Abstract
Obstructive sleep apnea causes cardiovascular disease via chronic intermittent hypoxia (IH), which may be related to oxidative stress. Nuclear factor-erythroid 2-related factor 2 (Nrf2) is an important cellular defense mechanism against oxidative stress by regulating its down-stream multiple antioxidants. The present study was to define whether IH can induce renal pathogenic damage and if so, whether Nrf2 and its down-stream antioxidants are involved in IH-induced pathogenic changes. Mice were culled for exposure to intermittent air as control or IH that consisted of 20.9% O2/ 8% O2 FIO2 alternation cycles (30 episodes per h) with 20 seconds at the nadir FIO2 for 12 h a day during daylight. Short-term IH exposure (3 – 7 days) induced significant increases in renal inflammatory response and antioxidant levels along with a reduction of the spontaneous content of malondialdehyde while long-term IH exposure (8 weeks) induced a significant decrease of antioxidant levels and significant increases of renal inflammation, oxidative damage, cell death, and fibrosis. This study suggests that IH induces a hormetic response, i.e.: short-term IH exposure is able to induce a protective response to protect the kidney from oxidative damage while long-term IH exposure is able to induce a damage effect on the kidney.
Keywords: Intermittent hypoxia, kidney hypoxic damage, Nrf2, metallothionein
INTRODUCTION
As a highly prevalent chronic disease, obstructive sleep apnoea (OSA) is characterized by recurrent episodes of partial or complete upper airway collapse and obstruction during sleep. OSA affects about 9% of adult women and 24% of adult men (Young et al. 1993). The intermittent hypoxia (IH) has been considered as a main cause of OSA pathogenesis since IH is able to induce oxidative stress, inflammation, atherosclerosis, endothelial dysfunction and hypertension (Dematteis et al. 2009).
In a cross-sectional study of 35 patients with chronic kidney diseases, the majority of patients (54%) had OSA (Markou et al. 2006). Despite a high overall oxygen supply, the tissue oxygen tension in the kidney is comparatively low that renders the kidney prone to hypoxic injury (Eckardt et al. 2003). Compared to subjects without nocturnal hypoxia, subjects with nocturnal hypoxia demonstrated an almost three-fold increase in the risk of accelerated loss of kidney function (Ahmed et al. 2011). However, there was also report that declining kidney function increased the prevalence of sleep apnea too (Nicholl et al. 2012). Therefore, it was unclear whether OSA-related IH induces kidney damage or kidney dysfunction causes sleep apnea. Can IH directly affect the kidney function in the subject who does not have any abnormality? To answer this question, we have to use animal studies. However, there was no a detail time-course study on the kidney response to IH in terms of structural and functional changes as well as cellular pathogenic changes with animal models.
Inflammation-related oxidative stress that has been assumed as a major cause for the cardiovascular damage and dysfunction in patients with OSA (Khayat et al. 2009) may also contribute to chronic kidney disease progression. Oxidative stress describes an imbalance between the production of reactive oxygen or nitrogen species (ROS or RNS) and the antioxidant capacity.
Cellular ROS and RNS levels and their effects are regulated by a variety of specific antioxidant systems. Nuclear factor-erythroid 2-related factor 2 (Nrf2) is one of the important cellular defense mechanisms against oxidative stress (Tkachev et al. 2011). It regulates many phase II detoxifying enzymes and proteins that detoxify xenobiotics and neutralize ROS and/or RNS to maintain cellular redox homeostasis. Hemeoxygenase-1 (HO-1), NAD(P)H quinone oxidoreductase-1 (NQO-1), superoxide dismutase (SOD), and metallothionein (MT) are among the well-studied Nrf2 target genes (Tkachev et al. 2011). It has been reported that hypoxia can induce Nrf2 activation (Papaiahgari et al. 2006).
The present study was to investigate the dynamic responses of the kidney to short- and long-term exposure to IH for 3 days to 8 weeks, which was followed by renal functional, structural and biochemical examinations. To study whether oxidative stress is involved in the pathophysiological changes, Nrf2 expression and its down-stream antioxidants were examined dynamically.
MATERIALS AND METHODS
Animals
FVB mice were used for this study and housed in the University of Louisville Research Resources Center at 22 °C with a 12-h light/dark cycle with free access to standard rodent chow and tap water. All animal procedures were approved by the Institutional Animal Care and Use Committee, which is certified by the American Association for Accreditation of Laboratory Animal Care.
IH and sham exposure
The murine model of IH exposures during sleep has been established and extensively utilized in the study of sleep apnea-associated morbidities (Cai et al. 2012). In this study, 8 – 10 weeks old male mice were culled for exposure by two groups – IH group and intermittent air control group. Selection of male mice only for the present study was because several measurements induced by IH were significantly protected in female mice (Li et al. 2012). The outcome from the present study will not reflect the response of female mice to the same challenge. The animals were randomly allocated into four control groups and four hypoxia groups: 3 days, 1 week, 2 weeks, and 8 weeks. Animal numbers were 3 in control groups (except for 2 weeks control, n = 5) and 5 in hypoxia groups (except for 2 weeks hypoxia group, n = 8). The IH paradigm consisted of 20.9% O2/8% O2 FIO2 alternation cycles (30 episodes per h) with 20 seconds at the nadir FIO2 for 12 h a day during daylight. Pulse oxyhemoglobin saturation (SpO2) changed in a recurrent manner with the nadir hemoglobin oxygen saturations mainly ranging between 60% and 70% to mimic hypoxia/reoxygenation events occurring in moderate to severe OSA patients. The IH exposure lasted different times from 3 days to 8 weeks. All animals were assigned to identical custom-designed chambers (Oxycycler model A84XOV, BioSpherix, Lacona, NY, USA) to be operated under 12-h light-dark cycle. After IH exposures, mice were transferred to room air until sacrifice for tissue collection. At the end of study, animal renal function was measured by individually measuring 24-h urinary protein and then sacrificed to collect the kidney individually from each animal for perform various biochemical measurements.
Analysis of the kidney function
Mice were placed in metabolic cages for 24-h urine collection. Urine protein was measured by Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA).
Western blots
MT expression was detected by a modified Western blotting protocol (Wang et al. 2006). Briefly, kidney proteins were treated with DTT at a final concentration of 20 mM at 56 °C for 30 min, followed by addition of iodoacetamide (Sigma Chemical Co. St. Louis, MO, USA) at 50 mM at room temperature for 1 h in the dark. In addition, proteins were electrophoretically separated and transferred to nitrocellulose membrane with the transfer buffer including 2 mM CaCl2. The monoclonal antibody against human’s MT (Dako North America, Carpentaria, CA, USA) was used at 1:1000 dilutions in 3% BSA at 4 °C overnight. Since in the transfer buffer contains CaCl2, these blots for MT could not be stripped and reprobed for β-actin analysis. Therefore, another parallel gel was used for β-actin analysis with regular process as did for other proteins (see below).
The regular Western blotting was performed as described in our previous studies (Cai et al. 2005; Wang et al. 2006). Briefly, kidney tissue were homogenized and fractionated on 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gels, and proteins were transferred to a nitro-cellulose membrane. The membrane was incubated overnight at 4 °C with primary antibodies, which included HO-1 (1:500), connective tissue growth factor (CTGF, 1:500), intracellular adhesion molecule-1 (ICAM-1, 1:250), vascular cell adhesion molecule-1 (VCAM-1, 1:250), hypoxia-inducible factor 1-α (HIF-1α, 1:500), extracellular signal-regulated kinases (ERK1/2, 1:1000), Nrf2 (1:500), NQO-1 (1:500), SOD-1 (1:1000, all from Santa Cruz, CA, USA), plasminogen activator inhibitor 1 (PAI-1, 1:2000, BD Biosciences, Franklin Lakes, NJ, USA), and β-actin (1:5000, Cell Signaling). The rest of Western blotting and quantitative densitometry procedure was described in previous studies (Cai et al. 2005; Wang et al. 2006).
Histopathology examination
Kidneys were collected and immersion-fixed in 10% neutral formalin, embedded in paraffin and sectioned into 5-μm-thick sections onto glass slides. After deparaffinization, tissue sections were rehydrated and stained with 0.1% Sirius-red F3BA and 0.25% Fast green FCF. Sirius-red stained sections were assessed for the proportion of fibrosis (collagen) in the kidney tissues. Apoptotic cell death in the kidney was measured by terminal deoxynucleotidyl transferase-mediated dUTP nick end labelling (TUNEL) staining with the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Chemicon, Temecula, CA, USA), as described before (Cai et al. 2005).
Quantitative analysis of lipid peroxides
The lipid peroxide concentration was determined by a previously described method (Cai et al. 2005) which measures the amount of thiobarbituric acid reactivity by the amount of malondialdehyde (MDA) formed during acid hydrolysis of the lipid peroxide compound. After weigh out approximately 20 mg of tissue into a 1.5 ml centrifuge tube, add 200 μl of RIPA Buffer and sonicate for 15 seconds at 40 V over ice. Centrifuge the tube at 1600×g for 10 minutes at 4°C. Use the supernatant for analysis. The reaction mixture contained 50 μl sample, 20 μl 8.1% sodium dodecyl sulfate, 150 μl 20% acetic acid solution (buffered to pH3.5), and 210 μl 0.571% thiobarbituric acid. The mixture was then incubated at 90 °C for 1 h, cooled in an ice bath, mixed with 100 μl distilled water and then shaken vigorously. After centrifugation at 4000 rpm for 15 min., absorbance of the solvent layer was measured at 540 nm. Tetraethoxypropane was used as an external standard, and the lipid peroxide level expressed in terms of nanomoles MDA per gram wet weight.
Statistical analysis
Data were collected from multiple mice and presented as mean ± SD. Two-way ANOVA was used for analysis for the differences exist and if so, a post hoc Tukey‘s test was used for analysis for the difference between groups, with Origin 7.5 laboratory data analysis and graphing software. Statistical significance was considered as P < 0.05.
RESULTS
Effects of IH on the kidney weight, renal function, and morphological change
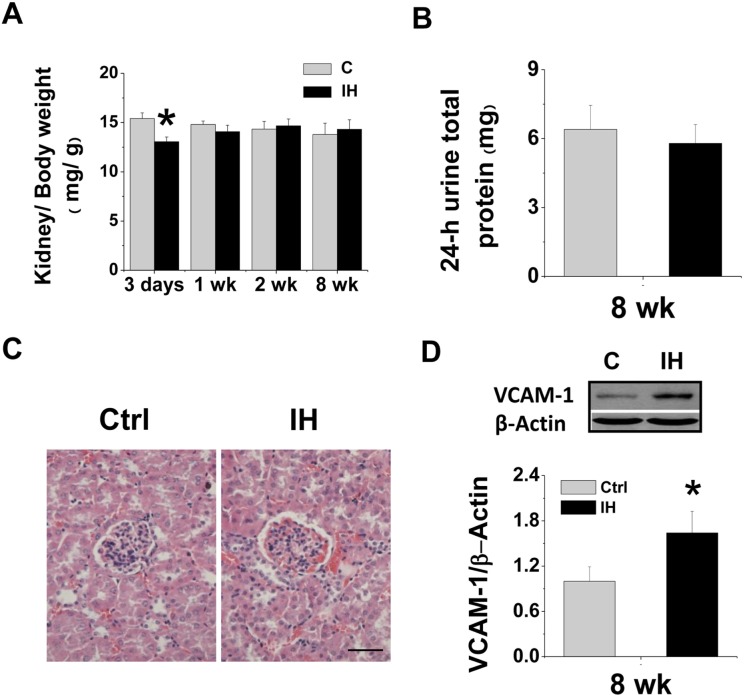
The ratio of kidney weight to body weight significantly decreased at the 3rd day after IH and did not change at the 1st, 2nd and 8th weeks after IH (Fig. 1A). Renal function analysis was examined by measuring 24-h urinary albumin level for mice exposed to IH for 8 weeks, which was not significantly changed (Fig. 1B). Morphological examination showed a significant increase of blood cells in the kidney of mice treated with IH for 8 weeks (Fig. 1C). In addition, there was also a significant increase of renal VCAM-1 expression, examined by Western blotting (Fig. 1D).
FIGURE 1.
Effects of IH on kidney weight, 24-h urine protein, morphological changes and renal VCAM-1 expression. Eight-10 weeks old male FVB mice were exposed to intermittent air (Control, C) or IH for 3 days, 1 week, 2 weeks and 8 weeks. Body weight and kidney weight was measured at the end of experiments at indicated exposure times. The ratio of kidney weight to body weight (A) was calculated and presented as mg kidney weight to g body weight. Urine was collected in metabolic cages during a 24-h time interval at the end of experiments (8 weeks). Microalbunminuria (B) was detected. Kidney morphology was examined with hematoxylin & eosin staining (C). Original magnification × 400. Bars: 50 μm. Renal tissues were subject to Western blotting assay for VCAM-1 expression (D). β-actin was used for loading control. Data are presented as mean ± SD (n = 3 – 8). *, p < 0.05 vs control group.
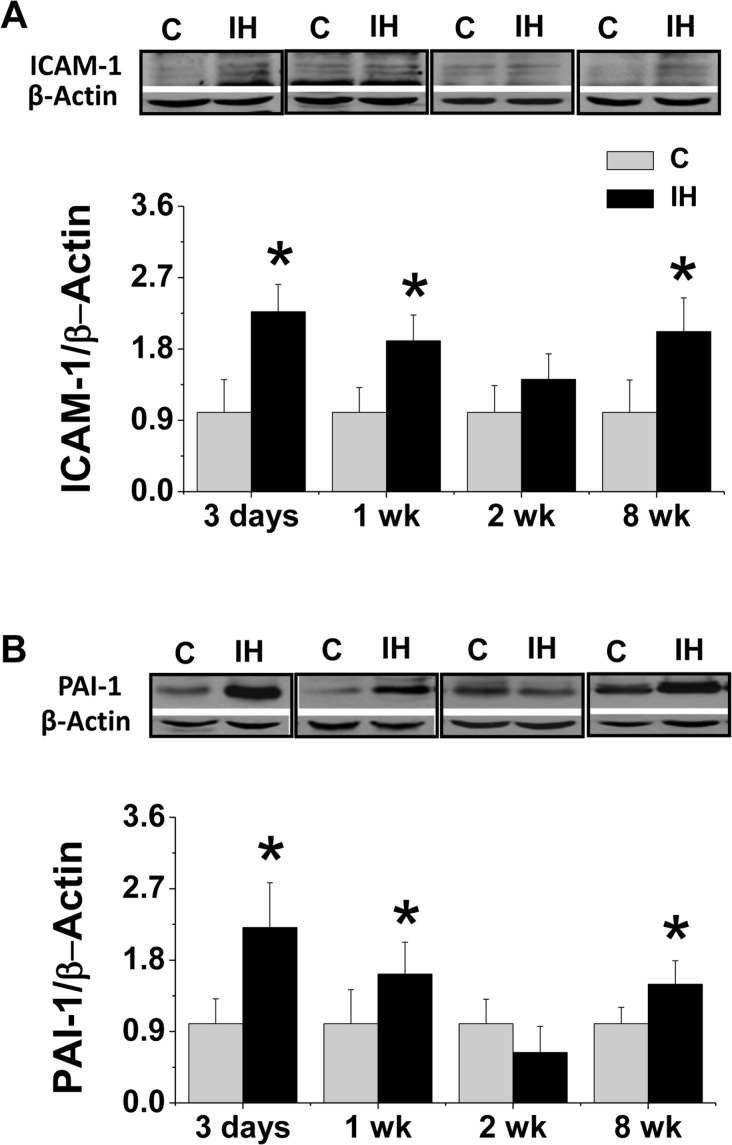
Next a detail time-course effect of IH on renal inflammatory responses was investigated by examining inflammatory cytokines such as ICAM-1 since it is one of the major markers of inflammation in tissue and PAI-1 since it acts as both pro-fibrotic mediator and inflammatory mediator (Cesari et al. 2010; Samarakoon et al. 2010). We found that IH induced significant increase of the renal expression of ICAM-1 (Fig. 2A) and PAI-1 (Fig. 2B) after exposure to IH for 3 and 7 days (1 week), no change for 2 weeks, but significant increase again for 8 weeks. Therefore it appears that there were two phases of inflammatory responses in the kidney in response to acute exposure and chronic exposure to IH.
FIGURE 2.
Effects of IH on renal inflammatory responses. Eight-10 weeks old male FVB mice were exposed to intermittent air (C) or IH for 3 days, 1 week, 2 weeks and 8 weeks. Renal tissue was collected for examining ICAM-1 (A) and PAI-1 (B) expression by Western blotting assay. β-actin was used for loading control. Data are presented as mean ± SD (n = 3 – 8). *, p < 0.05 vs corresponding controls.
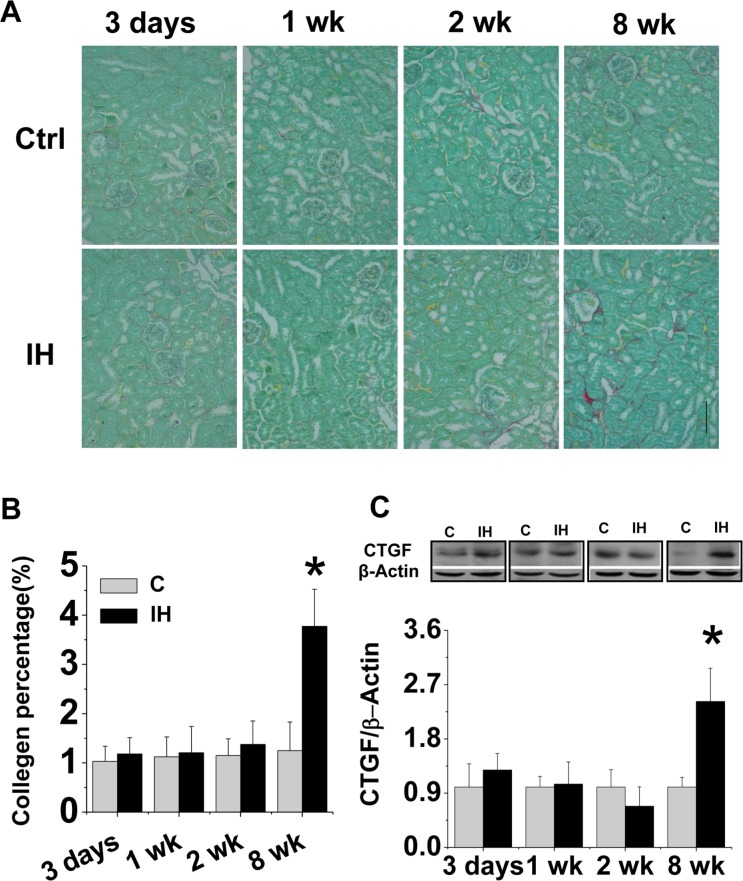
Long-term exposure to IH induced renal fibrotic effect
The increased expression of PAI-1 as both inflammatory and pro-fibrotic cytokine in the kidney of mice exposed to IH for 8 weeks suggests the possible existence of renal fibrosis. Sirius-red staining for collagen, followed by semi-quantitative analysis, showed no significantly fibrotic response in the kidney of mice exposed to IH for 3 days until 2 weeks, but a significant positive staining in interstitial area of the kidney of mice exposed to IH for 8 weeks (Fig. 3A, B). The fibrotic effect of 8-week IH exposure on the kidney was confirmed by the increased expression of CTGF, an important pro-fibrotic mediator, by Western blotting assay (Fig. 3C).
FIGURE 3.
Effects of IH on renal fibrosis. Kidney tissue was stained with Sirius-red for collagen (A). Original magnification x 200. Bars: 50 μm. Semi-quantitative analysis was done by computer imaging system (B). Renal expression of CTGF was examined by Western blotting assay(C). Data are presented as mean ± SD (n = 3 – 8). *, p < 0.05 vs corresponding controls.
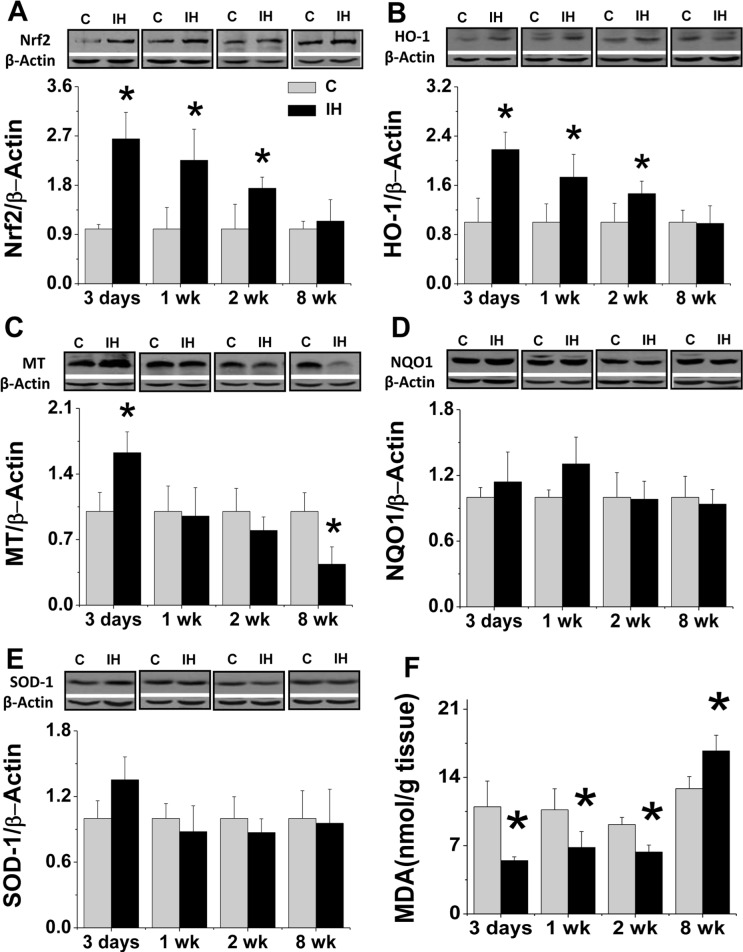
Effects of IH on renal antioxidant contents, oxidative damage and cell death
The renal Nrf2 expression and its downstream target genes HO-1, NQO1, MT and SOD1 expression were examined in the following studies with Western blotting assay. Renal expressions of Nrf2 (Fig. 4A) and HO-1 (Fig. 4B) significantly increased at early as the 3rd day and then gradually returned to normal level at the 8th week after exposure to IH. Renal expression of MT was increased at the 3rd day and significantly decreased at the 8th week (Fig. 4C). Neither renal NQO-1 (Fig. 4D) nor SOD1 (Fig. 4E) expression was changed during the period of IH exposure from 3 days to 8 weeks.
FIGURE 4.
Effects of IH on renal antioxidants and renal lipid peroxidation. Renal tissue from FVB mouse exposed to intermittent air (C) or IH were subject to Western blotting assay for the expression of Nrf2 (A), HO-1 (B), MT (C), NQO-1 (D), and SOD1 (E). Concentration of renal lipid peroxides (F) was determined by TBA assay, which measures the amount of thiobarbituric acid reactivity with malondialdehyde (MDA) formed during the acid hydrolysis of lipid peroxide compound. Lipid peroxide level was expressed in terms of nanomoles MDA per gram wet weight. Data are presented as mean ± SD (n = 3 – 8). *, p < 0.05 vs corresponding controls.
To functionally evaluate the effect of increased antioxidants in the kidney of IH-treated mice, MDA as an index of lipid peroxidation in response to oxidative stress was measured and showed that spontaneous contents of renal MDA was significantly decreased in the kidney of mice exposed to IH for 3 days to 2 weeks, but there was a significant increase in the kidney of mice exposed to IH for 8 weeks (Fig. 4F).
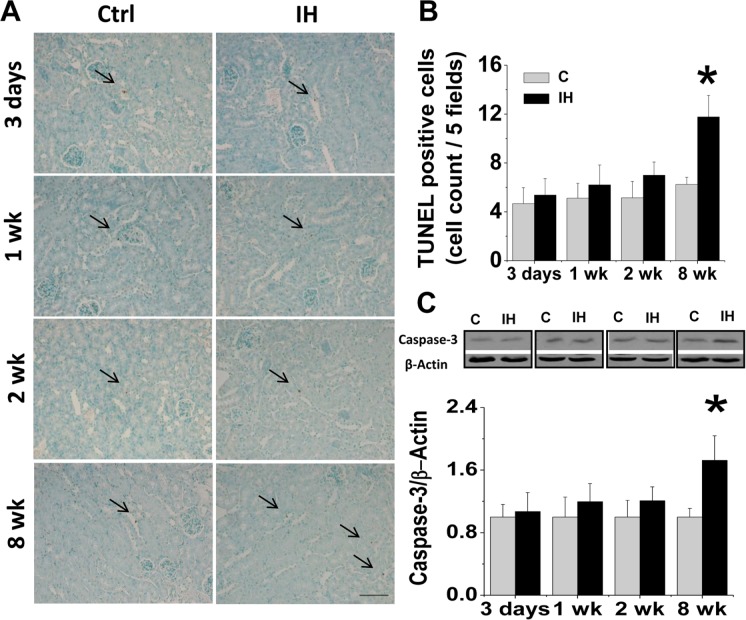
Next apoptotic cell death in the kidney of IH-treated mice was also examined by TUNEL staining (Fig. 5A, B) and caspase-3 cleavage with Western blotting assay (Fig. 5C). Both measurements showed that exposure to IH for 3 days to 2 weeks did not induce, but exposure to IH for 8 weeks induced a significant increase of apoptotic cell death.
FIGURE 5.
Effects of IH on renal apoptotic cell death. Renal apoptotic cell death was examined by TUNEL staining (A), followed by semi-quantitative analysis with expression of an average number of TUNEL positive cells per five high-power fields (B). Arrows indicate TUNEL positive cells, which are expressed as average number of TUNEL positive stained cells per five high power fields. * P < 0.05 vs. control group. Original magnification x 200. Bars: 50 μm. Renal tissue was also subject to Western blotting assay for cleaved-caspase-3 expression (C). Data are presented as mean ± SD (n = 3 – 8). *, p < 0.05 vs corresponding controls.
Effects of IH on renal ERK1/2 phosphorylation and HIF-1α
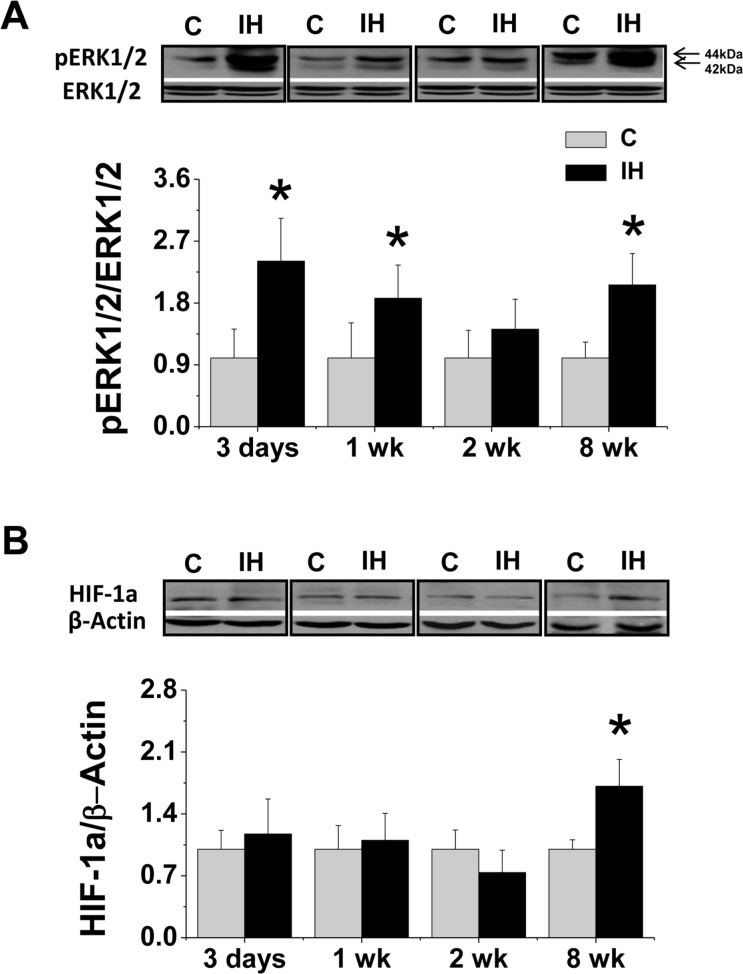
To explore signal mechanism, renal expression of phosphorylated ERK1/2 was examined with Western blotting assay, which revealed that ERK1/2 phosphorylation was significantly increased in the kidney of mice exposed to IH for 3 days or 1 week as early phase and also for 8 weeks as the late phase (Fig. 6A), which is comparable inflammatory response (Fig. 2).
FIGURE 6.
Effects of IH on renal ERK1/2 and HIF-1α phosphorylation. Renal tissue from FVB mouse exposed to intermittent air (C) or IH were subject to Western blotting assay for total or phosphorylated ERK1/2 (A) and HIF-1α (B) expression. Data are presented as mean ± SD (n = 3 – 8). *, p < 0.05 vs corresponding controls.
HIF-1α is a key mediator of cellular responses to hypoxia. Therefore, we examined renal expression of HIF-1α protein with Western blotting assay (Fig. 6B) and found that exposure of mice to IH for 3 days to 2 weeks did not significantly affect, but to IH for 8 weeks significantly increased renal HIF-1α expression.
DISCUSSION
In the present study, we have provided the first evidence that short-term IH exposure can induce a renal inflammatory response without significant pathophysiological abnormality while chronic IH exposure induced a significant renal inflammation along with significant increases of renal cell death and fibrosis. The early inflammatory response was accompanied with significant up-regulation of antioxidants, including Nrf2 and its down-stream antioxidants such as HO-1 and MT, while the late inflammatory response was accompanied with decreased antioxidants and increased oxidative damage. In terms of signaling pathways, the early protective inflammatory response and up-regulation of antioxidant may be related to the ERK1/2 phosphorylation while the late detrimental inflammation along with cell death and fibrosis may be related to the late up-regulated both ERK1/2 phosphorylation and HIF-1α expression.
Induction of a hormetic response in the heart by short-term IH has been documented (Beguin et al. 2007), but there was no any information for the renal response to IH. Hypoxia can induce inflammation (Hartmann et al. 2000; Eltzschig et al. 2011). In the present study we demonstrated that quick induction of renal inflammation after 3-day to 1-week IH exposure, shown by increased expression of ICAM and PAI-1, was associated with a quick up-regulation of renal antioxidants including Nrf2, HO-1 and MT. This may represent body’s stress response to the sudden IH, and the acute stress responses may include acute inflammatory response and quick up-regulation of renal antioxi-dant contents. The up-regulated expression of antioxidants (Fig. 4A–C) was associated with a significant decrease of renal lipid peroxidation (Fig. 4F). However, when mice were long-termly exposed to IH, the kidney would become incapable to tolerate IH-induced changes, reflected by the second increase of renal inflammation along with lipid peroxidation, cell death and mild renal fibrosis. Although there was no similar study to the present study, a previous study has shown the up-regulation of Nrf2 expression and activation of its down-stream genes in the kidney of mice subjected to renal ischemia and reperfusion (Leonard et al. 2006). Short IH exposure also up-regulated pro-inflammatory and antioxidant genes such as Nrf2 and HO-1 in human aortic endothelia cells, to provide a protective effect against oxidative stress (Polotsky et al. 2010). MT as a potent antioxidant plays an important role in preventing oxidative damage in the kidney under different conditions (Dorian et al. 1995; Sharma et al. 2002). However, when mice were exposed to a long-term IH (8 weeks), renal antioxidants such as Nrf2 and HO-1 were not up-regulated anymore and MT protein expression was even decreased compared to control, which may be the major mechanism for the induction of renal oxidative damage and cell death that both can lead to the mild fibrotic response in the kidney of exposed to 8-week IH.
Signaling mechanisms for the early adaptive response may be related to the activation of ERK1/2. Emerging evidence has indicated that ERK1/2, one of the best-characterized members of the mitogen-activated protein kinase (MAPK) family, mediates a range of activity from metabolism, motility, and inflammation to cell death and survival. The phosphorylated ERK1/2 level is usually increased in response to various stresses, but whether an increase in ERK1/2 phosphorylation is protective or detrimental is highly variable. The intensity and duration of stress along with the individual response determine the final outcome. Several studies indicated that activation of ERK/MAPK signaling pathways with protective genes during short-term hypoxia might represent a rapid activation of anti-apoptotic pathways (Milton et al. 2008) while the delayed and sustained activation of ERK/MAPK might contribute to detrimental effects (Shinozaki et al. 2006; Lu et al. 2011). There was a report that exposure to IH for 24 h induced a significant increase of Erk1/2 phosphorylation (Beguin et al. 2007). In agreement with these early studies, we found here that the early activation of ERK1/2 MAPK is associated with a prevention of oxidative damage and up-regulation of several antioxidants, including Nrf2, HO-1 and MT. Indeed, induction of HO-1 and even Nrf2 is also dependent on ERK1/2 activation under many conditions (Manandhar et al. 2007; Yang et al. 2011).
However, long-term and sustained activation of ERK /MAPK signaling pathway has been reported to contribute to oxidative damage and apoptotic cell death (Shinozaki et al. 2006; Lu et al. 2011). Furthermore, HIF-1α was found to be activated by an ERK-dependent pathway in response to hypoxia under certain conditions (Minet et al. 2000); therefore, we assumed that the later increase of ERK1/2 MAPK phosphorylation activates HIF-1α that leads to the pathogenic effects we observed in the present study in the kidney of mice exposed to IH.
HIF-1 is the major regulator of oxygen homeostasis within the cell, affecting and regulating dozens of genes as cellular oxygen concentrations change. HIF-1 activity is induced when mice or cultured cells are subjected to IH, an effect that is related to oxidative stress (Semenza et al. 2007). For instance, carotid bodies from mice that are heterozygous for a null allele at the locus encoding HIF-1 appeared histologically normal but did not respond to continuous hypoxia or CIH. In contrast to wild-type littermates, when heterozygous-null mice are subjected to CIH, they do not develop hypertension or increased levels of HIF-1 and ROS. This report suggests the existence of a feed-forward mechanism in which CIH-induced ROS activate HIF-1, which then promotes persistent oxidative stress and damage (Semenza and Prabhakar 2007). In addition, a recent study has established a pathogenic mechanism linking HIF-1, ROS generation, and cardiovascular pathology in response to IH, i.e.: HIF-1 mediates increased expression of NADPH oxidase-2 in response to IH to generate ROS and RNS (Yuan et al. 2011). Overexpression of HIF-1 in alveolar epithelial cells resulted in increased apoptosis (Krick et al. 2005). Several studies also demonstrated the induction of PAI-1 and CTGF by HIF-1α in response to hypoxia and other stresses (Higgins et al. 2007; Kimura et al. 2008).
There may be a limitation that the matured younger (8 – 10 weeks old) animals were used in the present study whereas it is well known that conditions like sleep apnea increase or worsen with age (Hwang et al. 1994; Martin et al. 2002). For instance, the influence of a short-time isobaric hypoxia as well as reoxygenation on markers of oxidative stress (MDA, total SOD, GSH) and on the mRNA expression of the antioxidative enzymes (Cu/Zn- and Mn-SOD, catalase, GSH reductase and GSH peroxidase) were differentially affected by age of animals between liver and kidney (Martin et al. 2002). Therefore, animals with different ages will be used to investigate the effect of age on the hormetic dose response in terms of renal antioxidant expression and oxidative damage in response to different period of IH, as performed in the present study.
In summary, here we provided the first evidence that short-term IH exposure induced renal acute inflammatory response with significant up-regulation of antioxidants including Nrf2, HO-1 and MT with a significant inhibition of oxidative damage while long-term IH exposure induced significant renal inflammatory and down-regulation of antioxidants such as MT along with significant increases of renal oxidative damage, cell death and fibrosis. This study suggests that IH is able to induce a renal hormetic response, i.e.: short-term IH induces a protective response against the renal oxidative damage, but long-term IH exposure induces a damage effect on the kidney.
Acknowledgments
The work was supported in part by grants from American Diabetes Association (1-11-BS-17 to Dr. L. Cai), Sleep Research Society Foundation/J. Christian Gillin M.D. Research Grant (001GN09 to Dr. J. Cai), and from NSF of China (No.81070189 to Dr. Y. Wang), and also by Start-Up fund for Chinese-American Research Institute for Diabetic Complications from Wenzhou Medical College (to Drs. Y. Tan & L. Cai).
REFERENCES
- Ahmed SB, Ronksley PE, Hemmelgarn BR, Tsai WH, Manns BJ, Tonelli M, Klarenbach SW, Chin R, Clement FM, Hanly PJ. Nocturnal hypoxia and loss of kidney function. PLoS One. 2011;6(4):e19029. doi: 10.1371/journal.pone.0019029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beguin PC, Belaidi E, Godin-Ribuot D, Levy P, Ribuot C. Intermittent hypoxia-induced delayed cardioprotection is mediated by PKC and triggered by p38 MAP kinase and Erk1/2. J Mol Cell Cardiol. 2007;42(2):343–351. doi: 10.1016/j.yjmcc.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Cai J, Tuong CM, Zhang Y, Shields CB, Guo G, Fu H, Gozal D. Mouse intermittent hypoxia mimicking apnoea of prematurity: effects on myelinogenesis and axonal maturation. J Pathol. 2012;226(3):495–508. doi: 10.1002/path.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Wang J, Li Y, Sun X, Wang L, Zhou Z, Kang YJ. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54(6):1829–1837. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- Cesari M, Pahor M, Incalzi RA. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28(5):e72–91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dematteis M, Godin-Ribuot D, Arnaud C, Ribuot C, Stanke-Labesque F, Pepin JL, Levy P. Cardiovascular consequences of sleep-disordered breathing: contribution of animal models to understanding the human disease. ILAR J. 2009;50(3):262–281. doi: 10.1093/ilar.50.3.262. [DOI] [PubMed] [Google Scholar]
- Dorian C, Klaassen CD. Protection by zinc-metallothionein (ZnMT) against cadmium-metallothionein-induced nephrotoxicity. Fundam Appl Toxicol. 1995;26(1):99–106. doi: 10.1006/faat.1995.1079. [DOI] [PubMed] [Google Scholar]
- Eckardt KU, Rosenberger C, Jurgensen JS, Wiesener MS. Role of hypoxia in the pathogenesis of renal disease. Blood Purif. 2003;21(3):253–257. doi: 10.1159/000070698. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann G, Tschop M, Fischer R, Bidlingmaier C, Riepl R, Tschop K, Hautmann H, Endres S, Toepfer M. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein. Cytokine. 2000;12(3):246–252. doi: 10.1006/cyto.1999.0533. [DOI] [PubMed] [Google Scholar]
- Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117(12):3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SM, Wilson PD, Laskin JD, Denhardt DT. Age and development-related changes in osteopontin and nitric oxide synthase mRNA levels in human kidney proximal tubule epithelial cells: contrasting responses to hypoxia and reoxygenation. J Cell Physiol. 1994;160(1):61–68. doi: 10.1002/jcp.1041600108. [DOI] [PubMed] [Google Scholar]
- Khayat R, Patt B, Hayes D., Jr Obstructive sleep apnea: the new cardiovascular disease. Part I: Obstructive sleep apnea and the pathogenesis of vascular disease. Heart Fail Rev. 2009;14(3):143–153. doi: 10.1007/s10741-008-9112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y. Stable expression of HIF-1a in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol. 2008;295(4):F1023–1029. doi: 10.1152/ajprenal.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krick S, Eul BG, Hanze J, Savai R, Grimminger F, Seeger W, Rose F. Role of hypoxia-inducible factor-1a in hypoxia-induced apoptosis of primary alveolar epithelial type II cells. Am J Respir Cell Mol Biol. 2005;32(5):395–403. doi: 10.1165/rcmb.2004-0314OC. [DOI] [PubMed] [Google Scholar]
- Leonard MO, Kieran NE, Howell K, Burne MJ, Varadarajan R, Dhakshinamoorthy S, Porter AG, O’Farrelly C, Rabb H, Taylor CT. Reoxygenation-specific activation of the antioxidant transcription factor Nrf2 mediates cytoprotective gene expression in ischemia-reperfusion injury. FASEB J. 2006;20(14):2624–2626. doi: 10.1096/fj.06-5097fje. [DOI] [PubMed] [Google Scholar]
- Li QY, Li M, Feng Y, Guo Q, Gu SY, Liu JL, Zhang RF, Wan HY. Chronic Intermittent Hypoxia Induces Thioredoxin System Changes in a Gender-Specific Fashion in Mice. AM J MED SCI. 2012;343(6):458–461. doi: 10.1097/MAJ.0b013e318235b03e. [DOI] [PubMed] [Google Scholar]
- Lu TH, Hsieh SY, Yen CC, Wu HC, Chen KL, Hung DZ, Chen CH, Wu CC, Su YC, Chen YW, Liu SH, Huang CF. Involvement of oxidative stress-mediated ERK1/2 and p38 activation regulated mitochondria-dependent apoptotic signals in methylmercury-induced neuronal cell injury. Toxicol Lett. 2011;204(1):71–80. doi: 10.1016/j.toxlet.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Manandhar S, Cho JM, Kim JA, Kensler TW, Kwak MK. Induction of Nrf2-regulated genes by 3H-1, 2-dithiole-3-thione through the ERK signaling pathway in murine keratinocytes. Eur J Pharmacol. 2007;577(1–3):17–27. doi: 10.1016/j.ejphar.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Markou N, Kanakaki M, Myrianthefs P, Hadjiyanakos D, Vlassopoulos D, Damianos A, Siamopoulos K, Vasiliou M, Konstantopoulos S. Sleep-disordered breathing in non-dialyzed patients with chronic renal failure. Lung. 2006;184(1):43–49. doi: 10.1007/s00408-005-2563-2. [DOI] [PubMed] [Google Scholar]
- Martin R, Fitzl G, Mozet C, Martin H, Welt K, Wieland E. Effect of age and hypox-ia/reoxygenation on mRNA expression of antioxidative enzymes in rat liver and kidneys. Exp Gerontol. 2002;37(12):1481–1487. doi: 10.1016/s0531-5565(02)00168-7. [DOI] [PubMed] [Google Scholar]
- Milton SL, Dirk LJ, Kara LF, Prentice HM. Adenosine modulates ERK1/2, PI3K/Akt, and p38MAPK activation in the brain of the anoxia-tolerant turtle Trachemys scripta. J Cereb Blood Flow Metab. 2008;28(8):1469–1477. doi: 10.1038/jcbfm.2008.45. [DOI] [PubMed] [Google Scholar]
- Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, Remacle J, Michiels C. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 2000;468(1):53–58. doi: 10.1016/s0014-5793(00)01181-9. [DOI] [PubMed] [Google Scholar]
- Nicholl DD, Ahmed SB, Loewen AH, Hemmelgarn BR, Sola DY, Beecroft JM, Turin TC, Hanly PJ. Declining Kidney Function Increases the Prevalence of Sleep Apnea and Nocturnal Hypoxia. Chest. 2012;141(6):1422–1430. doi: 10.1378/chest.11-1809. [DOI] [PubMed] [Google Scholar]
- Papaiahgari S, Zhang Q, Kleeberger SR, Cho HY, Reddy SP. Hyperoxia stimulates an Nrf2-ARE transcriptional response via ROS-EGFR-PI3K-Akt/ERK MAP kinase signaling in pulmonary epithelial cells. Antioxid Redox Signal. 2006;8(1–2):43–52. doi: 10.1089/ars.2006.8.43. [DOI] [PubMed] [Google Scholar]
- Polotsky VY, Savransky V, Bevans-Fonti S, Reinke C, Li J, Grigoryev DN, Shimoda LA. Intermittent and Sustained Hypoxia Induce a Similar Gene Expression Profile in the Human Aortic Endothelial Cells. Physiol Genomics. 2010 Mar 2; doi: 10.1152/physiolgenomics.00091.2009. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarakoon R, Goppelt-Struebe M, Higgins PJ. Linking cell structure to gene regulation: signaling events and expression controls on the model genes PAI-1 and CTGF. Cell Signal. 2010;22(10):1413–1419. doi: 10.1016/j.cellsig.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL, Prabhakar NR. HIF-1-dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9(9):1391–1396. doi: 10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- Sharma R, Sharma M, Datta PK, Savin VJ. Induction of metallothionein-I protects glomeruli from superoxide-mediated increase in albumin permeability. Exp Biol Med (Maywood) 2002;227(1):26–31. doi: 10.1177/153537020222700105. [DOI] [PubMed] [Google Scholar]
- Shinozaki Y, Koizumi S, Ohno Y, Nagao T, Inoue K. Extracellular ATP counteracts the ERK1/2-mediated death-promoting signaling cascades in astrocytes. Glia. 2006;54(6):606–618. doi: 10.1002/glia.20408. [DOI] [PubMed] [Google Scholar]
- Tkachev VO, Menshchikova EB, Zenkov NK. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry (Mosc) 2011;76(4):407–422. doi: 10.1134/s0006297911040031. [DOI] [PubMed] [Google Scholar]
- Wang J, Song Y, Elsherif L, Song Z, Zhou G, Prabhu SD, Saari JT, Cai L. Cardiac metallothionein induction plays the major role in the prevention of diabetic cardiomyopathy by zinc supplementation. Circulation. 2006;113(4):544–554. doi: 10.1161/CIRCULATIONAHA.105.537894. [DOI] [PubMed] [Google Scholar]
- Yang YC, Lii CK, Lin AH, Yeh YW, Yao HT, Li CC, Liu KL, Chen HW. Induction of glutathione synthesis and heme oxygenase 1 by the flavonoids butein and phloretin is mediated through the ERK/Nrf2 pathway and protects against oxidative stress. Free Radic Biol Med. 2011;51(11):2073–2081. doi: 10.1016/j.freeradbiomed.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- Yuan G, Khan SA, Luo W, Nanduri J, Semenza GL, Prabhakar NR. Hypoxia-inducible factor 1 mediates increased expression of NADPH oxidase-2 in response to intermittent hypoxia. J Cell Physiol. 2011;226(11):2925–2933. doi: 10.1002/jcp.22640. [DOI] [PMC free article] [PubMed] [Google Scholar]