Abstract
Objective
Mortality associated with acute lung injury (ALI) remains high. Early identification of ALI prior to onset of respiratory failure may provide a therapeutic window to target in future clinical trials. The recently validated Lung Injury Prediction Score (LIPS) identifies patients at risk for ALI but may be limited for routine clinical use. We sought to empirically derive clinical criteria for a pragmatic definition of Early Acute Lung Injury (EALI) to identify patients with lung injury prior to the need for positive pressure ventilation.
Design
Prospective observational cohort study.
Setting
Stanford University Hospital.
Patients
We prospectively evaluated 256 patients admitted to Stanford University Hospital with bilateral opacities on chest radiograph without isolated left atrial hypertension.
Interventions
None.
Measurements and Main Results
Of the 256 patients enrolled, 62 (25%) progressed to ALI requiring positive pressure ventilation. Clinical variables (through first 72 hours or up to 6 hours prior to ALI) associated with progression to ALI were analyzed by backward regression. Oxygen requirement, maximal respiratory rate, and baseline immune suppression were independent predictors of progression to ALI. A simple 3 component EALI score (1 point for oxygen requirement > 2 to 6 liters/min or 2 points for > 6 liters/min; and 1 point each for a respiratory rate ≥ 30 and immune suppression) accurately identified patients who progressed to ALI requiring positive pressure ventilation (AUC 0.86) and performed similarly to the LIPS. An EALI score ≥ 2 identified patients who progressed to ALI with 89% sensitivity and 75% specificity. Median time of progression from EALI criteria to ALI requiring positive pressure ventilation was 20 hours.
Conclusions
This pragmatic definition of EALI accurately identified patients who progressed to ALI prior to requiring positive pressure ventilation. Pending further validation, these criteria could be useful for future clinical trials targeting early treatment of ALI.
Keywords: acute lung injury, acute respiratory distress syndrome, early diagnosis, cohort study, critical care, emergency medicine
INTRODUCTION
The American-European Consensus Conference (AECC) defines acute lung injury (ALI) as acute respiratory failure with bilateral pulmonary infiltrates and PaO2/FiO2 (P/F) ratio < 300 in the absence of left atrial hypertension.[1] Clinical trials in ALI have been primarily limited to mechanically ventilated patients.[2-8] Likewise, the two most rigorous studies of the incidence and outcomes of ALI only included patients receiving positive pressure ventilation via an endotracheal tube or face mask.[9, 10] However, there has been increasing recognition that the process of ALI is often occurring in spontaneously breathing patients outside of the intensive care unit (ICU).[11-13]
Despite extensive investigation over the past 15 years, a lung protective strategy of mechanical ventilation remains the only disease-specific therapy shown to improve survival.[2] Numerous pharmacologic treatments have failed to improve survival in multicenter trials.[14, 15] While limiting the diagnosis of ALI to patients receiving mechanical ventilation helps standardize patients for clinical trials, it may prevent initiation of therapies in an earlier and, potentially more treatable phase of acute lung injury. Following the paradigm of early goal-directed therapy for severe sepsis[16], identifying patients and initiating treatment prior to the need for positive pressure ventilation may improve clinical outcomes. However, direct extrapolation of the AECC criteria to spontaneously breathing patients may not identify a sufficiently high risk or homogenous population to warrant enrollment in clinical trials.
Recently, the United States Critical Illness and Investigation (USCIIT) group derived and validated the Lung Injury Prediction Score (LIPS) to identify patients at an increased risk for developing ALI.[17] However, the LIPS requires inclusion of a broad array of risk factors and risk modifiers that may be challenging to calculate in clinical practice. Also, the LIPS was designed to identify at-risk patients prior to the onset of lung injury and thus identified a relatively low-risk patient population (positive predictive value for developing ALI only 18% at the recommended cut-off of > 4). In contrast, our goal was to empirically derive pragmatic criteria for Early Acute Lung Injury (EALI) which identifies patients with early but existing lung injury. These patients maybe at higher risk of developing ALI requiring positive pressure ventilation and thus, be more appropriate targets for future clinical research. We previously published criteria for EALI based only on assessment in the emergency department.[18] However, physiologic markers of developing lung injury may not be apparent at time of admission, thus limiting their predictive value and increasing reliance on other risk factors and risk modifiers. We hypothesized that following patients prospectively beyond hospital admission would improve the performance of a simple physiology based scoring system and still allow identification of patients prior to the need for positive pressure ventilation. Therefore, we conducted a prospective cohort study evaluating clinical variables predictive of progression to ALI for up to 72 hours in patients with evidence of lung injury on admission chest radiograph.
MATERIALS AND METHODS
Study Population
Study physicians screened all adult chest radiographs done in the emergency department at Stanford University hospital. A qualifying chest radiograph was defined as bilateral opacities (including equivocal findings of interstitial opacities consistent with pulmonary edema, bibasilar opacities consistent with either atelectasis or consolidation, and/or effusions with possible adjacent consolidation) present for less than 7 days. The formal interpretation by chest radiologists was used for screening. The primary author (JEL), who completed the ARDS Network online training, reviewed all films prior to enrollment (as well as all films to qualifying for ALI). Patients admitted with an abnormal chest radiograph not meeting criteria (i.e. unilateral abnormalities, or a reading of minimal bibasilar opacities without other signs or symptoms of lung injury) were followed and enrolled if they progressed to a subsequent qualifying film within 72 hours. Other inclusion criteria were age ≥ 18 years and hospital admission.
Exclusion criteria were endotracheal intubation or meeting ALI criteria with noninvasive ventilation prior to leaving the emergency department, clinical evidence of left atrial hypertension (pulmonary capillary wedge pressure >18 or a right atrial pressure > 14 mmHg, echocardiographic evidence of new or worsening left ventricular dysfunction; N-terminal pro-B-type natriuretic peptide > 400 pg/ml; or criteria for acute coronary syndrome[19]); severe chronic lung or neuromuscular disease with respiratory failure as defined by the NHLBI ARDS Network[5]; pregnancy; and patient/family refusal of positive pressure ventilation.
Because up to 30% of patients with ALI may have concomitant volume overload[5], patients with suspected left atrial hypertension (by the above criteria) were eligible if they had an admission diagnosis of pneumonia [defined by focal airspace opacities on chest radiograph or purulent sputum and an abnormal temperature (< 36 or > 38°C) or white blood cell count (WBC > 12,000, < 4,000 or > 10% bands)] or sepsis (defined by criteria for the systemic inflammatory response syndrome[20] and a known infectious etiology).
Sixty-five patients in the current 256 patient cohort were also included in our previous study of 100 patients assessed at presentation to the emergency department.[18] However, our prior analysis only included data available within the first six hours of presentation. We performed a sensitivity analysis by removing these patients from the analysis.
Data Collection
Demographic characteristics, comorbidities (defined in online supplement), and admission diagnoses were collected at the time of enrollment. Physiologic [highest heart rate, highest respiratory rate, oxygen requirement, abnormal temperature (< 36 ° C or > 38° C), sepsis and shock] and laboratory [abnormal white blood cell count (< 4,000, > 12,000, or > 10% bands) and culture data] variables were collected prospectively up to 6 hours prior to progression to ALI or for the first 72 hours following the first qualifying chest radiograph (whichever came first). We selected this time interval because most patients progress to ALI within 72 hours, and we thought 6 hours was the minimal clinically relevant time period to allow initiation of treatment to prevent progression. ALI time was defined as the first time patients met AECC criteria with a P/F ratio less than < 300 while receiving positive pressure ventilation. As previously reported, oxygen requirement was defined categorically as the level of supplemental oxygen (room air, ≤ 2, > 2 to 6, and > 6 liters/min) required to maintain a peripheral oxygen saturation ≥ 90%.[18] For patients whose peripheral oxygen saturation was consistently ≥ 90% while receiving between 2 and 6 liters/min, study physicians went to the bedside once daily to titrate down the level of supplemental oxygen (over approximately 5 – 10 minutes) to accurately determine the minimum oxygen requirement. For safety reasons, patients already receiving > 6 liters/min or facemask oxygen were categorized as > 6 liters/min and not titrated. For prospectively collected physiologic variables, we selected the most abnormal value or category observed over the data collection period for inclusion in both univariable and multivariable analyses. For the composite EALI score, independent physiologic predictors (respiratory rate and oxygen requirement) were analyzed as the most abnormal value occurring in the same calendar day with the composite score being the highest daily score. After establishing an optimal cut-off, we subsequently identified the EALI time as the earliest time an EALI score ≥ 2 occurred with all contributing components met simultaneously. Subjects were followed until hospital discharge for the primary outcome of progression to ALI (defined by AECC criteria while receiving positive pressure ventilation through an endotracheal tube or face mask). Following the recent publication of the Lung Injury Prediction Score (LIPS), a LIPS score was retrospectively calculated for all patients for whom sufficient data was available in the medical record (248/256 patients).
The institutional review board at Stanford University Medical Center approved the study with a waiver of consent for the available clinical data analyzed in this study. Informed consent was obtained for patients requiring bedside oxygen titration.
Statistical Analysis
Categorical variables were analyzed by chi square and Fisher exact tests. Continuous variables were analyzed by t-tests for normally distributed data (mean ± standard deviation) and Wilcoxon rank sum tests for non-normally distributed data (median, interquartile range). Independent predictors of progression to ALI were identified by backward stepwise regression (significance for selection p < 0.05) of all variables associated (p < 0.05) with progression to ALI on univariable analysis. For the composite EALI score, patients received 1 point for the presence of each independent risk factor. Respiratory rate was included as a dichotomous variable at a previously validated cut-off of ≥ 30 breaths/min [17, 21] and oxygen requirement as a categorical variable (1 point for between 2 and 6 liters/min and an additional point for > 6 liters/min) since these categories were both significant on multivariable regression. This simplified scoring system was compared to a score with points assigned by the coefficients (rounded to the nearest integer) from the multivariable regression. While calibration was predictably better with the coefficient based score (Hosmer-Lemeshow chi square 0.11 vs. 3.5) both scores showed adequate calibration (p = 0.99 and 0.32, respectively) (Figure E1 - Electronic Supplement) and similar discrimination (AUC 0.85 for both). Since our focus was identifying cases of ALI (i.e., discrimination) and to avoid potential overfitting with the coefficient based score, only the simplified score results are presented here. Discrimination and calibration of the EALI score for identifying patients who progressed to ALI were compared to the LIPS and the Acute Physiology and Chronic Health Evaluation (APACHE) II score by the area under the receiver-operator characteristic curve (AUC) and Hosmer-Lemeshow statistics, respectively. Predicted probabilities for all model discrimination and calibration were calculated using 10-fold cross validation. Primary analyses were performed using SAS Enterprise Guide 4.2 (Cary, North Carolina). Prediction assessment was performed in R 2.14. Study data were collected and managed using REDCap electronic data capture tools hosted at Stanford University.
RESULTS
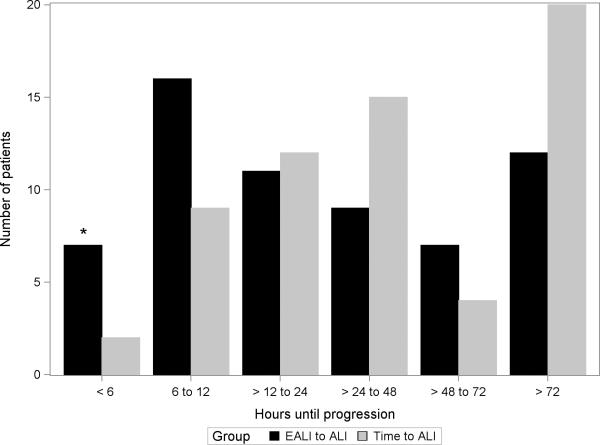
During 803 days of screening, we reviewed 32,341 chest radiographs. Of 5,545 patients with an abnormal chest radiograph, 256 were enrolled with bilateral opacities not due exclusively to left atrial hypertension. Of these, 62 (25%) progressed to ALI requiring positive pressure ventilation (Figure 1). Median time of progression to ALI from the initial qualifying chest radiograph was 37 (IQR 15 – 81) hours; however, 20 patients (31%) progressed after 72 hours (Figure 2).
Figure 1.
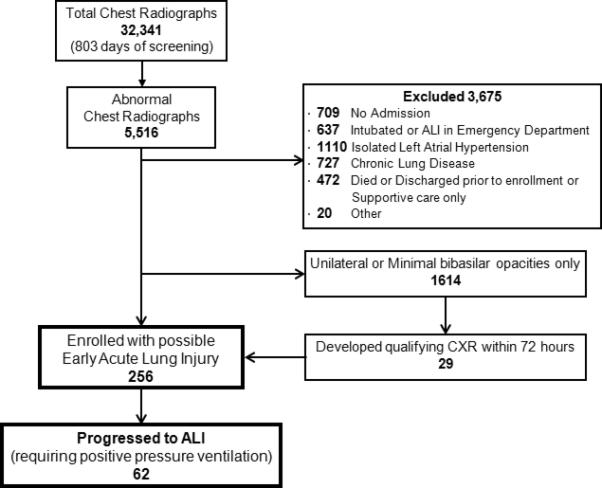
Flow diagram of patient selection. ALI, acute lung injury
Figure 2.
Histogram of time of progression to acute lung injury (ALI) for the 62 patients who progressed. Time to ALI, time from enrollment with a qualifying chest radiograph to meeting ALI criteria while receiving positive pressure ventilation; EALI to ALI, time from meeting early acute lung injury criteria (EALI score ≥ 2) to ALI (*the “< 6 hours” column includes the 7 patients who did not qualify for EALI at least 6 hours prior to meeting ALI criteria, i.e., false negatives).
Baseline characteristics are shown in Table 1. Rates of immune suppression (as defined by APACHE II[22]), do not intubate (DNI) status (allowed if patients were willing to receive noninvasive ventilation), and LIPS and APACHE II scores were higher among patients who progressed to ALI. There was no significant difference in admission diagnosis between groups, with pneumonia and non-pulmonary infections accounting for 84% of all diagnoses. Clinical variables associated with progression to ALI are highlighted in Table 2. Analyzed as a dichotomous variable, oxygen requirement cut-offs of > 2 liters/min and > 6 liters/min showed similar discrimination (AUC 0.77 and 0.78, respectively) with > 2 liters/min being more sensitive (90% vs. 69%) and > 6 liters/min more specific (89% vs. 64%).
Table 1.
Baseline characteristics
| Characteristic | No ALI (n = 194) | ALI (n = 62) | p value |
|---|---|---|---|
| Age (n ± SD) | 63 ± 18 | 64 ± 17 | 0.73 |
| Male (n, %) | 109 (56%) | 34 (55%) | 0.85 |
| Hispanic (n, %) | 19 (10%) | 9 (15%) | 0.18 |
| Race (n, %) | 0.43 | ||
| White | 130 (67%) | 34 (55%) | |
| African American | 18 (9%) | 7 (11%) | |
| Asian | 20 (10%) | 11 (18%) | |
| Hawaiian/PI | 4 (2%) | 2 (3%) | |
| Unknown | 22 (11%) | 5 (8%) | |
| Comorbidities (n, %) | |||
| Cardiac | 37 (19%) | 17 (27%) | 0.16 |
| COPD | 20 (10%) | 3 (5%) | 0.2 |
| Diabetes | 45 (23%) | 16 (26%) | 0.67 |
| CRI | 30 (16%) | 14 (23%) | 0.19 |
| ESLD | 7 (5%) | 5 (8%) | 0.14 |
| Immune suppression | 63 (32%) | 31 (50%) | 0.01 |
| Volume Overload | 26 (13%) | 13 (20%) | 0.15 |
| DNI (n, %) | 12 (6%) | 12 (19%) | 0.002 |
| Diagnosis (n, %) | 0.92 | ||
| Pneumonia | 119 (61%) | 40 (65%) | |
| Aspiration | 10 (5%) | 2 (3%) | |
| Non-pulmonary Infection | 45 (23%) | 12 (19%) | |
| Trauma | 5 (3%) | 2 (3%) | |
| Transfusion reaction | 1 (0.5%) | 0 | |
| Idiopathic | 1 (0.5%) | 1 (2%) | |
| Other | 13 (7%) | 6 (10%) | |
| LIPS | 5 ± 2 | 8 ± 2 | < 0.001 |
| APACHE II | 13 ± 6 | 18 ± 8 | < 0.001 |
COPD, chronic obstructive pulmonary disease; CRI, chronic renal insufficiency; ESLD, end-stage liver disease; DNI, do not intubate order; TRALI, transfusion relate acute lung injury; LIPS, lung injury prediction score; APACHE II, acute physiology and chronic health evaluation II score
Table 2.
Prospectively collected physiologic and laboratory variables
| Characteristic | No ALI (n = 194) | ALI (n = 62) | P value |
|---|---|---|---|
| Oxygen Requirement | < 0.001 | ||
| Room air | 62 (32%) | 2 (3%) | |
| < 2 liters/min O2 | 62 (32%) | 4 (6%) | |
| > 2 to 6 liters/min O2 | 48 (25%) | 13 (21%) | |
| > 6 liters/min O2 | 22 (11%) | 43 (69%) | |
| Sepsis (n, %) | 149 (77%) | 46 (74%) | 0.67 |
| Shock (n, %) | 16 (8%) | 17 (28%) | < 0.001 |
| Respiratory Rate (n ± SD) | 26 ± 6 | 32 ± 7 | < 0.001 |
| Heart Rate (n ± SD) | 108 ± 21 | 117 ± 21 | 0.003 |
| Abnormal white blood cell (< 4,000 or >12,000) | 135 (70%) | 41 (66%) | 0.61 |
| Positive Cultures | |||
| Blood | 38 (20%) | 16 (25%) | 0.3 |
| Sputum | 14 (7%) | 10 (16%) | 0.04 |
| Urine | 30 (15%) | 8 (12%) | 0.62 |
| Any | 69 (36%) | 29 (47%) | 0.11 |
In multivariable analysis, supplemental oxygen requirement, maximal respiratory rate and baseline immune suppression were independently predictive of progression to ALI. When respiratory rate was included as a dichotomous variable (≥ 30 breaths/min) and oxygen requirement as a three-level categorical variable (≤ 2 liters/min; > 2 to 6 liters/min; and > 6 liters/min) along with baseline immune suppression, the model retained similar discrimination (AUC 0.886 vs. 0.894) compared to the full model (Table 3). When the 65 patients who were included in our prior analysis of emergency department data only were excluded, results were similar. However, while baseline immune suppression was associated with progression to ALI in univariable analysis (p = 0.04) and retained similar magnitude of effect in the multivariable model (odds ratio 2.1 vs. 2.4), it did not retain significance in the multivariable model restricted to 191 patients (p = 0.12 compared to p = 0.02 for all 256 patients).
Table 3.
Multivariable analysis of risk factors for progression to acute lung injury
| Risk Factor | Odds Ratio | 95% CI | P value |
|---|---|---|---|
| Supplemental Oxygen | |||
| ≤ 2 liters/min | reference | ||
| > 2 to 6 liters/min | 5.2 | 1.8 – 15 | 0.002 |
| > 6 liters/min | 33.7 | 12 – 93 | <0.0001 |
| Respiratory Rate ≥ 30 breaths/min | 2.9 | 1.4 – 6.2 | 0.006 |
| Immune suppression | 2.5 | 1.2 – 5.5 | 0.02 |
Variables selected by backward stepwise regression (significance ≤ 0.05) of significant variables on univariable analysis (respiratory rate, oxygen requirement, immune suppression, heart rate, abnormal temperature, DNI status, positive sputum culture and shock); 95% CI, 95% confidence interval
Clinical Prediction Models
A 3 component EALI score incorporating the independent risk factors for progression to ALI on multivariable regression (1 point for an oxygen requirement > 2 to 6 liters/min or 2 points for > 6 liters/min; and 1 point each for a respiratory rate ≥ 30 breaths/min and baseline immune suppression) accurately identified patients who progressed to ALI requiring positive pressure ventilation (AUC 0.85, 95% CI 0.80-0.91). Discrimination of the EALI score was similar to the LIPS and significantly outperformed the APACHE II by AUC analysis (Table 4 and Figure 3). An EALI score ≥ 2 identified patients who progressed to ALI with 89% sensitivity and 75% specificity. In this cohort (with a 25% incidence of ALI), this corresponded to positive and negative predictive values of 53% and 95%. By comparison, the positive and negative predictive values were 33% and 97% for a LIPS > 4 (recommended cut-off) and 46% and 92% for a LIPS > 6 (best performance in this cohort) (Table 4). Median time from EALI to meeting ALI criteria while receiving positive pressure ventilation was 20 (IQR 8 – 66) hours (Figure 2).
Table 4.
Comparison of scores for predicting progression to acute lung injury
| Continuous Score a | ||||
|---|---|---|---|---|
| Model | AUC (95% CI) | p valueb | Odds Ratioc (95% CI) | Calibrationd Hosmer-Lemeshow chi square (p value) |
| EALI | 0.85 (0.80 - 0.91) | Ref | 5.2 (3.5 - 7.9) | 3.5 (p = 0.32) |
| LIPS | 0.82 (0.76 - 0.88) | > 0.25 | 4.1 (2.7 - 6.3) | 5.8 (p = 0.12) |
| APACHE II | 0.66 (0.58 - 0.74) | < 0.001 | 2.0 (1.4 - 2.7) | 5.8 (p = 0.12) |
| Dichotomous Score | |||||
|---|---|---|---|---|---|
| Model | Sensitivity | Specificity | PPV | NPV | AUC |
| EALI score ≥ 2 | 89% | 75% | 53% | 95% | 0.82 |
| LIPS > 4e | 97% | 37% | 33% | 97% | 0.67 |
| LIPS > 6f | 82% | 70% | 46% | 92% | 0.76 |
EALI, Early Acute lung Injury; LIPS, APACHE II, Acute physiology and chronic health evaluation II score; Lung Injury Prediction Score; AUC, area under receiver-operator characteristic curve; PPV, positive predictive value; NPV, negative predictive value
Discrimination and calibration calculated using 10-fold cross validation
p value for AUC relative to EALI
Odds ratio per 1 standard deviation increase in risk score
Hosmer-Lemeshow chi square (lower score indicates higher calibration)
recommended LIPS cut-off[35]
best performance of LIPS in current cohort
Figure 3.
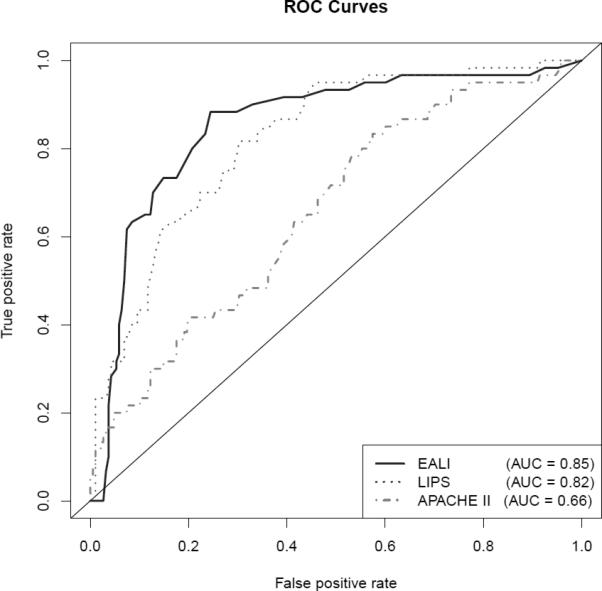
Comparison of receiver-operator characteristic (ROC) curves of scoring systems for predicting progression to acute lung injury while receiving positive pressure ventilation. Area under the curve (AUC) calculated using 10-fold cross validation. Analysis excludes 6 patients for whom sufficient data was not available to calculate a Lung Injury Prediction Score (LIPS). EALI, Early Acute Lung Injury score; APACHE II, Acute Physiology and Chronic Health Evaluation II score.
Outcomes
Outcomes of patients’ hospital admissions are shown in Table 5. Sixty-one of the 62 patients who progressed to ALI required ICU admission (one received noninvasive ventilation and had a qualifying blood gas outside the ICU). Of the patients who progressed to ALI, 42 (68%) received mechanical ventilation through an endotracheal tube while 20 patients (32%) received noninvasive ventilation only. Among patients who did not progress to ALI, 20% required ICU admission, and 2 (3%) received noninvasive ventilation (none were intubated). Direct admission from the emergency department to the ICU was more common among patients who progressed to ALI (53% vs. 16%, p < 0.0001), but nearly half of ALI patients were initially admitted to a non-ICU service. In-hospital mortality was 35% among patients who progressed to ALI compared with 3 deaths (2%) among non-progressors (p < 0.0001). Similarly, hospital length of stay was longer (14 vs. 5 days, p < 0.001) in patients who progressed to ALI and survivors were less likely to be discharged to home than non-progressors (63% vs. 86%, p = 0.002).
Table 5.
Outcomes of hospitalization by acute lung injury status
| Outcome | No ALI (n = 194) | ALI (n = 62) | P value |
|---|---|---|---|
| Time to ALI, hours (median, IQR) | NA | 37 (15, 81) | |
| ICU admission (n, %) | 39 (20%) | 61 (98%) | < 0.0001 |
| Direct ED to ICU | 31 (16%) | 33 (53%) | <0.0001 |
| Positive Pressure Ventilation | 2 (3%) | 62 (100%) | <0.0001 |
| Noninvasive Mask only | 2 (3%) | 20 (32%) | <0.0001 |
| Endotracheal tube | 0 | 42 (69%) | <0.0001 |
| Length of Stay, days (median, IQR) | 5 (3, 8) | 14 (8, 25) | < 0.0001 |
| Disposition | <0.001 | ||
| Home | 164 (85%) | 25 (40%) | |
| Died | 3 (2%) | 22 (35%) | |
| Skilled nursing | 25 (13%) | 11(18%) | |
| Other acute care | 2 (1%) | 4 (6%) |
ALI, acute lung injury; ICU, intensive care unit; ED, emergency department; IQR, interquartile range
DISCUSSION
We conducted a prospective cohort study to test whether patients with evidence of early lung injury could be accurately identified prior to progression to positive pressure ventilation. In contrast to previous work[11, 12, 17, 23, 24], our primary aim was not to identify risk factors for developing ALI, but instead to establish empiric criteria for a clinically relevant syndrome of Early Acute Lung Injury (EALI). In establishing a clinical definition of EALI, we attempted to preserve the principal components of the AECC criteria for ALI, minus mechanical ventilation and the need to calculate a P/F ratio. Thus, we only evaluated patients with pre-existing bilateral abnormalities on the chest radiograph. In this patient population, EALI defined by hospital admission with bilateral opacities on chest radiograph in the absence of isolated left atrial hypertension and an EALI score ≥ 2 identified patients who progressed to ALI requiring positive pressure ventilation with a sensitivity of 89% and a specificity of 75% and corresponded to positive and negative predictive values of 53% and 95%. Median time from meeting EALI criteria to progression to ALI was 20 hours suggesting a meaningful interval to allow initiation of early interventions. Interestingly, nearly a third of the cohort progressed to ALI > 72 hours after their initial qualifying chest radiograph. We hypothesize that many of these cases represent a “second-hit” phenomenon triggering progression.
Recently, the LIPS has been validated for risk stratification of patients presenting to the emergency department with risk factors for ALI. However, the LIPS may be difficult to calculate and, in a low risk patient population, the recommended cut-off of a LIPS > 4 had a positive predictive value for identifying cases of ALI of only 18%.[17] In contrast, our EALI score contains only 3 components (oxygen requirement, respiratory rate and immune suppression) and is designed for ease of use at the bedside. The success of the EALI score likely derives from the longitudinal evaluation of physiologic variables for potentially up to 6 hours prior to the onset of ALI. In contrast, the LIPS only includes variables present within the first 6 hours of admission. In this context, it is understandable that a scoring system would be more heavily influenced by multiple baseline risk factors and risk modifiers and less by acute pulmonary physiology predicting impending respiratory failure. However, the requirement for real-time recognition of qualifying chest radiographic abnormalities may add complexity to identifying the target population to which the EALI score is applicable relative to broadly applying LIPS to all emergency department patients with an identifiable risk factor.
We chose to evaluate supplemental oxygen requirements by novel but pre-defined criteria.[18] Rice et al have established criteria for ALI in mechanically ventilated patients based on an oxygen saturation to FiO2 (S/F) ratios.[25] However, accurate estimation of the FiO2 remains problematic in patients breathing in a non-closed system (i.e., not via a tight fitting facemask or endotracheal tube) and direct extrapolation of the P/F or S/F ratio to spontaneously breathing patients ignores the beneficial effects of positive pressure ventilation on lung recruitment and oxygenation. Instead, we classified the degree of oxygenation impairment by the level of supplemental oxygen required to maintain an oxygen saturation ≥ 90%. This pragmatic classification strongly predicted progression to ALI. Dichotomous cut-offs of > 2 liters/min and > 6 liters/min had similar discrimination (AUC 0.77 and 0.78, respectively) with > 2 liters/min being more sensitive and > 6 liters/min more specific. We suspect these levels of oxygen requirement are particularly useful because > 2 liters/min reflects a sufficient oxygenation impairment to exclude subjects with chest radiograph abnormalities primarily due to atelectasis but retains sensitivity for mild early lung injury, while > 6 liters/min accurately identifies patients failing supplemental oxygen therapy alone and at high risk for needing positive pressure ventilation. Our prospective data collection allowing bedside assessment to accurately determine minimum oxygen requirements likely contributes to the predictive value of this variable relative to other cohorts and is a major strength of our study. However, this methodology may pose significant challenges to research personnel trying to implement this score as a research tool and disparities in oxygen delivery practices across hospitals may further limit the generalizability of this risk factor to other patient populations.
We defined ALI as meeting AECC criteria while receiving positive pressure ventilation as defined in best validated epidemiologic cohort of ALI.[9] In addition, the recently published Berlin criteria only considered patients receiving at least 5 mmHg of positive pressure for the classification of “mild ARDS” (roughly the equivalent of non-ARDS ALI).[10] Other studies have extrapolated the AECC criteria for ALI to spontaneously breathing patients outside of the intensive care unit but not assessed the sensitivity and specificity of these criteria for identifying patients who progress to ALI requiring positive pressure ventilation. A pediatric study retrospectively identified emergency department patients with acute hypoxic respiratory failure defined as a P/F < 300 (using a PaO2 derived from recorded saturations and charted FiO2).[12] However, only 5% of these patients were intubated during the follow-up period. Ferguson et al prospectively followed 815 patients admitted with at least one pre-defined risk factor for ALI.[11] Fifty-three patients were identified as developing ALI, however, of 17 patients not in an intensive care unit at the time of ALI diagnosis, 11 were discharged without ever requiring ICU admission. A third study enrolled patients admitted to respiratory isolation rooms outside the intensive care unit and compared patients with ALI (defined by bilateral infiltrates and hypoxemia) to patients without one or both.[13] Respiratory distress was more frequent in the group considered to have ALI, but mortality was low and similar between groups (12% vs. 10%). These studies suggest that simple extrapolation of the AECC criteria for ALI to spontaneously breathing patients outside the intensive care unit may not be valid. In comparison, our empirically derived criteria for EALI accurately identified patients at high risk for progressing to ALI requiring positive pressure ventilation. Patients who progressed to ALI by these criteria had substantially increased mortality and hospital lengths of stay and lower rates of independent functional status at the time of hospital discharge (Table 5).
Our study was conducted at a single university teaching hospital, which may limit generalizability. The high rate of baseline immune suppression (37%) may particularly limit extrapolation to more standard community-based populations. The relatively small number of cases of ALI limits our power to assess the importance of recently identified risk modifiers such as receipt of blood products[26], alcohol abuse[27, 28] and smoking[27, 29] and the potential protective effects of outpatient medications such as aspirin[30], statins[31] and inhaled corticosteroids and beta agonists[32]. Also, this cohort contains 65 patients included in our prior emergency department based assessment of EALI. However, since the current analysis includes additional data collected for up to 72 hours beyond admission, these patients are not likely to bias our results. Immune suppression was not significant in multivariable analysis when these patients were excluded, but the magnitude of the effect of this risk factor was similar, suggesting a type II error due to limited power of the smaller sample.
Finally, we have not validated our findings in a prospectively collected external cohort and our modest sample size is subject to over-fitting. To limit over-fitting, we used previously validated criteria for supplemental oxygen[18], tachypnea[17, 21] and immune suppression[22]. Also, in a cohort including 64 cases of ALI, we identified only 3 independent predictors of ALI suggesting that our model is not over-fit. We have attempted to validate the performance of our model using 10-fold cross validation. Nevertheless, the performance of the score likely benefited from testing in its derivation cohort and validation in an independent multicenter cohort will be needed before we can recommend widespread adoption of this definition of Early Acute Lung Injury.
Selection of strategies targeting prevention or early treatment of acute lung injury depends on several factors including generalizablity to relevant subgroups, ease of use in clinical practice, and the relative positive and negative predictive values as they pertain to the inherent additional cost and potential harms of treating at-risk patients who may otherwise not develop ALI. We limited enrollment to patients with evidence of lung injury on chest radiograph and excluded patients intubated in the emergency department. This approach likely led to a bias toward pulmonary etiologies of ALI in our cohort and to an underrepresentation of high risk surgical patients (who may not have lung injury prior surgery) and severe trauma patients (who are likely to be intubated in the emergency department). Our methodology does not allow us to comment on patients who may develop ALI without an interval qualifying chest radiograph or patients who develop ALI after intubation (potentially 24% of patients without ALI at the time of intubation[33]). Thus, we cannot be certain of the fraction of patients at-risk for developing ALI who could potentially be identified by our EALI score. Also, recognition of radiographic abnormalities is inherently subjective and requiring radiographic evidence of lung injury will likely reduce sensitivity relative to the LIPS for identifying high-risk patients for optimizing prevention of ALI. However, requiring bilateral radiographic abnormalities is in agreement with current consensus criteria for ALI[34] and likely contributed the higher prevalence of ALI (25%) in this cohort relative to the LIPS (7%). These patients may be higher yield targets for some future clinical trials targeting early treatment.
CONCLUSIONS
This study empirically derived clinical criteria for a novel and pragmatic definition of Early Acute Lung Injury (EALI) based on supplemental oxygen requirement, respiratory rate and baseline immune suppression in patients with bilateral infiltrates on chest radiograph. In this cohort, these criteria identified patients who progressed to ALI requiring positive pressure ventilation with 89% sensitivity and 75% specificity. In contrast to the LIPS, our study evaluated at-risk patients longitudinally beyond hospital admission to identify criteria for the early phase of acute lung injury prior to progression to respiratory failure requiring positive pressure ventilation. Following further validation, application of this definition could identify patients for inclusion in future clinical trials targeting the early treatment of ALI.
Supplementary Material
Acknowledgments
This publication was supported by research grant K23HL091334 (Dr. Levitt) from the National Heart, Lung and Blood Institute.
Footnotes
Dr. Levitt has no conflicts of interest to disclose.
Dr. Calfee has no conflicts of interest to disclose.
Dr. Goldstein has no conflicts of interest to disclose
Ms. Vojnik has no conflicts of interest to disclose
Dr. Matthay has no conflicts of interest to disclose.
Institution where research performed: Stanford University Hospital
The authors have not disclosed any potential conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joseph E. Levitt, Division of Pulmonary/Critical Care, Stanford University 300 Pasteur Drive, MC 5236 Stanford, CA 94305 Ph: (650) 723-6381, Fax: (650) 725-5489 jlevitt@stanford.edu.
Carolyn S. Calfee, Departments of Medicine and Anesthesia, Cardiovascular Research Institute, University of California, San Francisco
Benjamin A Goldstein, Department of Medicine, Stanford University.
Rosemary Vojnik, Division of Pulmonary/Critical Care, Stanford University.
Michael A. Matthay, Departments of Medicine and Anesthesia, Cardiovascular Research Institute, University of California, San Francisco.
REFERENCES
- 1.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. The New England journal of medicine. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 3.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. The New England journal of medicine. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, deBoisblanc B, Connors AF, Jr., Hite RD, Harabin AL. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 5.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr., Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. The New England journal of medicine. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 6.Liu KD, Levitt J, Zhuo H, Kallet RH, Brady S, Steingrub J, Tidswell M, Siegel MD, Soto G, Peterson MW, et al. Randomized Clinical Trial of Activated Protein C for the Treatment of Acute Lung Injury. Am J Respir Crit Care Med. 2008 doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, Holets S, Kallet RH, Liu KD, MacIntyre N, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta-agonist for treatment of acute lung injury. American journal of respiratory and critical care medicine. 184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith FG, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, Khan Z, Lamb SE. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. The New England journal of medicine. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 10.Rubenfeld GD. Acute Respiratory Distress Syndrome. The Berlin Definition. J Am Med Inform Assoc. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 11.Ferguson ND, Frutos-Vivar F, Esteban A, Gordo F, Honrubia T, Penuelas O, Algora A, Garcia G, Bustos A, Rodriguez I. Clinical risk conditions for acute lung injury in the intensive care unit and hospital ward: a prospective observational study. Critical care (London, England) 2007;11(5):R96. doi: 10.1186/cc6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freishtat RJ, Mojgani B, Mathison DJ, Chamberlain JM. Toward early identification of acute lung injury in the emergency department. J Investig Med. 2007;55(8):423–429. doi: 10.2310/6650.2007.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quartin AA, Campos MA, Maldonado DA, Ashkin D, Cely CM, Schein RM. Acute lung injury outside of the ICU: incidence in respiratory isolation on a general ward. Chest. 2009;135(2):261–268. doi: 10.1378/chest.08-0280. [DOI] [PubMed] [Google Scholar]
- 14.Calfee CS, Matthay MA. Nonventilatory treatments for acute lung injury and ARDS. Chest. 2007;131(3):913–920. doi: 10.1378/chest.06-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levitt JE, Matthay MA. Treatment of acute lung injury: historical perspective and potential future therapies. Semin Respir Crit Care Med. 2006;27(4):426–437. doi: 10.1055/s-2006-948296. [DOI] [PubMed] [Google Scholar]
- 16.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. The New England journal of medicine. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 17.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H, 3rd, Hoth JJ, Mikkelsen ME, Gentile NT, et al. Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. American journal of respiratory and critical care medicine. 2010;183(4):462–470. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levitt JE, Bedi H, Calfee CS, Gould MK, Matthay MA. Identification of early acute lung injury at initial evaluation in an acute care setting prior to the onset of respiratory failure. Chest. 2009;135(4):936–943. doi: 10.1378/chest.08-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36(3):959–969. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 20.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–874. [PubMed] [Google Scholar]
- 21.Iscimen R, Cartin-Ceba R, Yilmaz M, Khan H, Hubmayr RD, Afessa B, Gajic O. Risk factors for the development of acute lung injury in patients with septic shock: an observational cohort study. Critical care medicine. 2008;36(5):1518–1522. doi: 10.1097/CCM.0b013e31816fc2c0. [DOI] [PubMed] [Google Scholar]
- 22.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 23.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Critical care medicine. 2005;33(6):1191–1198. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 24.Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. American journal of respiratory and critical care medicine. 1995;151(2 Pt 1):293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 25.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 26.Gajic O, Rana R, Winters JL, Yilmaz M, Mendez JL, Rickman OB, O'Byrne MM, Evenson LK, Malinchoc M, DeGoey SR, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. American journal of respiratory and critical care medicine. 2007;176(9):886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iribarren C, Jacobs DR, Jr., Sidney S, Gross MD, Eisner MD. Cigarette smoking, alcohol consumption, and risk of ARDS: a 15-year cohort study in a managed care setting. Chest. 2000;117(1):163–168. doi: 10.1378/chest.117.1.163. [DOI] [PubMed] [Google Scholar]
- 28.Moss M, Bucher B, Moore FA, Moore EE, Parsons PE. The role of chronic alcohol abuse in the development of acute respiratory distress syndrome in adults. Jama. 1996;275(1):50–54. [PubMed] [Google Scholar]
- 29.Calfee CS, Matthay MA, Eisner MD, Benowitz N, Call M, Pittet JF, Cohen MJ. Active and passive cigarette smoking and acute lung injury after severe blunt trauma. American journal of respiratory and critical care medicine. 2011;183(12):1660–1665. doi: 10.1164/rccm.201011-1802OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erlich JM, Talmor DS, Cartin-Ceba R, Gajic O, Kor DJ. Prehospitalization antiplatelet therapy is associated with a reduced incidence of acute lung injury: a population-based cohort study. Chest. 139(2):289–295. doi: 10.1378/chest.10-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Neal HR, Jr., Koyama T, Koehler EA, Siew E, Curtis BR, Fremont RD, May AK, Bernard GR, Ware LB. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Critical care medicine. 39(6):1343–1350. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enrique Ortiz-Diaz, Guangxi Li, Daryl Kor, Ognjen Gajic, Ozan Akca, Adebola Adesanya, Jason Hoth, Festic aE. Chest: 2011. American College of Chest Physicians; Honolulu, Hawaii: 2011. Preadmission Use of Inhaled Corticosteroids Is Associated With a Reduced Risk of Direct Acute Lung Injury/Acute Respiratory Distress Syndrome. [Google Scholar]
- 33.Gajic O, Dara SI, Mendez JL, Adesanya AO, Festic E, Caples SM, Rana R, St Sauver JL, Lymp JF, Afessa B, et al. Ventilator-associated lung injury in patients without acute lung injury at the onset of mechanical ventilation. Crit Care Med. 2004;32(9):1817–1824. doi: 10.1097/01.ccm.0000133019.52531.30. [DOI] [PubMed] [Google Scholar]
- 34.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. Journal of critical care. 1994;9(1):72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 35.Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson Iii H, Hoth JJ, Mikkelsen ME, Gentile NT, et al. Early Identification of Patients at Risk of Acute Lung Injury: Evaluation of Lung Injury Prediction Score in a Multicenter Cohort Study. Am J Respir Crit Care Med. doi: 10.1164/rccm.201004-0549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.