primary care physician at each growth assessment. Families were provided with research staff contact information and encouraged to contact research staff with any questions about information in the letter.
Each trial also had two additional interventions that combined GROWTH MONITORING with a behavioral, parent-only program. The additional interventions targeted two different energy-balance behaviors to examine which approach may be more efficacious in improving weight status. Trial 1 focused on eating behaviors. DECREASE used a restrictive approach, commonly used in pediatric weight control interventions, and reduced intake of non-nutrient-dense, energy-dense foods. As restricting foods may enhance liking of and preference for these foods (7), a second approach, INCREASE, that increased healthy foods was also investigated. Increasing intake of these foods may help shape food preferences for healthy foods in children (8), and lower the energy density of the diet (9).
In Trial 2, one eating and one leisure-time activity were targeted. TRADITIONAL focused on behaviors typically targeted in pediatric weight management programs, decrease sugar sweetened beverage intake and increase physical activity, while SUBSTITUTES, used a behavioral economics approach and changed substitute behaviors for sugar sweetened beverages (i.e., increase low-fat milk intake) and physical activity (i.e., decrease TV watching). According to behavioral economics theory, changing a substitute behavior of a target behavior enhances the feeling of choice for engaging in and liking the targeted behavior (10), which could increase long-term adherence (11).
Following the 6-month intervention, all families received feedback on growth at 9 months, and final assessments were conducted at 12 months. The primary dependent variable in both trials for the child was standardized BMI (ZBMI). Secondary outcomes were dietary intake, and leisure-time activity behaviors (Trial 2 only) assessed at 0, 6, and 12 months. Families received $20 for completing each of the 6- and 12-month assessments.
Behavioral Parent-only Interventions
The parent-only intervention was delivered in small groups and consisted of biweekly meetings for 2 months, and then monthly meetings for months 3 to 6, for a total of 8 meetings, with each meeting lasting 45 minutes. Most (93%) meetings were held in a medical-school research setting and the remainder in a group room in a primary care setting. Meetings were led by an experienced research-staff therapist (either master or doctoral-level) with expertise in nutrition or exercise science, and behavior modification. Sessions covered behavioral lessons and emphasized monitoring of targeted behaviors, pre-planning, problem-solving, shaping, setting goals, positive reinforcement, stimulus control, and parental modeling of targeted behaviors (12). These behavioral strategies are endorsed in both the 1997 and 2007 recommendations (3, 4). Children and their parents self-monitored the targeted behaviors and turned in records at each meeting.
In Trial 1, children and parents in DECREASE reduced intake of sweet and salty snack foods (i.e., candy, cookies, ice cream, chips, nuts) to ≤ three servings/week, and sugar sweetened beverages (i.e., soda, Kool-aid, sweetened tea, non-100% fruit juice, sports drinks) to ≤ three servings/week. INCREASE was encouraged to consume two servings/day of whole fruit, three servings/day of vegetables, and two servings/day of low-fat dairy products (13). In Trial 2, TRADITIONAL encouraged children to reach 60 min/day (parents 30 min/day) of moderate-intensity physical activity most days of the week (14) and for children and parents to consume ≤ 3 servings of sugar sweetened beverages/week. SUBSTITUTES encouraged children and parents to watch ≤ two hours of TV/day (15) and to consume two servings of low-fat milk/day (13).
Measures
Dependent measures were collected in a medical-school research setting (97%) or primary care setting (3%), by trained research-staff blinded to treatment assignment. Measures were collected at 0, 6, and 12 months from both the child and the parent.
Demographic characteristics (baseline only)
Basic demographic information (e.g., child’s gender and age; parent’s gender, age, and education level) was obtained by self-report.
Weight, height, BMI, and ZBMI
Child’s and parent’s weight were assessed by a balance beam scale, and height was assessed using a stadiometer, using standard procedures (16) with participants wearing light clothing and no shoes. BMI was calculated with the following formula: BMI = weight in kg/height in m2. For children, standardized BMI (ZBMI) scores were calculated based upon the value of the 50th BMI percentile and the standard deviation of the age and sex appropriate sample from the Centers for Disease Control growth charts (5).
Dietary intake
Energy and targeted food group intake was assessed by 3-day food diaries (1 weekend day, 2 weekdays), completed for both the child and parent. Under the age of 8 years, children do not have the cognitive capabilities to self-report food intake (17), thus parents were asked to complete the diaries for their children. During the 3-day period, if the child was under the supervision/care of another adult, the parent was instructed to obtain information from this other adult about what the child consumed. Diaries were reviewed with families for completion. Nutrition data was analyzed using the Nutrition Data System Software for Research (NDS-R) developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, Minnesota.
Leisure-time activity
The Previous Day Physical Activity Recall (PD-PAR) (18) is a self-report questionnaire that assesses leisure-time activity in children and adolescents. The PD-PAR has an interrater reliability of .98, and correlates significantly with accelerometer and heart rate estimates of physical activity (18). The PD-PAR was administered on the same three days, (1 weekend day, 2 weekdays) as the food diary in Trial 2 for both the child and parent. As with the food diary, parents were asked to complete the PD-PAR for their children. PD-PARs were reviewed with families for completion. Dependent variables included percent of time engaged in moderate-to-vigorous physical activity, mean daily metabolic equivalent (MET) values as determined from the Compendium of Physical Activities (19), and mean daily hours spent watching TV.
Compliance and Retention at Follow-up
Attendance rates for growth assessments at 3, 6, and 9 months were calculated. Compliance for the parent-only interventions was calculated as the percentage of actual completed child diaries and actual sessions attended by parents. Retention rates were the percentage of families with anthropometric data at 6- and 12-month follow-ups.
Serious adverse effects
At each assessment, parents were asked to report any injury, allergy, or growth issue potentially related to the intervention.
Statistical Analyses
Analyses were conducted on each trial, and for child and parent measures, separately. Baseline group differences were examined using chi-square tests and analysis of variance (ANOVA) for nominal and interval/ratio data, respectively. Mixed-factor ANOVAs were conducted on dietary and leisure-time activity variables, and included children or parents with complete data on these variables.
The primary analysis for children examined group differences in ZBMI at 0, 6, and 12 months using a mixed-factor ANOVA. These analyses were conducted using an intention-to-treat analysis, with missing data filled using a multiple imputation strategy (20). Specifically, for each participant with a missing ZBMI value, five random variables from a normal distribution that has a mean equal to the baseline ZBMI and variance equal to the estimated variance for ZBMI of other participants at the time where ZBMI is missing. This process led to five complete datasets; each of which was analyzed using the ANOVA model, and effects were computed by averaging the appropriate regression coefficient across models. Significant outcomes of analyses were followed up with pair-wise comparisons using bonferroni corrections. The same analysis was conducted for parents, but used BMI as the primary dependent variable.
Compliance rates for attending the growth monitoring assessment, attending parenting sessions and turning in child monitoring records, and retention rates at follow-up assessments were analyzed with chi-square analyses. Analyses were conducted using SPSS 19.0 (SPSS, Inc). Alpha-level was set at 0.05.
Results
Trial 1
Baseline Characteristics
Children were 61.4% female, 86.1% white, 18.8% Hispanic, and 7.2 ± 1.7 years of age, with a ZBMI of 2.32 ± 0.64. Parents were 92.9% female, 90.9% white, 19.2% Hispanic, 78.8% married, 90.9% with some education beyond high school, and 38.1 ± 5.7 yrs of age, with a BMI of 33.4 ± 8.4 kg/m2 (82.8% were overweight/obese). No differences between conditions were found for baseline characteristics or anthropometrics (see Table 1).
Table 1.
Baseline Characteristics of Children and Parents in Trial 1 and Trial 2 (M ± SD)
| TRIAL 1 | TRIAL 2 | |||||
|---|---|---|---|---|---|---|
|
Growth Monitoring (n = 33) |
Decrease (n = 35) |
Increase (n = 33) |
Growth Monitoring (n = 29) |
Traditional (n = 26) |
Substitutes (n = 26) |
|
| Age (yrs) | 6.8 ± 1.8 | 7.2 ± 1.6 | 7.6 ± 1.6 | 6.7 ± 1.6 | 7.2 ± 1.5 | 7.4 ± 1.3 |
| Gender (M/F) | 13/20 | 13/22 | 13/20 | 12/17 | 9/17 | 11/15 |
| Race - White (%) | 90.9 | 80.0 | 87.9 | 93.1 | 84.6 | 92.3 |
| Ethnicity - Hispanic (%) | 21.2 | 20.0 | 15.2 | 13.8 | 11.5 | 7.7 |
| ZBMI | 2.45 ± 0.86 | 2.15 ± 0.44 | 2.34 ± 0.52 | 2.27 ± 0.71 | 2.25 ± 0.38 | 2.28 ± 0.67 |
| Parent | ||||||
| Age (yrs) | 38.0 ± 5.6 | 37.7 ± 6.7 | 38.7 ± 4.6 | 38.5 ± 5.1 | 37.8 ± 7.0 | 37.6 ± 5.2 |
| Gender (M/F) | 5/28 | 2/35 | 1/32 | 4/25 | 0/26 | 4/22 |
| Race - White (%) | 96.8 | 85.7 | 90.9 | 96.4 | 92.3 | 92.6 |
| Ethnicity - Hispanic (%) | 22.6 | 17.1 | 18.2 | 14.3 | 11.5 | 7.4 |
| Married (%) | 90.3 | 68.6 | 78.8 | 89.3 | 57.7 | 77.8 |
| Some education beyond high school (%) | 87.9 | 88.6 | 90.9 | 79.3 | 76.9 | 88.5 |
| BMI (kg/m2) | 34.6 ± 9.7 | 33.4 ± 8.3 | 32.2 ± 7.2 | 33.2 ± 9.1 | 30.5 ± 7.2 | 33.6 ± 8.5 |
| Overweight/obese (%) | 80.6 | 82.9 | 84.8 | 82.1 | 80.8 | 84.6 |
Note. M = male; F = female; ZBMI = standardized body mass index; BMI = body mass index.
Dietary Intake
Child and parent dietary intake for Trial 1 is shown in Table 2. There were no significant differences between the interventions at baseline in dietary intake for either the child or parent.
Table 2.
Child and Parent Dietary Intake in Trial 1 at 0, 6, and 12 months (M ± SD)
| CHILD | PARENT | |||||
|---|---|---|---|---|---|---|
|
Growth Monitoring (n = 24) |
Decrease (n = 26) |
Increase (n = 26) |
Growth Monitoring (n = 22) |
Decrease (n = 25) |
Increase (n = 25) |
|
| Fruit and Vegetables (servings/day) | ||||||
| 0 months | 2.0 ± 1.2a | 1.9 ± 1.3a | 2.1 ± 1.5a | 2.7 ± 1.5 | 3.5 ± 1.9 | 3.4 ± 1.6 |
| 6 months | 2.7 ± 2.5b | 2.3 ± 2.1b | 3.3 ± 2.2b | 3.5 ± 1.6 | 3.0 ± 2.1 | 3.5 ± 2.1 |
| 12 months | 2.3 ± 1.3a | 2.4 ± 1.6a | 3.1 ± 2.8a | 3.2 ± 2.5 | 3.4 ± 1.7 | 3.1 ± 1.8 |
| Low-fat Dairy (servings/day) | ||||||
| 0 months | 1.0 ± 0.7 | 0.7 ± 0.7 | 1.1 ± 0.7 | 0.1 ± 0.3 | 0.1 ± 0.1 | 0.2 ± 0.3 |
| 6 months | 0.8 ± 0.8 | 0.9 ± 1.1 | 1.1 ± 1.0 | 0.2 ± 0.3 | 0.0 ± 0.1 | 0.1 ± 0.3 |
| 12 months | 1.0 ± 0.9 | 0.8 ± 0.7 | 1.1 ± 0.8 | 0.1 ± 0.2 | 0.2 ± 0.3 | 0.2 ± 0.2 |
| Sweet and Salty Snack Foods (servings/day) | ||||||
| 0 months | 2.2 ± 1.6a | 2.0 ± 1.5a | 2.0 ± 1.5a | 2.2 ± 1.9a | 1.7 ± 1.6a | 1.6 ± 1.5a |
| 6 months | 1.4 ± 1.1b | 1.0 ± 0.8b | 1.7 ± 1.7b | 1.8 ± 1.5a,b | 1.1 ± 1.2a,b | 1.2 ± 1.4a,b |
| 12 months | 1.8 ± 1.3a | 1.3 ± 1.0a | 1.9 ± 1.9a | 1.7 ± 1.3b | 0.9 ± 1.0b | 1.2 ± 0.9b |
| Sugar Sweetened Beverages (servings/day) | ||||||
| 0 months | 0.9 ± 0.7a | 1.0 ± 1.3a | 0.4 ± 0.5a | 1.5 ± 2.0a | 1.0 ± 1.3a | 0.9 ± 1.1a |
| 6 months | 0.8 ± 0.9a | 0.4 ± 0.5b | 0.5 ± 0.7a | 1.1 ± 1.3b | 0.3 ± 0.4b | 0.8 ± 1.4b |
| 12 months | 0.8 ± 0.9a | 0.7 ± 0.9a | 0.8 ± 1.2a | 1.1 ± 1.6a | 0.6 ± 0.9a | 0.9 ± 1.5a |
| Energy (kcals/day) | ||||||
| 0 months | 1687 ± 488a | 1716 ± 524a | 1632 ± 400a | 1674 ± 445a | 1749 ± 478a | 1765 ± 562a |
| 6 months | 1534 ± 394b | 1395 ± 388b | 1595 ± 448b | 1531 ± 454b | 1545 ± 581b | 1575 ± 474b |
| 12 months | 1574 ± 377b | 1523 ± 363b | 1560 ± 459b | 1595 ± 392b | 1578 ± 488b | 1422 ± 470b |
Note. Values with different letters in superscripts are significantly (p < 0.05) different from each other. For example, values with a superscript of “a” are not significantly different from other values with a superscript of “a” but are significantly different from values with a superscript of “b;” values with a superscript of “ab” are not significantly different from values with a superscript of “a” or “b.”
Child
For fruit and vegetable and snack food intake, there was a main effect of time, with a significant (p < 0.01) increase in fruits and vegetable consumption from 0 to 6 months, while snack food intake significantly (p < 0.01) decreased from 0 to 6 months. There were no differences found between the interventions in change in fruit and vegetable, or snack food intake. For sugar sweetened beverages, an interaction of intervention x time was found, with DECREASE significantly (p < 0.01) reducing intake from 0 to 6 months. A main effect of time occurred for energy intake, with energy intake significantly (p < 0.05) decreasing from 0 to 6 months and 0 to 12 months. There were no differences found between the interventions in change in energy intake. No change in intake in low-fat dairy was found.
Parent
There was a significant main effect of time for snack food and sugar sweetened beverage intake. Snack food intake significantly (p < 0.05) decreased from 0 to 12 months and sugar sweetened beverage intake significantly (p < 0.05) decreased from 0 to 6 months. A main effect of time was also found for energy intake, with energy intake significantly (p < 0.01) decreasing from 0 to 6 months and 0 to 12 months. There were no differences found between the interventions in change in dietary intake. No change in intake in fruits and vegetables, and low-fat dairy was found.
Anthropometrics
Child
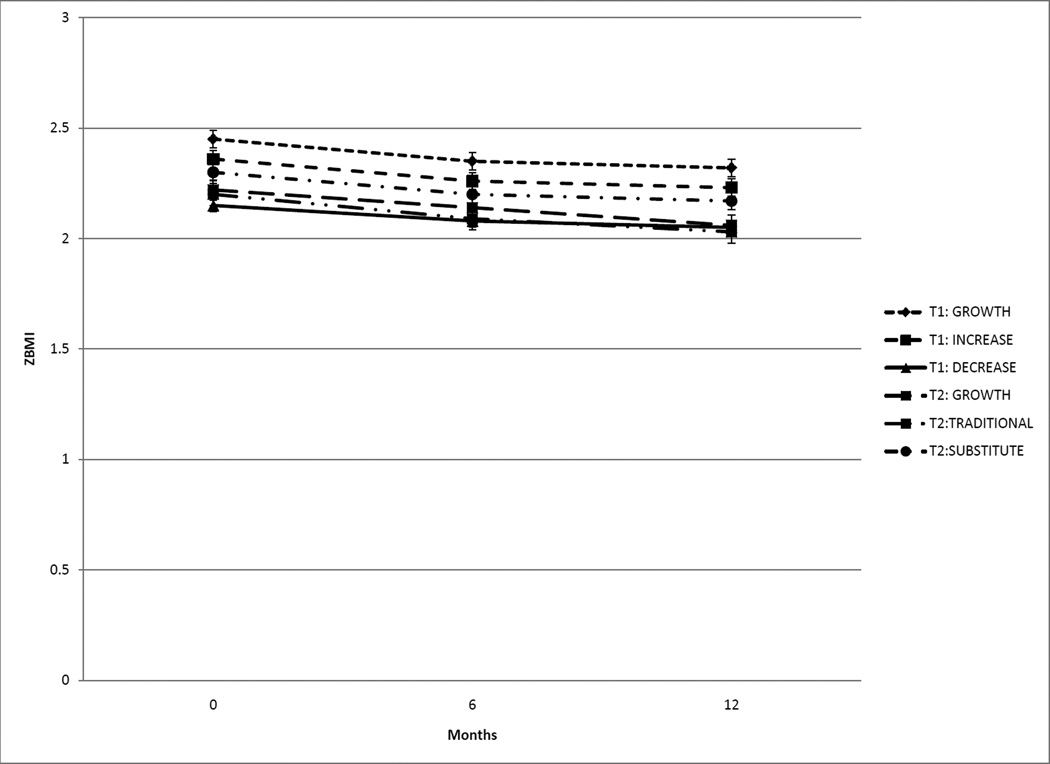
See Figure 2 for child anthropometric outcomes. There was no intervention x time interaction. A significant main effect of time was found, with baseline ZBMI significantly (p < 0.001) greater than 6- and 12-months. Mean reduction in ZBMI from 0 to 12 months was −0.12 ± 0.22. Effect sizes for change in ZBMI from 0 to 12 months were: GROWTH MONITORING = 0.17; INCREASE = 0.25; DECREASE = 0.24.
Figure 2.
In Trial 1 a significant (p < 0.001) reduction in ZBMI occurred from 0 to 6 months and 0 to 12 months, and Trial 2 had a significant (p < 0.01) reduction in ZBMI at each time point (M ± SD).
Parent
There were no changes in parent BMI in the trial.
Compliance and Retention at Follow-up
Attendance at growth monitoring appointments did not differ among the groups. Compliance with attendance (5.8 ± 2.5 sessions) and turning in monitoring dairies was 72.5%, with no intervention difference occurring. Retention at 6- and 12-month follow-up for ZBMI was 91.9% and 90.1%, respectively, with no intervention difference occurring.
Trial 2
Baseline Characteristics
Children were 60.5% female, 90.1% white, 11.1% Hispanic, and 7.1 ± 1.5 yrs of age, with a ZBMI of 2.27 ± 0.60. Parents in Trial 2 were 90.1% female, 93.8% white, 11.1% Hispanic, 75.3% married, 66.0% with some education beyond high school, and 38.0 ± 5.7 yrs of age, with a BMI of 32.4 ± 8.3 kg/m2 (82.5% were overweight/obese). No differences in baseline characteristics and anthropometrics were found (see Table 1).
Dietary Intake and Leisure-time Activity
Child and parent dietary intake and leisure-time activity for Trial 2 is shown in Table 3. There were no baseline differences in these measures between the interventions for both child and parent.
Table 3.
Child and Parent Dietary Intake and Leisure-time Behaviors in Trial 2 at 0, 6, and 12 months (M ± SD)
| CHILD | PARENT | |||||
|---|---|---|---|---|---|---|
|
Growth Monitoring (n = 24) |
Traditional (n = 17) |
Substitutes (n = 19) |
Growth Monitoring (n = 23) |
Traditional (n = 17) |
Substitutes (n = 19) |
|
| Sugar Sweetened Beverages (servings/day) | ||||||
| 0 months | 0.7 ± 0.8 | 1.0 ± 1.3 | 1.0 ± 0.9 | 1.3 ± 2.0 | 0.6 ± 0.8 | 2.8 ± 5.6 |
| 6 months | 0.7 ± 0.8 | 0.5 ± 0.8 | 0.8 ± 0.8 | 1.2 ± 1.4 | 1.3 ± 4.6 | 1.5 ± 1.5 |
| 12 months | 0.7 ± 0.9 | 0.4 ± 0.7 | 1.0 ± 1.1 | 1.2 ± 1.8 | 1.3 ± 4.2 | 1.5 ± 1.6 |
| Low-fat Milk (servings/day)a | ||||||
| 0 months | 0.7 ± 0.8 | 0.6 ± 0.6 | 0.8 ± 0.8 | 0.3 ± 0.4c | 0.5 ± 0.9c | 0.4 ± 0.5c |
| 6 months | 0.7 ± 0.8 | 0.7 ± 0.7 | 1.3 ± 0.7 | 0.4 ± 0.6c | 0.4 ± 0.7c | 0.9 ± 0.8d |
| 12 months | 0.6 ± 0.7 | 0.7 ± 0.6 | 1.2 ± 0.9 | 0.5 ± 1.0c | 0.2 ± 0.4c | 0.6 ± 0.5cd |
| Energy (kcals/day)b | ||||||
| 0 months | 1661 ± 434c | 1693 ± 502c | 1823 ± 416c | 1680 ± 467c | 1664 ± 622c | 2002 ± 526c |
| 6 months | 1598 ± 371d | 1346 ± 459d | 1791 ± 411d | 1577 ± 517d | 1250 ± 373d | 1769 ± 381d |
| 12 months | 1577 ± 411c | 1394 ± 413c | 1803 ± 414c | 1565 ± 484d | 1211 ± 298d | 1779 ± 301d |
| Time in Moderate-to-Vigorous Physical Activity (%) | ||||||
| 0 months | 18.9 ± 25.1 | 31.1 ± 80.3 | 9.8 ± 5.9 | 8.1 ± 11.7 | 15.0 ± 12.2 | 15.8 ± 17.0 |
| 6 months | 13.5 ± 8.9 | 19.3 ± 13.8 | 11.8 ± 6.5 | 14.3 ± 19.0 | 10.1 ± 13.1 | 11.7 ± 15.1 |
| 12 months | 13.9 ± 10.0 | 12.9 ± 10.6 | 10.4 ± 10.0 | 10.6 ± 12.2 | 9.8 ± 8.1 | 7.3 ± 12.0 |
| Daily MET | ||||||
| 0 months | 1.7 ± 0.5 | 1.6 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.4 | 1.8 ± 0.4 | 1.8 ± 0.4 |
| 6 months | 1.8 ± 0.4 | 2.0 ± 0.9 | 1.6 ± 0.2 | 1.7 ± 0.4 | 1.7 ± 0.4 | 1.7 ± 0.4 |
| 12 months | 1.8 ± 0.6 | 1.7 ± 0.4 | 1.6 ± 0.4 | 1.7 ± 0.3 | 1.6 ± 0.3 | 1.6 ± 0.3 |
| TV watching (hrs/day) | ||||||
| 0 months | 1.6 ± 1.5 | 2.0 ± 0.9 | 1.8 ± 0.9 | 1.2 ± 0.9 | 1.8 ± 1.4 | 1.7 ± 0.9 |
| 6 months | 1.5 ± 0.9 | 1.7 ± 1.7 | 1.8 ± 1.2 | 1.3 ± 1.0 | 1.2 ± 1.2 | 1.5 ± 0.9 |
| 12 months | 1.7 ± 1.1 | 1.3 ± 1.8 | 1.8 ± 1.2 | 1.5 ± 1.1 | 1.0 ± 1.1 | 1.2 ± 0.9 |
Note. MET = metabolic equivalent; values with different letters in superscripts are significantly (p < 0.05) different from each other. For example, values with a superscript of “a” are not significantly different from other values with a superscript of “a” but are significantly different from values with a superscript of “b;” values with a superscript of “ab” are not significantly different from values with a superscript of “a” or “b.”
For children, SUBSTITUTES consumed more low-fat milk than GROWTH MONITORING (p < 0.05);
For parents, TRADITIONAL was significantly lower than SUBSTITUTES (p < 0.01).
Child
A main effect of time occurred for energy intake, with energy intake significantly (p < 0.05) decreasing from 0 to 6 months, with no difference found between the interventions. A significant (p < 0.05) main effect of group was found for low-fat milk, with SUBSTITUTES consuming more low-fat milk than GROWTH MONITORING. No change in sugar sweetened beverage intake was found. There were no changes in any leisure-time variables found.
Parent
A main effect of time was occurred for energy intake, with energy intake significantly (p < 0.01) decreasing from 0 to 6 months, and 0 to 12 month. There were no differences found between the interventions in change in energy intake. A significant (p < 0.05) main effect of group was found for energy intake, with TRADITIONAL consuming less than SUBSTITUTES. For low-fat milk, an interaction of intervention x time was found, with SUBSTITUTES significantly (p < 0.01) increasing low-fat milk consumption from 0 to 6 month. No change in sugar sweetened beverage intake was found. There were no changes in any leisure-time variables found.
Anthropometrics
Child
See Figure 2 for child anthropometric outcomes. There was no intervention x time interaction. All time points were significantly (p < 0.01) different, and ZBMI decreased over time. Mean reduction in ZBMI from 0 to 12 months was −0.16 ± 0.31. Effect sizes for change in ZBMI from 0 to 12 months were: GROWTH MONITORING = 0.24; TRADITIONAL = 0.41; SUBSTITUTES = 0.21.
Parent
There were no changes in parent BMI in the trial.
Compliance and Retention at Follow-up
Attendance at growth monitoring appointments did not differ among the groups. Compliance with attendance (5.1 ± 2.6 sessions) and turning in weekly monitoring dairies was 64.2%, with no intervention difference occurring. Retention at 6- and 12-month follow-up for ZBMI were 87.6% and 91.4%, respectively, with no intervention difference occurring.
Serious Adverse Effects
No serious adverse effects were reported in either trial.
Discussion
These trials examined the efficacy of U.S. pediatric obesity treatment recommendations for primary care on reductions in ZBMI in children aged 4 to 9 years. Although these strategies are recommended for use in primary care, as their efficacy has not been examined, these trials were conducted in research settings with intervention delivered by trained research staff to increase the internal validity of the trials. Results indicated that in both trials, all interventions showed significant improvements in child weight status from 0 to 6 months that were either maintained from 6 to 12 months (Trial 1) or continued to improve from 6 to 12 months (Trial 2). Changes in weight status of the parents did not occur.
Additionally, participants in all groups in both trials showed improvements in dietary intake. In Trial 1, both children and parents reduced intake of snacks, sugar sweetened beverages, and energy. Children also increased intake of fruits and vegetables. In Trial 2, both children and parents reduced energy intake, and parents in the SUBSTITUTES intervention increased low-fat milk intake.
In both trials all interventions improved child weight status and decreased child energy intake over time, with no differences among the conditions. This finding suggests that the frequent monitoring of and feedback about weight status provided to families and physicians may have been the most active component of these interventions. There is strong support for the use of monitoring, a strategy commonly used in cognitive behavioral therapy, in interventions designed to improve health (21). Self-monitoring is believed to assist with increasing awareness of behaviors and provides feedback on achievement of goals (21). Monitoring weight status over time allows for responses in behaviors related to energy balance to occur to address changes in weight status. In adults, more frequent self-monitoring of weight is associated with improved weight gain prevention and weight loss (22), and successful weight loss maintenance (23). In these trials, the more frequent monitoring of growth with provided feedback may have influenced changes in dietary intake that assisted with reducing energy intake. Across the trials, the reduction in energy intake in the children was approximately 100 kcals/day, while it was about 200 kcals/day for the parents. The reduction in energy intake combined with the growth in height for the children appeared to improve weight status, but the reduction in energy intake was not sufficient to significantly improve weight status of the parents.
Surprisingly, the addition of the behavioral parent-only intervention did not strengthen outcomes in either trial. However, changes in ZBMI found in this investigation were similar to those found in other recent, less-intensive, family-based pediatric obesity interventions only targeting parents (24, 25). While a no-treatment control group was not included in either of the two trials in the current investigation, Janicke and colleagues and West and colleagues had waitlist control groups that had no improvements in ZBMI (24, 25). Although improvements in weight status were found in this investigation, in comparison to the more intensive family-based interventions, the improvements were not as great (1). This may be due to the decreased intensity of the present investigation’s interventions as compared to more intensive family-based interventions.
Trial 2 did not find changes in any of the leisure-time behaviors in either children or parents. While it is not clear why these behaviors did not change, reasons for the lack of change may be due to greater need for increased frequency of contact to change these specific behaviors, lack of perceived need to change these behaviors (i.e., TV watching was below recommended levels at baseline), and lower motivation for focusing on these behaviors (i.e., families may have been more motivated to make changes in the diet as compared to leisure-time activities).
Limitations of the study include the lack of a no-intervention control, the self-reported diet and physical activity data, the homogenous sample, enrollment limited to parents who could read English, and the short-term follow-up. Also, it is important to note that participating families predominantly had an overweight/obese parent, which is common in pediatric obesity intervention trials (1), as children with at least one overweight/obese parent are at greater risk for overweight/obesity (26). However, this higher prevalence of adult overweight/obesity indicates that these families may not be reflective of the general population. Data were not collected on how many visits families may have made to their child’s primary care physician and if the child’s growth was discussed at any of these visits. Finally, the sample size of each of the interventions was fairly small. Future research is needed to compare these interventions to an assessment with no feedback control group, using larger samples, and following families for a longer time. Importantly, as these trials were designed to test the efficacy of the recommendations, future trials are needed to examine the effectiveness and translatability of the recommendations being delivered in primary care settings.
In conclusion, this investigation found that the behavioral parent-only interventions did not differ in reductions in ZBMI from the GROWTH MONITORING conditions. However significant reductions in ZBMI were seen in all conditions in both trials. Whether this reduction in ZBMI was related to the influence of growth monitoring and feedback or secondary to participation in a clinical trial cannot be determined. Given that the effectiveness of the recommendations needs to be further tested, future research should examine the cost-effectiveness and sustainability of the approaches examined in these trials to determine how best to translate the recommendations into a primary care setting. Moreover, as this investigation was conducted in young children, future research is required to ascertain if these recommendations are efficacious in older children.
Acknowledgements
This research was supported by grants DK074919 from the National Institute of Diabetes and Digestive and Kidney Diseases and ADA 7-05-HFC-27 from the American Diabetes Association.
Footnotes
Conflict of Interest Statement: The authors have no conflicts of interest to declare.
Contributor Information
Hollie A. Raynor, Associate Professor, Department of Nutrition, University of Tennessee, 1215 W. Cumberland Avenue, JHB 341, Knoxville, TN 37996-1920, Tel: 865-974-6259, Fax: 865-974-3491, hraynor@utk.edu.
Kathrin M. Osterholt, Contact information during trial: Project Coordinator, The Weight Control and Diabetes Research Center, The Miriam Hospital, 196 Richmond Street, Providence, RI 02903, Tel. 401-793-8951, Fax. 401-793-8944, kosterholt@lifespan.org.
Chantelle N. Hart, Assistant Professor (Research), Psychiatry & Human behavior, The Weight Control and Diabetes Research Center, The Miriam Hospital/Brown Medical School, 196 Richmond Street, Providence, RI 02903, Tel. 401-793-9727, Fax: 401-793-8944, chart@lifespan.org.
Elissa Jelalian, Associate Professor (Research), Psychiatry & Human behavior, The Weight Control and Diabetes Research Center, The Miriam Hospital/Brown Medical School, 196 Richmond Street, Providence, RI 02903, Tel. 401-444-8945, Fax: 401-793-8944, jelalian@lifespan.org.
Patrick Vivier, Associate Professor, Community Health and Pediatrics, Brown Medical School Box G-S121, Providence, RI 02903 Tel: 401 863 2034Fax: 401 863-3533 Patrick_Vivier@Brown.EDU.
Rena R. Wing, Professor, Psychiatry & Human behavior, The Weight Control and Diabetes Research Center, The Miriam Hospital/Brown Medical School, 196 Richmond Street, Providence, RI 02903, Tel. 401-793-8959, Fax. 401-793-8944, rwing@lifespan.org.
References
- 1.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: Twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26:381–391. doi: 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stettler N. Comment: The global epidemic of childhood obesity: Is there a role for the pediatrician? Obesity Rev. 2004;5:1–3. doi: 10.1111/j.1467-789X.2004.00132.x. [DOI] [PubMed] [Google Scholar]
- 3.Barlow SE, Dietz WH. Obesity evlauation and treatment: Expert committee recommendations. Pediatrics. 1998;102:e29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed] [Google Scholar]
- 4.Spear BA, Barlow SE, Ervin C, et al. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120:S254–S288. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- 5.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM. CDC growth charts: United States. Hyattsville, MD: National Center for Health Statistics; 2000. [Google Scholar]
- 6.Epstein LH, Wing RR, Woodall K, Penner BC, Kress MJ, Koeske R. Effects of family-based behavioral treatment on obese 5-to 8-year-old children. Behavior Therapy. 1985;16:205–212. [Google Scholar]
- 7.Fisher JO, Birch LL. Restricting access to foods and children’s eating. Appetite. 1999;32:405–419. doi: 10.1006/appe.1999.0231. [DOI] [PubMed] [Google Scholar]
- 8.Birch LL. Development of food preferences. Ann Rev Nutr. 1999;19:41–62. doi: 10.1146/annurev.nutr.19.1.41. [DOI] [PubMed] [Google Scholar]
- 9.Rolls BJ. The relationship between dietary energy density and energy intake. Physiol Behav. 2009;97:609–615. doi: 10.1016/j.physbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein LH, Roemmich JN, Saad FG, Handley EA. The value of sedentary alternatives influences child physical activity choice. Int J Behavioral Med. 2004;11:236–242. doi: 10.1207/s15327558ijbm1104_7. [DOI] [PubMed] [Google Scholar]
- 11.Epstein LH, Valoski AM, Vara LS, et al. Effects of decreasing sedentary behavior and increasing activity on weight change in obese children. Health Psychol. 1995;14:109–115. doi: 10.1037//0278-6133.14.2.109. [DOI] [PubMed] [Google Scholar]
- 12.Epstein LH, Paluch RA, Kilanowski CK, Raynor HA. The effect of reinforcement or stimulus control to reduce sedentary behavior in the treatment of pediatric obesity. Health Psychol. 2004;23:71–380. doi: 10.1037/0278-6133.23.4.371. [DOI] [PubMed] [Google Scholar]
- 13.USDA. The food guide pyramid, vol. Washington, D.C: Home and Garden Bulletin Number 252; 1996. [Google Scholar]
- 14.Department of Health and Human Services US. Dietary Guidelines for Americans, 2005. 6th edition. Washington, DC: U.S. Government Printing Office; 2005. [Google Scholar]
- 15.American Academy of Pediatrics. Committee on Public Education: Media education. Pediatrics. 1999;104:341–343. [PubMed] [Google Scholar]
- 16.Lohman TR, Roche AF, Martorell R. Anthropometric Standardization Reference Manual. Champaign, Illinois: Human Kinetics Books; 1988. [Google Scholar]
- 17.Livingstone MB, Robson PJ. Measurement of dietary intake in children. Proc Nutr Soc. 2000;59:279–293. doi: 10.1017/s0029665100000318. [DOI] [PubMed] [Google Scholar]
- 18.Westin AT, Petosa R, Pate RR. Validation of an instrument for measurement of physical activity in youth. Med Sci Sports Exer. 1997;29:138–143. doi: 10.1097/00005768-199701000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: An update of activity codes and MET intensities. Med Sci Sports Exer. 2000;32:S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 20.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: Wiley & Sons; 1987. [Google Scholar]
- 21.Spahn JM, Reeves RS, Keim KS, et al. State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. J Amer Diet Assoc. 2010;110:879–891. doi: 10.1016/j.jada.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Annals of Behav Med. 2005;30:210–216. doi: 10.1207/s15324796abm3003_5. [DOI] [PubMed] [Google Scholar]
- 23.Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: A key component of successful weight loss maintenance. Obesity. 2007;15:3091–3096. doi: 10.1038/oby.2007.368. [DOI] [PubMed] [Google Scholar]
- 24.Janicke DM, Sallinen BJ, Perri MG, Lutes LD, Silverstein JH, Brumback B. Comparison of program costs for parent-only and family-based interventions for pediatric obesity in medically underserved rural settings. J Rural Health. 2009;25:326–330. doi: 10.1111/j.1748-0361.2009.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West F, Sanders MR, Cleghorn GJ, Davies PSW. Randomised clinical trial of a family-based lifestyle intervention for childhood obesity involving parents as the exclusive agents of change. Behav Res Ther. 2010;48:1170–1179. doi: 10.1016/j.brat.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. New Eng J Med. 1997;25:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]