Abstract
Objective
Obesity is associated with insulin resistance, chronic low-grade inflammation and atherosclerosis. Toll-like receptor 4 (TLR4) participates in the cross-talk between inflammation and insulin resistance, being activated by both lipopolysaccharide and saturated fatty acids. This study was undertaken to determine whether TLR4 deficiency has a protective role in inflammation, insulin resistance and atherosclerosis induced by a diabetogenic diet.
Methods and Results
TLR4 and LDL receptor double knockout (Tlr4−/−Ldlr−/−) mice and Ldlr−/− mice were fed either a normal chow or a diabetogenic diet for 24 weeks. Tlr4−/−Ldlr−/− mice fed a diabetogenic diet showed improved plasma cholesterol and triglyceride levels but developed obesity, hyperinsulinemia and glucose intolerance equivalent to obese Ldlr−/− mice. Adipocyte hypertrophy, macrophage accumulation and local inflammation were not attenuated in intra-abdominal adipose tissue in Tlr4−/−Ldlr−/− mice. However, TLR4 deficiency led to markedly decreased atherosclerosis in obese Tlr4−/−Ldlr−/− mice. Compensatory up-regulation of TLR2 expression was observed both in obese TLR4 deficient mice and in palmitate-treated TLR4-silenced 3T3-L1 adipocytes.
Conclusions
TLR4 deficiency decreases atherosclerosis without affecting obesity-induced inflammation and insulin resistance in LDL receptor deficient mice. Alternative pathways may be responsible for adipose tissue macrophage infiltration and insulin resistance that occurs in obesity.
Keywords: Toll-like receptor 4, insulin resistance, atherosclerosis, inflammation, diabetogenic diet
Obesity is a low-grade chronic inflammatory disease associated with an increase of circulating inflammatory markers1. This inflammation is associated with insulin resistance in tissues such as adipose tissue and liver. Hallmarks of this inflammatory process are early infiltration of intra-abdominal adipose tissue with immune cells (mainly macrophages) and autocrine and paracrine secretion of pro- and anti-inflammatory cytokines2–5, which have been postulated to lead to insulin resistance. Insulin resistance and inflammation in turn have been suggested to play a causal role in cardiovascular disease6–8.
Toll-like receptors (TLR) elicit innate immune responses and inflammation when activated by either exogenous microbial products or endogenous molecules with similar structures, and they are candidate mediators of atherogenic inflammation9. In recent years, the activation of immune cells via toll-like receptor 4 (TLR4) has received considerable attention. TLR4, the best characterized TLR, is an essential receptor for lipopolysaccharide (LPS). The lipid A moiety of LPS contains acylated hydroxyl saturated fatty acids (SFAs). Several different groups have shown that SFAs could be natural ligands for TLR4, culminating in inflammatory gene expression by nuclear factor–κB (NF-κB) activation10–14. Moreover, we have previously reported that palmitate increases SAA3 and MCP-1 expression via a TLR4-dependent mechanism in 3T3-L1 adipocytes15. The responsiveness of TLR4 to fatty acids makes TLR4 an appealing intermediate between obesity and recruitment of macrophages to adipose tissue and the artery wall.
Indeed, others have shown that TLR4 deficient/mutated mice are protected against inflammation and insulin resistance when challenged with high-fat diets10,16–18. However, conflicting results regarding to the role of TLR4 in adiposity and macrophage infiltration of intra-abdominal adipose tissue also have been obtained19,20. Moreover recent studies showed that TLR4 deletion promotes obesity21, insulin resistance22, hyperlipidemia23, and a diabetic phenotype24 in mice. Therefore, more research is needed to understand the uncertain effects of TLR4 on obesity-associated local inflammation, insulin resistance, influx of macrophages to intra-abdominal adipose tissue and atherosclerosis.
To determine whether inactivation of TLR4 would reduce obesity-induced macrophage infiltration into adipose tissue and subsequently prevent insulin resistance and atherosclerosis, we used TLR4-deficient (Tlr4−/−) mice on the atherosclerosis-prone low-density lipoprotein receptor-deficient (Ldlr−/−) background. A diabetogenic diet (DD) rich in SFAs and refined carbohydrate was used since we have previously shown that this diet leads to multiple features of the metabolic syndrome such as insulin resistance, adipose inflammation, chronic systemic inflammation, and atherosclerosis in Ldlr−/− mice25. Our data demonstrate that lack of TLR4 decreases atherosclerosis without affecting obesity-induced inflammation and insulin sensitivity in this mouse model.
Methods
Please see supplemental materials for additional details.
Animals and diets
10-week old adult male Tlr4−/−Ldlr−/− and littermate control Ldlr−/− mice were fed rodent chow diet and a “diabetogenic” high SFA-rich and carbohydrate-rich diet (DD) for a total of 24 weeks25. All experimental procedures were undertaken with approval from the Institution Animal Care and Use Committee of the University of Washington.
Analytical procedures
Total plasma cholesterol and triglycerides, plasma insulin, and hepatic triglycerides were measured using commercially available assay kits. Lipoprotein distribution was analyzed by fast protein liquid chromatography (FPLC) as described previously26.
Immunohistochemistry and adipocyte sizing
Single-label immunohistochemistry was performed on adipose tissues as previously described27. Adipocyte cross-sectional area was measured by computer image analysis using a modification of techniques described previously28. All analyses were performed by a blinded observer.
In vitro TLR4 gene silencing
To test the role of TLR4 or TLR2-mediated SAA3 and MCP-1 expression, 3T3-L1 adipocytes were transiently transfected (2 days after completion of the differentiation protocol) with small-interfering RNA (siRNA) duplexes for TLR4 (Ambion DeliverX system, Panomics), as described previously29,30.
Real-time quantitative PCR analysis
Total RNA was extracted according to the manufacturer’s protocols. Relative quantities of mRNA were calculated using GAPDH as the reference gene. The amount of target gene was calculated using the ΔΔCt formula.
Atherosclerotic quantification
The extent of aortic atherosclerosis was measured by the en face technique as described previously31. Total surface area and lesion areas in the studies sections of aortas were quantified by Image J software (NIH, Bethesda, MD, USA).
Statistical analysis
Data were analyzed using GraphPad Prism 5 program (GraphPad Software Inc) and represent means ± standard errors unless noted otherwise. Analysis of variance (ANOVA) with Tukey post hoc testing was used to detect differences among groups. Data not normally distributed were analyzed using the Kruskal-Wallis test with Dunn post test analysis. A p value <0.05 was considered as statistically significant.
Results
Hyperlipidemia is attenuated in Tlr4−/−Ldlr−/− mice fed the diabetogenic diet
As expected from our previous study25, body weight gain in mice fed DD significantly outpaced that in chow-fed mice. Body weights at the end of 24 weeks did not differ between Tlr4−/−Ldlr−/− mice and their controls either in DD or chow groups (Supplemental Figure SIA), and no differences in food intake were detected (data not shown). Body composition analysis revealed a comparable increase in fat mass in DD versus chow-fed mice (Supplemental Figure SIB–IC). These results demonstrate that the loss of TLR4 did not affect the body weight and the distribution of fat in the obese animals. Thus, the Tlr4−/−Ldlr−/− and Ldlr−/− groups were well matched for body weight, obesity and all the other aspects of adiposity at the end of the study.
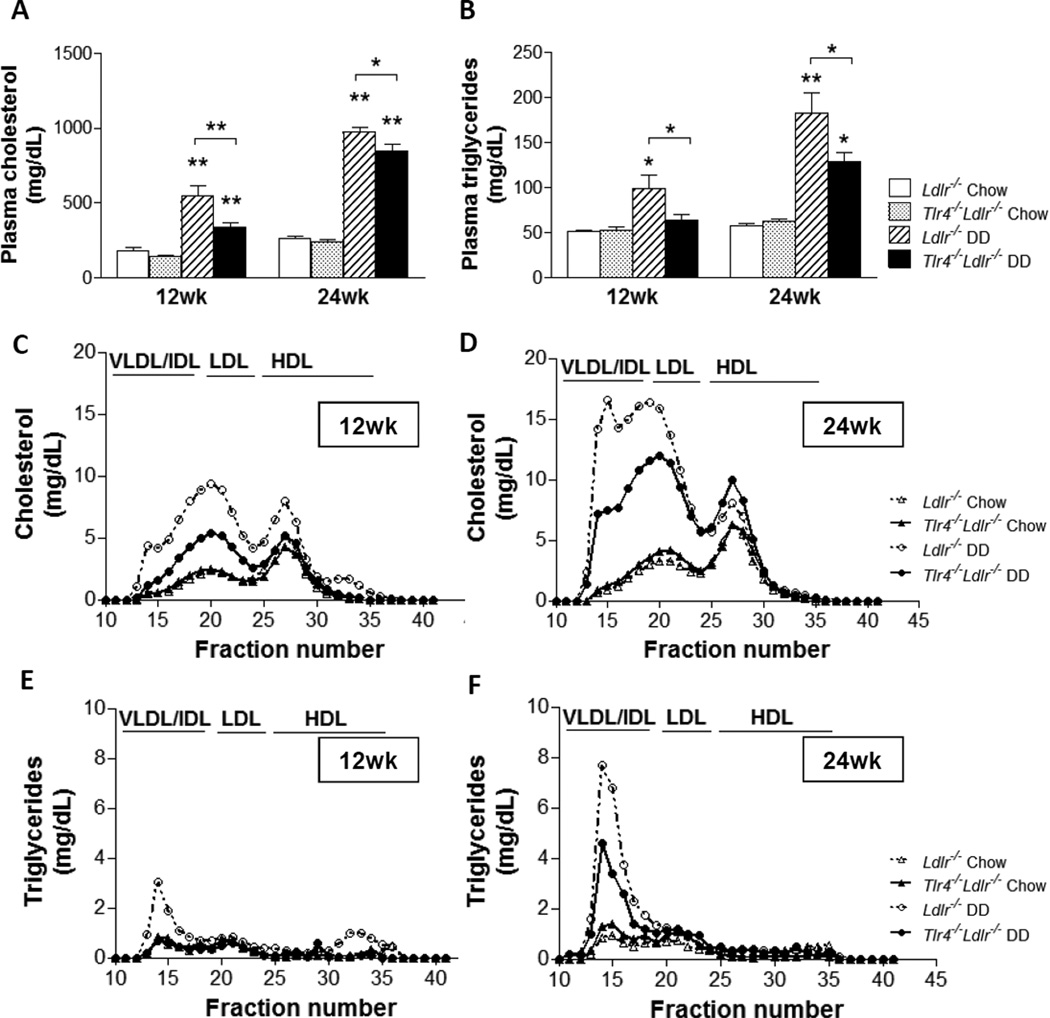
We previously showed that Ldlr−/− mice fed DD for 24 weeks develop hypercholesterolemia and hypertriglyceridemia25. In the present study we confirmed these findings. Furthermore, we found that the magnitude of hypercholesterolemia and especially hypertriglyceridemia were significantly lower in the Tlr4−/−Ldlr−/− group fed DD for 12 and 24 weeks compared to control mice fed DD (Figure 1A–1B, p<0.05). Lipoprotein profiles showed lower VLDL/IDL and LDL cholesterol (Figure 1C) and triglycerides levels (Figure 1D) in the obese Tlr4−/−Ldlr−/− mice fed the diabetogenic diets. These data suggest that TLR4 deficiency reduces obesity-induced hyperlipidemia in Ldlr−/− mice.
Figure 1. Hyperlipidemia is improved in Tlr4−/−Ldlr−/− mice fed a diabetogenic diet.
Plasma cholesterol (A), plasma triglycerides (B), cholesterol (C) and triglycerides (D) levels in plasma FPLC fractions at 12 week and 24 weeks of diet in Tlr4−/−Ldlr−/− mice fed DD (black bars and solid circle lines) and chow (grey bars and solid triangle lines), Ldlr−/− control mice fed DD (hatched bars and open circle dash lines) and chow (open bars and open triangle dash lines);(n=10–15, *P<0.05, **P<0.01 vs DD or chow groups).
Hyperglycemia and insulin resistance are not improved in Tlr4−/−Ldlr−/− mice fed the diabetogenic diet
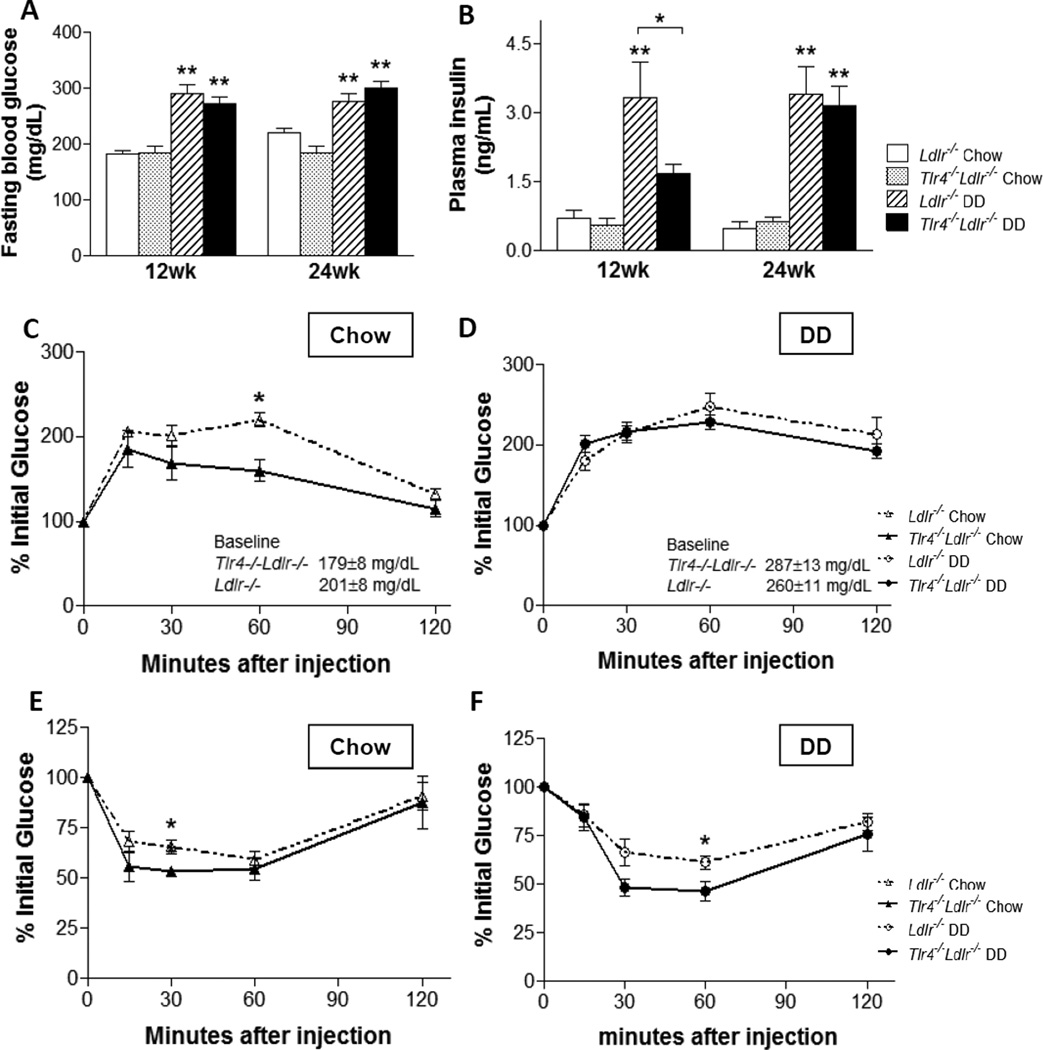
Mice on DD developed hyperglycemia and hyperinsulinemia compared to the chow-fed mice (Figure 2A–2B). Fasting glucose and insulin levels did not differ in the DD and chow groups at the end of the study, despite the plasma fasting insulin being significantly reduced in the Tlr4−/−Ldlr−/− DD group at 12 weeks (Figure 2B, p<0.05), .
Figure 2. Hyperglycemia and insulin resistance are not improved in Tlr4−/−Ldlr−/− mice fed a diabetogenic diet.
Plasma fasting glucose (A) and insulin levels (B), glucose tolerance tests (C–D), and insulin tolerance tests (E–F); (n=10–15, *P<0.05, **P<0.01 vs DD or chow groups). Tlr4−/−Ldlr−/− mice fed DD (black bars and solid circle lines) and chow (grey bars and solid triangle lines), Ldlr−/− control mice fed DD (hatched bars and open circle dash lines) and chow (open bars and open triangle dash lines).
The glucose response during a glucose tolerance test (GTT) was increased in DD-fed mice (Figure 2C–2D). On DD, the baseline glucose levels were higher than the chow-fed animals (287.3 ± 12.9 mg/dL in Tlr4−/−Ldlr−/− DD, 260.0 ± 10.8 mg/dL in Ldlr−/− DD, 179.1 ± 7.7 mg/dL in Tlr4−/−Ldlr−/− chow and 201.4 ± 8.1 mg/dL in Ldlr−/− chow). Also, the presence or absence of TLR4 had no effect on the insulin response at the 30 minute time point during the GTT in either the DD or the chow groups (Supplemental Figure SII). Moreover, the area under the curve was not statistically different between Ldlr−/− and Tlr4−/−Ldlr−/− obese or lean animals (data not shown). Whereas DD caused insulin resistance on insulin tolerance testing (ITT), the hypoglycemic response in the Tlr4−/−Ldlr−/− group was comparable to that in Ldlr−/− mice, with only one data point showing a borderline difference (Figure 2E–2F, p<0.05). Taken together, these findings reveal no overall improvement in glucose tolerance or insulin resistance as a result of TLR4 deficiency in this mouse model.
Macrophage infiltration in intra-abdominal adipose tissue does not decrease in Tlr4−/−Ldlr−/− mice fed the diabetogenic diet
To evaluate changes in intra-abdominal adipose tissue morphology and macrophage accumulation that occurs with obesity, epididymal (intra-abdominal) adipose tissue harvested at the time of sacrifice was studied. As expected, adipocyte size mirrored epididymal fat pad weights. Both adipose tissue weight and adipocyte hypertrophy were higher in mice fed the DD versus the control diet, but did not differ between Tlr4−/−Ldlr−/− and Ldlr−/− groups (Supplemental Figure SIIIA–IIIB). Interestingly, adipose tissue cholesterol content was significantly decreased (p<0.05) in Tlr4−/−Ldlr−/−mice fed DD compared to Ldlr−/− mice fed DD (Supplemental Figure SIIIC).
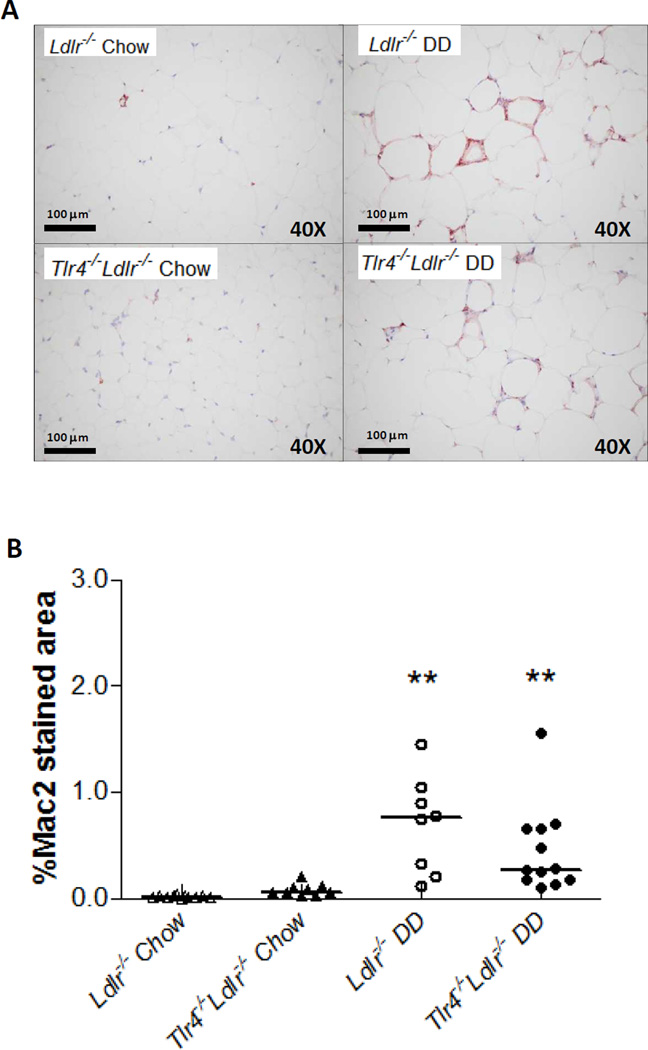
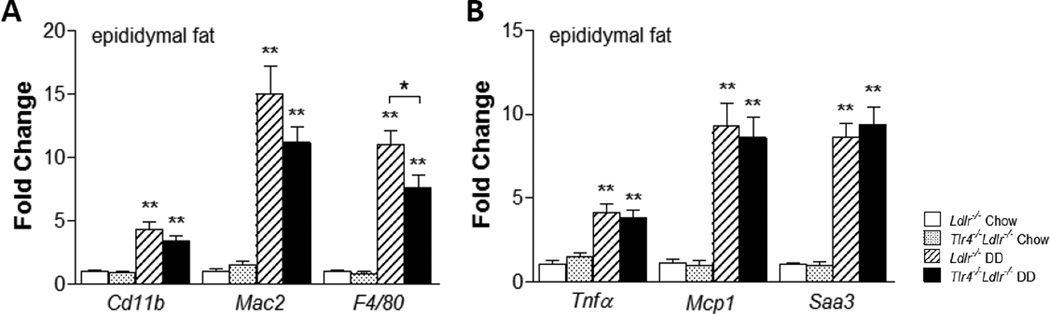
We performed single-labeled immunohistochemistry to identify the presence of macrophages within adipose tissue. Immunostaining for the macrophage-specific marker, Mac2, showed DD induced a significant increase of macrophages in epididymal adipose tissue (Figure 3A–3B, p<0.01). Although the DD-fed Tlr4−/−Ldlr−/− mice tended to have decreased Mac2 staining compared to DD-fed Ldlr−/− mice, these changes did not achieve statistical significance. Both the expression of Mac2 and CD11b, the monocyte-macrophage marker, did not show statistical differences in the DD and chow groups (Figure 4A). However, mRNA levels of another macrophage marker, F4/80, was significantly decreased in DD-fed Tlr4−/−Ldlr−/− mice in both liver (data not shown) and adipose tissue (Figure 4A, p<0.05). Furthermore, we measured the levels of Nos2 and Il6 as M1 markers, Arg1 and Fizz1 as M2 markers in the adipose tissue of Tlr4−/−Ldlr−/− mice and its control Ldlr−/− mice fed DD, and found no difference in the above genes between groups (Supplemental Figure SIV). Therefore, it is less likely that the macrophages represent an alternatively-activated type of macrophage. These findings are consistent with an overall lack of effect of TLR4 deletion on macrophage accumulation in both adipose tissue and liver.
Figure 3. Intra-abdominal adipose tissue macrophage accumulation is not reduced in Tlr4−/−Ldlr−/− mice.
Representative photomicrographs of epididymal adipose tissue stained with a macrophage-specific antibody Mac2 (1:2000 dilution, red), 40×magnification, n=10–13 per group (A), and quantification of Mac2 staining (B); (**P<0.01 vs DD or chow groups).
Figure 4. Differential expression of genes in epididymal white adipose tissue.
Macrophage-related inflammatory genes (A–B; n=10–15, *P<0.05, **P<0.01 vs DD or chow groups).
Local inflammation in intra-abdominal adipose tissue and liver in is not altered in Tlr4−/−Ldlr−/− mice
Given that macrophages are the cell type with the highest surface expression of TLR4 and, as such, are potent sensors and effectors for TLR4-mediated local inflammatory responses, we assessed the extent of local inflammation by examining expression of selective genes involved in inflammation in intra-abdominal adipose tissue and liver by real-time quantitative RT-PCR.
mRNA levels of pro-inflammatory chemokines and cytokines in intra-abdominal adipose tissue were significantly increased in Ldlr−/− mice on DD, as has been shown previously25. SAA3, the extrahepatic isoform of SAA produced by adipocytes and macrophages in mice, is a locally synthesized molecule with chemoattractant properties. Saa gene expression in intra-abdominal adipose tissue (Figure 4B) and liver (data not shown) did not differ between Tlr4−/−Ldlr−/− and Ldlr−/− mice. Similarly, the monocyte chemoattractant gene, Mcp1, and the pro-inflammatory cytokine, Tnfα, also did were not different between groups (Figure 4B). These data suggests that TLR4 deficiency did not affect local inflammation in adipose tissue or liver.
To investigate if cholesterol metabolism is affected by TLR4 deficiency, the expression of Apob, Hmgcr, Acat1, Srebp1c, Dgat1 and Mttp was measured in the liver of Tlr4−/−Ldlr−/− mice fed DD and Ldlr−/− mice fed DD. However, none of these genes were significantly different between the two groups (Supplemental Figure SVA–VF). Triglyceride content of liver was increased in both groups of mice fed DD, but again, no differences were observed between the DD-fed obese Tlr4−/−Ldlr−/− and Ldlr−/− mice (19.6 ± 1.5 mg/g liver in DD-fed Tlr4−/−Ldlr−/−, 19.8 ± 2.4 mg/g in DD-fed Ldlr−/−, 8.9 ± 0.6 mg/g liver in chow-fed Tlr4−/−Ldlr−/− and 8.6 ± 1.2 mg/g liver in chow-fed Ldlr−/−). These findings indicate that the absence of TLR4 is not associated with a decrease in hepatic triglycerides and cholesterol synthesis despite improvement of DD-induced lipid levels in plasma. It is possible that TLR4 deficiency affects triglyceride removal via LDL receptor-independent pathways since these mice were deficient in LDL receptors.
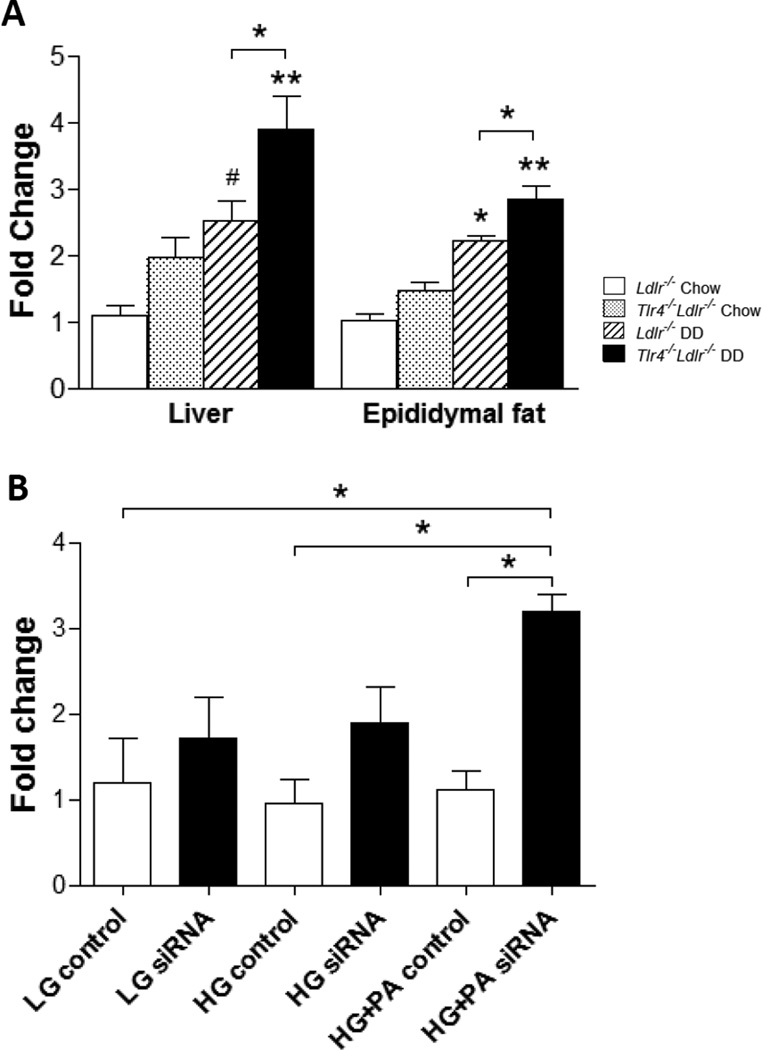
TLR2 expression is increased in both Tlr4−/−Ldlr−/− mice fed DD and TLR4 silenced FFA-treated adipocytes
Considering that TLR2 is closely related to TLR4 and is also shown to be activated by SFAs32, we next examined the Tlr2 mRNA abundance in intra-abdominal adipose tissue and liver from Tlr4−/−Ldlr−/− mice. On chow diets, TLR4 deficiency did not alter the mRNA level of Tlr2 expression; however, Tlr2 expression was significantly increased in Tlr4−/−Ldlr−/− mice fed DD as compared to obese Ldlr−/− mice in both intra-abdominal adipose tissue and liver (Figure 5A).
Figure 5. TLR2 expression is increased in DD-fed TLR4 deficient mice and TLR4-silenced differentiated 3T3-L1 adipocytes treated with FFA.
Tlr2 mRNA expression in liver and epididymal adipose tissue from TLR4-deficient mice (A); (n=10–15, *P<0.05, **P<0.01 vs DD or chow groups). Tlr4 mRNA expression in differentiated 3T3-L1 cells (B). 3T3-L1 adipocytes were transfected with a siRNA specific for TLR4 or a scrambled siRNA as control. 24 hours later, the cells were exposed to palmitate (PA, 250 µmol/L) in 5 (LG) and 25 mmol/l (HG) for 7 days with daily medium changes (*P<0.05 as tested by one way ANOVA compared with LG control). Tlr4−/−Ldlr−/− mice fed DD (black bars) and chow (grey bars), Ldlr−/− control mice fed DD (hatched bars) and chow (open bars).
We have previously shown that silencing TLR4 in differentiated 3T3-L1 adipocytes with a TLR4-specific siRNA markedly reduced Saa3 and Mcp1 expression in response to palmitate but not glucose15. TLR4-siRNA transfected adipocytes were used in this study to measure Tlr2 expression in response to palmitate exposure in both 5 and 25 mmol/L glucose. The success of transfection was confirmed by markedly silenced Tlr4 expression and reduced expression of Saa3 and Mcp1 (data not shown). Similarly to what we found in the in vivo experiment, mRNA level of Tlr2 was significantly increased in palmitate-treated TLR4-silenced cells (Figure 5B, p<0.05). Taken together, these results imply that TLR4 deletion leads to a compensatory increase of Tlr2 expression, which could in part contribute to the lack of change in adipose tissue inflammation and insulin resistance in this study.
Atherosclerosis is significantly decreased in Tlr4−/−Ldlr−/− mice fed dfiabetogenic diets
TLR4 deficiency has been shown to reduce intimal lipid by ~75% in apoE deficient mice33. Because Ldr−/− mice are particularly susceptible to the DD-induced adipose inflammation, insulin resistance and atherosclerosis, whereas apoE deficient mice do not have similar changes31,34, we assessed the effect of TLR4 deficiency on atherosclerosis in Ldlr−/− mice.
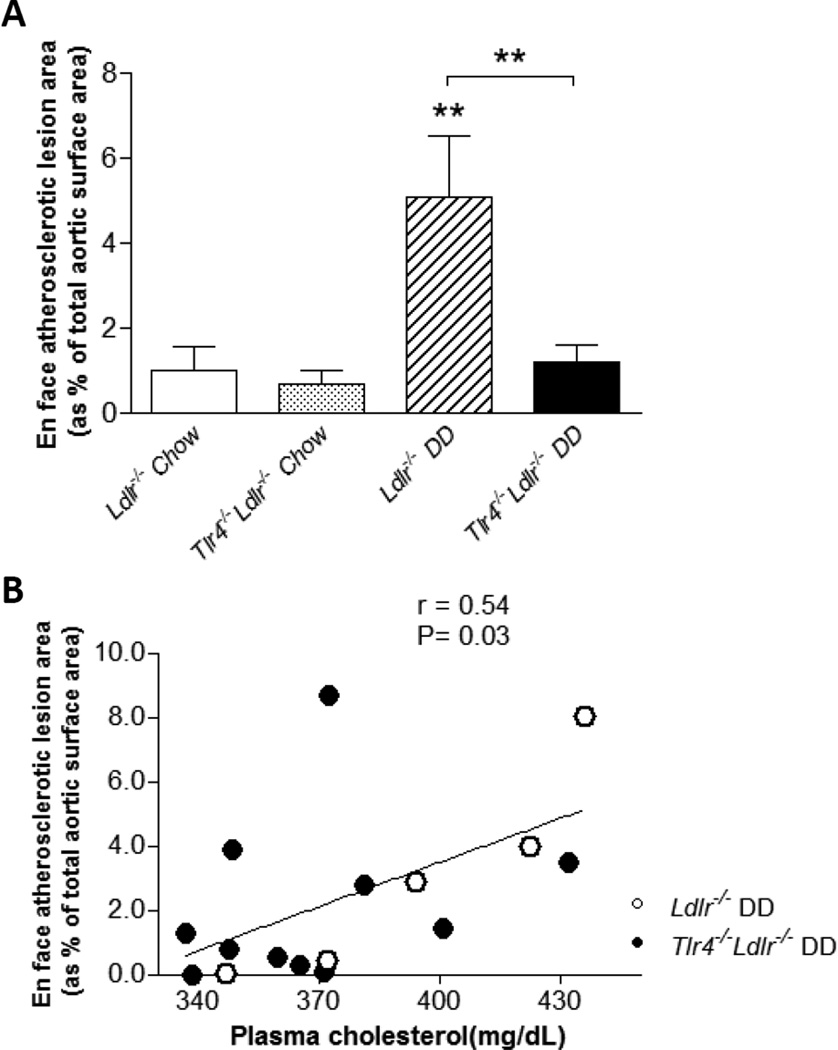
We confirmed that Ldlr−/− mice fed DD for 24 weeks have significantly increased atherosclerotic lesion area as compared to chow-fed animals25 (Figure 6A, p<0.01). However, DD-fed Tlr4−/−Ldlr−/− mice had markedly reduced (by 76%) aortic atherosclerotic lesion area compared to obese Ldlr−/− mice (Figure 6A, p<0.01).
Figure 6. Aortic atherosclerosis is significantly decreased in obese TLR4 deficient mice and correlates with plasma cholesterol level.
By the en face method, aortic atherosclerosis, expressed as % of total aortic surface area, does not differ between chow-fed Ldlr−/− (open bar) or chow-fed Tlr4−/−Ldlr−/− (grey bar) mice (A). However, in Ldlr−/− mice fed DD, aortic lesion area is significantly reduced in the Tlr4−/− group (black bar) as compared to Tlr4+/+ mice (hatched bar). (n=10–15, **P<0.01 vs DD or chow groups); For DD-fed mice Ldlr−/− (open circles) and Tlr4−/−Ldlr−/− (filled circles), en face atherosclerotic lesion area correlated with plasma total cholesterol level (B).
To evaluate the contribution of plasma cholesterol and triglycerides to atherosclerosis in these two groups of DD-fed animals, we performed linear regression analysis. Atherosclerotic lesion area correlated with plasma cholesterol (Figure 6B, r = 0.54, p = 0.03), but not with plasma triglycerides level (data not shown), suggesting improved plasma cholesterol might be one of the factors that contribute to the reduced atherosclerosis in Tlr4−/−Ldlr−/− mice fed DD.
Discussion
Our findings demonstrate that TLR4 deficiency led to ~75% reduction in the extent of atherosclerosis without parallel changes in adipose tissue inflammation and insulin sensitivity in LDL receptor deficient mice fed a diabetogenic diet high in saturated fat and sucrose. Activation of TLR4 by saturated fatty acids has been suggested to play a key role in causing insulin resistance mediated by saturated fat-rich diets. Such diets, when fed to LDL receptor deficient mice, lead to many features of metabolic syndrome such as obesity, tissue inflammation, insulin resistance and atherosclerosis. The reduction in atherosclerosis seen in TLR4/LDL receptor double deficient mice in the present study was associated with improved hyperlipidemia and increased expression of TLR2, but not with reduced adipose tissue inflammation or improved insulin sensitivity.
Chronic low-grade tissue inflammation has recently garnered considerable attention as an important contributor to insulin resistance in obesity. This inflammation is associated with macrophage accumulation in intra-abdominal adipose tissue and increased expression of various cytokines produced by these macrophages. Toll-like receptors play a critical role in activating the innate immune response and have been implicated in the induction of insulin resistance and atherosclerosis in obesity. In particular, TLR4 is an attractive candidate because it is highly expressed in macrophages and adipose tissue; TLR4 also is activated by fatty acids, which are increased in obesity11. Moreover, whole body TLR4 deficiency has been reported to be associated with improved insulin sensitivity in mice fed a high fat diet, without affecting body weight10. We therefore hypothesized that TLR4 deletion would attenuate obesity, adipose tissue macrophage accumulation, insulin resistance and subsequently, reduce atherosclerosis in LDL receptor deficient mice. While loss of TLR4 led to a significant reduction in atherosclerosis and hyperlipidemia, it did not decrease adipose tissue macrophage accumulation, nor did it significantly improve glucose or insulin tolerance. These findings suggest that the reduction of atherosclerosis is unlikely to be due to amelioration of whole-body insulin sensitivity or adipose tissue inflammation, but rather is due to a reduction in lipid levels and a direct effect of TLR4 on atherosclerosis per se.
Whether TLR4 deletion has a beneficial effect on obesity-related inflammation is unclear. Some early studies showed that mice with TLR4 deficiency or a loss-of-function mutation in TLR4 are protected against obesity-induced insulin resistance10,16–18,21. However, later studies have failed to confirm these findings with respect to the effect of TLR4 deficiency on body weight, insulin sensitivity and macrophage accumulation in adipose tissue19,20,22–24. Davis et al. found that TLR4 deficiency does not completely attenuate adipose tissue inflammation as indicated by the TNF-α or interleukin-6 expression in adipose tissue20. By transplanting Tlr4−/− bone marrow into non-obese Ldlr−/− Agouti mice, Coenen et al. reported a moderate decrease in both atherosclerosis and adipose tissue macrophage content only in low-fat fed female mice, without any changes in body composition, insulin sensitivity and plasma lipids19. In addition, several recent studies report an adverse effect of TLR4 deficiency on insulin resistance and other metabolic disease22–24. TLR4 mutated mice fed with a diet rich in trans-fatty acids were found to actually have exacerbated phenotypes of obesity, hyperglycemia and hyperinsulinemia22. Also, the same authors showed that loss of TLR5 in mice already deficient in either TLR4 or TLR2 still led to obesity, hyperlipidemia and insulin resistance23. Furthermore, Hosoi et al. reported a quite unexpected diabetic phenotype in MyD88-deficient mice fed high-fat diet24. Potential reasons for the variability between these studies include differences in weight, gender or strain of mice used, the nature of the genetic alteration in TLR4, differences in the composition or duration of the high fat diet, or difference in body weight or fat mass. All of these might alter assessment of insulin resistance and/ or adipose tissue. Since insulin levels were reduced at 12 but not 24 weeks in our study, it is conceivable that an effect of TLR4 deficiency on adipose tissue and liver might have been missed by our focusing our studies on this later time point. Future studies should include a time course to insure that TLR4 deficiency does not lead changes in adipose tissue inflammation and insulin resistance at early time points. However, other investigators also have failed to show a beneficial effect of TLR4 deficiency after 12 weeks of feeding a high fat diet19,22. The diet used in our study also contained fructose (in the form of sucrose). Since fructose can cause significant insulin resistance in rodents35,36, it is possible that any potential beneficial effects of TLR4 deficiency on insulin resistance might have been offset by adverse effects of the fructose in the diet. The cholesterol content of the diet also might matter, since dietary cholesterol itself can contribute to macrophage accumulation in white adipose tissue25, and the interaction between dietary cholesterol and fatty acids is still largely unexplored. Another limitation of our study is that we did not specifically measure the phenotypes of macrophages in adipose tissue by FACS analysis. Although the lack of difference in a limited number of mRNA M1 and M2 macrophage markers in whole adipose tissue suggested that the macrophages observed are not of the alternatively-activated phenotype, future studies should include of macrophage phenotyping by FACS analysis of the stromal vascular fraction of adipose tissue.
In the present study, TLR4 deficient mice fed DD had increased expression of TLR2 in adipose tissue compared to controls. We confirmed this finding in TLR4-silenced 3T3-L1 adipocytes in vitro after exposure to conditions of excess glucose and palmitate. Similar results have been shown by others20,37. TLR2 is the most likely alternative toll-like receptor that may contribute to diet-induced inflammation in adipose tissue, because it is closely related to TLR4, and also has been reported to be activated by SFAs, although to a lesser extent than TLR432,38. Recently, others reported that TLR2 deficient mice show improvement in metabolic features associated with obesity induced by a high fat diet39–41. We speculate that the up-regulation of TLR2 expression in the absence of TLR4 might be a compensatory pathway that contributes to adipose tissue inflammation and insulin resistance observed in obese TLR4 deficient mice. Future studies in TLR4/TLR2 double deficient mice should help address this issue. It also is possible that the saturated fatty acid-rich diet may have altered the microflora in the gut, which in turn may have altered the intestinal barrier allowing translocation of microbial products leading to the activation of TLR4 independent pathways associated with inflammation and insulin resistance23.
To our knowledge, this is the first study to date to show a beneficial effect of TLR4 deficiency on atherosclerosis in LDL receptor deficient mice fed a diet rich in SFAs. The diabetogenic diet resulted in hypercholesterolemia and hypertriglyceridemia in these mice. TLR4 deficiency led to a significant reduction in plasma cholesterol and triglycerides levels due to reduced accumulation of lipoproteins in both LDL and, particularly, the VLDL/IDL density range. Similar findings have been reported by others in apoE deficient mice33,42, by unknown mechanisms, which should be addressed in future studies. Furthermore, in our study, the extent of atherosclerosis of the aortas showed a positive correlation with plasma cholesterol levels. Therefore, it is likely that reduction of these cholesterol-rich VLDL/IDL particles contributes to the effect of TLR4 deficiency on preventing atherosclerotic lesion formation in LDL receptor deficient mice. However, it is conceivable that TLR4 deficiency might in addition have direct effects on cells of the artery wall. Although we did not measure TLR expression in the artery wall in the present studies, evaluation of TLR expression in vascular tissues in future studies should help differentiate the effects observed on atherosclerosis from those observed in adipose tissue and liver, and help differentiate direct effects of TLR deficiency in arterial wall cells from those that result from alterations in plasma lipoproteins.
Our findings point to a pro-atherogenic role of TLR4, raising the important question of how TLR4 becomes activated and interferes with lipid metabolism in LDL receptor deficient mice. One possibility is that circulating LPS provides a chronic baseline stimulus to TLR4 signaling, as low levels of LPS can be found in the serum of apparently healthy humans and laboratory mice, particularly those consuming high fat diets43,44. Administration of exogenous LPS, the TLR4 agonist, promoted atherosclerosis in rabbits and mice45,46 and in humans47. An alternative hypothesis is that nonmicrobial, endogenous TLR4 agonists contribute to a “sterile inflammatory response”. Candidate TLR4 agonists include minimally or moderately oxidized forms of LDL, which stimulate TLR4-dependent fluid phase LDL uptake, a potential mechanism of foam cell formation and expression of multiple chemokines48,49. Further investigations will be needed to evaluate these possibilities.
In summary, we have demonstrated that TLR4 deficiency decreases atherosclerosis without affecting macrophage accumulation and insulin resistance in adipose tissue and liver in LDL receptor deficient mice. Collectively these data indicate the importance of innate immunity and plasma cholesterol in the induction of atherosclerosis in obesity. Our data also identify TLR4 as a potential target for the therapeutic treatment of atherosclerosis.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge Drs. Linda Curtiss and Peter Tobias for providing the Tlr4−/−Ldlr−/− mice.
Sources of Funding
This work was supported by an NIH grants HL007028, HL94352, HL092969. Body composition and lipoprotein analysis were supported by the Nutrition Obesity Research Center, University of Washington (DK035816).
Abbreviations
- TLR
toll-like receptor
- TLR4
toll-like receptor 4
- TLR2
toll-like receptor 2
- LDLR
low density lipoprotein-receptor
- DD
diabetogenic diet
- FFA
free fatty acids
- SFAs
saturated fatty acids
- RT-PCR
real time polymerase chain reaction
- Tnfα
tumor necrosis factor-α
- Il6
interleukin-6
- Saa
serum amyloid A
- Mcp1
monocyte chemoattractant protein 1
- Nos2
nitric oxide synthase 2
- Arg1
arginase 1
- Fizz1(RETNLB)
resistin like beta
- Hmgcr
3-hydroxy-3-methylglutaryl-CoA reductase
- Dgat1
diacylglycerol O-acyltransferase 1
- Acat1
acetyl-CoA acetyltransferase 1
- Srebp1c
sterol regulatory element binding transcription factor 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None
References
- 1.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 2.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 4.Pouliot MC, Després JP, Nadeau A, Moorjani S, Prud'Homme D, Lupien PJ, Tremblay A, Bouchard C. Visceral obesity in men: associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–834. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- 5.Carey VJ, Walters EE, Colditz GA, Solomon CG, Willett WC, Rosner BA, Speizer FE, Manson JE. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women: the nurse's health study. Am J Epidemiol. 1997;145:614–619. doi: 10.1093/oxfordjournals.aje.a009158. [DOI] [PubMed] [Google Scholar]
- 6.Rocha VZ, Lippy P. Obesity, inflammation and atherosclerosis. Nat Rev Cardiol. 2009;6:399–409. doi: 10.1038/nrcardio.2009.55. [DOI] [PubMed] [Google Scholar]
- 7.Shah A, Mehta N, Reilly MP. Adipose inflammation, insulin resistance, and cardiovascular disease. JPEN J Parenter Enteral Nutr. 2008;32:638–644. doi: 10.1177/0148607108325251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razani B, Chakravarthy MV, Semenkovich CF. Insulin resistance and atherosclerosis. Endocrinol Metab Clin North Am. 2008;37:603–621. doi: 10.1016/j.ecl.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 12.Huang D. Modulation of the expression of cyclooxygenase-2 by fatty acids mediated through toll-like receptor4-derived signaling pathways. Faseb J. 2001;15:2556–2564. doi: 10.1096/fj.01-0432com. [DOI] [PubMed] [Google Scholar]
- 13.Lee JY, Ye J, Gao Z, Youn HS, Lee WH, Zhao L, Sizemore N, Hwang DH. Reciprocal modulation of toll-like receptor 4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. J Biol Chem. 2003;278:37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 14.Suganami T, Tanimoto-Koyama K, Nishida J, Itoh M, Yuan X, Mizuarai S, Kotani H, Yamaoka S, Miyake K, Aoe S, Kamei Y, Ogawa Y. Role of the toll-like receptor 4/NF-kappaB pathway in saturated fatty acid-induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler Thromb Vasc Biol. 2007;27:84–91. doi: 10.1161/01.ATV.0000251608.09329.9a. [DOI] [PubMed] [Google Scholar]
- 15.Han CY, Kargi AY, Omer M, Chan CK, Wabitsch M, O'Brien KD, Wight TN, Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes- Dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59:386–396. doi: 10.2337/db09-0925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C, Peiretti F, Verdier M, Juhan-Vague I, Tanti JF, Burcelin R, Alessi MC. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a higjh-fat diet. Diabetologia. 2007;50:1267–1276. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- 17.Saberi M, Woods NB, de Luca C, Schenk S, Lu JC, Bandyopadhyay G, Verma IM, Olefsky JM. Hematopoietic cell-specific deletion of toll-like receptor 4 ameliorates hepatic and adipose tissue insulin resistance in high-fat-fed mice. Cell Metab. 2009;10:419–429. doi: 10.1016/j.cmet.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsukumo DM, Carvalho-Filho MA, Carvalheira JB, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassallo J, Curi R, Velloso LA, Saad MJ. Loss-of-function mutation in toll-like receptor 4 prevents diet-induced obesity and insulin resistance. Diabetes. 2007;56:1986–1998. doi: 10.2337/db06-1595. [DOI] [PubMed] [Google Scholar]
- 19.Coenen KR, Gruen ML, Lee-Young RS, Puglisi MJ, Wasserman DH, Hasty AH. Impact of macrophage toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia. 2009;52:318–328. doi: 10.1007/s00125-008-1221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity. 2008;16:1248–1255. doi: 10.1038/oby.2008.210. [DOI] [PubMed] [Google Scholar]
- 21.Suganami T, Mieda T, Itoh M, Shimoda Y, Kamei Y, Ogawa Y. Attenuation of obesity-induced adipose tissue inflammation in C3H/HeJ mice carrying a toll-like receptor 4 mutation. Biochem Biophys Res Commun. 2007;354:45–49. doi: 10.1016/j.bbrc.2006.12.190. [DOI] [PubMed] [Google Scholar]
- 22.Vijay-Kumar M, Aitken JD, Carvalho FA, Ziegler TR, Gewirtz AT, Ganji V. Loss of function mutation in toll-like receptor-4 does not offer protection against obesity and insulin resistance induced by a diet high in trans fat in mice. J Inflamm. 2011;8:2. doi: 10.1186/1476-9255-8-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosoi T, Yokoyama S, Matsuo S, Akira S, Ozawa K. Myeloid differentiation factor 88 (MyD88) - deficiency increases risk of diabetes in mice. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0012537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A, 3rd, Kirk EA, O'Brien KD, Chait A. Dietary cholesterol worsens adipose tissue inflammation accumulation and atherosclerosis in obese LDL receptor deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:685–691. doi: 10.1161/ATVBAHA.107.157685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis KE, Kirk EA, McDonald TO, Wang S, Wight TN, O'Brien KD, Chait A. Increase in serum amyloid a evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 2004;110:540–545. doi: 10.1161/01.CIR.0000136819.93989.E1. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien KD, McDonald TO, Kunjathoor V, Eng K, Knopp EA, Lewis K, Lopez R, Kirk EA, Chait A, Wight TN, deBeer FC, LeBoeuf RC. Serum amyloid A and lipoprotein retention in murine models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:785–790. doi: 10.1161/01.ATV.0000158383.65277.2b. [DOI] [PubMed] [Google Scholar]
- 28.Chen HC, Farese RV., Jr Determination of adipocyte size by computer image analysis. J Lipid Res. 2002;43:986–989. [PubMed] [Google Scholar]
- 29.Han CY, Subramanian S, Chan CK, Omer M, Chiba T, Wight TN, Chait A. Adipocyte-derived serum amyloid A3 and hyaluronan play a role in monocyte recruitment and adhesion. Diabetes. 2007;56:2260–2273. doi: 10.2337/db07-0218. [DOI] [PubMed] [Google Scholar]
- 30.Han CY, Chiba T, Campbell JS, Fausto N, Chaisson M, Orasanu G, Plutzky J, Chait A. Reciprocal and coordinate regulation of serum amyloid A versus apolipoprotein A–I and paraoxonase-1 by inflammation in murine hepatocytes. Arterioscler Thromb Vasc Biol. 2006;26:1806–1813. doi: 10.1161/01.ATV.0000227472.70734.ad. [DOI] [PubMed] [Google Scholar]
- 31.Schreyer SA, Lystig TC, Vick CM, LeBoeuf RC. Mice deficient in apolipoprotein E but not LDL receptors are resistant to accelerated atherosclerosis associated with obesity. Atherosclerosis. 2003;171:49–55. doi: 10.1016/j.atherosclerosis.2003.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Lee JY, Zhao L, Youn HS, Weatherill AR, Tapping R, Feng L, Lee WH, Fitzgerald KA, Hwang DH. Saturated fatty acid activates but polyunsaturated fatty acid inhibits toll-like receptor 2 dimerized with toll-like receptor 6 or 1. J Biol Chem. 2004;279:16971–16979. doi: 10.1074/jbc.M312990200. [DOI] [PubMed] [Google Scholar]
- 33.Higashimori M, Tatro JB, Moore KJ, Mendelsohn ME, Galper JB, Beasley D. Role of toll-like receptor 4 in intimal foam cell accumulation in apolipoprotein E deficient mice. Arterioscler Thromb Vasc Biol. 2010;31:50–57. doi: 10.1161/ATVBAHA.110.210971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schreyer SA, Vick C, Lystig TC, Mystkowski P, LeBoeuf RC. LDL receptor but not apolipoprotein E deficiency increases diet-induced obestiy and diabetes in mice. Am J Physiol Endocrinol Meb. 2002;282:E207–E214. doi: 10.1152/ajpendo.2002.282.1.E207. [DOI] [PubMed] [Google Scholar]
- 35.Samuel VT. Fructose induced lipogenesis: from sugar to fat to insulin resistance. Trends Endocrinol Metab. 2011;22:60–65. doi: 10.1016/j.tem.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev. 2010;90:23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 37.Devaraj S, Tobias P, Kasinath BS, Ramsamooj R, Afify A, Jialal I. Knockout of toll-like receptor 2 attenuates both the proinflammatory state of diabetes and incipient diabetic nephropathy. Arterioscler Thromb Vasc Biol. 2011;31:1796–1804. doi: 10.1161/ATVBAHA.111.228924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via toll-like receptor 2 and 4 and JNK-dependent pathways. J Bio Chem. 2007;282:35279–35292. doi: 10.1074/jbc.M706762200. [DOI] [PubMed] [Google Scholar]
- 39.Himes RW, Smith CW. Tlr2 is critical for diet-induced metabolic syndrome in a murine model. Faseb J. 2010;24:731–739. doi: 10.1096/fj.09-141929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ehses JA, Meier DT, Wueest S, Rytka J, Boller S, Wielinga PY, Schraenen A, Lemaire K, Debray S, Van Lommel L, Pospisilik JA, Tschopp O, Schultze SM, Malipiero U, Esterbauer H, Ellingsgaard H, Rütti S, Schuit FC, Lutz TA, Böni-Schnetzler M, Konrad D, Donath MY. Toll-like receptor 2-deficient mice are protected from insulin resistance and beta cell dysfunction induced by a high-fat diet. Diabetologia. 2010;53:1795–1806. doi: 10.1007/s00125-010-1747-3. [DOI] [PubMed] [Google Scholar]
- 41.Mullick AE, Tobias PS, Curtiss LK. Modulation of atherosclerosis in mice by Toll-like receptor 2. J Clin Invest. 2005;115:3149–3156. doi: 10.1172/JCI25482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plague phenotype in mice deficient in apolipoprotein E. Proc Natl Acad Sci U S A. 2004;101:10679–10684. doi: 10.1073/pnas.0403249101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 44.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferriéres J. Energy intake is associated with endotoxemia in apparetly health men. Am J Clin Nutr. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 45.Ostos MA, Recalde D, Zakin MM, Scott-Algara D. Implication of natural killer T cells in atherosclerosis development during a LPS-induced chronic inflammation. FEBS Lett. 2002;519:23–29. doi: 10.1016/s0014-5793(02)02692-3. [DOI] [PubMed] [Google Scholar]
- 46.Lehr HA, Sagban TA, Ihling C, Zähringer U, Hungerer KD, Blumrich M, Reifenberg K, Bhakdi S. Immunopathogenesis of atherosclerosis: endotoxin accelerates atherosclerosis in rabbits on hypercholesterolemic diet. Circulation. 2011;104:914–920. doi: 10.1161/hc3401.093153. [DOI] [PubMed] [Google Scholar]
- 47.Stoll LL, Denning GM, Weintraub NL. Potential role of endotoxin as a proinflammatory mediator of atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:2227–2236. doi: 10.1161/01.ATV.0000147534.69062.dc. [DOI] [PubMed] [Google Scholar]
- 48.Choi SH, Harkewicz R, Lee JH, Boullier A, Almazan F, Li AC, Witztum JL, Bae YS, Miller YI. Lipoprotein accumulation in macrophages via toll-like receptor 4-dependent fluid phase uptake. Circ Res. 2009;104:1355–1363. doi: 10.1161/CIRCRESAHA.108.192880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, El Khoury J, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.