Abstract
During illnesses caused by infectious disease or other sources of inflammation, a suite of brain-mediated responses called the “sickness syndrome” occurs, including fever, anorexia, sleepiness, hyperalgesia, and elevated corticosteroid secretion. Much of the sickness syndrome is mediated by prostaglandins acting on the brain, and can be prevented by non-steroidal anti-inflammatory drugs, such as aspirin or ibuprofen, that block prostaglandin synthesis. By examining which prostaglandins are produced at which sites and how they interact with the nervous system, researchers have identified specific neural circuits that underlie the sickness syndrome.
Most people experience several episodes of acute infectious disease each year. Despite the multiplicity of organisms that cause these illnesses, and the different body tissues that they infect (e.g., upper respiratory, urinary, gastrointestinal systems) a similar set of constitutional symptoms typically occurs. These include fever, achiness, loss of appetite, and sleepiness so that most people want to crawl into bed, pull the covers over their heads, and go to sleep. Known as the “sickness syndrome”, these autonomic, endocrine, and behavioral changes are an adaptive CNS response to help fight infectious diseases1–4. Fever (elevation of body temperature, Tb) helps the immune system fight infections by increasing activity of white blood cells and inhibiting growth of many microorganisms1, 5; achiness, fatigue, and sleepiness conserve energy that is needed to fight the infection and elevate Tb; and loss of appetite minimizes blood glucose6 which is a preferred fuel for many microorganisms.
Over the last two decades, it has become clear that the sickness syndrome is mediated by the brain response to inflammatory molecules produced during infection and other inflammatory diseases (such as arthritis, autoimmune disorders, and some types of cancers). Researchers have found that prostaglandins play a key role in linking systemic inflammation to brain response by their ability to signal across the blood-brain barrier (BBB). The availability of molecular tools and mouse models for manipulating prostaglandin synthetic enzymes and receptors has allowed investigators to begin mapping the brain circuitries that are engaged by prostaglandins to cause the sickness syndrome. In this review, we will first provide an overview of how immune mediators affect the brain, and then examine in detail the circuitry mediating each aspect of the sickness syndrome.
Many aspects of the sickness syndrome are mediated by prostaglandins
During an infection, a number of signals activate immune response. First, many immune system cells bear receptors for common macromolecules present on invading organisms. For example, CD14 is a protein that binds lipopolysaccharide (LPS), a common constituent of bacterial cell walls, and activates Toll-like receptor 4 which triggers an intracellular cascade of events activating immune responses7. In this innate immune response the activated cells release an array of hormones called cytokines such as interleukin1 (IL-1β), interleukin 6, and tumor necrosis factor α (TNFα). Some cytokines attract other immune cells to the site of the inflammation, while others act on a variety of other peripheral tissues to release additional chemical mediators of inflammation.
Among these mediators are the prostaglandins. Prostaglandins are derivatives of arachidonic acid, which is converted to prostaglandin H2 by cyclo-oxygenase (COX). There are two forms of COX: COX1 is constitutively active in many tissues, and COX2 is mainly induced during inflammatory responses8. Although COX2 is expressed constitutively by some neurons in the brain, systemic LPS induces COX2 in the brain only in perivascular and endothelial cells along small venules, and IL-1β primarily activates COX2 in perivascular cells9–11. These venules are found throughout the brain, but are densest in the preoptic area, the base of the hypothalamus, and in the ventrolateral medulla and nucleus of the solitary tract. A number of additional enzymes then convert prostaglandin H2 to other prostaglandins, prostacyclins, or leukotrienes. For the purpose of this review, we will concentrate on two of these molecules, prostaglandin E2 (PGE2, produced by microsomal PGE synthase 1, or mPGES1) and prostaglandin D2 (PGD2, produced by lipocalin PGD synthase, or L-PGDS), as for reasons reviewed below, these molecules appear to be most relevant for producing the sickness syndrome. PGE2 acts on four different EP receptors (EP1-4), all of which are expressed in different parts of the central nervous system12. PGD2 acts on the DP1 receptor, which is mainly found in the meninges along the surface of the brain, especially in the area ventral to the hypothalamus. A DP2 receptor has recently been described, but is present at only very low levels in the brain, and as yet no functions have been attributed to it in that site.
For many years, researchers have debated whether the sickness syndrome is due to the action of cytokines directly on the brain or via an intermediary such as prostaglandins. Inflammatory cytokines such as IL-1β, interleukin-6, and TNFα, which can cause fever responses (and are therefore sometimes called endogenous pyrogens) and other aspects of the sickness syndrome, are too bulky to cross the BBB in large amounts directly13, 14. Thus, although they can cause local inflammation if injected directly into the brain, these responses are more akin to encephalitis, whereas in this review we will concentrate on central nervous system (CNS) responses caused by systemic immune activation.
Cytokines can, however, enter the brain in small quantities at the circumventricular organs, small islands of neural tissue along the surface of the cerebral ventricles that lack a BBB, allowing circulating proteins to interact with nearby neurons13. In addition, there is evidence that the inflammatory cytokines can be actively transported into the CNS14, but the physiological significance of this process is not clear. The strongest argument that cytokines require prostaglandin intermediates to activate many of the components of the sickness syndrome is one that is exploited by millions of people on a daily basis: COX inhibitors such as aspirin, ibuprofen, or naproxen can substantially reduce fever, sleepiness, pain, and anorexia. The neural circuits underlying these prostaglandin-dependent components of the sickness syndrome have been intensively studied, and will be the focus of the remainder of this review.
Fever
Fever is a brain-regulated elevation of Tb that occurs during an inflammatory response. After peripheral administration of LPS, fever occurs in several phases, each representing a distinct burst of activity of thermoregulatory effectors and a distinct rise in Tb15. Fever responses are blocked by systemic administration of COX inhibitors and are absent in animals in which the mPGES1 gene has been deleted16, indicating that they are mediated by PGE2. However, the PGE2 that mediates different phases of fever may come from different sources. The first (early) phase of LPS fever is triggered by PGE2 of peripheral origin, as neutralization of circulating PGE2 by an anti-PGE2 antibody that does not cross the BBB blocks this response17. LPS acts on Toll-like receptor 4 on hepatic (Kupffer cells) and pulmonary macrophages, in which there is upregulation of mRNA and protein for phospholipase A2 (which produces arachidonic acid), COX-2, and mPGES1, which in turn results in an increase in PGE2 in both venous and arterial blood17, 18. The PGE2 binds to albumin, which transports it and may protect it from enzymatic inactivation18. PGE2 may dissociate from albumin and then be carried across the BBB to its site of action by specialized transporters that are expressed in the hypothalamus18.
The more prominent later phases of fever, which start at about 1 hour after LPS administration and last for several hours15, are mediated by PGE2 produced by COX-2 in perivascular and endothelial cells2, 9, 10, 19. IL-1βand low doses of LPS upregulate COX2 expression in perivascular cells, whereas higher doses of LPS also increase COX2 in endothelial cells, mainly along venules9, 11. However, only endothelial cells have been found to produce mPGES1 in adult brain20, so they are the source of PGE2 in the later phases of fever. Induction of COX-2 and mPGES1 in brain occurs simultaneously with the later febrile phases18, 21. Whereas studies in knockout mice have demonstrated the indispensable roles of COX-222 and mPGES116 in LPS fever, studies with cell type-specific modulation of expression of these enzymes are still needed to determine the cellular source of febrigenic PGE218. The interplay of PGE2-degrading and transporting systems may also play an important role in regulating brain levesl of PGE2 during fever18.
PGE2 acts on neuronal receptors of the EP family within the thermoregulation circuitry to trigger fever. The EP3 receptor is now viewed as the principle type responsible for fever23–25, but EP1 receptors may also contribute under some conditions25. The EP3 receptor occurs in several isoforms, and in rodents isoforms α and γ are strongly expressed in the median preoptic nucleus (MnPO)24–26 and are thought to mediate fever27. The MnPO is also the most responsive part of the brain to both the pyrogenic action of PGE228 and the antipyretic action of intracerebral injection of ketorolac (a COX inhibitor) after LPS administration29. Focal deletion of EP3 receptors in the MnPO has shown that their presence is required for fever following systemic LPS or intracerebroventricular (i.c.v.) PGE224. Preoptic EP3-expressing neurons produce γ-aminobutyric acid (GABA)30, and are thought to inhibit downstream neurons that drive increases in Tb. Typically, α and γ EP3 receptors inhibit neuronal function through Gimediated inhibition of adenylate cyclase. Hence, it is likely that PGE2 binding to EP3 receptors reduces the activity of MnPO neurons, resulting in disinhibition of downstream targets that elevate Tb31.
What targets do EP3-expressing preoptic neurons engage to increase Tb during fever? The autonomic contribution to fever after systemic LPS32or i.c.v. PGE33 relies primarily on two responses: cutaneous vasoconstriction (particularly of the tail skin, the principle means by which rodents elevate Tb in a warm environment), and activation of thermogenesis in brown adipose tissue (BAT, which rodents recruit to elevate Tb in a cool environment). These responses are controlled by two populations of GABAergic preoptic neurons that are warm-sensitive (i.e., fire faster when warmed). One population located mainly in the MnPO regulates tail skin vasoconstriction by means of projections to the rostral medullary raphe (RMR), which in turn directly innervates sympathetic preganglionic neurons. These EP3-expressing MnPO neurons are probably also involved in baseline thermoregulation34. The other group is located more caudally and laterally in the dorsolateral preoptic area and regulates BAT thermogenesis through projections to the dorsomedial hypothalamus (DMH), which also projects to the RMR35, 36. Hence, in rodents, PGE2 triggers autonomic heat-conservation and heat-production responses by acting on EP3 receptors to reduce activity of preoptic GABAergic neurons, thus disinhibiting sympathetic activation of tail skin vasoconstriction and BAT thermogenesis.
The fever response to LPS is opposed by central pathways containing α -melanocyte stimulating hormone (α-MSH) acting on melanocortin (MC) 3 and 4 receptors37. The MC3/4 blocker SHU-9119 increases fever due to intraperitoneal (i.p.) LPS but blocks anorexia (see below), suggesting that there is tonic action of α-MSH on MC3/4 receptors that opposes hyperthermia but mediates anorexia. As the DMH neurons that project to the RMR have high levels of MC4 receptors38, which are inhibitory, this is a likely site where α-MSH might inhibit fever. LPS fever also involves several thermoregulatory behaviors, including selection of a higher ambient temperature, or warmth seeking39. Neural pathways controlling thermoregulatory behaviors differ from those controlling autonomic thermoeffectors31, 36, 40. They do not travel through the preoptic area but instead may follow thalamo-cortical pathways for discriminatory thermal sensitivity41. Rats with large electrolytic lesions of the preoptic area exhibit excellent behavioral thermoregulation, including the ability to generate fever after LPS administration by moving to a higher ambient temperature, even though the same rats are incapable of mounting autonomic thermoregulatory responses39. The locus of PGE2 action for behavioral thermoregulation remains unknown.
Sleepiness
Irresistible, prolonged sleep is a major aspect of the sickness syndrome. Normally, about 25% of the sleep period in humans is spent in rapid eye movement (REM) sleep, a state characterized by dreaming, fast cortical activity, and muscle paralysis, and the remaining 75% is spent in non-REM (NREM) sleep, a state of slow cortical activity and low metabolism. Wakefulness is driven by monoaminergic and cholinergic neurons that activate the forebrain, but during sleep these wake-promoting systems are inhibited by GABAergic neurons in the ventrolateral preoptic nucleus (VLPO) and adjacent parts of the preoptic area42. This action of the VLPO is necessary to produce adequate amounts of sleep, and a key target of the VLPO is the histaminergic tuberomammillary nucleus (TMN), which is a major wake-promoting site. Researchers are now beginning to understand how inflammatory mediators act through these pathways to alter sleep.
In healthy human subjects, as in rats and mice, low to moderate doses of intravenous LPS increase deep NREM sleep and reduce REM sleep43–45. Some components of the sleep response to systemic inflammation may be mediated by direct effects of cytokines on neurons or via other mediators such as nitric oxide on the brain46, 47. However, the importance of prostaglandins as mediators of the sleep response is demonstrated by the fact that even after i.c.v. administration of TNF i.c.v. injection of a COX2 inhibitor blocks the increase in NREM sleep48, 49.
Hayaishi, Urade, and colleagues have identified PGD2 as a major sleep-promoting factor. Incubation of preoptic brain slices with LPS induces production of PGD250, and infusion of PGD2 in the subarachnoid space just ventral to the VLPO produces large and sustained increases in NREM sleep51. The origin of PGD2 is likely to be in the meninges, as the synthetic enzyme L-PGDS is found in the meninges and choroid plexus, but not in the brain parenchyma52. PGD2 then activates target cells through DP1 receptors, which are most abundant in the meninges just ventral to the VLPO53.
DP1 signaling then induces release of adenosine which acts as a paracrine signaling molecule to increase sleep54. Adenosine may act in part by inhibiting wake-active neurons through A1 receptors55–57, but excitatory adenosine A2a receptors also play a role. Infusion of an adenosine A2a agonist in the subarachnoid space just ventral to the preoptic area promotes sleep, and the sleep-promoting response to PGD2 can be reduced by an A2a antagonist58, 59. It remains unclear how adenosine activates VLPO neurons, which show fewer inhibitory synaptic inputs but no direct response to adenosine56, but this question can now be addressed by focally expressing or deleting the A2a receptor. For example, deletion of the A2a receptors in the nucleus accumbens was recently shown to block the wake-promoting effects of caffeine, which is an adenosine antagonist60. These observations suggest that A2a receptors in the nucleus accumbens shell promote sleep, but it has not been explored whether this signaling pathway mediates the increase in NREM sleep with systemic inflammation.
Whereas PGD2 ultimately activates sleep-promoting neurons in the VLPO, PGE2 activates histaminergic, wake-promoting TMN neurons. Infusion of PGE2 in the TMN markedly increases wakefulness61. In addition, TMN neurons express EP4 receptors, and infusion of an EP4 agonist in this region increases histamine release and wakefulness62. While this effect may seem to run counter to the sleep-promoting effects of PGD2, sleep becomes more fragmented in people treated with relatively high doses of LPS43. Hence, with mild to moderate levels of inflammation, PGD2 may activate the VLPO sufficiently to suppress activity in the TMN and increase sleep, whereas with more severe inflammation, PGE2 may activate TMN neurons sufficiently to overcome VLPO inhibition, and produce fragmented, fitful sleep, or even an agitated delirium.
Anorexia
In the 24 hours after a single dose of i.p. LPS, both rats and mice eat and drink less and lose weight63–65. The anorexia and weight loss are eliminated by systemic administration of a COX inhibitor (e.g., indomethacin) prior to the LPS, so these responses are likely to be mediated predominantly if not exclusively by prostaglandins. Interestingly, a relatively small, early component of the anorexia appears to be sensitive to COX1 inhibitors and is lost in COX1-/- mice, while the later and larger component is sensitive to COX2 inhibitors63. Similar to LPS, IL-1 administered i.p. produces anorexia which is attenuated in COX2 -/- mice63. Thus, the bulk of the response (i.e., after the first hour) is likely to be due to COX2-mediated production of prostaglandins.
The anorexic response can be replicated by i.c.v. injection of PGE2, and the response to i.p. IL-1β administration is eliminated in mice that lack mPGES166, thus suggesting involvement of PGE2. The anorexic effect of PGE2 i.c.v. can be reversed with the EP4 receptor antagonist, ONO-AE3-208, whereas i.c.v administration of the EP4 agonist ONO-AE1-329 mimics the effect of PGE2 on feeding67. Because the EP4 receptor is present at high levels in the paraventricular nucleus of the hypothalamus (PVH), and its expression is increased by intravenous (i.v.) LPS68, some of the CNS effects of PGE2 may be mediated by the PVH. Because i.c.v. PGD2 causes an increase in feeding in mice69, it is unlikely to play a role in the anorexia seen after systemic LPS (except perhaps to limit it).
After i.v. LPS, there is activation of expression of cFos (an early-response protein that is used as a marker of neuronal activity) in neurons in the PVH, as well as in the arcuate nucleus, the DMH, and the lateral hypothalamic area70. Within the PVH, many of the cFos+ neurons are immunoreactive for corticotropin-releasing hormone (CRH), whereas in the arcuate nucleus many of the cFos+ neurons stain with antibodies against pro-opiomelanocortin (POMC) peptides71. The POMC neurons are thought to inhibit feeding during the first few hours after LPS, whereas the CRH neurons are thought to mediate an increase in corticosteroid production (see below) which may increase feeding at later times.
Recent studies have emphasized the presence of two populations of neurons in the arcuate nucleus with opposite effects on feeding. The POMC neurons express cFos after administration of leptin, a hormone that signifies adequate availability of energy substrates72. They project to the PVH, the DMH, and the lateral hypothalamic area, where they act on MC4 receptors which inhibit feeding. Other arcuate neurons, which produce neuropeptide Y (NPY) and agouti-related protein (AgRP), project to the same targets73, where AgRP acts as an endogenous antagonist of the MC4 receptor74. The arcuate NPY/AgRP neurons, which are activated by high levels of the appetite-promoting hormone ghrelin, help drive feeding75. They also contain GABA, with which they directly inhibit the POMC neurons and other brainstem feeding circuitry76. This network of structures is thought to modulate feeding as well as energy expenditure and body weight through a variety of CNS projections to autonomic, endocrine, and behavioral targets. Thus the activation of neurons in these structures after i.v. LPS likely reflects their roles in regulating feeding. For example, the blockade of MC3/4 receptors by SHU-9119 is known to prevent the anorexia after i.p. LPS37, indicating that the POMC/α-MSH projections may play a key role in the response. It will be important to explore the roles of the targets of this pathway that express MC3/4 receptors, and to identify other chemically-defined populations of neurons to determine which of them contribute to the anorexia.
On the other hand, i.p. LPS also causes a fall in levels of ghrelin, a peptide secreted by gastric cells that increases feeding77. Replacing the ghrelin restores feeding after i.p. LPS. The effect of LPS on ghrelin release could be blocked by i.p. indomethacin77, so it is prostaglandindependent, but whether the prostaglandins that suppress ghrelin secretion act on the brain or periphery has not been studied. Hence it is possible that the anorexia associated with i.p. LPS administration may be mediated by PGE2 acting at a number of different levels within the system that regulates feeding and energy metabolism.
Hyperalgesia
Like anorexia, the influence of inflammation on pain perception may also be mediated at different levels of the neuraxis. The classic signs of inflammation, “tumor, calor, rubor, and dolor” (swelling, warmth, redness, and pain), are due to the local effects of inflammatory mediators, which cause vasodilation, edema, and can sensitize the terminals of nociceptive neurons causing local pain. However, the central response to pain is also susceptible to modulation at multiple levels, from the spinal cord to the brainstem and the forebrain. The tests used to measure pain responses are dependent upon different levels of the neuraxis, and therefore can produce different results, even in the same model of inflammation. In addition, the effects of inflammation on pain are dynamic, showing hyperalgesia initially, but in some assays a later analgesic effect4. The later analgesia may be due to cascades of additional mediators, such as corticosteroids, and so we will focus here on the initial, hyperalgesic response to acute inflammation.
Some aspects of the hyperalgesic response to inflammation apparently are not mediated by prostaglandins. For example, after i.p. injection of LPS, there is a reduction in the latency of the tail-flick response, a pain assay that measures tail movement in response to local heating and is generally agreed to be spinally mediated. This response is unaffected by systemic administration of the COX inhibitor indomethacin78. This is understandable, as i.p. LPS causes expression of inflammatory mediators that can irritate local peripheral nerves. Thus COX inhibitors may be unable to eliminate the local pain and the effects on the associated spinal reflexes. On the other hand, the latency to paw withdrawal from noxious heat, which is sensitive to supraspinal influences, is also reduced after i.p. LPS, but it is restored to near-normal levels by systemic administration of drugs that inhibit prostaglandin synthesis79.
The i.v. LPS model is better for examining the effect of inflammation on overall CNS response to pain, as there is no local site of inflammation, and LPS enters the circulation immediately, rather than by gradual hematogenous and lymphatic uptake80. In this model, initial hyperalgesia is followed in a few hours by hypoalgesia4, 81. After i.v. LPS even at relatively low doses (e.g., 10–100µg/kg), there is initially a reduction in the latency to paw withdrawal to noxious heating82. This hyperalgesic response is abolished by injecting a non-selective COX inhibitor (diclofenac) or a COX2 selective inhibitor (NS-398) into the preoptic area (but not other parts of the hypothalamus). The medial preoptic region of the hypothalamus has extensive projections to the midbrain periaqueductal gray matter83, 84, which is known to mediate antinociceptive responses.
There is some evidence that LPS-induced hyperalgesia may be reduced in animals with deletion of EP3 (but not EP1, 2, or 4) receptors85, but whether this effect was central vs peripheral was not examined. On the other hand, after i.c.v. injection of PGE2, there is also a reduction in latency to paw withdrawal, and an increase in the firing rate of spinal trigeminal neurons to noxious toe pinching81. This hyperalgesic effect was replicated by i.c.v. injection of an EP3 agonist (M&B28767), but not an EP1 (17-phenyl-ω-trinorPGE2) or EP2 (butaprost) agonist, and the most sensitive site for either PGE2 or the EP3 agonists to cause hyperalgesia was the preoptic area. Given that EP3 receptors in the preoptic area are highly concentrated in the MnPO24, 68, which along with the adjacent medial preoptic nucleus projects extensively to the periaqueductal gray matter83, this site is a likely locus for PGE2 to produce not only fever, but also hyperalgesia. However, this hypothesis remains to be tested.
Elevated corticosteroid levels
Corticosteroid secretion is elevated by a variety of stressful stimuli, including acute inflammation. However, the degree to which this response is dependent upon prostaglandins has been controversial, as some studies have found complete inhibition by systemic administration of COX inhibitors86, and others only partial inhibition71, 87, or none at all88–90. These discrepancies may be explained by differences in route of administration or species, as well as the time course of the effect. For example, in mice lacking either EP1 or EP3 receptors, the expected elevation of adrenocorticotropic hormone (ACTH) secretion mice was absent at 1 hour and 3 hours after i.p. LPS administration, but present at 2 hours after administration91. Other workers found that i.c.v. administration of COX1 (but not COX2) inhibitors caused reductions in ACTH and corticosterone in rats only at 30 minutes, but not at later time points92. Such findings suggest a complex dynamic response, in which some components are prostaglandin-dependent and some are not. Most of the work on neural circuitry has been done on this prostaglandin-dependent early component of the corticosteroid response, which will be the focus here.
Sawchenko and colleagues avoided peritoneal irritation by examining the mechanisms for elevation of CRH mRNA in neurons in the PVH after acute administration of i.v. LPS or IL-1β in rats93, 94. They found increased cFos expression in catecholaminergic neurons in the ventrolateral medulla (VLM), and showed that the elevation of CRH mRNA was blocked by interrupting noradrenergic and adrenergic afferents from the VLM to the PVH. They then showed increased expression of COX1 and COX2 mRNA in blood vessels in the VLM after IL- 1β administration, and that injection of PGE2 into the VLM could increase CRH mRNA expression in the PVH. However, i.c.v. administration of a COX1 inhibitor only partially blocked the early ACTH and corticosterone responses to i.v. LPS or IL-1β, and a COX2 inhibitor had no effect92. These findings are consistent with the observations that a COX1 inhibitor prevents i.v. LPS from activating cFos expression in VLM catecholamine neurons that express EP3 receptors, and which in turn activate PVH CRH neurons92, 95, but indicate that there are additional steps, at least some of which do not depend on prostaglandins, to cause secretion of ACTH during an inflammatory response.
Matsuoka and colleagues examined the roles of different EP receptors in the corticosteroid response to LPS by measuring levels of ACTH in blood one hour after injecting i.p. LPS in mice91. They found that the elevation of ACTH after LPS injection was absent in EP1 and EP3 knockout mice, but unaffected in EP2 or EP4 knockout mice. LPS also increased cFos expression in the PVH, especially in CRH neurons. Surprisingly, the increased c-Fos in the PVH still occurred in EP1 and EP3 knockout mice, although it was prevented by administering an EP1 antagonist to EP3 knockout mice, suggesting that either EP1 or EP3 receptors can activate pathways that cause cFos expression in PVH neurons, but neither one alone is sufficient to increase CRH secretion. EP1, but not EP3 receptors, were found in the central nucleus of the amygdala, a source of afferents to the PVH.
Another potential site of action for PGE2 would be the MnPO, which contains both EP1 and EP3 receptors, and which projects to the PVH83. Both amygdala and MnPO neurons that project to the PVH also receive extensive noradrenergic inputs from the medulla. However, whether the cells projecting to the PVH from these sites bear EP receptors, and what neurotransmitters they use are not known.
In summary, the cascade of mediators that are released during inflammation can act at multiple levels of the nervous system. Though peripheral nerves trigger some responses, there is strong evidence that the penetration of the inflammatory signal into the CNS requires the mediation of prostaglandins, especially for fever and feeding responses, and that prostaglandin-activated circuitry plays an important role in regulating sleep, hyperalgesia, and corticosteroid responses as well. This linkage provides an opportunity to probe the CNS circuitry that is engaged by the different prostaglandin receptors. Studies in the last decade have identified many of these circuits, and suggest testable hypotheses about others. In addition, drugs that can differentially alter these responses may be of value in alleviating suffering due to the sickness syndrome, without diminishing its valuable adaptive effects.
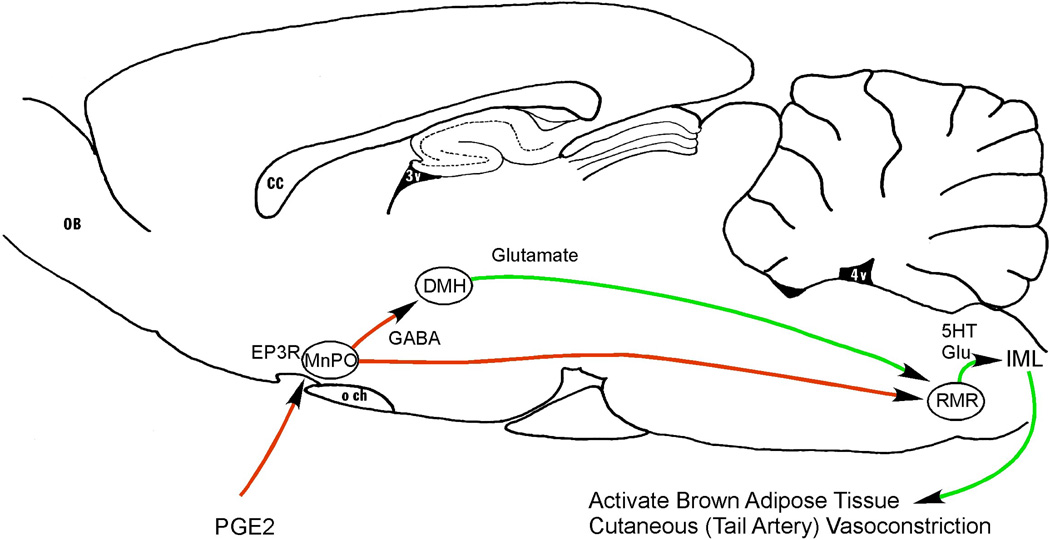
Figure 1. Neuronal pathways causing fever during systemic inflammation.
Prostaglandin E2 (PGE2) is produced by endothelial and perivascular cells along small venules at the edges of the brain, particularly in the preoptic area. It acts on EP3 receptors (EP3R) to inhibit neurons in the median preoptic nucleus (MnPO). Many of these neurons are GABAergic, and they in turn inhibit neurons in the dorsomedial nucleus of the hypothalamus (DMH) and rostral medullary raphe (RMR) that act to increase body temperature. RMR neurons use glutamate and serotonin (5HT) to excite sympathetic preganglionic neurons in the intermediolateral column of the spinal cord (IML), which activate brown adipose tissue (which produces heat) and cutaneous vasoconstriction (particularly in the tail artery, which conserves heat). Thus, PGE2 in the MnPO disinhibits these heat producing and conserving pathways and elevates body temperature. CC, corpus callosum; OB, olfactory bulb; och, optic chiasm; 4V, fourth ventricle.
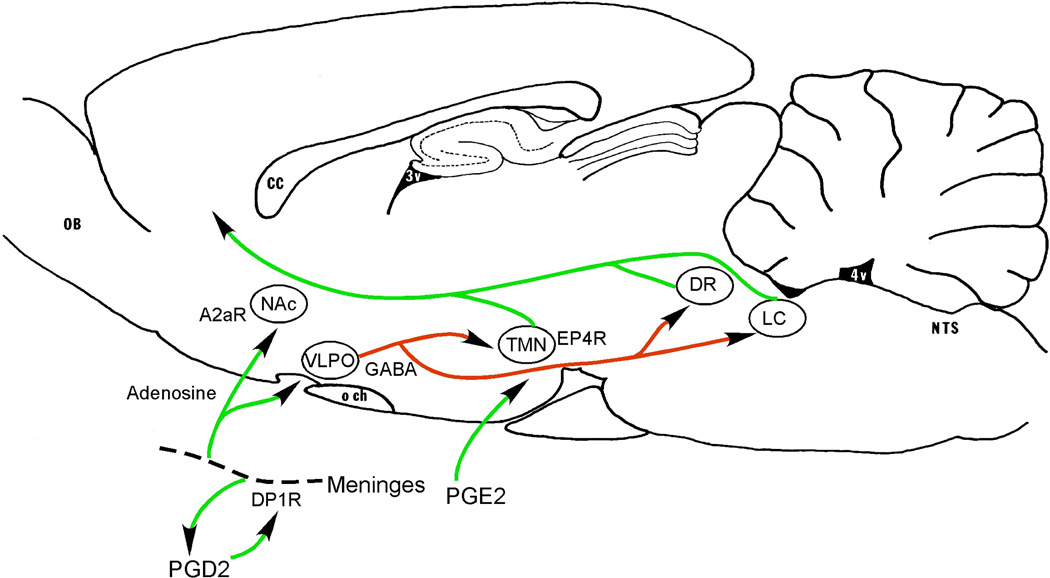
Figure 2. Neuronal pathways promoting sleep during systemic inflammation.
Leptomeningeal cells produce prostaglandin D2 (PGD2) which acts via DP1 receptors to induce local production of adenosine. Adenosine acts on A2a receptors in the forebrain to activate neurons in the nucleus accumbens (NAc) and the ventrolateral preoptic nucleus (VLPO). The VLPO neurons directly inhibit the arousal system, including the histaminergic tuberomammillary nucleus (TMN), the serotoninergic dorsal raphe nucleus (DR), and the noradrenergic locus coeruleus (LC), thus promoting sleep. The mechanism by which the nucleus accumbens causes sleepiness is not yet established, but it has extensive GABAergic projections to the perifornical region containing the orexin neurons. PGE2 produced during inflammation may have a counteracting effect, by exciting TMN neurons via EP4 receptors, perhaps explaining why the excess sleep observed during inflammation tends to be fragmented by frequent awakenings. Other abbreviations as in Fig. 1
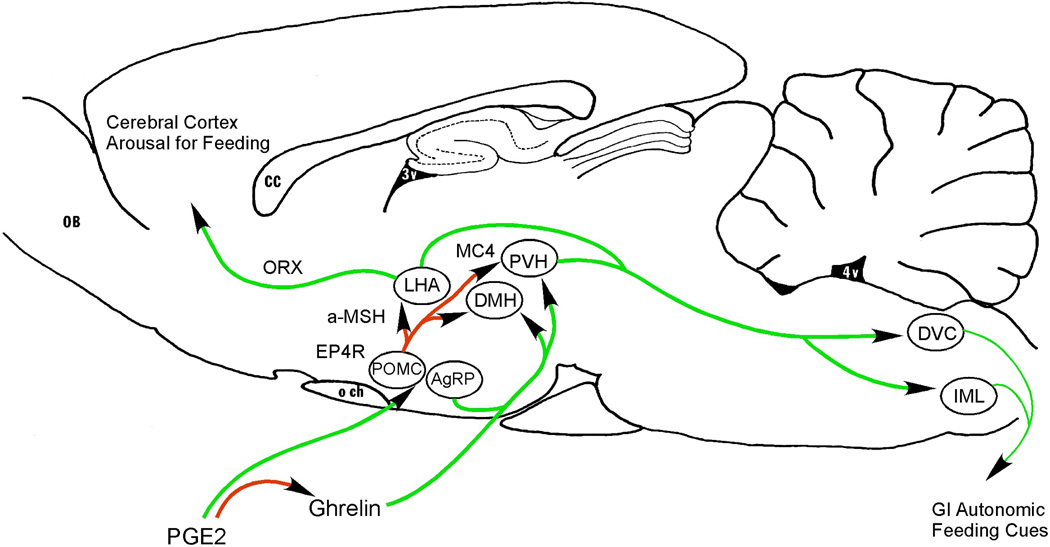
Figure 3. Neuronal pathways that may cause anorexia during systemic inflammation.
PGE2 produced by vascular and perivascular cells in the region of the median eminence acts via EP4 receptors to activate pro-opiomelanocortin (POMC) expressing neurons in the arcuate nucleus. These neurons produce α-melanocyte stimulating hormone (a-MSH) which acts via melanocortin 4 (MC4) receptors to inhibit neurons in the DMH, paraventricular nucleus (PVH), and lateral hypothalamic area (LHA) that otherwise promote feeding, by means of descending projections to autonomic regions that control the gastrointestinal system, and by ascending orexin (ORX) containing projections to the cerebral cortex. During systemic inflammation, there is also a prostaglandin-mediated fall in levels of serum ghrelin, a peptide made in the stomach that promotes feeding by acting on neurons in the DMH and PVH as well as the arcuate nucleus neurons containing agouti-related peptide (AgRP). Other abbreviations as in previous figures.
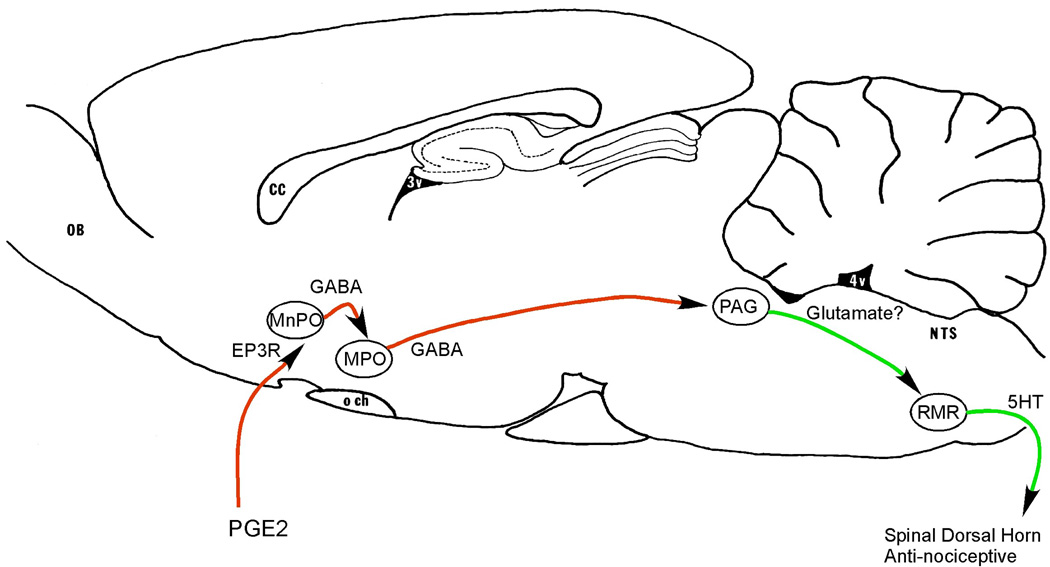
Figure 4. Neuronal pathways that may cause hyperalgesia in the first few hours of systemic inflammation.
PGE2 made by vascular and perivascular cells along venules in the preoptic area acts on EP3 receptors in the MnPO. This results in disinhibition of descending inhibitory projections from the medial preoptic area (MPO) to the brainstem anti-nociceptive system, including neurons in the periaqueductal gray matter (PAG) that promote analgesia by activating descending serotoninergic neurons in the RMR, which in turn inhibit nociceptive neurons in the spinal dorsal horn. Other abbreviations as in previous figures.
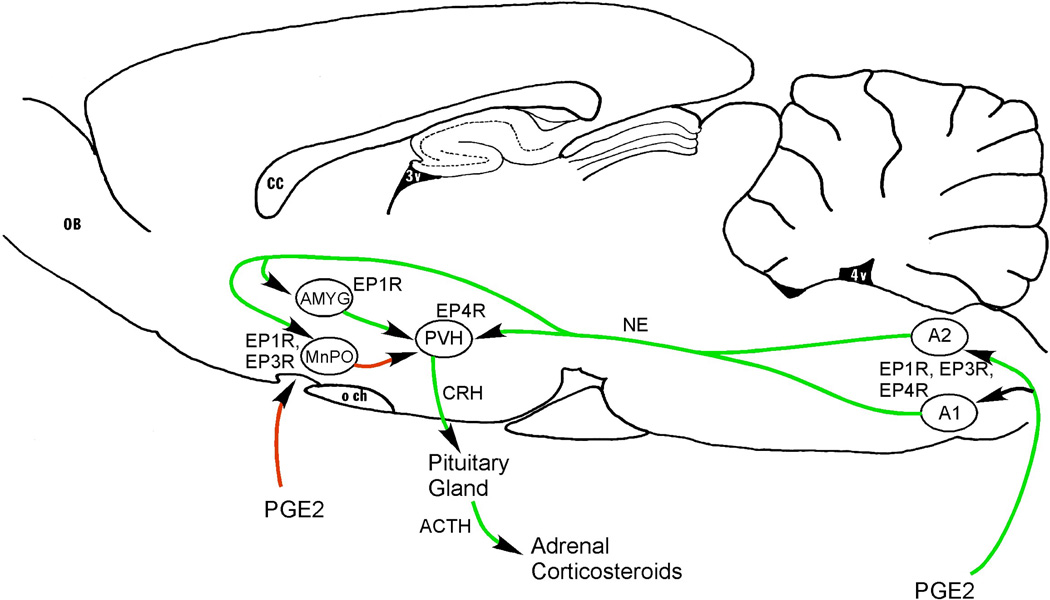
Figure 5. Neuronal pathways that may increase secretion of corticosteroids in response to systemic inflammation.
PGE2 may act at multiple locations to facilitate release of corticotropin-releasing hormone (CRH) from PVH neurons, which in turn causes secretion of adrenal corticotropic hormone (ACTH) from the pituitary, and hence adrenal corticosteroids. PGE2 activates medullary noradrenergic neurons in the A1 and A2 cell groups, which express EP1, EP3, and EP4 receptors, an input that is required for increased cFos and CRH expression in PVH neurons. In addition, EP1 or EP3 receptors are found on neurons in the MnPO, and EP1 receptors are found on neurons in the central nucleus of the amygdala (AMYG), both of which provide inputs to the CRH cells in the PVH. Other abbreviations as in previous figures.
Box 1 Multiple Models of Inflammation.
In this review, we emphasize the sickness syndrome, a remarkably common pattern of CNS response to inflammation from a variety of different causes, which includes fever, sleepiness, anorexia, hyperalgesia, and corticosteroid release. On the other hand, infections can occur in any organ of the body, and each part of the body is differentially susceptible to specific pathogens. These may be bacteria, viruses, or fungal infections, and each species and strain of pathogen activates the immune system differently. Similarly, in experimental models of inflammation, specific details of the sickness syndrome vary depending upon the species of host animal that is studied; the route of administration (intramuscular, intraperitoneal, intravenous, intracerebral); the response that is measured and the timing of the measurement; and the nature of the inflammatory stimulus (e.g., responses to bacterial molecules such as lipopolysaccharide, may differ for each strain of bacteria, and responses to some products not discussed here, such as staphylococcus enterotoxin B, may be quite different from the response to LPS). Because these infectious stimuli set off a cascade of hormones, called cytokines, that are made by white blood cells and other tissues, some models use injections of specific cytokines, such as interleukin-1β or tumor necrosis factor α, to initiate the response. Even such factors as the strain of rat or mouse that is used, the time of day of administration, and the time point at which the response is measured can affect the outcome.
In this review, we are focusing on the acute (1–4 hour) response to intravenous or intraperitoneal challenge with LPS during the daytime, to examine the brain response to a generalized systemic inflammatory event, in the absence of an infection. We further are concentrating on mouse and rat models, as the largest numbers of studies about neural circuitry engaged by inflammation have been done in these species. Finally, we are limiting our review to the effects that are mediated by prostaglandins, which tend to play a prominent role in many of the components of the sickness syndrome. Because the prostaglandin synthetic enzymes and receptors are known, have been mapped within the brain, and specific knockout animals and drugs exist for most of them, the effects of the prostaglandin-mediated responses on specific brain circuits have been studied in the greatest detail. On the other hand, it is likely that we will identify additional CNS circuitry mediating the effects of inflammation as other models and non-prostaglandin- dependent responses come under more intense investigation in the future.
Box 2 The Role of the Vagus Nerve in Immune Signaling.
The role played by the vagus nerve in signaling the presence of peripheral inflammatory mediators has been controversial. As we have reviewed elsewhere (for references see96), there was a burst of enthusiasm for the vagal theory in the early 1990’s based upon studies by several labs demonstrating that subdiaphragmatic vagotomy prevented fever responses to i.p. LPS or IL- 1β However, it soon became apparent that surgical vagotomy caused gastric stasis, slow bowel transit, and poor absorption of nutrients, resulting in malnutrition and inability to mount a thermogenic response97. Most studies of the effects of vagotomy in rats that were done after 1997 involved placing animals on a liquid diet to prevent malnutrition and associated disorders. In these later studies, surgical vagotomy was found to have little effect on the febrile response to LPS or IL-1β98–100, except after very low doses of pyrogens (e.g., 500-2,000 ng/kg for i.v. LPS and 100–500 ng/kg for i.v. IL-1β in rats). Similarly, subdiaphragmatic vagotomy followed by liquid diet in mice and rats has been reported to have minimal effects on anorexia induced by i.p. LPS or IL-1β, open field investigation after IL-1β, or corticosteroid secretion after IL-1βor LPS64, 80. Hence, while vagal circuitry certainly is activated by systemic LPS or IL-1β70, the contribution of vagal signaling to the sickness syndrome is not entirely clear, and is not discussed in this review.
Acknowledgements
The authors wish to acknowledge support from USPHS grants NS055367 (TES), HL095491 (CBS, TES), NS072337 (CBS), and NS41233 (AAR).
Reference List
- 1.Kozak W, Conn CA, Kluger MJ. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am. J. Physiol. 1994;266:R125–R135. doi: 10.1152/ajpregu.1994.266.1.R125. [DOI] [PubMed] [Google Scholar]
- 2.Elmquist JK, Scammell TE, Saper CB. Mechanisms of CNS response to systemic immune challenge: the febrile response. Trends Neurosci. 1997;20:565–570. doi: 10.1016/s0166-2236(97)01138-7. [DOI] [PubMed] [Google Scholar]
- 3.Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- 4.Romanovsky AA, et al. First and second phases of biphasic fever: two sequential stages of the sickness syndrome? Am. J. Physiol. 1996;271:R244–R253. doi: 10.1152/ajpregu.1996.271.1.R244. [DOI] [PubMed] [Google Scholar]
- 5.Kluger MJ, Kozak W, Conn CA, Leon LR, Soszynski D. Role of fever in disease. Ann. N. Y. Acad. Sci. 1998;856:224–233. doi: 10.1111/j.1749-6632.1998.tb08329.x. 224–233. [DOI] [PubMed] [Google Scholar]
- 6.Yates DT, et al. Effects of bacterial lipopolysaccharide injection on white blood cell counts, hematological variables, and serum glucose, insulin, and cortisol concentrations in ewes fed low- or high-protein diets. J. Anim Sci. 2011;89:4286–4293. doi: 10.2527/jas.2011-3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bode JG, Ehlting C, Haussinger D. The macrophage response towards LPS and its control through the p38(MAPK)-STAT3 axis. Cell Signal. 2012 doi: 10.1016/j.cellsig.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Kalinski P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2012;188:21–28. doi: 10.4049/jimmunol.1101029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiltz JC, Sawchenko PE. Distinct brain vascular cell types manifest inducible cyclooxygenase expression as a function of the strength and nature of immune insults. J. Neurosci. 2002;22:5606–5618. doi: 10.1523/JNEUROSCI.22-13-05606.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breder CD, Saper CB. Expression of inducible cyclooxygenase mRNA in the mouse brain after systemic administration of bacterial lipopolysaccharide. Brain Res. 1996;713:64–69. doi: 10.1016/0006-8993(95)01474-8. [DOI] [PubMed] [Google Scholar]
- 11.Serrats J, et al. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron. 2010;65:94–106. doi: 10.1016/j.neuron.2009.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woodward DF, Jones RL, Narumiya S. International Union of Basic and Clinical Pharmacology. LXXXIII: classification of prostanoid receptors, updating 15 years of progress. Pharmacol. Rev. 2011;63:471–538. doi: 10.1124/pr.110.003517. [DOI] [PubMed] [Google Scholar]
- 13.Maness LM, Kastin AJ, Banks WA. Relative contributions of a CVO and the microvascular bed to delivery of blood-borne IL- 1alpha to the brain. Am. J. Physiol. 1998;275:E207–E212. doi: 10.1152/ajpendo.1998.275.2.E207. [DOI] [PubMed] [Google Scholar]
- 14.Banks WA, Erickson MA. The blood-brain barrier and immune function and dysfunction. Neurobiol. Dis. 2010;37:26–32. doi: 10.1016/j.nbd.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 15.Romanovsky AA, Simons CT, Kulchitsky VA. "Biphasic" fevers often consist of more than two phases. Am. J. Physiol. 1998;275:R323–R331. doi: 10.1152/ajpregu.1998.275.1.R323. [DOI] [PubMed] [Google Scholar]
- 16.Engblom D, et al. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat. Neurosci. 2003;6:1137–1138. doi: 10.1038/nn1137. [DOI] [PubMed] [Google Scholar]
- 17.Steiner AA, et al. Cellular and molecular bases of the initiation of fever. PLoS. Biol. 2006;4:e284. doi: 10.1371/journal.pbio.0040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci. 2004;9:1977–1993. doi: 10.2741/1383. 1977–1993. [DOI] [PubMed] [Google Scholar]
- 19.Matsumura K, et al. Brain endothelial cells express cyclooxygenase-2 during lipopolysaccharide-induced fever: light and electron microscopic immunocytochemical studies. J. Neurosci. 1998;18:6279–6289. doi: 10.1523/JNEUROSCI.18-16-06279.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamagata K, et al. Coexpression of microsomal-type prostaglandin E synthase with cyclooxygenase-2 in brain endothelial cells of rats during endotoxin-induced fever. J. Neurosci. 2001;21:2669–2677. doi: 10.1523/JNEUROSCI.21-08-02669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inoue W, et al. Brain-specific endothelial induction of prostaglandin E(2) synthesis enzymes and its temporal relation to fever. Neurosci. Res. 2002;44:51–61. doi: 10.1016/s0168-0102(02)00083-4. [DOI] [PubMed] [Google Scholar]
- 22.Steiner AA, et al. Expanding the febrigenic role of cyclooxygenase-2 to the previously overlooked responses. Am. J. Physiol Regul. Integr. Comp Physiol. 2005;289:R1253–R1257. doi: 10.1152/ajpregu.00371.2005. [DOI] [PubMed] [Google Scholar]
- 23.Ushikubi F, et al. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- 24.Lazarus M, et al. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- 25.Oka T, et al. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J. Physiol. 2003;551:945–954. doi: 10.1113/jphysiol.2003.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura K, et al. Immunohistochemical localization of prostaglandin EP3 receptor in the rat nervous system. J. Comp Neurol. 2000;421:543–569. doi: 10.1002/(sici)1096-9861(20000612)421:4<543::aid-cne6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Vasilache AM, Andersson J, Nilsberth C. Expression of PGE2 EP3 receptor subtypes in the mouse preoptic region. Neurosci. Lett. 2007;423:179–183. doi: 10.1016/j.neulet.2007.06.048. [DOI] [PubMed] [Google Scholar]
- 28.Scammell TE, Elmquist JK, Griffin JD, Saper CB. Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J. Neurosci. 1996;16:6246–6254. doi: 10.1523/JNEUROSCI.16-19-06246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scammell TE, Griffin JD, Elmquist JK, Saper CB. Microinjection of a cyclooxygenase inhibitor into the anteroventral preoptic region attenuates LPS fever. Am. J. Physiol. 1998;274:R783–R789. doi: 10.1152/ajpregu.1998.274.3.R783. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K, et al. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J. Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakamura K. Central circuitries for body temperature regulation and fever. Am. J. Physiol Regul. Integr. Comp Physiol. 2011;301:R1207–R1228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- 32.Szekely M, Szelenyi Z. Endotoxin fever in the rat. Acta Physiol Acad. Sci. Hung. 1979;53:265–277. [PubMed] [Google Scholar]
- 33.Szelenyi Z, Bartho L, Szekely M, Romanovsky AA. Cholecystokinin octapeptide (CCK-8) injected into a cerebral ventricle induces a fever-like thermoregulatory response mediated by type B CCK-receptors in the rat. Brain Res. 1994;638:69–77. doi: 10.1016/0006-8993(94)90634-3. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M, McKinley MJ, McAllen RM. Roles of two preoptic cell groups in tonic and febrile control of rat tail sympathetic fibers. Am. J. Physiol Regul. Integr. Comp Physiol. 2009;296:R1248–R1257. doi: 10.1152/ajpregu.91010.2008. [DOI] [PubMed] [Google Scholar]
- 35.Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J. Neurosci. 2009;29:11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison SF. 2010 Carl Ludwig Distinguished Lectureship of the APS Neural Control and Autonomic Regulation Section: Central neural pathways for thermoregulatory cold defense. J. Appl. Physiol. 2011;110:1137–1149. doi: 10.1152/japplphysiol.01227.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang QH, Hruby VJ, Tatro JB. Role of central melanocortins in endotoxin-induced anorexia. Am. J. Physiol. 1999;276:R864–R871. doi: 10.1152/ajpregu.1999.276.3.R864. [DOI] [PubMed] [Google Scholar]
- 38.Kishi T, et al. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol. 2003;457:213–235. doi: 10.1002/cne.10454. [DOI] [PubMed] [Google Scholar]
- 39.Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur. J. Neurosci. 2006;23:3359–3367. doi: 10.1111/j.1460-9568.2006.04854.x. [DOI] [PubMed] [Google Scholar]
- 40.Romanovsky AA. Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am. J. Physiol Regul. Integr. Comp Physiol. 2007;292:R37–R46. doi: 10.1152/ajpregu.00668.2006. [DOI] [PubMed] [Google Scholar]
- 41.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 42.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–1042. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullington J, et al. Dose-dependent effects of endotoxin on human sleep. Am. J. Physiol Regul. Integr. Comp Physiol. 2000;278:R947–R955. doi: 10.1152/ajpregu.2000.278.4.R947. [DOI] [PubMed] [Google Scholar]
- 44.Morrow JD, Opp MR. Diurnal variation of lipopolysaccharide-induced alterations in sleep and body temperature of interleukin-6-deficient mice. Brain Behav. Immun. 2005;19:40–51. doi: 10.1016/j.bbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Krueger JM, Kubillus S, Shoham S, Davenne D. Enhancement of slow-wave sleep by endotoxin and lipid A. Am. J. Physiol. 1986;251:R591–R597. doi: 10.1152/ajpregu.1986.251.3.R591. [DOI] [PubMed] [Google Scholar]
- 46.Krueger JM, et al. Involvement of cytokines in slow wave sleep. Prog. Brain Res. 2011;193:39–47. doi: 10.1016/B978-0-444-53839-0.00003-X. 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imeri L, Opp MR. How (and why) the immune system makes us sleep. Nat. Rev. Neurosci. 2009;10:199–210. doi: 10.1038/nrn2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshida H, Kubota T, Krueger JM. A cyclooxygenase-2 inhibitor attenuates spontaneous and TNF-alpha-induced non-rapid eye movement sleep in rabbits. Am. J. Physiol Regul. Integr. Comp Physiol. 2003;285:R99–R109. doi: 10.1152/ajpregu.00609.2002. [DOI] [PubMed] [Google Scholar]
- 49.Terao A, Matsumura H, Yoneda H, Saito M. Enhancement of slow-wave sleep by tumor necrosis factor-alpha is mediated by cyclooxygenase-2 in rats. Neuroreport. 1998;9:3791–3796. doi: 10.1097/00001756-199812010-00005. [DOI] [PubMed] [Google Scholar]
- 50.Ueno R, et al. Role of prostaglandin D2 in the hypothermia of rats caused by bacterial lipopolysaccharide. Proc. Natl. Acad. Sci. U. S. A. 1982;79:6093–6097. doi: 10.1073/pnas.79.19.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsumura H, et al. Prostaglandin D-2-sensitive, sleep-promoting zone defined in the ventral surface of the rostral basal forebrain. Proc Natl Acad Sci USA. 1994;91:11998–12002. doi: 10.1073/pnas.91.25.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urade Y, et al. Dominant expression of mRNA for prostaglandin D synthase in leptomeninges, choroid plexus, and oligodendrocytes of the adult rat brain. Proc. Natl. Acad. Sci. U. S. A. 1993;90:9070–9074. doi: 10.1073/pnas.90.19.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mizoguchi A, et al. Dominant localization of prostaglandin D receptors on arachnoid trabecular cells in mouse basal forebrain and their involvement in the regulation of non-rapid eye movement sleep. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11674–11679. doi: 10.1073/pnas.201398898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urade Y, Hayaishi O. Prostaglandin D2 and sleep/wake regulation. Sleep Med. Rev. 2011;15:411–418. doi: 10.1016/j.smrv.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 55.Bjorness TE, Greene RW. Adenosine and sleep. Curr. Neuropharmacol. 2009;7:238–245. doi: 10.2174/157015909789152182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chamberlin NL, et al. Effects of adenosine on gabaergic synaptic inputs to identified ventrolateral preoptic neurons. Neuroscience. 2003;119:913–918. doi: 10.1016/s0306-4522(03)00246-x. [DOI] [PubMed] [Google Scholar]
- 57.Oishi Y, Huang ZL, Fredholm BB, Urade Y, Hayaishi O. Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19992–19997. doi: 10.1073/pnas.0810926105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scammell TE, et al. An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience. 2001;107:653–663. doi: 10.1016/s0306-4522(01)00383-9. [DOI] [PubMed] [Google Scholar]
- 59.Satoh S, Matsumura H, Suzuki F, Hayaishi O. Promotion of sleep mediated by the A2a-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5980–5984. doi: 10.1073/pnas.93.12.5980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lazarus M, et al. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J. Neurosci. 2011;31:10067–10075. doi: 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Onoe H, Watanabe Y, Ono K, Koyama Y, Hayaishi O. Prostaglandin E2 exerts an awaking effect in the posterior hypothalamus at a site distinct from that mediating its febrile action in the anterior hypothalamus. J. Neurosci. 1992;12:2715–2725. doi: 10.1523/JNEUROSCI.12-07-02715.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huang ZL, et al. Prostaglandin E2 activates the histaminergic system via the EP4 receptor to induce wakefulness in rats. J. Neurosci. 2003;23:5975–5983. doi: 10.1523/JNEUROSCI.23-14-05975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Swiergiel AH, Dunn AJ. Distinct roles for cyclooxygenases 1 and 2 in interleukin-1-induced behavioral changes. J. Pharmacol. Exp. Ther. 2002;302:1031–1036. doi: 10.1124/jpet.102.036640. [DOI] [PubMed] [Google Scholar]
- 64.Wieczorek M, Swiergiel AH, Pournajafi-Nazarloo H, Dunn AJ. Physiological and behavioral responses to interleukin- 1beta and LPS in vagotomized mice. Physiol Behav. 2005;85:500–511. doi: 10.1016/j.physbeh.2005.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kozak W, et al. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1 beta-deficient mice. Am. J. Physiol. 1995;269:R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- 66.Pecchi E, et al. Involvement of central microsomal prostaglandin E synthase- 1 in IL-1beta-induced anorexia. Physiol Genomics. 2006;25:485–492. doi: 10.1152/physiolgenomics.00306.2005. [DOI] [PubMed] [Google Scholar]
- 67.Ohinata K, Suetsugu K, Fujiwara Y, Yoshikawa M. Activation of prostaglandin E receptor EP4 subtype suppresses food intake in mice. Prostaglandins Other Lipid Mediat. 2006;81:31–36. doi: 10.1016/j.prostaglandins.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 68.Oka T, et al. Relationship of EP(1–4) prostaglandin receptors with rat hypothalamic cell groups involved in lipopolysaccharide fever responses. J. Comp Neurol. 2000;428:20–32. doi: 10.1002/1096-9861(20001204)428:1<20::aid-cne3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 69.Ohinata K, et al. Central prostaglandin D(2) stimulates food intake via the neuropeptide Y system in mice. FEBS Lett. 2008;582:679–684. doi: 10.1016/j.febslet.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 70.Elmquist JK, Scammell TE, Jacobson CD, Saper CB. Distribution of Fos-like immunoreactivity in the rat brain following intravenous lipopolysaccharide administration. J. Comp Neurol. 1996;371:85–103. doi: 10.1002/(SICI)1096-9861(19960715)371:1<85::AID-CNE5>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 71.Rorato R, et al. Prostaglandin mediates endotoxaemia-induced hypophagia by activation of pro-opiomelanocortin and corticotrophin-releasing factor neurons in rats. Exp. Physiol. 2009;94:371–379. doi: 10.1113/expphysiol.2008.045435. [DOI] [PubMed] [Google Scholar]
- 72.Elias CF, et al. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- 73.Elias CF, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 74.Williams G, et al. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 75.Wang L, Saint-Pierre DH, Tache Y. Peripheral ghrelin selectively increases Fos expression in neuropeptide Y - synthesizing neurons in mouse hypothalamic arcuate nucleus. Neurosci. Lett. 2002;325:47–51. doi: 10.1016/s0304-3940(02)00241-0. [DOI] [PubMed] [Google Scholar]
- 76.Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur. J. Pharmacol. 2011;660:21–27. doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang L, et al. LPS inhibits fasted plasma ghrelin levels in rats: role of IL-1 and PGs and functional implications. Am. J. Physiol Gastrointest. Liver Physiol. 2006;291:G611–G620. doi: 10.1152/ajpgi.00533.2005. [DOI] [PubMed] [Google Scholar]
- 78.Watkins LR, et al. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- 79.Schmelzer KR, et al. Enhancement of antinociception by coadministration of nonsteroidal anti-inflammatory drugs and soluble epoxide hydrolase inhibitors. Proc. Natl. Acad. Sci. U. S. A. 2006;103:13646–13651. doi: 10.1073/pnas.0605908103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Romanovsky AA, et al. Lipopolysaccharide transport from the peritoneal cavity to the blood: is it controlled by the vagus nerve? Auton. Neurosci. 2000;85:133–140. doi: 10.1016/S1566-0702(00)00232-0. [DOI] [PubMed] [Google Scholar]
- 81.Hori T, Oka T, Hosoi M, Abe M, Oka K. Hypothalamic mechanisms of pain modulatory actions of cytokines and prostaglandin E2. Ann. N. Y. Acad. Sci. 2000;917:106–120. doi: 10.1111/j.1749-6632.2000.tb05375.x. 106–120. [DOI] [PubMed] [Google Scholar]
- 82.Abe M, Oka T, Hori T, Takahashi S. Prostanoids in the preoptic hypothalamus mediate systemic lipopolysaccharide-induced hyperalgesia in rats. Brain Res. 2001;916:41–49. doi: 10.1016/s0006-8993(01)02861-x. [DOI] [PubMed] [Google Scholar]
- 83.Uschakov A, Gong H, McGinty D, Szymusiak R. Efferent projections from the median preoptic nucleus to sleep- and arousal-regulatory nuclei in the rat brain. Neuroscience. 2007;150:104–120. doi: 10.1016/j.neuroscience.2007.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rizvi TA, Murphy AZ, Ennis M, Behbehani MM, Shipley MT. Medial preoptic area afferents to periaqueductal gray medullo-output neurons: a combined Fos and tract tracing study. J. Neurosci. 1996;16:333–344. doi: 10.1523/JNEUROSCI.16-01-00333.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ueno A, et al. Major roles of prostanoid receptors IP and EP(3) in endotoxin-induced enhancement of pain perception. Biochem. Pharmacol. 2001;62:157–160. doi: 10.1016/s0006-2952(01)00654-2. [DOI] [PubMed] [Google Scholar]
- 86.Parsadaniantz SM, et al. Effects of the inhibition of cyclo-oxygenase 1 or 2 or 5- lipoxygenase on the activation of the hypothalamic-pituitary-adrenal axis induced by interleukin-1beta in the male Rat. J. Neuroendocrinol. 2000;12:766–773. doi: 10.1046/j.1365-2826.2000.00517.x. [DOI] [PubMed] [Google Scholar]
- 87.Gadek-Michalska A, Spyrka J, Bugajski J. Psychosocial stress affects the involvement of prostaglandins and nitric oxide in the lipopolysaccharide-induced hypothalamic-pituitary-adrenal response. J. Physiol Pharmacol. 2005;56:287–298. [PubMed] [Google Scholar]
- 88.Dunn AJ, Chuluyan HE. The role of cyclo-oxygenase and lipoxygenase in the interleukin-1-induced activation of the HPA axis: dependence on the route of injection. Life Sci. 1992;51:219–225. doi: 10.1016/0024-3205(92)90078-4. [DOI] [PubMed] [Google Scholar]
- 89.Roth J, Hubschle T, Pehl U, Ross G, Gerstberger R. Influence of systemic treatment with cyclooxygenase inhibitors on lipopolysaccharide-induced fever and circulating levels of cytokines and cortisol in guinea-pigs. Pflugers Arch. 2002;443:411–417. doi: 10.1007/s004240100718. [DOI] [PubMed] [Google Scholar]
- 90.Nadjar A, Sauvant J, Combe C, Parnet P, Konsman JP. Brain cyclooxygenase-2 mediates interleukin-1-induced cellular activation in preoptic and arcuate hypothalamus, but not sickness symptoms. Neurobiol. Dis. 2010;39:393–401. doi: 10.1016/j.nbd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 91.Matsuoka Y, et al. Impaired adrenocorticotropic hormone response to bacterial endotoxin in mice deficient in prostaglandin E receptor EP1 and EP3 subtypes. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4132–4137. doi: 10.1073/pnas.0633341100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garcia-Bueno B, Serrats J, Sawchenko PE. Cerebrovascular cyclooxygenase-1 expression, regulation, and role in hypothalamic-pituitary-adrenal axis activation by inflammatory stimuli. J. Neurosci. 2009;29:12970–12981. doi: 10.1523/JNEUROSCI.2373-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schiltz JC, Sawchenko PE. Specificity and generality of the involvement of catecholaminergic afferents in hypothalamic responses to immune insults. J. Comp Neurol. 2007;502:455–467. doi: 10.1002/cne.21329. [DOI] [PubMed] [Google Scholar]
- 94.Ericsson A, Kovacs KJ, Sawchenko PE. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J. Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang YH, Lu J, Elmquist JK, Saper CB. Specific roles of cyclooxygenase-1 and cyclooxygenase-2 in lipopolysaccharide-induced fever and Fos expression in rat brain. J. Comp Neurol. 2003;463:3–12. doi: 10.1002/cne.10743. [DOI] [PubMed] [Google Scholar]
- 96.Romanovsky AA. Signaling the brain in the early sickness syndrome: are sensory nerves involved? Front Biosci. 2004;9:494–504. doi: 10.2741/1247. [DOI] [PubMed] [Google Scholar]
- 97.Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N, Szekely M. Febrile responsiveness of vagotomized rats is suppressed even in the absence of malnutrition. Am. J. Physiol. 1997;273:R777–R783. doi: 10.1152/ajpregu.1997.273.2.R777. [DOI] [PubMed] [Google Scholar]
- 98.Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am. J. Physiol. 1997;273:R407–R413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- 99.Luheshi GN, et al. Vagotomy attenuates the behavioural but not the pyrogenic effects of interleukin-1 in rats. Auton. Neurosci. 2000;85:127–132. doi: 10.1016/S1566-0702(00)00231-9. [DOI] [PubMed] [Google Scholar]
- 100.Hansen MK, et al. Subdiaphragmatic vagotomy does not block intraperitoneal lipopolysaccharide-induced fever. Auton. Neurosci. 2000;85:83–87. doi: 10.1016/S1566-0702(00)00224-1. [DOI] [PubMed] [Google Scholar]