Summary
SIRT1 is a NAD+-dependent protein deacetylase that governs many physiological pathways, including circadian rhythm in peripheral tissues. Here we show that SIRT1 in the brain governs central circadian control by activating transcription of the two major circadian regulators, BMAL1 and CLOCK. This activation itself comprises an amplifying circadian loop involving SIRT1, PGC-1α and Nampt. In aged wild type mice, SIRT1 levels in the suprachiasmatic nucleus are decreased, as are levels of BMAL1 and PER2, giving rise to a longer intrinsic period, a more disrupted activity pattern, and an inability to adapt to changes in the light entrainment schedule. Young mice lacking brain SIRT1 pheno-copy these aging-dependent circadian changes, while mice that over-express SIRT1 in brain are protected from the effects of aging. Our findings indicate that SIRT1 activates the central pacemaker to maintain robust circadian control in young animals, and a decay in this activity may play an important role in aging.
Introduction
In response to the daily, 24-hour light-dark cycle, living organisms have developed an evolutionarily conserved program that allows appropriate physiology and behavior to be coordinated with the environment. To achieve circadian regulation, genes exist in cells to generate an oscillating transcriptional network, which coordinates expression of pathways involved in metabolism and physiology (Asher and Schibler, 2011). In mammals, systemic circadian regulation is accomplished through the central oscillator in the suprachiasmatic nucleus (SCN) of the anterior hypothalamus. The SCN responds to light-dark cycles and coordinates rhythms of all aspects of circadian control, including locomotor activity, hormone secretion, body temperature maintenance, and feeding. Peripheral tissues, such as liver, use the same circadian oscillatory machinery. SCN-driven processes are important to phase-align peripheral tissues, but these tissues can also respond to feeding-fasting cycles (Welsh et al., 2010). Importantly, SCN implantation is able to restore circadian rhythms in SCN-lesioned animals, or in non-rhythmic genetic models (Lehman et al., 1987; Ralph et al., 1990; Sujino et al., 2003). The restored period is determined by the genotype of the SCN donor, not the SCN-lesioned host, underscoring the dominating role of the SCN in circadian physiology (Lehman et al., 1987; Ralph et al., 1990; Sujino et al., 2003).
The link of clock genes and circadian regulation to health has been recognized in many disease-associated fields including sleep disorders, diabetes and cancer. A number of pathologies can be triggered by circadian disruptions via genetic or environmental perturbations (Sahar and Sassone-Corsi, 2009; Takahashi et al., 2008), suggesting that proper maintenance of circadian control is crucial to maintain robust health.
The molecular mechanism for oscillation, in both SCN and peripheral tissues is generated by a transcriptional autoregulatory feedback loop (Bass and Takahashi, 2010; Dibner et al., 2010). The network involves the core transcriptional activators, BMAL1 and CLOCK, which form a heterodimer, to positively regulate the expression of target genes Cryptochrome (Cry1 and Cry2) and Period (Per1, Per2 and Per3). When PER and CRY proteins accumulate to critical levels, they translocate into the nucleus as dimers and repress transcription activity of CLOCK-BMAL1. Orphan nuclear receptors REV-ERBα/β and RORα proteins are also targets of CLOCK-BMAL1 and contribute to transcriptional control of the Bmal1, and Clock genes (Preitner et al., 2002; Sato et al., 2004). REV-ERBα and β act coordinately and shown to be crucial in sustaining circadian behavior and metabolism recently (Bugge et al., 2012; Cho et al., 2012). Post-translational events also regulate the molecular clock; i.e. the Skp1, Cullin1, F-box protein (SCF)/ β -TrCP ubiquitin ligase complex targets PER and CRY proteins for degradation (Lamia et al., 2009). These transcriptional and post-translational events ensure the fidelity of the circadian cycle.
The circadian machinery has recently been linked to the SIRT1 NAD-dependent deacetylase (Asher et al., 2008; Nakahata et al., 2008; Nakahata et al., 2009; Ramsey et al., 2009), illustrating one way in which circadian control is coupled to metabolism. SIRT1 is the mammalian homolog of yeast Sir2, an NAD-dependent protein deacetylase that is involved in aging, stress response, maintenance of genomic integrity and energy metabolism (Finkel et al., 2009). SIRT1 mediates the salutary metabolic effects of caloric restriction, and its activity is critical in the maintenance of health (Guarente, 2012). Besides histones, SIRT1 deacetylates a number of transcriptional regulatory proteins that govern major arms of metabolism; eg. FOXOs, LXR, PPARγ coactivator-1α (PGC-1α), HIF-1 α and NF-kβ. SIRT1 is also able to mitigate neurodegenerative diseases in mouse model systems, such as Alzheimer's, Huntington's and Parkinson's diseases (Donmez et al., 2012; Donmez et al., 2010; Jeong et al., 2012; Jiang et al., 2012), and was recently demonstrated to couple diet and metabolism to mood and behavior (Libert et al., 2011). SIRT1 can also regulate POMC and NF-Y neurons in the hypothalamus to regulate feeding behavior (Ramadori et al., 2011; Ramadori et al., 2010; Satoh et al., 2010). Thus its effects on the brain are pervasive.
It has been shown that SIRT1 in peripheral tissues like liver can influence circadian rhythm in a cell-autonomous fashion by multiple mechanisms. It can deacetylate BMAL1 to affect its activity (Nakahata et al., 2008) or PER2 to alter its stability (Asher et al., 2008). Interestingly, one of the metabolic output targets of CLOCK-BMAL1 is Nampt, an enzyme required for biosynthesis of the SIRT1 essential cofactor NAD+, which ensures the rhythmic accumulation of NAD+ and SIRT1 activity (Nakahata et al., 2009; Ramsey et al., 2009). Since all studies on SIRT1 regulation of circadian rhythm to date have been conducted in these cell-autonomous systems, we were interested in investigating a role of SIRT1 in central circadian control of physiology and behavior. Here we show that altering SIRT1 levels in the brain can exert moderate changes in the intrinsic circadian periods of mice. Moreover, SIRT1 appears to be at the center of aging-dependent decline in central circadian function. Our findings trace a novel circadian regulated loop in the brain involving Nampt, SIRT1 and PGC-1α, and may lead to strategies to treat aging-dependent decline in circadian function.
Results
Brain SIRT1 regulates the central circadian clock
We wished to test whether SIRT1 regulates the central clock in the brain and could thus alter circadian behaviors in vivo. It is known that the suprachiasmatic nucleus (SCN) at the anterior hypothalamus is the central circadian pacemaker in the brains of mammals (Bass and Takahashi, 2010; Welsh et al., 2010). Our strategy involves knocking out and overexpressing SIRT1 in the brain primarily using nestin-cre, because an SCN-specific system is not available. We cannot therefore rule out the possibility that some of the phenotypes we describe below may have contributions from brain regions besides the SCN. All experiments use C57BL/6 mice; wild type (WT), brain specific SIRT1 knockout (BSKO) (Cohen et al., 2009), and transgenic lines that over-express brain SIRT1 two-fold (Sir2d) (Bordone et al., 2007) or 10-fold (BSTG) (Firestein et al., 2008).
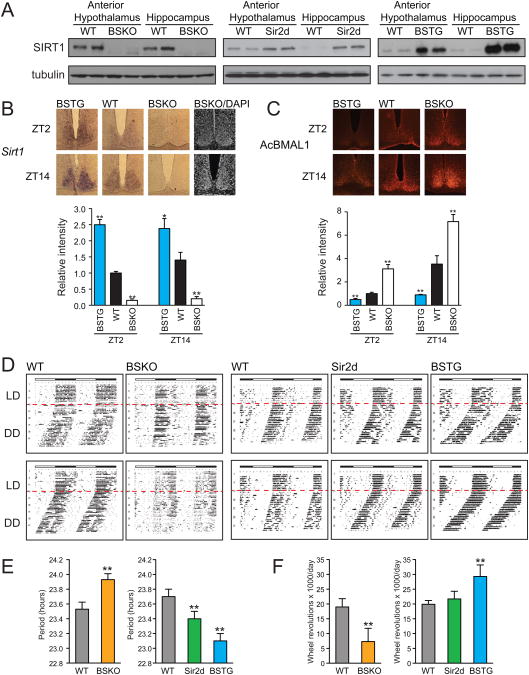
Western blots of punch out biopsies of the SCN region in the anterior hypothalamus showed SIRT1 protein was deleted in BSKO and appropriately over-expressed in transgenic mice (Figure 1A). RNA in situ hybridization of the SCN also showed that SIRT1 RNA was undetectable in SCN of BSKO mice and over-expressed in SCN of BSTG mice (Figure 1B). To determine whether SIRT1 activity in the SCN was affected in genetically altered mice, we carried out immunohistochemistry (IHC) using a specific antibody to acetylated Lys537 of BMAL1, a validated SIRT1 substrate in peripheral tissues (Nakahata et al., 2008). This assay revealed increased BMAL1 acetylation in SCN of BSKO mice compared to wild type and decreased acetylation in SCN of BSTG mice (Figure 1C), even though we later show that total BMAL1 protein levels are lower in BSKO SCN and higher in BSTG SCN.
Figure 1. Circadian phenotypes of brain specificSirt1KO and transgenic mice.
(A) Immunoblot of SIRT1 in the anterior hypothalamus and hippocampus from the brain specific Sirt1 KO (BSKO), Sirt1 whole body transgenic (Sir2d) and brain specific Sirt1 transgenic (BSTG) mice. Tubulin was probed as a loading control. (B) Typical in situ hybridization images and relative signal intensities for Sirt1 mRNA in the SCN. Sections were prepared from 3 month-old mice that were sacrificed at ZT2 and ZT14 (n≥3 mice/genotype). Signal intensities were quantitated and are shown relative to wild type (≥6 sections). DAPI stained images were shown to indicate SCN in the BSKO sections. (C) Typical immunohistochemical staining results and relative signal intensities for acetylated BMAL1-K537 in the SCN. Sections were prepared from 3 month-old mice that were sacrificed at ZT2 and ZT14 (n=3 mice/genotype). Signal intensities of protein levels were quantitated and are shown relative to wild type (6 sections). Values represent the mean ± standard deviation. *P<0.05; **P<0.01; t test. (D) Actograms showing wheel-running activity in constant darkness after two weeks of light/dark entrainment. Representative results of 6 month-old male BSKO (n=10) and the littermate wild-type control mice (n=10) are shown on the left panel. Sir2d (n=6) and BSTG (n=7) compared to wild-type control mice (n=7) are shown on the right. Red lines indicate the starting day for constant darkness. (E) Bar graphs of innate periods of animals during the initial three weeks in constant darkness. (F) Total wheel revolutions performed per day in animals. Values in (E) and (F) represent the mean ± standard deviation. **P<0.01; t test.
Activity assays for circadian period or actograms employed cohorts of 8-10 littermates, which were entrained on a 12 hour light/dark (LD) cycle, and then placed in an all dark (DD) environment for 30 days. Their intrinsic periods were revealed by monitoring free-running activity on running wheels, which normally would occur during the dark cycle. Actograms show that WT mice had intrinsic periods of 23.6 hours (Figure 1D and E), close to reported values for C57BL/6 mice (Valentinuzzi et al., 1997). In contrast, BSKO mice had an elongated period or 23.9 hours. We also scored the activity of animals, as indicated by the density of black ticks in the actograms, and found that BSKO showed reduced activity (Figure 1F), also indicated by the interrupted running pattern evident in the actograms.
We next tested the two SIRT1 over-expression strains and found effects that were reciprocal to the BSKO for both period and activity. The Sir2d mice had a period of 23.4 hours and the BSTG of 23.1 hours, compared to 23.7 for WT (Figure 1 D and E). In addition, the activity level of BSTG mice was significantly higher than WT, while the Sir2d mice showed a trend in that direction (Figure 1F). The fact that SIRT1 BSKO and over-expressing mice showed opposite effects on period and activity compared to WT, along with the dosage-dependency of the effects of two over-expressing strains, argue strongly that SIRT1 in the brain governs central circadian rhythm.
Brain SIRT1 governs aging-dependent decline in circadian function
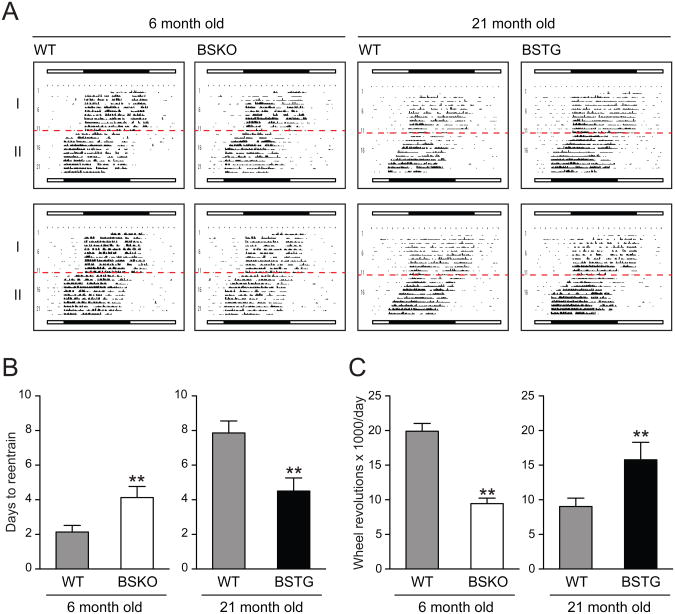
The increase in intrinsic period from 23.6 to 23.9 hours in our young BSKO mice was similar to what was reported in aged (22 months) WT C57BL/6 mice animals (Valentinuzzi et al., 1997), raising the possibility that like yeast Sir2p (Dang et al., 2009), SIRT1 function in the brain declines in aged mice. Critically, Valentinuzzi et al. also observed a decline in circadian function in aged mice, as measured by the ability to adapt to an abrupt change in the entrainment cycle. Young mice adapted to an abrupt advancement in the light cycle within 1-2 days, while aged mice took at least 8 days to adapt (Valentinuzzi et al., 1997). Thus, we tested young (6 months) or aged (21 months) WT, BSKO and BSTG mice in this so-called jet lag experiment by advancing the light entrainment abruptly by 4 hours and following adaptation (Figure 2). Like Valentinuzzi et al. (1997), we also found that aged WT mice took much longer to adapt than young WT mice. Strikingly, young BSKO partially pheno-copied aged WT mice, doubling the time required for re-entrainment (4.0 ± 0.6 d versus 2.1 ± 0.3 d) (Figure 2B). In 22 month-old BSKO mice, re-entrainment times were longer but the percentage difference in re-entrainment times between wild type and BSKO mice was reduced compared to young mice (Figure S2). More strikingly, we observed partial rescue of the ability to adapt in 21 month old BSTG mice compared to aged WT (4.0 ± 0.6 d versus 7.9 ± 0.3 d) (Figure 2B). This rescue was evident even in very old mice (30 months of age) (Figure S1). Old BSTG mice also showed rescue of the aging-dependent decline in activity observed in WT mice, while young BSKO again showed lower activity compared to young WT (Figure 2C). These findings indicate that loss of SIRT1 in the brain partially mimics the jet lag phenotype of old mice while SIRT1 over-expression rescues the defect. These findings are both consistent with the hypothesis that a loss of SIRT1 function in the brain is responsible for at least part of the decline in central circadian robustness in old animals.
Figure 2. Aging-induced deficit in re-entrainment is suppressed in brain specificSirt1transgenic mice.
(A) Jet lag experiment depicting actograms of 6 month-old young wild type (n=7) versus BSKO (n=7) (left) and 21 month-old wild type (n=6) versus BSTG (n=7) (right) subjected to a 4 hr phase advance. Red lines indicate the start of the new LD cycle. (B) Days required for re-entrainment of animals. (C) Total wheel revolutions per day of animals. Values in (B) and (C) represent the mean ± standard deviation. **P<0.01; t test. See also Figure S1 and S2
SIRT1 positively regulates circadian genes in SCN and declines with aging
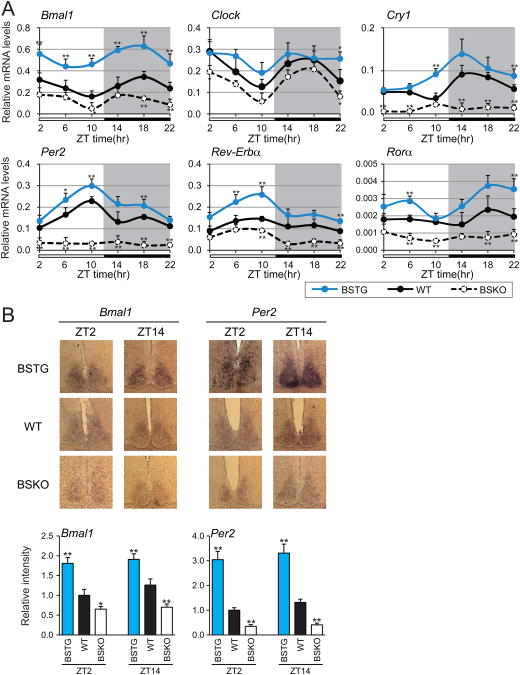
Since the SCN is the primary determinant of central circadian control in mammals (Bass and Takahashi, 2010; Welsh et al., 2010), we determined whether SIRT1 expression levels in the SCN could determine expression levels of circadian machinery components. Anterior hypothalamus samples that were enriched for SCN were collected at 4-hour time intervals. Quantitative real time PCR analysis of punch out SNC biopsies revealed that all circadian controlled genes tested, including the core transcription factor BMAL1 and CLOCK, were significantly down regulated in BSKO mice (Figure 3A). Conversely, expression of the circadian genes was markedly up-regulated in BSTG mice (Figure 3A). Expression of these genes varied over Zeitgebers (ZT) time was circadian. To confirm that the expression differences in the RT-PCR assays reflected expression specifically in the SCN, we carried out in situ hybridization of Bmal1 and Per2 mRNA in coronal brain slices through the SCN. Deletion of Sirt1 in BSKO mice resulted in a clear decrease in both Bmal1 and Per2 RNA levels in SCN, while SIRT1 over-expression in BSTG mice increased levels (Figure 3B). These findings are consistent with the idea that SIRT1 is a positive regulator of the Bmal1 and Clock genes in the SCN.
Figure 3. SIRT1 up-regulates circadian gene RNA levels in the SCN.
(A) Quantitative RT-PCR analysis of CLOCK-BMAL1 controlled genes. 6 month-old mice were sacrificed for brain samples at 4 hr intervals across the 12:12 light/dark cycle (n=3-4 mice/genotype/time point). Suprachiasmatic nucleus (SCN)-enriched anterior hypothalamus samples were later isolated for RNA preparation from frozen brain samples with needle punch collection. Target gene expression levels are shown relative to the ribosomal protein reference gene, rpl19. (B) Typical in situ hybridization images and relative signal intensities for Bmal1 and Per2 mRNA in the SCN. Sections were prepared from 3 month-old mice that were sacrificed at ZT2 and ZT14 (n≥3 mice/genotype). DAPI stained images were shown to indicate SCN in the BSKO sections. Signal intensities of protein levels were quantitated and are shown relative to wild type (≥ 6 sections). Values represent the mean ± standard deviation. *P<0.05; **P<0.01; t test.
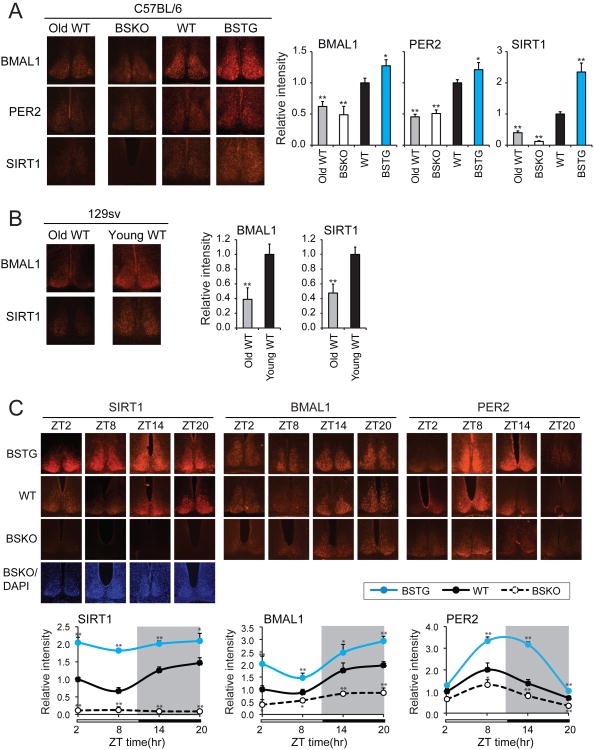
Having established a functional link between SIRT1 and circadian gene expression, we next addressed the possibility, raised above, that SIRT1 function declines in aged mice. Sections of brain were obtained by cryostat slicing to allow immunohistochemical staining in the SCN. In SCNs of WT C57BL/6 mice that were sacrificed at ZT4, a significant decline in SIRT1 staining was observed in 22 month-old mice compared to 5 month-olds (Figure 4A). Similarly, there was a large decline in expression of the circadian proteins BMAL1 and PER2, consistent with the conclusion above that SIRT1 is a positive regulator of the genes encoding these proteins. Since there appears to be some variation in circadian behavior among different mouse strains, we repeated this analysis in young and aged 129sv mice and made very similar observations (Figure 4B). To be certain these expression differences were not due to a circadian phase difference between genotypes, we repeated the experiment now sampling more time points around the circadian period (ZT2, ZT8, ZT14, and ZT20). Again, deletion of SIRT1 reduced BMAL1 and PER2 protein levels in SCN compared to WT, while over-expression of SIRT1 increased protein levels in a setting where we could observe the entire cycle of expression (Figure 4C).
Figure 4. SIRT1 up-regulates circadian proteins in the SCN and declines with aging.
(A) Typical immunohistochemical staining results and relative signal intensities for BMAL1, PER2 and SIRT1 proteins in the SCN. Sections were prepared from C57BL/6 background mice that were sacrificed at ZT4 (n≥3 mice/genotype). Aged WT mice (22 months) are compared to young BSKO, WT and BSTG mice (5 months). Signal intensities of protein levels were quantitated and are shown relative to wild type (≥ 6 sections). (B) Typical immunohistochemical staining images and relative signal intensities of BMAL1 and SIRT1 proteins in the SCN. Sections were prepared from aged (21 month) or young (4 month) 129 sv background mice that were sacrificed at ZT4 (n≥3). Signal intensities of protein levels were quantitated and are shown relative to young animals (≥ 6 sections). Values represent the mean ± standard deviation. *P<0.05; **P<0.01; t test. (C) Temporal analysis of BMAL1, PER2 and SIRT1 proteins in the SCN. SCN sections were prepared from 3 month-old mice that were sacrificed at indicated time (n≥3 mice/genotype/time point). DAPI stained images were shown to indicate SCN in the BSKO sections. Signal intensities of protein levels were quantitated and are shown relative to wild type (≥ 6 sections). Values represent the mean ± standard deviation. *P<0.05; **P<0.01; t test.
SIRT1 regulates circadian gene expression in neuronal cells
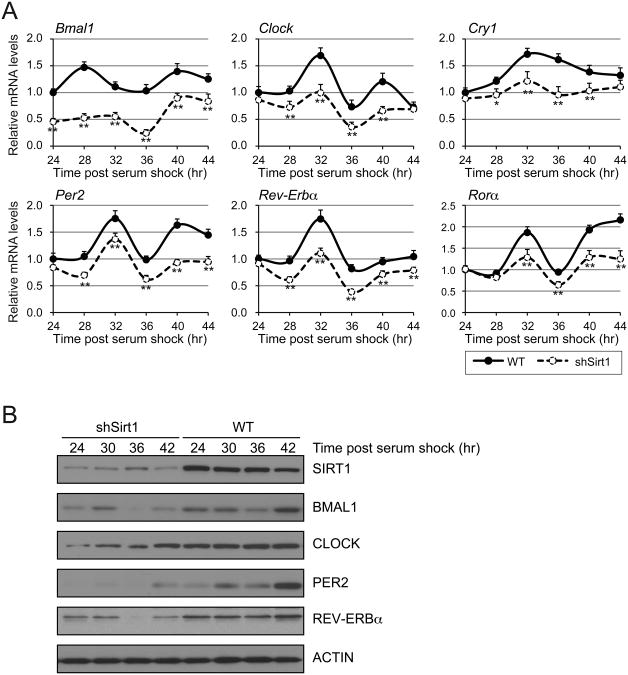
To study the mechanism of how SIRT1 regulates expression of BMAL1 and CLOCK in SCN, we employed N2a murine neuroblastoma cells, which are frequently used to support in vivo studies. Importantly, we found N2a cells can be synchronized by horse serum treatment for subsequent circadian studies; i.e. post horse serum shock, we found numerous circadian transcripts (Bmal1, Clock, Cry1, Per2, Rev-Erba, Rora) were regulated in a circadian manner (Figure 5A). We then examined the effect on circadian regulation of SIRT1 depletion by short hairpin RNA. Like in the SCN, SIRT1 depletion reduced levels of all of these transcripts. Protein analysis confirmed that SIRT1 was knocked down, and that levels of all the circadian proteins were also reduced (Figure 5B). These findings suggest that N2a cells faithfully recapitulate the regulation of circadian proteins by SIRT1 observed in vivo.
Figure 5. SIRT1 regulates endogenous circadian gene RNA and protein levels in neuroblastoma N2a cells.
(A) Quantitative RT-PCR analysis of clock controlled genes. Mouse N2a cells (WT) and Sirt1 shRNA knock down cells were synchronized with 50% horse serum and time points were taken at 4 hr intervals. Results are shown relative to WT after normalization to ribosomal reference gene, rpl19. Values represent the mean ± standard deviation. *P<0.05; **P<0.01; t test. (B) Immunoblots of SIRT1 and components of the circadian machinery. Time points were taken at 6 h intervals after serum shock.
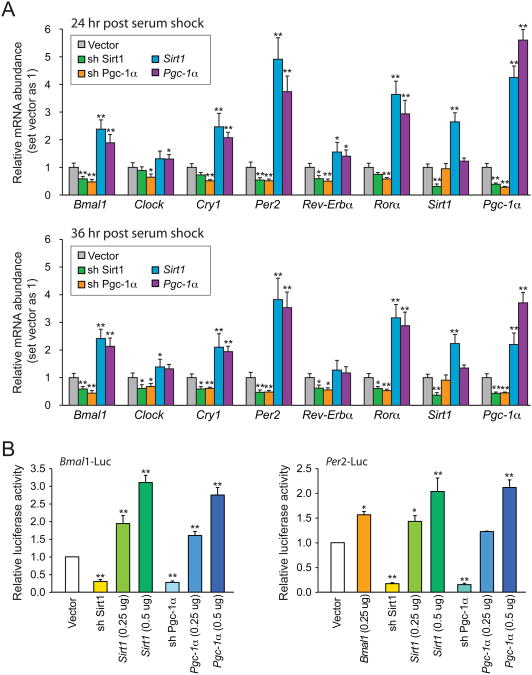
Next we began to search for the mechanism by which SIRT1 regulates circadian gene expression. Bmal1 transcription is positively regulated by the nuclear receptor RORα, and this activation requires the PPARγ coactivator-1α (PGC-1α) (Liu et al., 2007). Moreover, PGC-1α knockout mice show a similar shift in intrinsic period as we observed in BSKO mice (Liu et al., 2007), and PGC-1α is one of the best-characterized SIRT1 substrates (Rodgers et al., 2005). Thus it seemed possible that the mechanism by which SIRT1 regulates the circadian cycle in the SCN may involve PGC-1α. We queried whether PGC-1α knock down in N2a cells would recapitulate the effect of SIRT1 knock down on Bmal1 expression level and its downstream circadian genes. Indeed, knock down of PGC-1α by shRNA resulted in a decreased level of circadian targets in both 24hour and 36-hour time points post serum shock, similar to knocking down SIRT1 (Figure 6A and S3). Conversely, overexpression of PGC-1α enhanced circadian gene expression similar to SIRT1 overexpression. It is noteworthy that enforced expression of SIRT1 also drove higher expression of Pgc-1α, suggesting that the activities of these two proteins are highly coupled in neurons.
Figure 6. SIRT1 and PGC-1α mutually regulate circadian gene expression in N2a cells.
(A) Quantitative RT-PCR analysis of clock controlled genes. Sirt1 or Pgc-1α shRNA knock down cells and transient Sirt1 or Pgc-1α overexpression cells were synchronized with 50% horse serum 24 or 36 hr before harvesting for total RNA. Results are shown relative to a vector control. (B) Reporter assays using Bmal1- or Per2- luciferase under the indicated knock down or overexpression conditions. N2a cells were harvested for luciferase assays 24 hr after horse serum shock. Results are shown relative to a vector control. Values represent the mean ± standard deviation. *P<0.05; **P<0.01; t test. See also Figure S3 and S4.
To further support these findings, we tested whether SIRT1 could regulate a luciferase reporter driven by the Bmal1 or Per2 promoters (Nagoshi et al., 2004; Travnickova-Bendova et al., 2002). By transiently transfecting 250 or 500 ng of Sirt1 plasmid in N2a cells followed by horse serum shock, we found Bmal1-luciferase or Per2-luciferase were enhanced in a dose-dependent manner (Figure 6B). The effects of transfection of a vector expressing PGC-1α on Bmal1 and Per2-luciferase were very similar to SIRT1. Finally, depletion of either SIRT1 or PGC-1α caused a roughly 70% to 80% reduction in Bmal1- or Per2-driven luciferase (Figure 6B). Similar but milder effects were observed without serum shock (Figure S4). Together, these findings indicate that SIRT1 and PGC-1α function in an interdependent manner to activate circadian genes in neurons.
Cooperative binding of SIRT1 and PGC-1α to the Bmal1 Promoter
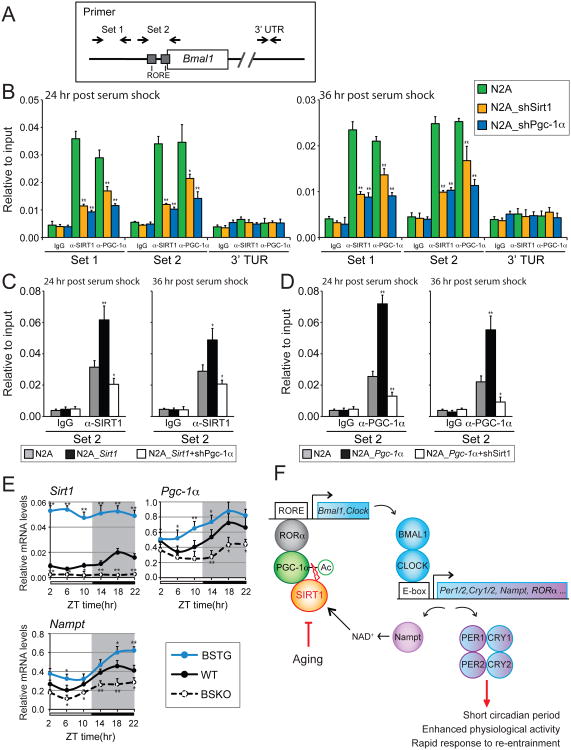
Since both in vivo and cell culture experiments indicated that SIRT1 activates expression of circadian genes including Bmal1, we hypothesized that it functioned at the Bmal1 promoter. We thus conducted a chromatin immunoprecipitation (ChIP) assay in N2a cells, to test whether SIRT1 binds to the RORα-binding sites (RORE) at the proximal Bmal1 promoter region. It's been demonstrated that PGC-1α binds to these sites through a synergistic action with RORa (Liu et al., 2007). Our ChIP results confirmed that PGC-1α binds to this Bmal1 promoter region spanning from -25 to +114, containing two RORE consensus sequences (Preitner et al., 2002) (Figure 7A). Interestingly, another set of primers revealed binding to the -698 to -502 region, but it is not clear whether this fragment has novel binding sites or is scoring positive due to linkage to the -25 to +114 sites. Importantly, primers in a control region in the Bmal1 3′UTR showed no binding.
Figure 7. Cooperative binding of SIRT1 and PGC-1α at theBmal1promoter.
(A) Schematic view of primer locations. Primers that flank two RORE sequences (Set 2) and another upstream region (Set 1) at the Bmal1 promoter are showed. Control primers were designed at the 3′UTR of Bmal1 gene (See Table S1). (B) Chromatin-immunoprecipitation (ChIP) assays in N2a cells. Cells were transfected with a vector control or shRNA knock down constructs for SIRT1 or PGC-1α (B). Overexpression of SIRT1 (C) and PGC-1α (D) were ChIP assayed as in (B) with indicated knock down of PGC-1α or SIRT1, respectively. Rabbit IgG was used as an immunoprecipitation control. Assays were performed 24 hr or 36 hr after synchronization with 50% horse serum. ChIP results were analyzed by qPCR. (E) Oscillation of Sirt1, Pgc-1α and Nampt in the SCN. SCN enriched samples were isolated from 6 month-old male mice at 4 hr intervals across the 12:12 light/dark cycle (n=3-4 mice/genotype/time point). Target gene expression levels are shown relative to a ribosomal reference gene, rpl19. Values represent the mean ± standard deviation. *P<0.05; **P<0.01; t test. (F) Schematic model of SIRT1 mediated circadian gene activation in the SCN. The model depicts an oscillating loop that amplifies expression of BMAL1, CLOCK, and their targets. Age-associated decline of SIRT1 results in circadian phenotypes such as prolonged period and a defect in re-entrainment. See also Figure S5.
A second ChIP experiment was performed using SIRT1 antibodies and revealed similar binding at Bmal1 promoter sites but not the 3′UTR (Figure 7B). This finding indicates that SIRT1 binds in close proximity to PGC-1α. Remarkably, knock down of PGC-1α markedly reduced SIRT1 binding to the Bmal1 promoter (as well as binding of itself), and knock down of SIRT1 reduced binding of PGC-1α (and itself) (Figure 7B). Furthermore, overexpressing either SIRT1 or PGC-1α increased the occupancy on the Bmal1 promoter, and the enhanced binding due to over-expression of one protein (e.g. SIRT1) was abolished by the shRNA knockdown of the other (e.g. PGC-1α) (Figure 7C and D). These studies show that neuronal SIRT1 and PGC-1α bind cooperatively and in close proximity at the Bmal1 promoter, and suggest that positive regulation of circadian genes occurs by the direct, cooperative action of SIRT1 and PGC-1α at the Bmal1 promoter.
Finally, to trace the SIRT1-mediated regulatory circuit back to the SCN, we measured Sirt1, Pgc-1α and Nampt RNA levels in vivo, as in Figure 3A (Figure 7E). We found that all three genes were expressed in a phased, circadian manner, and SIRT1 positively regulated the level of Pgc-1α transcription, as observed in N2a cells. This latter effect may be due to a heightened PGC-1α coactivation of FOXO at the PGC-1α promoter when the coactivator has been deacetylated by SIRT1 (Borniquel et al., 2010). All told, our in vivo and in vitro data suggest that an amplifying regulatory loop in the SCN comprising Nampt, SIRT1, and PGC-1α drives the expression of circadian genes in an aging-sensitive fashion (Figure 7F).
Discussion
In this report we show for the first time that SIRT1 regulates central circadian control in the brains of mice to determine period, activity levels, and ability to adjust to re-entrainment. SIRT1 directly activates transcription of Bmal1 via PGC-1α to increase the amplitude of gene expression in the SCN of BMAL and other circadian regulatory proteins. The components of this amplifying loop, SIRT1 and PGC-1α, and the NAD+ synthetic enzyme Nampt are themselves circadian in the SCN. This regulatory loop thus influences the intrinsic period of mice, which is shortened in transgenic mice with increased SIRT1 levels in the brain and elongated in brain-specific SIRT1 knockout mice.
Moreover, our findings provide the first molecular explanation of why robust central circadian control declines with aging. Aged mice exhibit a decrease in SIRT1 levels in the SCN and concomitant decreases in BMAL1 and other circadian regulatory proteins in this brain region. Thus, aged mice display an increase in their intrinsic period and an inability to adjust to abrupt light re-entrainment regimens, termed jet lag. Young mice lacking brain SIRT1 partially pheno-copy old wild type mice for period dilation and a decline in ability to re-entrain. Critically, old mice over-expressing brain SIRT1 are partially protected from the aging-associated decline in ability to re-entrain. These findings suggest that SIRT1 may be a linchpin in the aging-sensitive decline of central circadian function.
Mechanistic Implications
Studies in liver cells and mouse embryo fibroblasts revealed two mechanisms by which SIRT1 impinges on the circadian machinery in peripheral tissues. In the first, SIRT1 deacetylated the CLOCK-BMAL1 target PER2 to facilitate its ubiquitination and degradation by the proteosome (Asher et al., 2008). Thus, SIRT1 functioned as a positive regulator of BMAL1 and circadian components (Asher et al., 2008), as we observe in the SCN. In the second study, SIRT1 was shown to deacetylate BMAL1 and histones at circadian gene promoters, to facilitate repression by inhibitory components of the oscillator, thereby functioning as a negative regulator (Nakahata et al., 2008).
Our findings in SCN incorporate aspects of both of these mechanisms, but also appear to have distinct features. To wit, SIRT1 appears to deacetylate Lys 537 of BMAL1 in the SCN, as observed in peripheral tissues by Nakahata et al., but the effect of SIRT1 on the amplitude of the central circadian clock is positive, as observed in peripheral tissues by Asher et al. Thus, we wondered whether an alternative mechanism in SCN might reconcile all of these observations. One clue was that the deletion of the validated SIRT1 substrate, PGC-1α, in mice increased the intrinsic period from 23.5 to 24 hours (Liu et al., 2007), just what we observed for deleting SIRT1. Indeed, our studies in N2a neuroblastoma cells showed that SIRT1 and PGC-1α cooperatively bound to the Bmal1 promoter and functioned to activate transcription. Thus, we suggest that SIRT1 and PGC-1α function together in SCN to drive Bmal1 gene expression and the amplitude of the circadian machinery. We cannot rule out the possibility that deacetylation of BMAL1 by SIRT1 may play an additional role in regulating central clock.
In liver, the CLOCK-BMAL1 factor also drives circadian transcription of the NAD+ biosynthetic enzyme Nampt (Nakahata et al., 2009; Ramsey et al., 2009), which thus renders SIRT1 activity circadian. We find that SIRT1 and Nampt are also expressed in a circadian manner in the SCN, and SIRT1 activity appears necessary to drive normal PGC-1α levels. The Nampt/SIRT1/PGC-1α loop in the SCN is circadian most likely because it is driven by CLOCK-BMAL1, which themselves are subject to negative feedback by PER2 etc. We suggest that this Nampt/SIRT1/PGC-1α loop is what amplifies the expression of BMAL1 and other circadian proteins, and thus plays a critical role in the SCN central pacemaker (Figure 7). Consistent with this model, we observed that expression of Nampt is reduced in SCN of aged mice (Figure S5), similar to what we found for SIRT1 and BMAL1.
Implications for aging
Mutations in circadian genes have been associated with premature aging, cancer and other health maladies in mice (Fu et al., 2002; Kondratov et al., 2006; Marcheva et al., 2010). Many studies in humans also indicate that severe disruptions in circadian patterns are deleterious to health (Gallego and Virshup, 2007; Sehgal and Mignot, 2011). Two recent studies indicate that having an intrinsic period close to 24 hours is strongly associated with long life spans in rodents (Libert et al., 2012; Wyse and Coogan, 2010). This was interpreted as an indication that animals with innate periods different from 24 hours were forced to re-entrain daily in the 12LD cycle of the laboratory, and this imposed a life-shortening metabolic stress. However, most humans must re-entrain to daily changes in the diurnal cycle, which occur around the calendar. Were loss of SIRT1 and circadian proteins in the SCN to occur in aging humans, as we observe here in mice, it might trigger metabolic disruption and a decline in health due to an inability to re-entrain. Any intervention to maintain expression of SIRT1 and circadian proteins in the SCN during aging would therefore be salutary. Given the central nature of this regulation, it will be important to determine whether the role of SIRT1 in the SCN is even more important for healthy aging than other functions of this sirtuin in other tissues.
Linking circadian control to diet and drugs
Our findings suggest that dietary and pharmacological interventions that affect SIRT1 activity may also impinge on central circadian control. Many studies indicate that calorie restriction increases SIRT1 levels, while a high fat diet decreases levels in peripheral tissues. Were this to apply to the SCN, one would predict dietary effects on intrinsic periods and ability to adjust to changes in entrainment. For example, the progression through metabolic syndrome to diabetes might be expected to lengthen the intrinsic period and foster deterioration in circadian function. Conversely, calorie restriction or drugs that cause an increase in SIRT1 activity might have opposite effects. In this regard, it is noteworthy that resveratrol was recently reported to shorten the free-running circadian period in primates (Pifferi et al., 2011), consistent with our findings here. Several SIRT1 activators have been tested recently, and show repressive activity on circadian expression in an osteosarcoma cell line and in the liver (Bellet et al., 2013), suggesting again that there are differences between central and peripheral circadian control. It will be interesting to test the in vivo effects of SIRT1 activator/inhibitor compounds on central circadian control.
It is also interesting to reconsider a role for melatonin, which is produced in the pineal gland, declines with aging, and can re-entrain the central circadian clock (Dibner et al., 2010; Kondratova and Kondratov, 2012; Sack et al., 1986). Melatonin was initially reported to increase the life span in C57BL/6 mice (Pierpaoli and Regelson, 1994), but this finding was later discounted because C57BL/6 mice were shown to have relatively low melatonin levels (Grace et al., 1999). Interestingly, melatonin was more recently reported to preserve SIRT1 expression in hippocampus of sleep-deprived rats (Chang et al., 2009). It may thus be of interest to investigate whether falling melatonin levels in normal aging play any role in the decline of SIRT1 in the SCN.
Conclusion
Sirtuins play many roles in adapting organisms to the two critical features of calorie restriction; metabolic reprogramming and stress resistance. Our findings indicate a novel function of SIRT1 in the brain in coupling metabolic processes faithfully to circadian control, and maintaining central circadian function during aging. SIRT1 is part of a regulatory loop that is itself circadian and amplifies circadian gene expression. Our finding that SIRT1 in the SCN declines in aged animals helps explain the known aging-sensitive decline in circadian function, and is consistent with the idea that the uncoupling of metabolic processes from a diurnal cycle may itself be central in the aging process. Therefore, strategies that can maintain SIRT1 function in the SCN may slow the onset and progression of diseases of aging.
Materials and methods
Animals
SIRT1 brain-specific knockout mice (BSKO) were generated by crossing the loxP flanked exon 4 Sirt1 allele mice (Cheng et al., 2003) with the brain-specific Nestin-Cre expressing mice (Cohen et al., 2009). For SIRT1 brain-specific overexpressing mice (BSTG), a Sirt1STOP strain that harbors a loxP flanked transcriptional STOP element between the CAGGS promoter and the Sirt1 cDNA was used to breed with Nestin-Cre mice, to achieve a brain specific excision of the STOP sequence (Firestein et al., 2008). A Sir2d strain was obtained from mice that were heterozygous for the Sirt1-KI transgene (Bordone et al., 2007). Further detail for strains is listed in Table S2. All mice were maintained in the C57BL/6 background and were housed in a standard animal maintenance facility under a 12 h light:12 h dark cycle. All animal procedures were in accordance with the MIT animal care committee.
Plasmids
Constructs for Bmal1, Per2 and Sirt1 probes for in situ hybridization were cloned into pCR-Blunt II TOPO backbone with primers listed in Table S1. Bmal1 (1.1 kb) and Per2 (1.7 kb) promoter fragments were PCR amplified from mouse genomic DNA and cloned into pGL3 firefly luciferase vector (Promega) as described (Nagoshi et al., 2004; Travnickova-Bendova et al, 2002 and Table S1). pAd-Track mSirt1 and pLKO Pgc-1α for transient overexpression were kindly provided by Dr. P. Puigserver. Pgc-1α shRNA constructs were purchased from Sigma-Aldrich (Table S1).
Cell culture and transfections
N2a cells that stably knockdown for Sirt1 with shRNA was as previously described (Libert et al., 2011 and Table S1). For time point experiment, N2a cells at 5×105 cells/10-cm dish density were seeded and grown for 5 days in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, 2 mM glutamine, and 25 mM HEPES (pH 7.2) and cultured at 37°C in a humidified incubator with 5% CO2 until confluent. Cells were then synchronized with 50% horse serum for 2 h followed by replacing with low FBS (1%) containing DMEM for the subsequent 24 to 44 h time points. For transient overexpression experiment, Sirt1, Pgc-1α and reporter constructs were transfected in Day 4 and allowed for expression for 24 h before serum shock. All transient transfections were carried out with FuGENE HD Transfection Reagent (Roche) according to the manufacturer's instructions.
Locomotor activity
Wheel-running activity was accessed with wireless running wheel systems (MED Associates, ENV-044) and recorded by a sensor hub (MED Associates, DIG-804) according to the manufacturer's instructions. Male wild-type, SIRT1 BSKO, BSTG and sir2d mice at indicated ages were used for the voluntary activity assays. Mice were first maintained at 12:12 h LD cycle and recorded for their activities for at least 3 weeks, followed by releasing into 12:12 h DD free running condition (Siepka and Takahashi, 2005). 4 h phase shift were performed as previously described (Valentinuzzi et al, 1997). Actograms and chi-square periodogram were analyzed and generated by wheel manger software (MED Associates, SOF-860) and online circadian software (www.circadian.org).
Tissue collection and RNA analysis by real-time quantitative PCR
Brain samples were quickly harvested at indicated time points and kept in OCT compound (Tissue-Tek) at -80°C until use. To obtain SCN enriched sample, anterior hypothalamus at the optic chiasm level was cut coronally into two 150 μm thickness sections with a cryostat (Leica CM 1510S). SCN was then visualized under a phase contrast microscope and collected with a syringe needle. SCN samples from 3-4 mice were pooled for RNA extraction by using RNeasy Mini Kit (Qiagen). cDNAs were generated by RETROscript Kit (Ambion) according to the manufacturer's instructions. RT- PCR reactions were performed on a LightCycler 480 II (Roche) using iQ SYBR Green Supermix (Bio-Rad). The relative abundance of transcripts was calculated by normalizing to a ribosomal subunit gene, rpl19. Primers are listed in Table S1.
Immunohistochemistry
Mice were housed in all dark for 48h prior to the ZT collection experiments. Whole brain samples were quickly harvested at indicated time points under red dim light, then fixed in 4% phosphate buffered paraformaldehyde solution for overnight. After cryoprotected in 30% sucrose for 30 h, fixed tissues were embedded in OCT (Tissue-Tek) and stored at -80°C until staining. Detailed methods can be found in Extended Experimental Procedures.
In situ hybridization
Digoxigenin (DIG)-labeled antisense and sense RNA probes were made using linearized plasmids as templates and transcribed with T7 or SP6 RNA polymerases, respectively (Roche). Brain sections were fixed in 4% PFA, permeabilized with 5μg/ml proteinase K for 10 min before an acetylation step in triethanolamine/acetic anhydride solution. Detailed methods can be found in Extended Experimental Procedures.
Reporter assays
N2a cells were seeded into six well dishes at 1.5×106 cells/well density 24 h before transfections. Sirt1 and Pgc-1α overexpression constructs, reporter constructs and internal control Renilla-Luc plasmid were transfected at the ratio of 0.5 (or 0.25) μg: 0.2 μg: 0.1 μg per well. Cells were then synchronized with 50% horse serum for 2 h followed by replacing with low FBS containing DMEM for 24 h before harvested for luciferase assays. Cell lysis and luciferase measurements were performed according to the Promega Dual-Luciferase system instructions.
ChIP assays
N2a cells were cultured, transfected and synchronized with 50% horse serum as described. 24 or 36 h after synchronization, cells were fixed in 1% formaldehyde and collected for chromatin preparation according to SimpleChIP Enzymatic Chromatin IP Kit instructions (Cell Signaling Technology). Purified DNA was subjected to real-time PCR using primers listed in Table S1.
Supplementary Material
SIRT1 and PGC-1α bind directly to the BMAL promoter to activate transcription SIRT1 and the clock machinery decrease dramatically in the SCN in aged mice Over-expression of SIRT1 in the brain suppresses the decline in circadian function
SIRT1, PGC-1α and Nampt comprise a loop that amplifies circadian gene expressions
Acknowledgments
We thank P. Puigserver and C.-C. Lee for Pgc-1α constructs and PER2 antibody, respectively. We also thank E. Bell, E. Williams and Y.-C. Tang for reagents and comments on experiments, and members in the Guarente laboratory for discussions. This work was supported by grants from the NIH and the Glenn Foundation for Medical Research to L.G., H.-C. Chang is an Ellison Medical Foundation Fellow of the Life Science Research Foundation. L.G. consults for Sirtris (a GSK company).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, Mostoslavsky R, Alt FW, Schibler U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134:317–328. doi: 10.1016/j.cell.2008.06.050. [DOI] [PubMed] [Google Scholar]
- Asher G, Schibler U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011;13:125–137. doi: 10.1016/j.cmet.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet MM, Nakahata Y, Boudjelal M, Watts E, Mossakowska DE, Edwards KA, Cervantes M, Astarita G, Loh C, Ellis JL, et al. Pharmacological modulation of circadian rhythms by synthetic activators of the deacetylase SIRT1. Proc Natl Acad Sci U S A. 2013;110:3333–3338. doi: 10.1073/pnas.1214266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, et al. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Borniquel S, Garcia-Quintans N, Valle I, Olmos Y, Wild B, Martinez-Granero F, Soria E, Lamas S, Monsalve M. Inactivation of Foxo3a and subsequent downregulation of PGC-1 alpha mediate nitric oxide-induced endothelial cell migration. Mol Cell Biol. 2010;30:4035–4044. doi: 10.1128/MCB.00175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, Lazar MA. Rev-erbalpha and Rev-erbbeta coordinately protect the circadian clock and normal metabolic function. Genes Dev. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HM, Wu UI, Lan CT. Melatonin preserves longevity protein (sirtuin 1) expression in the hippocampus of total sleep-deprived rats. J Pineal Res. 2009;47:211–220. doi: 10.1111/j.1600-079X.2009.00704.x. [DOI] [PubMed] [Google Scholar]
- Cheng HL, Mostoslavsky R, Saito S, Manis JP, Gu Y, Patel P, Bronson R, Appella E, Alt FW, Chua KF. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100:10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Zhao X, Hatori M, Yu RT, Barish GD, Lam MT, Chong LW, DiTacchio L, Atkins AR, Glass CK, et al. Regulation of circadian behaviour and metabolism by REV-ERB-alpha and REV-ERB-beta. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DE, Supinski AM, Bonkowski MS, Donmez G, Guarente LP. Neuronal SIRT1 regulates endocrine and behavioral responses to calorie restriction. Genes Dev. 2009;23:2812–2817. doi: 10.1101/gad.1839209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang W, Steffen KK, Perry R, Dorsey JA, Johnson FB, Shilatifard A, Kaeberlein M, Kennedy BK, Berger SL. Histone H4 lysine 16 acetylation regulates cellular lifespan. Nature. 2009;459:802–807. doi: 10.1038/nature08085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Donmez G, Arun A, Chung CY, McLean PJ, Lindquist S, Guarente L. SIRT1 protects against alpha-synuclein aggregation by activating molecular chaperones. J Neurosci. 2012;32:124–132. doi: 10.1523/JNEUROSCI.3442-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Donmez G, Wang D, Cohen DE, Guarente L. SIRT1 suppresses beta-amyloid production by activating the alpha-secretase gene ADAM10. Cell. 2010;142:320–332. doi: 10.1016/j.cell.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein R, Blander G, Michan S, Oberdoerffer P, Ogino S, Campbell J, Bhimavarapu A, Luikenhuis S, de Cabo R, Fuchs C, et al. The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon cancer growth. PLoS One. 2008;3:e2020. doi: 10.1371/journal.pone.0002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111:41–50. doi: 10.1016/s0092-8674(02)00961-3. [DOI] [PubMed] [Google Scholar]
- Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–148. doi: 10.1038/nrm2106. [DOI] [PubMed] [Google Scholar]
- Grace MS, Chiba A, Menaker M. Circadian control of photoreceptor outer segment membrane turnover in mice genetically incapable of melatonin synthesis. Vis Neurosci. 1999;16:909–918. doi: 10.1017/s0952523899165106. [DOI] [PubMed] [Google Scholar]
- Guarente L. Sirtuins and calorie restriction. Nat Rev Mol Cell Biol. 2012;13:207. doi: 10.1038/nrm3308. [DOI] [PubMed] [Google Scholar]
- Jeong H, Cohen DE, Cui L, Supinski A, Savas JN, Mazzulli JR, Yates JR, 3rd, Bordone L, Guarente L, Krainc D. Sirt1 mediates neuroprotection from mutant huntingtin by activation of the TORC1 and CREB transcriptional pathway. Nat Med. 2012;18:159–165. doi: 10.1038/nm.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Wang J, Fu J, Du L, Jeong H, West T, Xiang L, Peng Q, Hou Z, Cai H, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington's disease through activation of multiple Sirt1 targets. Nat Med. 2012;18:153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13:325–335. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Sachdeva UM, DiTacchio L, Williams EC, Alvarez JG, Egan DF, Vasquez DS, Juguilon H, Panda S, Shaw RJ, et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science. 2009;326:437–440. doi: 10.1126/science.1172156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7:1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Bonkowski MS, Pointer K, Pletcher SD, Guarente L. Deviation of innate circadian period from 24 hours reduces longevity in mice. Aging Cell. 2012 doi: 10.1111/j.1474-9726.2012.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, Kapur K, Bergmann S, Preisig M, Otowa T, et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Li S, Liu T, Borjigin J, Lin JD. Transcriptional coactivator PGC-1alpha integrates the mammalian clock and energy metabolism. Nature. 2007;447:477–481. doi: 10.1038/nature05767. [DOI] [PubMed] [Google Scholar]
- Marcheva B, Ramsey KM, Buhr ED, Kobayashi Y, Su H, Ko CH, Ivanova G, Omura C, Mo S, Vitaterna MH, et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinaemia and diabetes. Nature. 2010;466:627–631. doi: 10.1038/nature09253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Xu Z, Chen-Goodspeed M, Liu M, Van Oort-Jansen A, Rea MA, Zhao Z, Lee CC, Chang KS. PML regulates PER2 nuclear localization and circadian function. EMBO J. 2012;31:1427–1439. doi: 10.1038/emboj.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi E, Saini C, Bauer C, Laroche T, Naef F, Schibler U. Circadian gene expression in individual fibroblasts: cell-autonomous and self-sustained oscillators pass time to daughter cells. Cell. 2004;119:693–705. doi: 10.1016/j.cell.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, Guarente LP, Sassone-Corsi P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134:329–340. doi: 10.1016/j.cell.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli W, Regelson W. Pineal control of aging: effect of melatonin and pineal grafting on aging mice. Proc Natl Acad Sci U S A. 1994;91:787–791. doi: 10.1073/pnas.91.2.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifferi F, Dal-Pan A, Menaker M, Aujard F. Resveratrol dietary supplementation shortens the free-running circadian period and decreases body temperature in a prosimian primate. J Biol Rhythms. 2011;26:271–275. doi: 10.1177/0748730411401788. [DOI] [PubMed] [Google Scholar]
- Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Anderson J, Berglund ED, Frazao R, Michan S, Vianna CR, Sinclair DA, Elias CF, Coppari R. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011;14:301–312. doi: 10.1016/j.cmet.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12:78–87. doi: 10.1016/j.cmet.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Sack RL, Lewy AJ, Erb DL, Vollmer WM, Singer CM. Human melatonin production decreases with age. J Pineal Res. 1986;3:379–388. doi: 10.1111/j.1600-079x.1986.tb00760.x. [DOI] [PubMed] [Google Scholar]
- Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886–896. doi: 10.1038/nrc2747. [DOI] [PubMed] [Google Scholar]
- Sato F, Kawamoto T, Fujimoto K, Noshiro M, Honda KK, Honma S, Honma K, Kato Y. Functional analysis of the basic helix-loop-helix transcription factor DEC1 in circadian regulation. Interaction with BMAL1. Eur J Biochem. 2004;271:4409–4419. doi: 10.1111/j.1432-1033.2004.04379.x. [DOI] [PubMed] [Google Scholar]
- Satoh A, Brace CS, Ben-Josef G, West T, Wozniak DF, Holtzman DM, Herzog ED, Imai S. SIRT1 promotes the central adaptive response to diet restriction through activation of the dorsomedial and lateral nuclei of the hypothalamus. J Neurosci. 2010;30:10220–10232. doi: 10.1523/JNEUROSCI.1385-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepka SM, Takahashi JS. Methods to record circadian rhythm wheel running activity in mice. Methods Enzymol. 2005;393:230–239. doi: 10.1016/S0076-6879(05)93008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sujino M, Masumoto KH, Yamaguchi S, van der Horst GT, Okamura H, Inouye ST. Suprachiasmatic nucleus grafts restore circadian behavioral rhythms of genetically arrhythmic mice. Curr Biol. 2003;13:664–668. doi: 10.1016/s0960-9822(03)00222-7. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9:764–775. doi: 10.1038/nrg2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travnickova-Bendova Z, Cermakian N, Reppert SM, Sassone-Corsi P. Bimodal regulation of mPeriod promoters by CREB-dependent signaling and CLOCK/BMAL1 activity. Proc Natl Acad Sci U S A. 2002;99:7728–7733. doi: 10.1073/pnas.102075599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273:R1957–1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyse CA, Coogan AN. Impact of aging on diurnal expression patterns of CLOCK and BMAL1 in the mouse brain. Brain Res. 2010;1337:21–31. doi: 10.1016/j.brainres.2010.03.113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.