Small molecule inhibitors of soluble epoxide hydrolase (sEH) have been shown to reduce blood pressure, inflammation, and pain in a number of mammalian disease models. In fact, an inhibitor of sEH has been moved into clinical trials for the treatment of hypertension. The beneficial effect of sEH inhibition is thought to be due to the role of sEH in the metabolism of anti-inflammatory, antihypertensive, and analgesic lipid signaling molecules such as the epoxyeicosatrienoic acids. Recently, application of sEH inhibitors in animal models of cardiac hypertrophy have produced promising results. In this review, we describe these results and discuss the effect of sEH inhibitors on the inflammatory NF-κB pathway and the implication this has for the treatment of cardiac hypertrophy.
Inhibitors sEH are in clinical trials as oral drugs for the treatment of hypertension. Based on animal models, sEHI appears to reduce the vascular inflammation and end-organ damage that commonly are associated with hypertension. There appears to be a dramatic reduction in renal damage in angiotensin and deoxycorticosterone acetate–salt models of hypertension. Moreover, sEH inhibitors have been shown to have an anti-inflammatory action1 and may be particularly useful in hypertensive patients, in whom it is important to control blood pressure in the presence of a systematic inflammatory disease. Animal models indicate that sEH inhibitors, presumably working through epoxylipid chemical mediators, reduce the progression of atherosclerotic plaque as well as infarct size associated with ischemic heart injury.2 An unexpected finding, however, was the dramatic reduction in cardiac hypertrophy in several model systems,3 which will be the focus of the present article. In addition, we will summarize recent literature on sEH and sEH inhibitors.
Endothelium-Derived Hyperpolarizing Factors
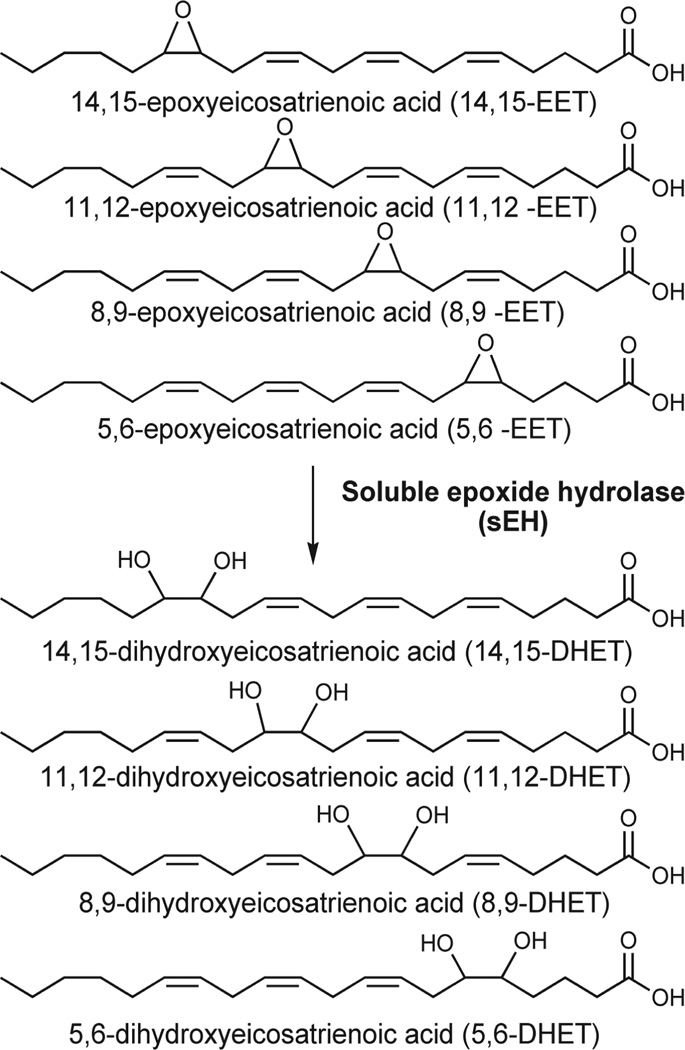
By inhibiting sEH, the levels of endogenous chemical mediators, including the epoxides of arachidonic acid (AA), epoxyeicosatrienoic acids (EETs),1 and other epoxylipids, are increased and the corresponding diol products of the enzyme are decreased (Figure 1). These epoxide-containing lipids are thought to be major contributors to the endothelium-derived hyperpolarizing factors (EDHFs) that lead to relaxation of vascular smooth muscle.1 Over the last few years, it has become clear that regulation of the EDHFs is intimately tied to the renin-angiotensin-aldosterone system (RAAS) for blood pressure regulation. The EDHF complex is a sufficiently important contributor to vascular biology and hypertension, and we often refer to the EDHF RAAS.
Figure 1.
Structures of epoxyeicosatrienoic acids (EETs) and dihydroxyeicosatrienoic acids (DHETs). The largely antihypertensive, anti-inflammatory, and analgesic EETs are converted to their corresponding diols through the action of soluble epoxide hydrolase (sEH). The diols are more easily conjugated, more water-soluble, and easier to excrete. The diols also have greatly reduced biologic activity. Thus the overall effect of sEH hydrolysis of these compounds is to decrease the epoxylipid signal.
There is growing evidence that the EDHF system is affected in cardiovascular disease states, such as hypertension, diabetes, chronic renal failure, and aging. Moreover, recent studies have suggested the link between EDHFs and the RAAS. For example, chronic treatment with RAAS inhibitors improves the age-related impairment of EDHF-mediated responses.4 Indeed, several clinical studies have shown that blocking the RAAS can improve endothelial function not only in hypertensive patients but also in normotensive patients with cardiovascular disease.5
Although the exploitation of a new mechanism for the treatment of hypertension is the major driver for moving sEH inhibitors into clinical trials, the compounds influence physiology in a number of ways. For example, they reduce inflammation and pain in addition to reducing blood pressure in numerous animal models.6,7 These additional attributes of sEH inhibitors may make them valuable in the treatment of a variety of disorders. They have been shown to be beneficial in animal models of adult respiratory distress syndrome, cisplatin overdose, glaucoma, chronic obstructive pulmonary disease, diabetes, erectile dysfunction, general inflammation, glaucoma, inflammatory bowel disease, inflammatory pain, ischemic stroke, neuropathic pain, Raynaud’s syndrome, and others. Many conditions on this long list share a focused mechanism. That is, the epoxylipid chemical mediators such as EETs appear to act to reduce inflammation and to move the eicosanoid cascade from a pattern of metabolites promoting propagation of inflammation toward one promoting resolution of inflammation.7
Soluble Epoxide Hydrolase
The mammalian sEH is an approximately 60 kDa enzyme of the α/β-hydrolase fold family, which forms a homodimer in solution.8 sEH contains two globular regions connected by a short pro-line-rich linker.8 The approximately 300-amino acid C-terminal region of mammalian sEH contains the enzyme’s epoxide hydrolase catalytic domain, while the approximately 200-amino acid N-terminal region has recently been shown to exhibit phosphatase activity using several phosphate lipid substrates, including polyisoprenyl phosphates.8
The structure of α/β-hydrolases consists of β sheets and α helices connected by loops that position catalytic residues. The “catalytic triad” contains a catalytic nucleophile (aspartate, cysteine, or serine) and an acid-base pair that activates a molecule of water.9 The sEH catalytic triad contains an aspartate nucleophile and an aspartate-histidine acid-base pair.9 In addition to this triad, two tyrosines have been implicated in polarizing the epoxide moiety, making the substrate more susceptible to nucleophilic attack.9
Epoxides are 3-member cyclic ethers. Compounds that contain epoxide moieties can be highly reactive and mutagenic, as is the case with arene oxides, but they can also be physiologically stable, as are most lipid epoxides.10 Epoxides such as cyclodiene epoxides can even be environmentally stable.10 Classes of lipid epoxides have been implicated as paracrine and autocrine signaling molecules in mammals. Two examples of epoxide-containing lipid signals are the EETs and the leukotoxins (EpOMEs). These are epoxides of AA (20:4) and linoleic acid (18:2), respectively, formed largely but not exclusively by cytochrome P450s (CYP).8,11 These lipid signaling molecules have been shown to play a role in regulation of inflammation and hypertension in various mammalian systems.6,7 Other metabolites of AA include the prostanoids, formed by cyclooxygenases, and the leukotrienes and prostaglandins, formed by lipoxygenases.12 Both of these branches of the AA cascade have been targeted by pharmaceutical companies for the treatment of inflammatory pain (eg, celecoxib) and asthma (eg, montelukast).
sEH converts the lipid signaling molecules, such as the EETs, to their corresponding diols. In the case of the EETs, the diols are called dihydroxyeicosatrienoic acids (DHETs).8 The DHETs may still possess some vasodilatory effect,8 but overall, the transformation of EETs to DHETs by sEH should reduce biologic activity, increase the excretion of the compound, and inhibit their incorporation into lipid membranes,8 thereby attenuating the vasodilatory or anti-inflammatory signal in those tissues. In addition to its action on the EETs, sEH metabolizes the EpOMEs to a more toxic diol species.8 These diols have been implicated in the onset of acute respiratory distress syndrome (ARDS), an inflammatory disease that causes sudden pulmonary failure.8 They may also be endogenous chemical mediators responsible, for example, for increasing vascular permeability.8 sEH also metabolizes the epoxides of the omega-3 fatty acids docosahexaenoic and eicosapentaenoic acid, which have been shown to be formed by CYPs and have anti-inflammatory effects in vitro.13,14
One example of the balance of lipid signals and the effect of sEH epoxide hydrolase activity occurs in the renal vasculature, where the CYP metabolite of AA 20-HETE has been shown to have a vasoconstrictive effect, while 14,15- and 11,12-EETs have a vasodilatory effect.15 Hypothetically, if the EETs are hydrolyzed to their corresponding diols by sEH and then excreted, there will be a relative increase of the vasoconstrictive signal over the vasodilatory signal, which should result in an increase in blood pressure. The situation is complicated by the fact that 8,9-EET has been shown to have a vasoconstrictive effect on the renal afferent arteriole.15 However, it has been shown that sEH hydrolyzes the 14,15-EET and 11,12-EET at a much faster rate than 8,9-EET,8 and so the total effect of sEH should be to increase the vasoconstrictive signal relative to the vasodilatory signal. This prediction has been supported by a number of animal studies.2,6,15
sEH Inhibitors
Small molecule inhibitors are valuable tools for the study of sEH in vivo. Based on knowledge of the mechanism of catalysis of the microsomal epoxide hydrolases and sEHs, potent transition state inhibitors for each enzyme class have been designed. Available inhibitors span a range of potency, solubility, and metabolic stability.9 Several inhibitors with IC50s in the high-picomolar/low-nanomolar range with mammalian sEH contain a central urea pharmacophore.9 The urea binds reversibly to the sEH active site as a transition state mimic.9 The carbonyl is polarized by the two catalytic tyrosines, while the urea participates in two hydrogen bonds with the catalytic aspartate in the active site. These inhibitors have been used to probe sEH function in cell culture and animal models in several mammalian species including rat, mouse, dog, and cat.
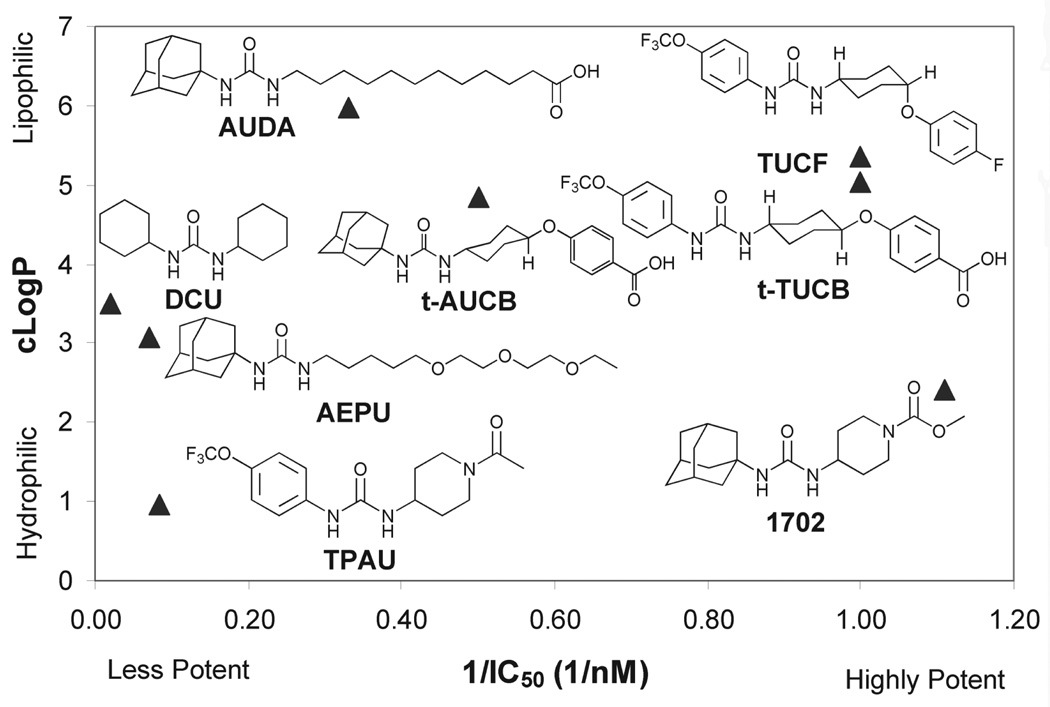
Earlier compounds such as AUDA (12-(3-adamantan-1-yl-ureido)-dodecanoic acid) were highly lipophilic and difficult to deliver as well as being metabolically unstable. AEPU (1-adamantan-3-(5-(2-(2-ethylethoxy)ethoxy) pentyl)urea) represents a compound that is more water-soluble and penetrates membranes well. Newer compounds such as t-AUCB and TPAU have better oral bioavailability and metabolic stability than their predecessors, possibly associated with greater water solubility. Some, like AUDA and t-AUCB, are very potent on the sEHs of many species. However, others such as TPAU can vary greatly in their potency. For example, TPAU has poor inhibitory activity on canine and feline sEHs (Figure 2).
Figure 2.
Structures, potency, and lipophilicity of representative soluble epoxide hydrolase (sEH) inhibitors. Early inhibitors of sEH such as DCU and AUDA employed a potent central urea pharmacophore, but they had low solubility. This pharmacophore was retained while the solubility was improved by adding more polar groups such as the polyethylene glycol tail in AEPU. These more soluble compounds are suitable for infusion, nebulization, or oral administration, whereas less soluble compounds such as t-TUCB are used for subcutaneous injection or slow-release formulations. Several compounds have been developed with IC50s in the low-nanomolar/high-picomolar range that remain in circulation for over a day, as is the case with tAUCB, or are metabolized in a matter of hours, as occurs with AEPU. The low blood level of AEPU does not reflect intercellular concentration because the compound easily penetrates plasma membranes. In fact, AEPU displayed high efficacy in several mammalian disease models. The incorporation of fluorides produces molecules such as TUCF, TPAU, and t-TUCB, which can be used to impregnate stents for slow release of the compound at sites that are susceptible to inflammation. IC50 assays were determined with the fluorescent substrate (3-phenyl-oxiranyl)-acetic acid cyano-(6-methoxy-naphthalen-2-yl)-methyl ester with purified recombinant human sEH. cLogP values are calculated estimates of the octanol/water partition coefficients commonly utilized in predicting a molecule’s properties. Algorithms for cLogPs are often poor indicators of logP for ureas, but they give a good indication of relative lipophilicity. These values were calculated with the ChemOffice suite (CambridgeSoft Corporation, Cambridge, MA).
sEH Inhibitors and Cardiac Hypertrophy and Failure
Cardiac hypertrophy is the heart’s compensatory response to a variety of extrinsic and intrinsic stimuli including pressure or volume overload, mutations of sarcomeric proteins, or loss of contractile mass from previous myocardial infarction. Cardiac hypertrophy is believed to have a compensatory function by diminishing wall stress. Yet, paradoxically, ventricular hypertrophy is associated with a significant increase in the risk of heart failure and malignant arrhythmia. To disentangle physiologic from pathologic hypertrophic growth, the molecular mechanisms that initiate the compensatory growth response or that precipitate the transition to heart failure have undergone intense investigations, and there is increasing evidence from antihypertensive agents that cardiac hypertrophy may represent a new therapeutic target to improve survival in patients.16
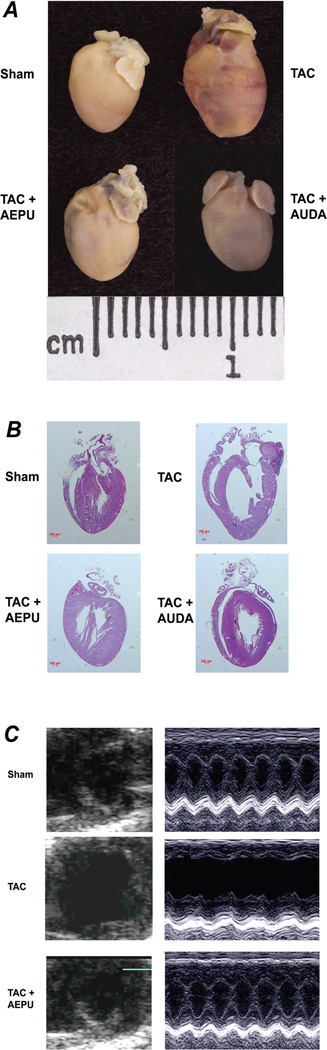
Motivated by the therapeutic potential, we recently tested the effects of sEH inhibitors on the development and reversal of cardiac hypertrophy using a murine model of thoracic aortic constriction. We showed that there is an almost complete resolution of cardiac hypertrophy by sEH inhibitors independent of the antihypertensive effects (Figure 3). The beneficial effects of sEH inhibitors were assessed using noninvasive cardiac echocardiography as well as histologic examination, as shown in Figure 3. Moreover, our study shows a beneficial effect of sEH inhibitors in the prevention of cardiac arrhythmias that occur in association with cardiac hypertrophy. In addition to the previously described anti-inflammatory and antihypertensive effects of sEH inhibitors, the increase in the endogenous levels of EETs by inhibiting the enzyme sEH exerts a beneficial effect by preventing the development of cardiac hypertrophy. This results in the preservation of the cardiac chamber, with no deterioration of cardiac contractility as assessed by noninvasive imaging, histologic examination, and biochemical analyses. Specifically, our findings support the notion that even though treatment with sEH inhibitors may result in a lack of normalization of wall tension in the short term, the treatment led to a preservation of cardiac contractility in the long term by preventing progressive cardiac deterioration associated with cardiac hypertrophy. Indeed, previous studies using gene-targeted mouse models with attenuated hypertrophic response have shown that despite inadequate wall tension normalization in the transgenic models with chronic pressure overload, the transgenic animals showed little cardiac deterioration over time compared with the wild-type animals.17
Figure 3.
Soluble epoxide hydrolase (sEH) inhibitors inhibit cardiac hypertrophy in TAC mice. (A) Mice were treated with AEPU or AUDA. Controls consisted of both TAC alone and sham-operated groups. The mice were sacrificed after 3 weeks of follow-up (scale, 1 cm). Treatment with AEPU and AUDA resulted in inhibition of the increase in TAC mouse heart size compared with controls as determined by heart/body weight ratios (mg/g). Heart/body weight ratios (mean ± SEM) were 5.7±0.4, 10.0±0.3, 5.9±0.4, and 5.4±0.3 mg/g in sham, TAC alone, TAC+AEPU, and TAC+AUDA groups, respectively (n=16; P<.05 comparing TAC alone to TAC+AEPU or TAC+AUDA). (B) Hematoxylin and eosin staining of histologic sections showing cardiac hypertrophy at 3 weeks in the TAC mouse. The images are oriented so that the atria is on top and the right ventricle is on the left (scale bars, 200 µm). (C) After 3 weeks of treatment, TAC mice showed evidence of cardiac failure with chamber dilation, while the AEPU-treated group did not. Heart condition was assessed with 2-dimensional and M-mode echocardiography. Fractional shortening (FS), a surrogate of systolic function, was calculated from left ventricle dimensions as follows: FS = ([EDD – ESD]/EDD) × 100% (FS for each group was 50.3±3.0, 23.9±3.3, 42.3±2.8, and 43.9±5.4 for sham, TAC alone, TAC+AEPU, and TAC+AUDA, respectively (n=10–16 for each group; P<.05 comparing TAC alone to TAC+AEPU or TAC+AUDA). Adapted with permission from Xu et al.3
Mechanisms Underlying the Observed Beneficial Effects of sEH Inhibitors
The exact mechanisms for the observed beneficial effects of sEH inhibitors in cardiac hypertrophy and failure remain uncertain. It has previously been shown that EETs can inhibit NF-κB-mediated gene transcription1 and produce an anti-inflammatory effect by inhibiting cytokine-induced NF-κB transcription. NF-κB is a pleiotropic transcription factor that can directly regulate the expression of immediate early genes and genes involved in the stress response following a variety of physiologic or pathologic stimuli.18 NF-κB is inactive when bound to IκB, an inhibitory protein that is phosphorylated by IκB kinase (IKK).19 Phosphorylation of IκB triggers its ubiquitination and degradation, which permits NF-κB translocation to the nucleus, where it activates transcription of inflammatory and immune response target genes.19 EETs inhibit IKK, preventing degradation of IκB and maintaining NF-κB in its inactive form.1 11,12-EET has been shown to produce the most potent effect in endothelial cells by inhibiting IKK-mediated phosphorylation of IκBα.1 Because sEH converts EETs to DHETs, inhibition of sEH may represent a potential approach for enhancing the biologic activity of EETs.1,9 On the other hand, the potential beneficial effects of sEH inhibitors in cardiac hypertrophy remain unexplored to date.
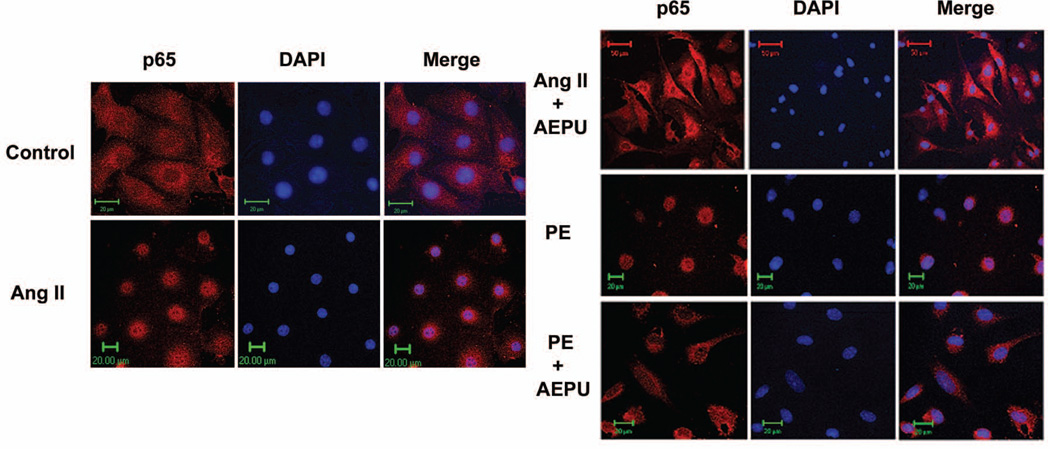
Using both in vivo and in vitro models of cardiac hypertrophy, our data suggest an inhibitory effect of sEH inhibitors on the NF-κB pathway (Figure 4). The NF-κB pathway has been implicated as a pivotal intracellular mediator of the inflammatory response associated with a variety of disease processes as well as cell growth and apoptosis.18
Figure 4.
Treatment with AEPU prevents nuclear translocation of NF-κB in primary murine neonatal cardiomyocytes. Cardiomyocytes treated with 50 µM Angiotensin II (Ang II) or phenylephrine (PE) were either cotreated with 20 µM AEPU or left untreated for 45 minutes. The nuclear translocation of p65 was visualized in fixed cells after staining with DAPI (blue) and anti-p65 mAb, followed by anti-mouse Texas red-conjugated secondary Ab (red). A Zeiss confocal microscope was used. Cells were serum starved for 24 hours and grown on coverslips. Adapted with permission from Xu et al.3
Recently, several studies have implicated NF-κB activation as a causal event in cardiac hypertrophic response, as modeled in cultured cardiac myocytes as well as in vivo model.20 Purcell and Molkentin20 demonstrated that viral mediated transfer of a “superrepressor” IκBα protein, a dominant negative NF-κB approach, prevented cardiomyocyte hypertrophy in response to G-protein coupled receptor (GPCR) agonists such as phenylephrine, endothelin-1, and angiotensin II. A crucial role for NF-κB activation in myotrophin-induced and GPCR-related cardiac hypertrophy in vivo has been demonstrated.21,22 Taken together, evidence now exists to support the notion that NF-κB plays a necessary role for myocyte hypertrophy in vitro and in vivo, at least downstream of GPCR agonist stimulation.
NF-κB signaling in the heart is extremely complex. Several signaling pathways implicated in cardiac hypertrophy and failure are capable of activating NF-κB including α-adrenergic stimulation, angiotensin II, PI3K/Akt, p38, ras, MEKK1/4, PKC, and gp130.23–26 Signaling by gp130 activates NF-κB via ras, Jak/Stat, and/or PI3K/Akt signaling and is required for pressure overload–induced cardiac hypertrophy.27 Several components of signaling cascades implicated in cardiac hypertrophy are also involved in cytokine-activated pathways that culminate in NF-κB activation. Conversely, cytokine-activated pathways activate many of the MAPK signaling cascades implicated in cardiac hypertrophy.28
The linkage between NF-κB and myocyte hypertrophy is especially intriguing given the long-standing hypothesis that inflammatory cytokines, for example, tumor necrosis factor (tumor necrosis factor α [TNFα] and interleukin-1 [IL1]) are relevant mediators of cardiomyopathic disease states and that NF-κB itself is dominantly regulated by the cytokine TNFα.29 Heart failure patients typically have elevated levels of circulating TNF-α in the blood and have significant myocardial NF-κB activation.29 NF-κB has been shown to be involved in the direct regulation of both proapoptotic and antiapoptotic genes, including antiapoptotic factors such as cIAPs, Bcl-2 family (Bcl xL), and FLICE inhibitory protein, as well as proapoptotic factors such as Fas, FasL, caspase-8, caspase-11, and TNFα.30 This is especially relevant given the hypothesis that cardiac myocyte apoptosis can exacerbate or even initiate heart failure. Taken together, the beneficial effects of blocking NF-κB by sEH inhibition in cardiac hypertrophy remain to be systematically investigated. Further experiments are needed to define the molecular mechanisms responsible for the observed inhibitory effects of sEH inhibitors on the development of cardiac hypertrophy. Finally, additional information is urgently needed to establish whether the observed beneficial effects are generalizable to other animal models of hypertrophy and especially to patients with cardiac hypertrophy and failure. There is consequently an enormous and exciting opportunity to uncover a potentially very powerful group of compounds that may be used effectively in the clinical setting and at the very least will deepen our understanding of the AA cascade and the development of cardiac hypertrophy.
References
- 1.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292(3):C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 2.Imig JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev. 2006;24(2):169–188. doi: 10.1111/j.1527-3466.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 3.Xu D, Li N, He Y, et al. Prevention and reversal of cardiac hypertrophy by soluble epoxide hydrolase inhibitors. Proc Natl Acad Sci U S A. 2006;103(49):18733–18738. doi: 10.1073/pnas.0609158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goto K, Fujii K, Kansui Y, et al. Changes in endothelium-derived hyperpolarizing factor in hypertension and ageing: response to chronic treatment with renin-angiotensin system inhibitors. Clin Exp Pharmacol Physiol. 2004;31(9):650–655. doi: 10.1111/j.1440-1681.2004.04054.x. [DOI] [PubMed] [Google Scholar]
- 5.Brewster UC, Setaro JF, Perazella MA. The renin-angiotensin-aldosterone system: cardiorenal effects and implications for renal and cardiovascular disease states. Am J Med Sci. 2003;326(1):15–24. doi: 10.1097/00000441-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Chiamvimonvat N, Ho CM, Tsai HJ, et al. The soluble epoxide hydrolase as a pharmaceutical target for hypertension. J Cardiovasc Pharmacol. 2007;50(3):225–237. doi: 10.1097/FJC.0b013e3181506445. [DOI] [PubMed] [Google Scholar]
- 7.Inceoglu B, Schmelzer KR, Morisseau C, et al. Soluble epoxide hydrolase inhibition reveals novel biological functions of epoxyeicosatrienoic acids (EETs) Prostaglandins Other Lipid Mediat. 2007;82(1–4):42–49. doi: 10.1016/j.prostaglandins.2006.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman JW, Morisseau C, Hammock BD. Epoxide hydrolases: their roles and interactions with lipid metabolism. Prog Lipid Res. 2005;44(1):1–51. doi: 10.1016/j.plipres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Morisseau C, Hammock BD. Epoxide hydrolases: mechanisms, inhibitor designs, and biological roles. Annu Rev Pharmacol Toxicol. 2005;45:311–333. doi: 10.1146/annurev.pharmtox.45.120403.095920. [DOI] [PubMed] [Google Scholar]
- 10.Wixtrom RN, Hammock BD. Membranebound and soluble fraction epoxide hydrolases. In: Zakim D, Vessey DA, editors. Biochemical Pharmacology and Toxicology. New York, NY: Wiley; 1985. pp. 1–93. [Google Scholar]
- 11.Capdevila JH, Falck JR, Estabrook RW. Cytochrome P450 and the arachidonate cascade. FASEB J. 1992;6(2):731–736. doi: 10.1096/fasebj.6.2.1537463. [DOI] [PubMed] [Google Scholar]
- 12.Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294(5548):1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- 13.Schwarz D, Kisselev P, Ericksen SS, et al. Arachidonic and eicosapentaenoic acid metabolism by human CYP1A1: highly stereoselective formation of 17(R),18(S)-epoxyeicosatetraenoic acid. Biochem Pharmacol. 2004;67(8):1445–1457. doi: 10.1016/j.bcp.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 14.VanRollins M. Epoxygenase metabolites of docosahexaenoic and eicosapentaenoic acids inhibit platelet aggregation at concentrations below those affecting thromboxane synthesis. J Pharmacol Exp Ther. 1995;274(2):798–804. [PubMed] [Google Scholar]
- 15.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol Renal Physiol. 2005;289(3):F496–F503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 16.Frey N, Katus HA, Olson EN, et al. Hypertrophy of the heart: a new therapeutic target? Circulation. 2004;109(13):1580–1589. doi: 10.1161/01.CIR.0000120390.68287.BB. [DOI] [PubMed] [Google Scholar]
- 17.Esposito G, Rapacciuolo A, Naga Prasad SV, et al. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation. 2002;105(1):85–92. doi: 10.1161/hc0102.101365. [DOI] [PubMed] [Google Scholar]
- 18.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 19.Karin M. How NF-kB is activated: the role of the IkB kinase (IKK) complex. Oncogene. 1999;18(49):6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- 20.Purcell NH, Molkentin JD. Is nuclear factor kB an attractive therapeutic target for treating cardiac hypertrophy? Circulation. 2003;108(6):638–640. doi: 10.1161/01.CIR.0000085362.40608.DD. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Purcell NH, Lin A, et al. Activation of nuclear factor-kappaB is necessary for myotrophin-induced cardiac hypertrophy. J Cell Biol. 2002;159(6):1019–1028. doi: 10.1083/jcb.200207149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirotani S, Otsu K, Nishida K, et al. Involvement of nuclear factor-kappaB and apoptosis signal-regulating kinase 1 in G-protein-coupled receptor agonist-induced cardiomyocyte hypertrophy. Circulation. 2002;105(4):509–515. doi: 10.1161/hc0402.102863. [DOI] [PubMed] [Google Scholar]
- 23.Craig R, Wagner M, McCardle T, et al. The cytoprotective effects of the glycoprotein 130 receptor-coupled cytokine, cardiotrophin-1, require activation of NF-kB. J Biol Chem. 2001;276(40):37621–37629. doi: 10.1074/jbc.M103276200. [DOI] [PubMed] [Google Scholar]
- 24.Force T, Kuida K, Namchuk M, et al. Inhibitors of protein kinase signaling pathways: emerging therapies for cardiovascular disease. Circulation. 2004;109(10):1196–1205. doi: 10.1161/01.CIR.0000118538.21306.A9. [DOI] [PubMed] [Google Scholar]
- 25.Rouet-Benzineb P, Gontero B, Dreyfus P, et al. Angiotensin II induces nuclear factor- kB activation in cultured neonatal rat cardiomyocytes through protein kinase C signaling pathway. J Mol Cell Cardiol. 2000;32(10):1767–1778. doi: 10.1006/jmcc.2000.1211. [DOI] [PubMed] [Google Scholar]
- 26.Zechner D, Craig R, Hanford DS, et al. MKK6 activates myocardial cell NF-kappaB and inhibits apoptosis in a p38 mitogenactivated protein kinase-dependent manner. J Biol Chem. 1998;273(14):8232–8239. doi: 10.1074/jbc.273.14.8232. [DOI] [PubMed] [Google Scholar]
- 27.Fischer P, Hilfiker-Kleiner D. Survival pathways in hypertrophy and heart failure: the gp130-STAT3 axis. Basic Res Cardiol. 2007;102(4):279–297. doi: 10.1007/s00395-007-0658-z. [DOI] [PubMed] [Google Scholar]
- 28.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91(9):776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 29.Matsumori A, Sasayama S. The role of inflammatory mediators in the failing heart: immunomodulation of cytokines in experimental models of heart failure. Heart Fail Rev. 2001;6(2):129–136. doi: 10.1023/a:1011457910659. [DOI] [PubMed] [Google Scholar]
- 30.Karin M, Lin A. NF-kB at the cross-roads of life and death. Nat Immunol. 2002;3(3):221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]