Abstract
STUDY QUESTION
Can biologically active vitamin D3 [1,25(OH)2D3] regulate the expression and activity of matrix metalloproteinases (MMPs) in human uterine fibroid cells?
SUMMARY ANSWER
1,25(OH)2D3 effectively reduced the expression and activities of MMP-2 and MMP-9 in cultured human uterine fibroid cells.
WHAT IS KNOWN ALREADY
Uterine fibroids (leiomyoma) express higher levels of MMP activity than adjacent normal myometrium, and this is associated with uterine fibroid pathogenesis. However, it is unknown whether 1,25(OH)2D3 can regulate the expression and activities of MMPs in human uterine fibroid cells.
STUDY DESIGN, SIZE, DURATION
Surgically removed fresh fibroid tissue was used to generate primary uterine fibroid cells.
PARTICIPANTS/MATERIALS, SETTING, METHODS
An immortalized human uterine fibroid cell line (HuLM) and/or primary human uterine fibroid cells isolated from fresh fibroid tissue were used to examine the expression of several MMPs, tissue inhibitors of metalloproteinases (TIMP) 1 and 2 and the activities of MMP-2 and MMP-9 after 1,25(OH)2D3 treatment. Real-time PCR and western blots analyses were used to measure mRNA and protein expression of MMPs, respectively. Supernatant cell culture media were analyzed for MMP-2 and MMP-9 activities using a gelatin zymography assay.
MAIN RESULTS AND THE ROLE OF CHANCE
1–1000 nM 1,25(OH)2D3 significantly reduced mRNA levels of MMP-2 and MMP-9 in HuLM cells in a concentration-dependent manner (P < 0.5 to P < 0.001). The mRNA levels of MMP-1, MMP-3, MMP-13 and MMP-14 in HuLM cells were also reduced by 1,25(OH)2D3. 1,25(OH)2D3 significantly reduced MMP-2 and MMP-9 protein levels in a concentration-dependent manner in both HuLM and primary uterine fibroid cells (P < 0.05 to P < 0.001). Moreover, 1,25(OH)2D3 increased the mRNA levels of vitamin D receptor (VDR) and TIMP-2 in a concentration-dependent manner in HuLM cells (P < 0.05 to P < 0.01). 1,25(OH)2D3 also significantly increased protein levels of VDR and TIMP-2 in all cell types tested (P < 0.05 to P < 0.001). Gelatin zymography revealed that pro-MMP-2, active MMP-2 and pro-MMP-9 were down-regulated by 1,25(OH)2D3 in a concentration-dependent manner; however, the active MMP-9 was undetectable.
LIMITATIONS, REASONS FOR CAUTION
This study was performed using in vitro uterine fibroid cell cultures and the results were extrapolated to in vivo situation of uterine fibroids. Moreover, in this study the interaction of vitamin D3 with other regulators such as steroid hormone receptors was not explored.
WIDER IMPLICATIONS OF THE FINDINGS
This study reveals an important biological function of 1,25(OH)2D3 in the regulation of expression and activities of MMP-2 and MMP-9. Thus, 1,25(OH)2D3 might be a potential effective, safe non-surgical treatment option for human uterine fibroids.
STUDY FUNDING/COMPETING INTEREST(S)
This study was primarily supported by Research Centers in Minority Institutions (RCMI)-pilot grant 2 G12 RR003032-26 to S.K.H. and supported in part by Meharry Translation Research Center/Clinical Research Center (MeTRC/CRC) award (RE: 202142-535001-20) to S.K.H. and NIH/NICHD 1 R01 HD046228 to A.A-H. The authors have no conflicts of interests.
TRIAL REGISTRATION NUMBER
Not applicable.
Keywords: vitamin D3, fibroids, VDR, MMPs, TIMP-2
Introduction
Uterine fibroids (leiomyoma) are highly prevalent benign tumors which are associated with symptoms including excessive vaginal bleeding, pelvic pain, recurrent spontaneous abortion, preterm labor and are the major indication for hysterectomy (Wilcox et al., 1994; Farhi et al., 1995). Steroid hormones are the major regulators of fibroid growth (Wilson et al., 1980; Rein et al., 1995). Uterine fibroids grow slowly by the deposition of a wide array of extracellular matrix (ECM) components, and the ECM is found to be abundant and disorganized in uterine fibroids (Leppert et al., 2004). Uterine fibroids are three to four times more prevalent among African American women when compared with Caucasian women. They also have 10 times higher risk of hypovitaminosis D (a condition in which the vitamin D3 level is lower than normal physiological levels) than Caucasian women (40 versus 4%) (Nesby-O'Dell et al., 2002; Baird and Dunson, 2003). However, the direct relationship between uterine fibroid pathogenesis and vitamin D effect is not yet well established.
The growth of uterine fibroids takes place due to an increase in cell proliferation and deposition of the ECM (Walker and Stewart, 2005). Uterine fibroids contain abnormal deposition of ECM components that play important role in the pathogenesis (Stewart et al., 1994; Sozen and Arici, 2002; Malik and Catherino, 2007). The degradation of ECM is an important characteristic of development, morphogenesis, tissue repair and remodeling. This degradation process is specifically regulated under normal physiological conditions; however, dysregulation of this process is a cause of several diseases such as arthritis nephritis, cancer, encephalomyelitis, chronic ulcers and fibrosis (Nagase et al., 2006). Although various types of proteinases are involved in ECM degradation, the major enzymes are considered to be matrix metalloproteinases (MMPs) (Visse and Nagase, 2003). There are 24 MMPs in humans, although the activities of most MMPs are very low or negligible under normal physiological condition. Their activities are primarily regulated by tissue inhibitors of matrix metalloproteinases (TIMPs), and thus the balance between MMPs and TIMPs are critical for the ultimate ECM remodeling in the tissue (Nagase et al., 2006). The MMP family of proteases has been characterized into several subgroups based on their ability to specifically degrade various interstitial matrix and basement membrane components, and those are collagenase, gelatinases, stromelysins, membrane-type (MT)-MMPs and several others (Nagase et al., 2006). Collagenases (MMP-1, MMP-8 and MMP-13) cleave interstitial collagens I, II and III, but they can also digest other ECM components and soluble proteins (Visse and Nagase, 2003). Gelatinases (MMP-2 and MMP-9) digest gelatin via their fibronectin type II repeats that binds to gelatin/collagen, and they can also digest a number of ECM molecules including type IV, V and XI collagens, laminin and others. MMP-2, but not MMP-9 can also digest collagens I, II and III in a similar manner to the collagenases (Aimes and Quigley, 1995; Patterson et al., 2001). Stromelysins (MMP-3, MMP-10 and MMP-11) are similar to that of collagenases, but they do not cleave interstitial collagens. MMP-3 and MMP-10 digest a number of ECM molecules and they participate in pro-MMP activation, while MMP-11 has very weak activity toward ECM molecules (Murphy et al., 1993). MT-MMPs (MMP-14, MMP-15, MMP-16 and MMP-24) are activated intracellularly and active enzymes are expressed on the cell surface. All MT-MMPs can activate pro-MMP-2. MMP-14 has collagenolytic activity on collagens I, II and III (Ohuchi et al., 1997).
MMP activities are specifically regulated by TIMPs. TIMP proteins consist of 184–194 amino acids and are specific inhibitors of MMPs. Four types of TIMP proteins have been identified in vertebrates and their expressions are regulated during development and tissue remodeling (Brew et al., 2000). Under pathological conditions that are associated with unbalanced expression and activities of MMPs, changes in TIMP levels are considered to be important due to their direct effect on MMP activity.
The MMPs are a family of zinc-dependent proteases that can degrade ECM components (Chambers and Matrisian, 1997). The MMP family of proteases is involved in the degradation of fibrillar collagen (Butler et al., 1997; Gioia et al., 2007). Soluble collagen type I is enzymatically digested by collagenases such as MMP-1, MMP-8, MMP-13 (Chung et al., 2004; Inada et al., 2004), MMP-14 (Ohuchi et al., 1997), gelatinase A (MMP-2) (Aimes and Quigley, 1995) and gelatinase B (MMP-9) (Allan et al., 1995). MMP-3 functions as an activator of other MMPs such as MMP-1 and MMP-9. One study has shown that fibroid growth is associated with increased activity of MMP-2 (Wolanska et al., 2004). An additional report demonstrated that MMP-1, MMP-2, MMP-3, MMP-9, TIMP-1, TIMP-2 and collagenase types I and III were highly expressed in uterine fibroids and myometrium during the progesterone-dominant secretory phase of the menstrual cycle (Dou et al., 1997). The expression of MMP-1, MMP-2 and MMP-3 are elevated in larger uterine fibroids (Wolanska et al., 2004). It also demonstrated that MMP-1 is elevated in large fibroids but not in small fibroids while the MMP-3 was elevated in both small and large fibroids. Moreover, microarray analysis showed alterations in a wide array of MMPs in uterine fibroids, suggesting that the deregulation of MMPs in uterine fibroids may play a critical role in excessive ECM deposition (Malik et al., 2010). In addition, it is also suggested that paradoxical increase in MMPs in uterine fibroids might reflect a dysfunction in ECM homeostasis and that a decrease in the expression of these MMPs, to the level noticed normal myometrium, might be associated with fibroid shrinkage and are a potential therapeutic strategy for the management of uterine fibroids.
Among the MMPs, MMP-2 and MMP-9 are the key enzymes that degrade major collagen in ECM component (Zucker et al., 1993). Increased activities of MMP-2 and MMP-9 have been associated with increasing tumor metastases in various human metastatic cancers (Mook et al., 2004). Most MMPs are secreted as inactive proenzymes and their proteolytic activities are regulated by TIMPs (Woessner, 1994). TIMPs inhibit the enzymatic activity by binding to the C-terminal catalytic domain of MMPs (Lambert et al., 2004). TIMP-1 and TIMP-2 are known to bind MMP-9 and MMP-2, respectively (Olson et al., 2000). Thus, the activities of MMPs are regulated by the expression levels of TIMPs. One study showed that MMPs and TIMPs are expressed in various normal tissues that undergo tissue remodeling, and their overexpression in certain pathological conditions is associated with extensive ECM degradation (Matrisian, 1992). Herein, we hypothesized that reduction of mRNA and/or protein expressions and the gelatinolytic activities of MMP-2 and MMP-9 in vitamin D3-treated human uterine fibroid cells might be very helpful to understand the therapeutic use of 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] in the non-surgical treatment option for uterine fibroids.
1,25(OH)2D3 is a biologically active vitamin D3, and in cell systems it functions through interacting with the vitamin D receptor (VDR) (Holick, 2003). The VDR is a nuclear transcription factor that plays major role in the modulation of gene expression by interacting with VDR response element in the promoter region of target genes. The effects of VDR in cell signaling include growth arrest, differentiation and/or induction of apoptosis demonstrating the involvement of vitamin D signaling in the inhibition of cell growth. Recently, we and others have shown that 1,25(OH)2D3 inhibits growth and induces apoptosis in vitro in human uterine fibroid cell culture (Blauer et al., 2009; Sharan et al., 2011). We also showed that 1,25(OH)2D3 treatment shrinks uterine fibroid tumor size in vivo in an Eker rat animal model (Halder et al., 2012). Herein, we examined the biological function of 1,25(OH)2D3 in the suppression of mRNA and protein expressions and activities of MMP-2 and MMP-9 in human uterine fibroid cells. This study proposes an important biological function of 1,25(OH)2D3 in the regulation of expression and activities of MMP-2 and MMP-9, which may play a key role in the pathogenesis of human uterine fibroids.
Materials and Methods
Reagents and antibodies
1,25(OH)2D3 was purchased from Sigma Biochemicals (St. Louis, MO, USA). Smooth muscle cell culture medium (SmBM) was purchased from Lonza (Walkersville, MD, USA). Antibodies were purchased as follows: anti-MMP-2, anti-MMP-9 and anti-TIMP-2 from BD Biosciences (San Jose, CA, USA); anti-VDR from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and anti-β-actin from Sigma Biochemicals.
Cell line and primary cell cultures
The immortalized human uterine fibroid cell line (HuLM) was a generous gift from Dr Darlene Dixon (National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA) (Carney et al., 2002). These HuLM cells were cultured and maintained as we described previously (Halder et al., 2011; Sharan et al., 2011). Primary human uterine fibroid cells were generated from uterine fibroid tissue specimens collected from Meharry Metro General Hospital under an approved IRB protocol (Protocol#090630WJR246 11). Uterine fibroid tissue sample was collected from an African American woman who underwent laparoscopic hysterectomy to surgically remove confirmed large (8 cm) uterine fibroids. The patient was not administered any hormone supplementations including vitamin D3 for at least 5 months before the hysterectomy was performed. The subject was 40-year-old women at the time of hysterectomy, and her serum vitamin D3 status was not measured. For preparation of the primary cell population, a portion (∼5 mm3) of the fresh uterine fibroid tissue was washed in culture medium to remove blood and then chopped into small pieces under sterile conditions, transferred into a 15 ml screw cap tube and suspended in Hank's Balanced Salt Solution (Cat. No. 14175; Life technologies, Grand Island, NY, USA) containing 1X antibiotic–antimycotic (Cat. No. 15240-096; Life technologies) and 300 units/ml collagenase type-4 (Cat. No. 4188; Worthington Biochemical Corp., Lakewood, NJ, USA). Suspended tissue pieces were incubated at 37°C for at least 12 h to obtain individual cells and/or clumps of few cells. The cell suspension was passed through a 100 µm pore size sterile nylon filter and the suspension of individual cells was plated out and incubated at 37°C allowing the cells to attach to the 90 mm sterile tissue culture treated plate (Cat. No. 40-229690; Krackeler Scientific, Albany, NY, USA) containing SmBm culture media as we described previously (Halder et al., 2011; Sharan et al., 2011). Several days later the monolayer of cells was trypsinized, collected and then split to maintain culture in above media.
Isolation of cellular RNA from HuLM cells
To determine the effects of 1,25(OH)2D3 on mRNA levels of MMPs, HuLM cells were serum starved and treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100 and 1000 nM) for 48 h. Cells were collected from the plates using cell scrapers and then cell pellets were obtained by centrifugation at 1500 rpm for 10 min at 4°C. Total cellular RNA was isolated using an RNeasy Protect kit according to the instruction provided by the manufacturers (Qiagen, Inc., Chatsworth, CA, USA). The concentration of total RNA was determined using a NanoVue system (GE Healthcare, USA).
Quantitative real-time PCR analysis
Real-time PCR analyses were performed to analyze mRNA expressions for MMPs, TIMPs and VDR using total RNA from 1,25(OH)2D3-treated HuLM cells extracted as described above. The first-strand cDNA was synthesized from 1 µg of total RNA using an Omniscript RT Kit (Qiagen, Inc., USA). Primer sequences were obtained from the published literature (Ottino et al., 2002; Gilad et al., 2006; Tempfer et al., 2009). Primer sequences were MMP-1, sense 5′-TAGAACTGTGAAGCATATCGATG-3′ and anti-sense 5′-AGTTGAACCAGCTATTAGCTTTC-3′; MMP-2, sense 5′-TCTACTCAGCCAGCACCCTGGA-3′ and anti-sense 5′-TGCAGGTCCACGACGGCATCCA-3′; MMP-3, sense 5′-TACTGGAGATTTGATGAGAAGAG-3′ and anti-sense 5′-TACAGATTCACGCTCAAGTTCC-3′; MMP-9, sense 5′-TTCGACGTGAAGGCGCAGATGGT-3′ and anti-sense 5′-TAGGTCACGTAGCCCACTTGGTC-3′; MMP-13, sense 5′-TGCAGCTGTTCACTTTGAGGA-3′ and anti-sense 5′-TGGCATGACGCGAACAATACG-3′; MMP-14, sense 5′-TACCGACAAGATTGATGCTGCTC-3′ and anti-sense 5′-TCTACCTTCAGCTTCTGGTTG-3′; TIMP-1 sense 5′-TGGACTCTTGCACATCACTACCTGC-3′ and anti-sense 5′-AGGCAAGGTGACGGGACTGGAA-3′; TIMP-2, sense 5′-GTAGTGATCAGGGCCAAAG-3′ and anti-sense 5′-TTCTCTGTGACCCAGTCCAT-3′; VDR, sense 5′- ATGCCATCTGCATCGTCTC-3′ and anti-sense 5′-GCACCGCACAGGCTGTCCTA-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH), sense 5′-AACATCATCCCTGCCTCTAC-3′ and anti-sense 5′-CTGCTTCACCACCTTCTTG-3′. Specific primer sets (sense and anti-sense) for MMP-1, MMP-2, MMP-3, MMP-9, MMP-13, MMP-14, VDR, TIMP-1, TIMP-2 and GAPDH were purchased from Qiagen, Inc. Briefly, 50 ng of each cDNA was added to the Mastermix containing appropriate primer sets and CYBR-green in a 25 µl reaction volume. All samples were analyzed in triplicate. Real-time PCR analyses were performed using a Bio-Rad MyiQ5. Cycling conditions included denaturation at 95°C for 10 min, and then followed by 40 cycles of 95°C for 30 s, 58°C for 30 s and 72°C for 30 s. Synthesis of a DNA product of the expected size was confirmed by melting curve analysis. GAPDH values (internal control) were used to normalize the expression data.
Western blot analyses
HuLM cells and primary human uterine fibroid cells were serum starved for overnight and then treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100 and 1000 nM) for 48 h. Preparation of cell lysates and western blot analyses were performed as we described previously (Halder et al., 2005, 2011). Briefly, equal amounts of each cell lysate (30–40 µg) were resolved in a 10% SDS–PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis), transferred onto PVDF membrane and then western blot analyses were performed using specific antibodies. The antigen–antibody complex was detected with an enhanced chemiluminescence detection system (Amersham Biosciences, Pittsburgh, PA, USA). Specific protein bands were visualized after exposure to autoradiography films and by developing the film using an automatic X-ray developer. The intensity of each protein band was quantified by image analysis software and normalized against corresponding β-actin. The normalized values were used to create data graphs.
Gelatin substrate gel zymography
Gelatinolytic activities of MMP-2 and MMP-9 were performed using zymography as described previously (Hibbs et al., 1985). In this gel zymography assay, MMP-2 and MMP-9 degrade gelatin incorporated into SDS–PAGE gels that results in negative (unstained) bands. This assay can detect the active and latent forms of gelatinases based on their proper molecular weight positions. Briefly, HuLM (400 000 cells/well) and primary human fibroid (250 000/well) cells were split into six-well plates. Twenty hours later cells were serum starved in DMEM/F12 medium (Life Technologies, Grand Island, NY, USA) for an additional 20 h and then treated with or without increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100 and 1000 nM) in 2.0 ml culture medium for 48 h. Subsequently, 40 µl of each clear conditioned medium was mixed with 5× SDS sample buffer containing no reducing agent and subjected to SDS–PAGE in a 7.5% SDS–PAGE gels containing 2 mg/ml gelatin (Sigma), in a cold room, using a Mini-PROTEAN II apparatus (Bio-Rad, Richmond, CA, USA). After electrophoresis, the gels were removed and washed in a 2.5% Triton X-100 for 60 min (3 times × 20 min each) at room temperature to remove SDS from gels. Gels were incubated at 37°C in incubation buffer containing 50 mM Tris–HCl, 5 mM CaCl2 and 0.02% NaN3 at pH 7.6 for about 48 h. The gels were stained with Coomassie Brilliant Blue R250 (0.25%) in 50% methanol and 10% acetic acid for about an hour, and then destained in 10% acetic acid and 30% methanol at room temperature to clearly visualize the digested bands. Proteolytic activities of MMP-2 and MMP-9 were visualized as clear bands against the blue background of stained gelatin.
Statistical analysis
All statistical data analyses (SDA) were performed using ANOVA procedure using WINKS SDA software version 7 (http://www.texasoft.com/upgrade). Results are presented as means ± SEM. Means were compared between untreated control and 1,25(OH)2D3-treatment data points using the Newman–Keuls test for multiple independent group comparisons. The significance of differences between consecutive points in the 1,25(OH)2D3-treated data were also calculated. Differences were considered statistically significant at the 95% confidence level when P value was <0.05 (P < 0.05).
Results
1,25(OH)2D3 reduced mRNA levels of MMPs in cultured HuLM cells
To first evaluate the effects of 1,25(OH)2D3 on mRNA expression of MMPs we performed real-time PCR analyses. We found that at 1–10 nM concentrations, 1,25(OH)2D3 significantly reduced MMP-2 and MMP-9 mRNA expressions in HuLM cells in a dose-dependent manner when compared with untreated control (Fig. 1A and B, P < 0.01 to P < 0.001). Similarly, at 1–10 nM concentrations, 1,25(OH)2D3 significantly reduced the mRNA expressions of MMP-1, MMP-3, MMP-13 and MMP-14 in cultured HuLM cells (Fig. 1C–F, P < 0.05 to P < 0.001). These results suggest that 1,25(OH)2D3 reduces mRNA levels, particularly of MMP-2 and MMP-9 in cultured HuLM cells.
Figure 1.
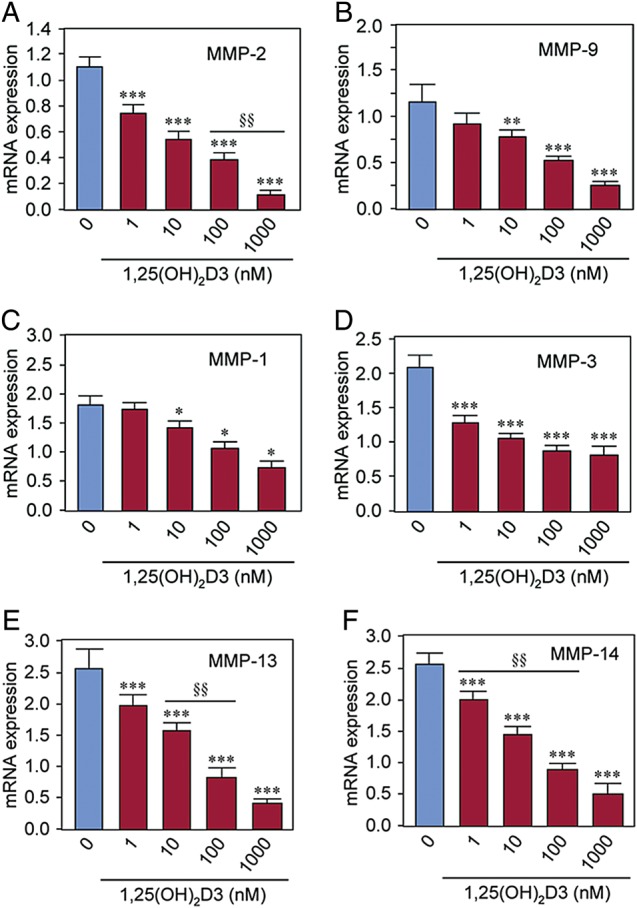
Effect of 1,25(OH)2D3 on mRNA expression of MMPs in cultured immortalized human uterine fibroid (HuLM) cells. Total RNA was isolated from HuLM cells treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100 and 1000 nM) for 48 h. Equal amounts of each total RNA was used to perform quantitative real-time PCR analyses as indicated. Total RNA (1 µg) was reverse transcribed to cDNA and then real-time PCR analyses were performed for MMP-2 (A) and MMP-9 (B), MMP-1 (C), MMP-3 (D), MMP-13 (E) and MMP-14 (F) using gene-specific forward and reverse primers as described in the section Materials and Methods. The mRNA expression levels of above MMPs were normalized with GAPDH (internal control) and the normalized values were used to generate the graphs. Data are means ± SEM, *P < 0.05, **P < 0.01 and ***P < 0.001 when compared with corresponding untreated control (0). §§P < 0.01 when compared between 1,25(OH)2D3-treated data points. These experiments were repeated twice with similar results.
1,25(OH)2D3 increased mRNA levels of VDR and TIMP-2 in cultured HuLM cells
1,25(OH)2D3 exerts its physiological function in cells by binding to and inducing endogenous VDR expression. To study the effect of 1,25(OH)2D3 on the VDR mRNA level, we performed quantitative real-time PCR analyses using total RNA prepared from HuLM cells as described above. We observed a low level of VDR mRNA in control HuLM cells, whereas treatment with 1,25(OH)2D3 induced VDR mRNA expression in a concentration-dependent manner (Fig. 2A). At ≥10 nM concentration, 1,25(OH)2D3 significantly induced VDR mRNA expression in HuLM cells when compared with untreated control (Fig. 2A, P < 0.01). To test the effect of 1,25(OH)2D3 on MMPs inhibitors, TIMP-1 and TIMP-2 we performed similar quantitative real-time PCR analyses as described above. Similar to VDR, 1,25(OH)2D3 induced the mRNA expression of TIMP-2 in a concentration-dependent manner. At ≥10 nM concentration, 1,25(OH)2D3 significantly induced TIMP-2 mRNA expression in HuLM cells when compared with untreated control (Fig. 2B, P < 0.01). However, the mRNA level of TIMP-1 in cultured HuLM cells was not affected by 1,25(OH)2D3 (Fig. 2C). These results suggest that 1,25(OH)2D3 can stimulate the mRNA expression of VDR and TIMP-2 in cultured HuLM cells in a concentration-dependent manner.
Figure 2.
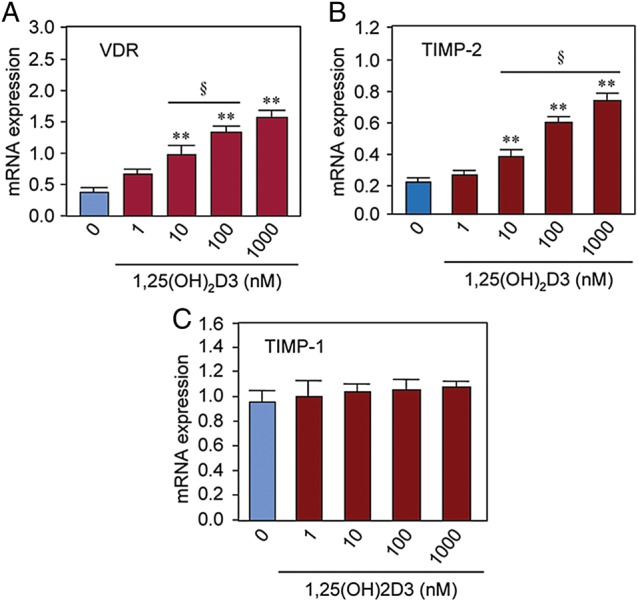
Effect of 1,25(OH)2D3 on mRNA expressions of the VDR and TIMP 1 and 2 in cultured immortalized human uterine fibroid (HuLM) cells. Real-time PCR analyses for mRNA expressions of VDR, TIMP-1 and TIMP-2 were performed using gene-specific sense and anti-sense primers as described in the section Materials and methods. The mRNA expression levels of VDR, TIMP-1 and TIMP-2 were normalized with GAPDH and the normalized values were used to generate the graphs. Data are means ± SEM, n = 3, **P < 0.01 when compared with corresponding untreated control. §P < 0.05 when compared between 1,25(OH)2D3-treated data points. This experiment was repeated twice with similar results.
1,25(OH)2D3 reduced protein levels of MMP-2 and MMP-9 in cultured human uterine fibroid cells
To further determine the effects of 1,25(OH)2D3 on protein expressions of MMP-2 and MMP-9 in uterine fibroid cells we performed western blot analyses using lysates from cultured HuLM cells treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100 and 1000 nM) for 48 h. We found that ≥10 nM 1,25(OH)2D3 significantly reduced the protein expression of MMP-2 and MMP-9 in HuLM cells when compared with untreated control (Fig. 3A, P < 0.01 to P < 0.001). To mimic these findings in human fibroid tumors, primary uterine fibroid cells were treated with 1,25(OH)2D3 and then cell lysates were analyzed for MMP-2 and MMP-9 as described above. Similar to HuLM cells, 1,25(OH)2D3 reduced both MMP-2 and MMP-9 protein expressions in a concentration-dependent manner in primary uterine fibroid cells (Fig. 3B). These results suggest that 1,25(OH)2D3 can suppress MMP-2 and MMP-9 protein expressions in cultured human uterine fibroid cells.
Figure 3.
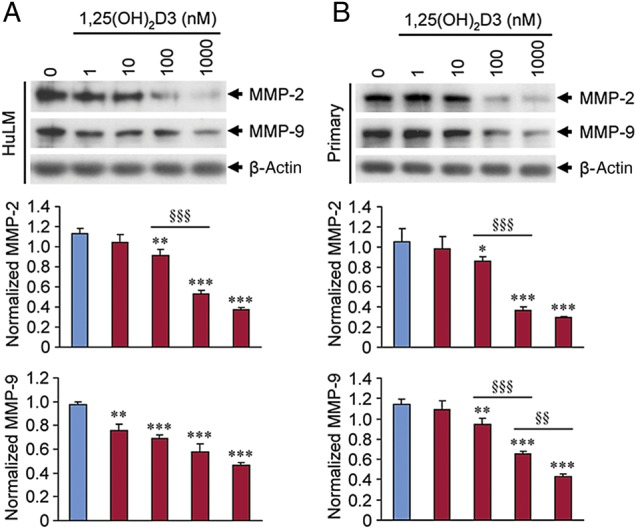
Effect of 1,25(OH)2D3 on protein expression of MMP 2 and 9 in cultured human uterine fibroid cells. Immortalized human uterine fibroid (HuLM) cells (A) and primary uterine fibroid cells (B) were serum starved and treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100 and 1000 nM) for 48 h. For each condition, an equal amount of protein lysate was analyzed by western blotting with anti-MMP-2 and anti-MMP-9 antibodies. Western blot with anti-β-actin antibody was used as the loading control. The intensity of each protein band was quantified and normalized with corresponding β-actin, and the normalized values were used to generate the graphs. Data are the mean ± SEM, n = 3, *P < 0.05, **P < 0.01 and ***P < 0.001 when compared with corresponding untreated control. §§P < 0.01 and §§§P < 0.001 when compared between 1,25(OH)2D3-treated data points. This experiment was repeated twice with similar results.
1,25(OH)2D3 increased VDR protein expression in cultured human uterine fibroid cells
Since 1,25(OH)2D3 induces VDR mRNA expression in HuLM cells, we further tested its effect on VDR protein expression after treating HuLM cells with 1,25(OH)2D3. We performed western blot analyses using lysates from HuLM cells treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100 and 1000 nM) for 48 h. We found that 1,25(OH)2D3 treatment sensitized HuLM cells via the induction of VDR protein (Fig. 4A). Moreover, 1,25(OH)2D3 induced VDR protein expression in a concentration-dependent manner in HuLM cells. At low concentrations (10 nM), 1,25(OH)2D3 significantly induced VDR protein level when compared with untreated control (Fig. 4A, P < 0.001). To mimic this finding in human uterine fibroids, we performed similar western blot analyses using lysates from primary uterine fibroid cells that were treated with 1,25(OH)2D3 as described above. 1,25(OH)2D3 at low concentrations (1–10 nM) induced VDR protein expression in primary uterine fibroid cells, and that induction of VDR was concentration dependent (Fig. 4B). These results suggest that 1,25(OH)2D3 treatment induces VDR protein expression in human uterine fibroid cells.
Figure 4.
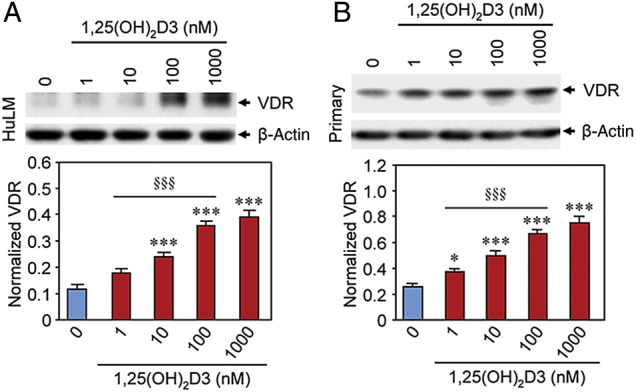
Effect of 1,25(OH)2D3 on protein expression of VDR in cultured human uterine fibroid cells. Immortalized human uterine fibroid (HuLM) cells (A) and primary human uterine fibroid cells (B) were serum starved and treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100 and 1000 nM) for 48 h. Equal amounts of each cell lysate were analyzed by western blotting using anti-VDR antibody. The intensity of each protein band was quantified and normalized with corresponding β-actin, and the normalized values were used to generate the graphs. *P < 0.05 and ***P < 0.001 when compared with corresponding untreated control. §§§P < 0.001 when compared between 1,25(OH)2D3-treated data points. This experiment was repeated twice with similar results.
1,25(OH)2D3 induced TIMP-2 protein expression in cultured human uterine fibroid cells
TIMP-2 plays important role in the regulation of most MMPs. We further tested the effect of 1,25(OH)2D3 on protein expression of TIMP-2 in cultured human uterine fibroid cells. We performed western blot analyses using lysates that were prepared from 1,25(OH)2D3-treated HuLM and human primary uterine fibroid cells. 1,25(OH)2D3 at the 10 nM concentration significantly induced TIMP-2 protein expression in HuLM cells when compared with untreated control (Fig. 5A, P < 0.01). To mimic these findings in human fibroid tumors we isolated primary fibroid cells from fibroid tumor tissues as described in the section Materials and methods. These primary uterine fibroid cells were treated with 1,25(OH)2D3 as described above. Similar to HuLM cells, 1,25(OH)2D3 significantly induced protein expression of TIMP-2 in primary uterine fibroid cells (Fig. 5B, P < 0.01 to P < 0.001). Moreover, the induction of TIMP-2 protein was dependent on the concentrations of 1,25(OH)2D3. These results suggest that 1,25(OH)2D3 can potentially induce TIMP-2 protein expression in cultured human uterine fibroid cells.
Figure 5.
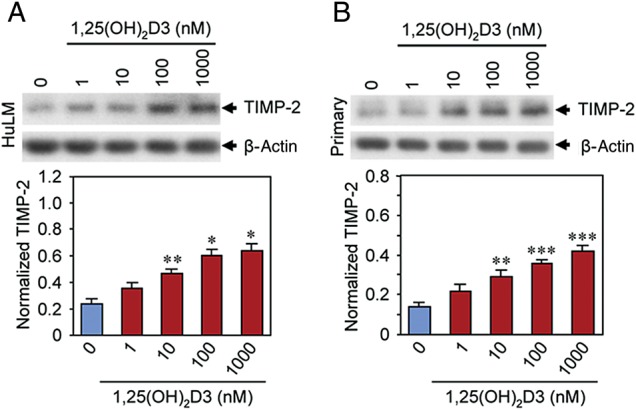
Effect of 1,25(OH)2D3 on protein expression of TIMP-2 in cultured human uterine fibroid cells. Lysates from immortalized human uterine fibroid (HuLM) cells (A) and primary uterine fibroid cells (B) treated with increasing concentrations of 1,25(OH)2D3 (as described above) were analyzed by western blots using anti-TIMP-2 antibody. Western blot with the anti-β-actin antibody was used as the loading control. The intensity of each protein band was quantified and normalized with corresponding β-actin, and the normalized values were used to generate the graphs. Data are mean ± SEM, n = 3, *P < 0.05, **P < 0.01 and ***P < 0.001 when compared with corresponding untreated control. This experiment was repeated twice with similar results.
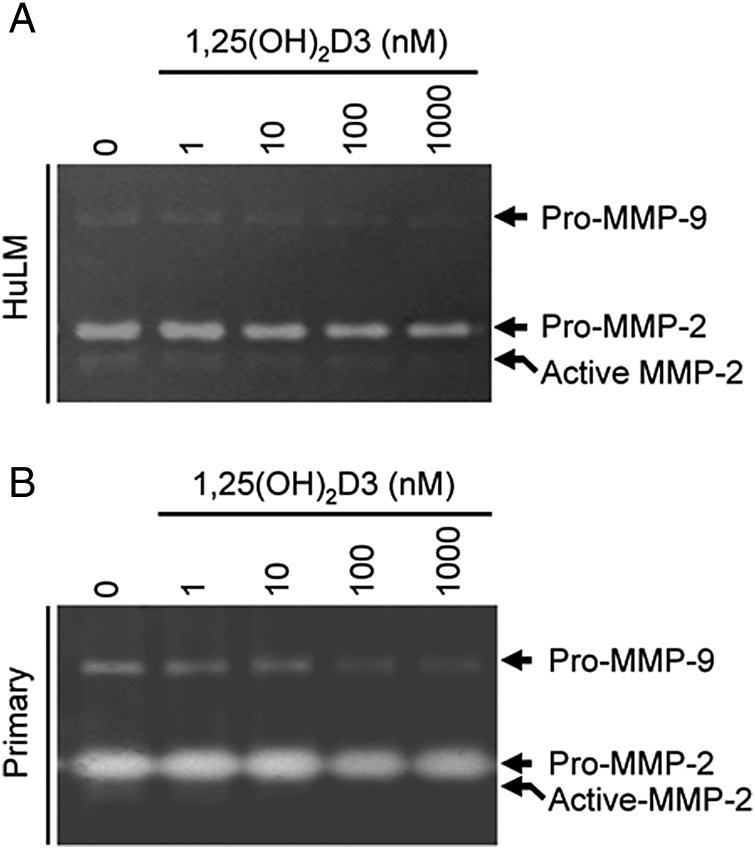
1,25(OH)2D3 reduced gelatinolytic activities of MMP-2 and MMP-9 in supernatant medium of cultured human uterine fibroid cells
To determine whether 1,25(OH)2D3 can affect gelatinolytic activities of MMP-2 and MMP-9, we performed a gelatin gel zymography assay. Supernatant media from cultured HuLM and primary human uterine fibroid cells treated with 1,25(OH)2D3 were analyzed in a 7.5% SDS–PAGE non-reducing gel. Gelatin zymography revealed that both latent (72 kDa) and active (62 kDa) forms of MMP-2 were present in HuLM cells while treatment with 1,25(OH)2D3 reduced their activities in a concentration-dependent manner (Fig. 6A). In contrast to MMP-2, we found weak pro-MMP-9 activity (92 kDa) in HuLM cells, while the active form of MMP-9 was undetectable (Fig. 6A). 1,25(OH)2D3 also reduced the pro-MMP-9 activity in a concentration-dependent manner (Fig. 6A). To mimic these findings in human fibroid tumors we used primary uterine fibroid cells. We observed that the pro-MMP-2 levels were much higher in primary uterine fibroid cells while the active MMP-2 was detected as a weak band. Moreover, the pro-MMP-9 levels were also detectable in primary uterine fibroid cells while the active form of MMP-9 was undetectable (Fig. 6B). Treatment with 1,25(OH)2D3 reduced the pro-MMP-2, active MMP-2 and pro-MMP-9 activities in primary human fibroid cells (Fig. 6B). These results suggest that 1,25(OH)2D3 can reduce the proteolytic activities of MMP-2 and MMP-9 in cultured human uterine fibroid cells.
Figure 6.
Effect of 1,25(OH)2D3 on gelatinolytic activities of MMP 2 and 9 in cultured human uterine fibroid cells. Both immortalized human uterine fibroid (HuLM) cells (A) and primary uterine fibroid cells (B) were serum starved and treated with increasing concentrations of 1,25(OH)2D3 (0, 1, 10, 100 M and 1000 nM) for 48 h. Each supernatant medium (40 µl/each sample) was analyzed in 7.5% SDS PAGE gelatin zymography as described in the section Materials and methods. The digestion of gelatin in the gel by MMP-2 and MMP-9 enzymes cause the negative (unstained) bands. The pro-MMP-2 (72 kDa) and active MMP-2 (65 kDa) digested bands were detected in the gels which were decreased by 1,25(OH)2D3. Similarly, the pro-MMP-9 (92 kDa) was also detected in supernatant media in both cell types, which were also decreased by 1,25(OH)2D3. The active form of MMP-9 was undetectable in supernatant media of both cell types. This experiment was repeated twice with similar results.
Discussion
The growth of uterine fibroids results from an increase in cell proliferation, deposition of ECM and growth promoting action of local growth factors acting in paracrine and/or autocrine manners (Walker and Stewart, 2005). Uterine fibroids contain abundant ECM which consists mainly of collagen, fibronectin and proteoglycan (Stewart et al., 1994; Berto et al., 2003; Catherino et al., 2004; Malik and Catherino, 2007). The ECM consists of fibrillar proteins that provide a structural scaffold for tissue support (Chirco et al., 2006). Collagens are the major components of the ECM which contribute to the stability and maintain the structural integrity of the tissues (Gelse et al., 2003). The fibrillar collagens such as type I, II, III, IV and V collagens are the most abundant structural proteins and in uterine fibroid, type I and III collagen mRNAs were found to be elevated (Stewart et al., 1994; Byers, 2000). In fibrosis, an imbalance of matrix synthesis and degradation leads to excessive deposition of the ECM, and eventually stimulates fibroid proliferation. The deregulated accumulation of the ECM represents an imbalance between synthesis and dissolution, and produces fibroproliferative conditions called keloids (Catherino et al., 2004). Fibronectin is an ECM protein which can undergo proteolytic cleavage to yield fragments that bind to integrin receptors and initiate signaling pathways that regulate cell growth, migration and survival. The excessive synthesis and deposition of the collagenic protein was found in fibroplasia, a condition where fibronectin production was elevated (Limper and Roman, 1992). Fibronectin expression is also up-regulated in uterine fibroids. Proteoglycans such as fibromodulin, biglycan and versican are important component in all ECMs. Fibromodulin is a collagen-binding proteoglycan that plays a key role in fibrogenesis by regulating collagen fibril spacing and thickness. Fibromodulin mRNA and protein levels were up-regulated in uterine fibroids (Levens et al., 2005). Biglycan is a small leucine-rich proteoglycan in ECM that influences differentiation and proliferation processes (Polgar et al., 2003). Versican is a large proteoglycan (chondroitin sulfate proteoglycan) in the ECM and is associated with cell proliferation and apoptosis (Ohara, 2009). Increased expression of versican is also involved in matrix assembly and structure (Catherino et al., 2004; Leppert et al., 2006). The mRNA and protein levels of versican were shown to be up-regulated in uterine fibroids (Norian et al., 2009).
MMPs are proteolytic enzymes that degrade nearly all components of ECM (Visse and Nagase, 2003). Under normal physiological conditions, the activity of MMPs is precisely regulated at the transcriptional and the post-transcriptional levels and is controlled at the protein level via their activators and inhibitors (Sternlicht and Werb, 2001). Most MMPs are secreted as inactive enzymes which are activated by serine proteases for their biological function. Recently, it has been shown that increased activities of MMP-2 and MMP-9 are associated with fibroid pathogenesis (Bogusiewicz et al., 2007). MMPs are a family of zinc-containing metalloproteinases that play critical roles in ECM degradation, synthesis and remodeling. Increased in MMP-2 and MMP-9 gelatinolytic activities, extensive collagen deposition and denaturation, and ECM remodeling have been observed in mice with heart failure (Li et al., 2002). Several studies have demonstrated a correlation between MMPs activation and the ventricular remodeling process, and that the inhibition of MMPs attenuates fibrosis and cardiac hypertrophy (Li et al., 2000; Spinale, 2002). Increased expression and activities of MMPs in animal models was associated with systolic heart failure (Mujumdar and Tyagi, 1999). One study has demonstrated that activation of MMP-2 and MMP-9 is associated with collagen deposition as well as fibrosis in the VDR knockout mice model (Rahman et al., 2007). In that study, a direct correlation has been shown between decreased expression of TIMPs and increased activation of MMPs (Rahman et al., 2007). The expression levels of MMPs and their activation processes are regulated by TIMP-2 (Nagase, 1998). Since uterine fibroids are characterized by the excessive synthesis and deposition of ECM, it is possible that the increased expression and activities of MMP-2 and MMP-9 in uterine fibroids might be due to the imbalance between ECM deposition and degradation process. We hypothesized that 1,25(OH)2D3 might regulate the expression and proteolytic activities of MMP-2 and MMP-9 in human uterine fibroid cells via inducing the nuclear VDR expression. Thus, this induced level of VDR can further stimulate TIMP-2, which inhibits MMP-2 and MMP-9 activation and eventually leading to the reduction of uterine fibroid pathogenesis.
The current study aims to evaluate the effects of 1,25(OH)2D3 on expression and activities of MMPs in both immortalized HuLM and primary human uterine fibroid cells. The VDR is a nuclear receptor that modulates gene expression when interacts with its biologically active ligand 1,25(OH)2D3. The cellular effects of VDR signaling include the growth arrest, differentiation and/or induction of apoptosis which demonstrated the involvement of vitamin D signaling in the inhibition of cell growth. Herein, we assessed the effect of 1,25(OH)2D3 on expression and activities of MMP-2 and MMP-9, and the expression levels of TIMP-2 in human cultured uterine fibroid cells. We found that 1,25(OH)2D3 significantly reduced mRNA levels of MMP-1, MMP-2, MMP-3, MMP-9, MMP-13 and MMP-14 in cultured HuLM cells (Fig. 1, P < 0.05 to P < 0.001). These observation demonstrates an important role of 1,25(OH)2D3 in the regulation of mRNA expression of MMPs in cultured HuLM cells. Since 1,25(OH)2D3 exerts its biological activities in cells by binding to and inducing nuclear VDR, we verified the levels of VDR expression after treating HuLM cells with increasing concentrations of 1,25(OH)2D3. Our results showed significant induction of VDR mRNA and protein expressions in HuLM cells (Figs 2A and 4A, P < 0.01 to P < 0.001). We also observed that the primary uterine fibroid cells respond to 1,25(OH)2D3 similar to HuLM cells and can induce VDR protein expression in a concentration-dependent manner (Fig. 4B). These finding suggests that 1,25(OH)2D3 functions in human uterine fibroid cells through the induction of VDR. Our finding is consistent with previous reports where an analog of 1,25(OH)2D3 functioned via the induction of VDR in both breast and prostate cancer cells (Sintov et al., 2013). Paricalcitol, an analogue of vitamin D3 has also been shown to function through the activation/induction of VDR and inhibits ECM-associated cardiac fibrosis in a mouse model (Meems et al., 2012). We further verified the mechanism by which 1,25(OH)2D3 regulates the expression and activity of MMPs. TIMP-2 has been demonstrated to be down-regulated in human uterine fibroids, while the activities of MMPs are up-regulated. It is possible that the decreased expression of TIMP-2 might be responsible for inducing the expression and activity of MMP-2 and MMP-9 in human uterine fibroids. It is also possible that the deposition of excessive collagen in uterine fibroids might be an outcome of MMP activation and an imbalance between ECM production and degradation. In Fig. 5, we have shown the concentration-dependent induction of TIMP-2 protein expression in both HuLM and primary human uterine fibroid cells after 1,25(OH)2D3 treatment, suggesting that TIMP-2 might be a potential target for 1,25(OH)2D3 function in the regulation of MMP-2 and MMP-9 in human uterine fibroid cells. This finding is consistent with the previous report where 1,25(OH)2D3 has been shown to induce the TIMP-2 expression in human breast cancer cells (Koli and Keski-Oja, 2000). Additionally, several studies have reported that 1,25(OH)2D3 suppresses the expression and activities of MMPs in other diseases such as rheumatoid lesion (Tetlow and Woolley, 1999), pulmonary tuberculosis (Anand and Selvaraj, 2009) and mycobacterium tuberculosis infection (Coussens et al., 2009). Since 1,25(OH)2D3 reduced MMP-2 and MMP-9 protein expression in a concentration-dependent manner in human uterine fibroid cells (Fig. 3), we further confirmed that the reduced protein expressions of MMP-2 and MMP-9 were correlated with their proteolytic activities upon treatment with 1,25(OH)2D3. Our results from gelatin zymography assay demonstrate that 1,25(OH)2D3 can reduce the proteolytic activities of MMP-2 and MMP-9 in both immortalized HuLM cells and primary uterine fibroid cells (Fig. 6A and B), and that reduction of enzyme activities might be an important function by which vitamin D3 inhibits fibroid proliferation. 1,25(OH)2D3 has also been shown to play important roles in suppressing MMP expression in cardiac tissue (Rahman et al., 2007). One study has demonstrated that lower serum levels of 1,25(OH)2D3 are associated with increased risk of tuberculosis (Nnoaham and Clarke, 2008). Furthermore, studies have shown that vitamin D deficiency influences the expression of MMPs that contribute to the pathogenesis of coronary heart disease (Chang et al., 1996; Timms et al., 2002). Moreover, we recently reported a direct correlation between low levels of serum 1,25(OH)2D3 and the risk associated with the development of uterine fibroids (Sabry et al., 2013). Low levels of VDR were also detected in uterine fibroid tumors when compared with adjacent normal myometrium (our unpublished data). Furthermore, our recent observations also indicate that 1,25(OH)2D3 has effect on reduction of ECM-associated fibronectin, collagen and proteoglycan protein expressions (our unpublished data). Therefore, it is possible that the loss or inadequate function of 1,25(OH)2D3 in human uterine fibroids might increase the expression and activities of MMP-2 and MMP-9, which can have impact on fibroid pathogenesis. Our study demonstrates that 1,25(OH)2D3 has the potential to suppress the expression and activities of MMP-2 and MMP-9, and these regulatory functions of 1,25(OH)2D3 might be potentially important for non-surgical treatment option for human uterine fibroids.
In summary, our results provide the first evidence that 1,25(OH)2D3 reduced mRNA and protein levels of MMP-2 and MMP-9 in a concentration-dependent manner in cultured HuLM cells. 1,25(OH)2D3 also significantly induced mRNA and protein expressions of VDR and TIMP-2 in cultured HuLM cells. Moreover, 1,25(OH)2D3 induced protein levels of MMP-2, MMP-9, VDR and TIMP-2 in cultured primary uterine fibroid cells. Furthermore, the gelatinolytic activities of MMP-2 and MMP-9 were reduced by 1,25(OH)2D3 in a concentration-dependent manner in cultured HuLM and primary human uterine fibroid cells. Together our results suggest that 1,25(OH)2D3 may have potential to reduce uterine fibroid growth by modulating the expression and activities of MMP-2 and MMP-9, and as a result supplementation of 1,25(OH)2D3 to the patients with low vitamin D status may be beneficial to reduce the difficulties associated with uterine fibroids. Thus, this study provide supports that 1,25(OH)2D3 is useful not only gaining insight into the biological roles of MMPs, but also might be useful for the development of novel therapeutic alternatives for an effective, safe and non-surgical treatment of human uterine fibroids that are associated with unbalanced ECM degradation.
Authors' roles
S.H. was primarily responsible for planning and conducting of the experiments, analyses of the results, writing and reading and revising of the manuscript. A.-H.A. and K.O. were responsible for reading and reviewing of the manuscript. All authors approved the revised version of the manuscript.
Funding
This study was primarily supported by Research Centers in Minority Institutions (RCMI)-pilot grant 2 G12 RR003032-26 to S.K.H., and supported in part by Meharry Translation Research Center/Clinical Research Center (MeTRC/CRC) award (RE: 202142-535001-20) to S.K.H. and NIH/NICHD 1 R01 HD046228 to A.A.-H.
Conflict of interest
The authors in this paper have no conflict of interest or nothing to disclose.
Acknowledgements
The authors thank Ms Takeisha Farmer (Lab Assistant) for her assistant in preparation of cellular RNA and performing quantitative real-time PCR analyses. The authors also thank the Center for Women's Health Research (CWHR) and the Department of Obstetrics and Gynecology at Meharry Medical College for supporting this research project.
References
- Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- Allan JA, Docherty AJ, Barker PJ, Huskisson NS, Reynolds JJ, Murphy G. Binding of gelatinases A and B to type-I collagen and other matrix components. Biochem J. 1995;309(Pt 1):299–306. doi: 10.1042/bj3090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand SP, Selvaraj P. Effect of 1, 25 dihydroxyvitamin D(3) on matrix metalloproteinases MMP-7, MMP-9 and the inhibitor TIMP-1 in pulmonary tuberculosis. Clin Immunol. 2009;133:126–131. doi: 10.1016/j.clim.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Baird DD, Dunson DB. Why is parity protective for uterine fibroids? Epidemiology. 2003;14:247–250. doi: 10.1097/01.EDE.0000054360.61254.27. [DOI] [PubMed] [Google Scholar]
- Berto AG, Sampaio LO, Franco CR, Cesar RM, Jr, Michelacci YM. A comparative analysis of structure and spatial distribution of decorin in human leiomyoma and normal myometrium. Biochim Biophys Acta. 2003;1619:98–112. doi: 10.1016/s0304-4165(02)00446-4. [DOI] [PubMed] [Google Scholar]
- Blauer M, Rovio PH, Ylikomi T, Heinonen PK. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil Steril. 2009;91:1919–1925. doi: 10.1016/j.fertnstert.2008.02.136. [DOI] [PubMed] [Google Scholar]
- Bogusiewicz M, Stryjecka-Zimmer M, Postawski K, Jakimiuk AJ, Rechberger T. Activity of matrix metalloproteinase-2 and -9 and contents of their tissue inhibitors in uterine leiomyoma and corresponding myometrium. Gynecol Endocrinol. 2007;23:541–546. doi: 10.1080/09513590701557416. [DOI] [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Butler GS, Will H, Atkinson SJ, Murphy G. Membrane-type-2 matrix metalloproteinase can initiate the processing of progelatinase A and is regulated by the tissue inhibitors of metalloproteinases. Eur J Biochem. 1997;244:653–657. doi: 10.1111/j.1432-1033.1997.t01-1-00653.x. [DOI] [PubMed] [Google Scholar]
- Byers PH. Collagens: building blocks at the end of the development line. Clin Genet. 2000;58:270–279. doi: 10.1034/j.1399-0004.2000.580404.x. [DOI] [PubMed] [Google Scholar]
- Carney SA, Tahara H, Swartz CD, Risinger JI, He H, Moore AB, Haseman JK, Barrett JC, Dixon D. Immortalization of human uterine leiomyoma and myometrial cell lines after induction of telomerase activity: molecular and phenotypic characteristics. Lab Invest. 2002;82:719–728. doi: 10.1097/01.lab.0000017499.51216.3e. [DOI] [PubMed] [Google Scholar]
- Catherino WH, Leppert PC, Stenmark MH, Payson M, Potlog-Nahari C, Nieman LK, Segars JH. Reduced dermatopontin expression is a molecular link between uterine leiomyomas and keloids. Genes Chromosomes Cancer. 2004;40:204–217. doi: 10.1002/gcc.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- Chang JC, Wysocki A, Tchou-Wong KM, Moskowitz N, Zhang Y, Rom WN. Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax. 1996;51:306–311. doi: 10.1136/thx.51.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- Chung L, Dinakarpandian D, Yoshida N, Lauer-Fields JL, Fields GB, Visse R, Nagase H. Collagenase unwinds triple-helical collagen prior to peptide bond hydrolysis. EMBO J. 2004;23:3020–3030. doi: 10.1038/sj.emboj.7600318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens A, Timms PM, Boucher BJ, Venton TR, Ashcroft AT, Skolimowska KH, Newton SM, Wilkinson KA, Davidson RN, Griffiths CJ, et al. 1alpha,25-dihydroxyvitamin D3 inhibits matrix metalloproteinases induced by Mycobacterium tuberculosis infection. Immunology. 2009;127:539–548. doi: 10.1111/j.1365-2567.2008.03024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Q, Tarnuzzer RW, Williams RS, Schultz GS, Chegini N. Differential expression of matrix metalloproteinases and their tissue inhibitors in leiomyomata: a mechanism for gonadotrophin releasing hormone agonist-induced tumour regression. Mol Hum Reprod. 1997;3:1005–1014. doi: 10.1093/molehr/3.11.1005. [DOI] [PubMed] [Google Scholar]
- Farhi J, Ashkenazi J, Feldberg D, Dicker D, Orvieto R, Ben Rafael Z. Effect of uterine leiomyomata on the results of in-vitro fertilization treatment. Hum Reprod. 1995;10:2576–2578. doi: 10.1093/oxfordjournals.humrep.a135748. [DOI] [PubMed] [Google Scholar]
- Gelse K, Poschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Gilad LA, Tirosh O, Schwartz B. Phytoestrogens regulate transcription and translation of vitamin D receptor in colon cancer cells. J Endocrinol. 2006;191:387–398. doi: 10.1677/joe.1.06930. [DOI] [PubMed] [Google Scholar]
- Gioia M, Monaco S, Fasciglione GF, Coletti A, Modesti A, Marini S, Coletta M. Characterization of the mechanisms by which gelatinase A, neutrophil collagenase, and membrane-type metalloproteinase MMP-14 recognize collagen I and enzymatically process the two alpha-chains. J Mol Biol. 2007;368:1101–1113. doi: 10.1016/j.jmb.2007.02.076. [DOI] [PubMed] [Google Scholar]
- Halder SK, Beauchamp RD, Datta PK. Smad7 induces tumorigenicity by blocking TGF-beta-induced growth inhibition and apoptosis. Exp Cell Res. 2005;307:231–246. doi: 10.1016/j.yexcr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Halder SK, Goodwin JS, Al-Hendy A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J Clin Endocrinol Metab. 2011;96:E754–E762. doi: 10.1210/jc.2010-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder SK, Sharan C, Al-Hendy A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol Reprod. 2012;86:116. doi: 10.1095/biolreprod.111.098145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs MS, Hasty KA, Seyer JM, Kang AH, Mainardi CL. Biochemical and immunological characterization of the secreted forms of human neutrophil gelatinase. J Biol Chem. 1985;260:2493–2500. [PubMed] [Google Scholar]
- Holick MF. Vitamin D: a millennium perspective. J Cell Biochem. 2003;88:296–307. doi: 10.1002/jcb.10338. [DOI] [PubMed] [Google Scholar]
- Inada M, Wang Y, Byrne MH, Rahman MU, Miyaura C, Lopez-Otin C, Krane SM. Critical roles for collagenase-3 (Mmp13) in development of growth plate cartilage and in endochondral ossification. Proc Natl Acad Sci USA. 2004;101:17192–17197. doi: 10.1073/pnas.0407788101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koli K, Keski-Oja J. 1alpha,25-dihydroxyvitamin D3 and its analogues down-regulate cell invasion-associated proteases in cultured malignant cells. Cell Growth Differ. 2000;11:221–229. [PubMed] [Google Scholar]
- Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit Rev Oncol Hematol. 2004;49:187–198. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Leppert PC, Baginski T, Prupas C, Catherino WH, Pletcher S, Segars JH. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril. 2004;82:1182–1187. doi: 10.1016/j.fertnstert.2004.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppert PC, Catherino WH, Segars JH. A new hypothesis about the origin of uterine fibroids based on gene expression profiling with microarrays. Am J Obstet Gynecol. 2006;195:415–420. doi: 10.1016/j.ajog.2005.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levens E, Luo X, Ding L, Williams RS, Chegini N. Fibromodulin is expressed in leiomyoma and myometrium and regulated by gonadotropin-releasing hormone analogue therapy and TGF-beta through Smad and MAPK-mediated signalling. Mol Hum Reprod. 2005;11:489–494. doi: 10.1093/molehr/gah187. [DOI] [PubMed] [Google Scholar]
- Li YY, McTiernan CF, Feldman AM. Interplay of matrix metalloproteinases, tissue inhibitors of metalloproteinases and their regulators in cardiac matrix remodeling. Cardiovasc Res. 2000;46:214–224. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- Li YY, Kadokami T, Wang P, McTiernan CF, Feldman AM. MMP inhibition modulates TNF-alpha transgenic mouse phenotype early in the development of heart failure. Am J Physiol Heart Circ Physiol. 2002;282:H983–H989. doi: 10.1152/ajpheart.00233.2001. [DOI] [PubMed] [Google Scholar]
- Limper AH, Roman J. Fibronectin. A versatile matrix protein with roles in thoracic development, repair and infection. Chest. 1992;101:1663–1673. doi: 10.1378/chest.101.6.1663. [DOI] [PubMed] [Google Scholar]
- Malik M, Catherino WH. Novel method to characterize primary cultures of leiomyoma and myometrium with the use of confirmatory biomarker gene arrays. Fertil Steril. 2007;87:1166–1172. doi: 10.1016/j.fertnstert.2006.08.111. [DOI] [PubMed] [Google Scholar]
- Malik M, Norian J, McCarthy-Keith D, Britten J, Catherino WH. Why leiomyomas are called fibroids: the central role of extracellular matrix in symptomatic women. Semin Reprod Med. 2010;28:169–179. doi: 10.1055/s-0030-1251475. [DOI] [PubMed] [Google Scholar]
- Matrisian LM. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- Meems LM, Cannon MV, Mahmud H, Voors AA, van Gilst WH, Sillje HH, Ruifrok WP, de Boer RA. The vitamin D receptor activator paricalcitol prevents fibrosis and diastolic dysfunction in a murine model of pressure overload. J Steroid Biochem Mol Biol. 2012;132:282–289. doi: 10.1016/j.jsbmb.2012.06.004. [DOI] [PubMed] [Google Scholar]
- Mook OR, Frederiks WM, Van Noorden CJ. The role of gelatinases in colorectal cancer progression and metastasis. Biochim Biophys Acta. 2004;1705:69–89. doi: 10.1016/j.bbcan.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Mujumdar VS, Tyagi SC. Temporal regulation of extracellular matrix components in transition from compensatory hypertrophy to decompensatory heart failure. J Hypertens. 1999;17:261–270. doi: 10.1097/00004872-199917020-00011. [DOI] [PubMed] [Google Scholar]
- Murphy G, Segain JP, O'Shea M, Cockett M, Ioannou C, Lefebvre O, Chambon P, Basset P. The 28-kDa N-terminal domain of mouse stromelysin-3 has the general properties of a weak metalloproteinase. J Biol Chem. 1993;268:15435–15441. [PubMed] [Google Scholar]
- Nagase H. Cell surface activation of progelatinase A (proMMP-2) and cell migration. Cell Res. 1998;8:179–186. doi: 10.1038/cr.1998.18. [DOI] [PubMed] [Google Scholar]
- Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Nesby-O'Dell S, Scanlon KS, Cogswell ME, Gillespie C, Hollis BW, Looker AC, Allen C, Doughertly C, Gunter EW, Bowman BA. Hypovitaminosis D prevalence and determinants among African American and white women of reproductive age: third National Health and Nutrition Examination Survey, 1988–1994. Am J Clin Nutr. 2002;76:187–192. doi: 10.1093/ajcn/76.1.187. [DOI] [PubMed] [Google Scholar]
- Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: a systematic review and meta-analysis. Int J Epidemiol. 2008;37:113–119. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- Norian JM, Malik M, Parker CY, Joseph D, Leppert PC, Segars JH, Catherino WH. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16:1153–1164. doi: 10.1177/1933719109343310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara N. A putative role of versican in uterine leiomyomas. Clin Exp Obstet Gynecol. 2009;36:74–75. [PubMed] [Google Scholar]
- Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- Olson MW, Bernardo MM, Pietila M, Gervasi DC, Toth M, Kotra LP, Massova I, Mobashery S, Fridman R. Characterization of the monomeric and dimeric forms of latent and active matrix metalloproteinase-9. Differential rates for activation by stromelysin 1. J Biol Chem. 2000;275:2661–2668. doi: 10.1074/jbc.275.4.2661. [DOI] [PubMed] [Google Scholar]
- Ottino P, Taheri F, Bazan HE. Platelet-activating factor induces the gene expression of TIMP-1, -2, and PAI-1: imbalance between the gene expression of MMP-9 and TIMP-1 and -2. Exp Eye Res. 2002;74:393–402. doi: 10.1006/exer.2001.1135. [DOI] [PubMed] [Google Scholar]
- Patterson ML, Atkinson SJ, Knauper V, Murphy G. Specific collagenolysis by gelatinase A, MMP-2, is determined by the hemopexin domain and not the fibronectin-like domain. FEBS Lett. 2001;503:158–162. doi: 10.1016/s0014-5793(01)02723-5. [DOI] [PubMed] [Google Scholar]
- Polgar A, Falus A, Koo E, Ujfalussy I, Sesztak M, Szuts I, Konrad K, Hodinka L, Bene E, Meszaros G, et al. Elevated levels of synovial fluid antibodies reactive with the small proteoglycans biglycan and decorin in patients with rheumatoid arthritis or other joint diseases. Rheumatology (Oxford) 2003;42:522–527. doi: 10.1093/rheumatology/keg168. [DOI] [PubMed] [Google Scholar]
- Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103:416–419. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- Rein MS, Barbieri RL, Friedman AJ. Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol. 1995;172:14–18. doi: 10.1016/0002-9378(95)90077-2. [DOI] [PubMed] [Google Scholar]
- Sabry M, Halder SK, Allah AS, Roshdy E, Rajaratnam V, Al-Hendy A. Serum vitamin D3 level inversely correlates with uterine fibroid volume in different ethnic groups: a cross-sectional observational study. Int J Womens Health. 2013;5:93–100. doi: 10.2147/IJWH.S38800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharan C, Halder SK, Thota C, Jaleel T, Nair S, Al-Hendy A. Vitamin D inhibits proliferation of human uterine leiomyoma cells via catechol-O-methyltransferase. Fertil Steril. 2011;95:247–253. doi: 10.1016/j.fertnstert.2010.07.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sintov AC, Berkovich L, Ben-Shabat S. Inhibition of cancer growth and induction of apoptosis by BGP-13 and BGP-15, new calcipotriene-derived vitamin D(3) analogs, in-vitro and in-vivo studies. Invest New Drugs. 2013;31:247–255. doi: 10.1007/s10637-012-9839-1. [DOI] [PubMed] [Google Scholar]
- Sozen I, Arici A. Interactions of cytokines, growth factors, and the extracellular matrix in the cellular biology of uterine leiomyomata. Fertil Steril. 2002;78:1–12. doi: 10.1016/s0015-0282(02)03154-0. [DOI] [PubMed] [Google Scholar]
- Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900–906. doi: 10.1210/jcem.79.3.8077380. [DOI] [PubMed] [Google Scholar]
- Tempfer H, Gehwolf R, Lehner C, Wagner A, Mtsariashvili M, Bauer HC, Resch H, Tauber M. Effects of crystalline glucocorticoid triamcinolone acetonide on cultered human supraspinatus tendon cells. Acta Orthop. 2009;80:357–362. doi: 10.3109/17453670902988360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetlow LC, Woolley DE. The effects of 1 alpha,25-dihydroxyvitamin D(3) on matrix metalloproteinase and prostaglandin E(2) production by cells of the rheumatoid lesion. Arthritis Res. 1999;1:63–70. doi: 10.1186/ar12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms PM, Mannan N, Hitman GA, Noonan K, Mills PG, Syndercombe-Court D, Aganna E, Price CP, Boucher BJ. Circulating MMP9, vitamin D and variation in the TIMP-1 response with VDR genotype: mechanisms for inflammatory damage in chronic disorders? QJM. 2002;95:787–796. doi: 10.1093/qjmed/95.12.787. [DOI] [PubMed] [Google Scholar]
- Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- Wilcox LS, Koonin LM, Pokras R, Strauss LT, Xia Z, Peterson HB. Hysterectomy in the United States, 1988–1990. Obstet Gynecol. 1994;83:549–555. doi: 10.1097/00006250-199404000-00011. [DOI] [PubMed] [Google Scholar]
- Wilson EA, Yang F, Rees ED. Estradiol and progesterone binding in uterine leiomyomata and in normal uterine tissues. Obstet Gynecol. 1980;55:20–24. [PubMed] [Google Scholar]
- Woessner JF., Jr The family of matrix metalloproteinases. Ann N Y Acad Sci. 1994;732:11–21. doi: 10.1111/j.1749-6632.1994.tb24720.x. [DOI] [PubMed] [Google Scholar]
- Wolanska M, Sobolewski K, Bankowski E, Jaworski S. Matrix metalloproteinases of human leiomyoma in various stages of tumor growth. Gynecol Obstet Invest. 2004;58:14–18. doi: 10.1159/000077177. [DOI] [PubMed] [Google Scholar]
- Zucker S, Lysik RM, Zarrabi MH, Moll U. M(r) 92,000 type IV collagenase is increased in plasma of patients with colon cancer and breast cancer. Cancer Res. 1993;53:140–146. [PubMed] [Google Scholar]