Abstract
STUDY QUESTION
Is targeted adenovirus vector, Ad-SSTR-RGD-TK (Adenovirus –human somatostatin receptor subtype 2- arginine, glycine and aspartate-thymidine kinase), given in combination with ganciclovir (GCV) against immortalized human leiomyoma cells (HuLM) a potential therapy for uterine fibroids?
SUMMARY ANSWER
Ad-SSTR-RGD-TK/GCV, a targeted adenovirus, effectively reduces cell growth in HuLM cells and to a significantly greater extent than in human uterine smooth muscle cells (UtSM).
WHAT IS KNOWN ALREADY
Uterine fibroids (leiomyomas), a major cause of morbidity and the most common indication for hysterectomy in premenopausal women, are well-defined tumors, making gene therapy a suitable and potentially effective non-surgical approach for treatment. Transduction of uterine fibroid cells with adenoviral vectors such as Ad-TK/GCV (herpes simplex virus thymidine kinase gene) decreases cell proliferation.
STUDY DESIGN, SIZE, DURATION
An in vitro cell culture method was set up to compare and test the efficacy of a modified adenovirus vector with different multiplicities of infection in two human immortalized cell lines for 5 days.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Immortalized human leiomyoma cells and human uterine smooth muscle cells were infected with different multiplicities of infection (MOI) (5–100 plaque-forming units (pfu)/cell) of a modified Ad-SSTR-RGD-TK vector and subsequently treated with GCV. For comparison, HuLM and UtSM cells were transfected with Ad-TK/GCV and Ad-LacZ/GCV. Cell proliferation was measured using the CyQuant assay in both cell types. Additionally, western blotting was used to assess the expression of proteins responsible for regulating proliferation and apoptosis in the cells.
MAIN RESULTS AND THE ROLE OF CHANCE
Transduction of HuLM cells with Ad-SSTR-RGD-TK/GCV at 5, 10, 50 and 100 pfu/cell decreased cell proliferation by 28, 33, 45, and 84%, respectively (P < 0.05) compared with untransfected cells, whereas cell proliferation in UtSM cells transfected with the same four MOIs of Ad-SSTR-RGD-TK/GCV compared with that of untransfected cells was decreased only by 8, 23, 25, and 28%, respectively (P < 0.01). Western blot analysis showed that, in comparison with the untargeted vector Ad-TK, Ad-SSTR-RGD-TK/GCV more effectively reduced expression of proteins that regulate the cell cycle (Cyclin D1) and proliferation (PCNA, Proliferating Cell Nuclear Antigen), and it induced expression of the apoptotic protein BAX, in HuLM cells.
LIMITATIONS, REASONS FOR CAUTION
Results from this study need to be replicated in an appropriate animal model before testing this adenoviral vector in a human trial.
WIDER IMPLICATIONS OF THE FINDINGS
Effective targeting of gene therapy to leiomyoma cells enhances its potential as a non-invasive treatment of uterine fibroids.
STUDY FUNDING/COMPETING INTEREST(S)
This work was supported by a grant from the National Institute of Child Health and Human Development, National Institutes of Health [R01 HD046228]. None of the authors has any conflict of interest to declare.
Keywords: uterine leiomyomas, gene therapy, adenovirus vectors, Ad-SSTR-RGD-TK
Introduction
Uterine leiomyomas, also known as uterine fibroids, are common benign pelvic tumors that occur in 20–25% of premenopausal women (Healy, et al., 1986; Walker, et al., 2000; Stewart, 2001). They cause major symptoms that drastically affect the quality of life in women and include prolonged, irregular, heavy menstrual bleeding, anemia, pelvic pain and bowel and bladder dysfunction. Treatment of uterine leiomyomas is based on the symptoms, the size of the tumors and the site of formation. Limited medical treatment options exist, and surgery is the mainstay. Among the treatment options, hysterectomy is the best surgical approach, although it affects the childbearing ability of the woman. Newer non-surgical approaches include uterine artery embolization and focused ultrasound surgery, both of which retain the uterus of the patients. These approaches have yet to achieve popularity due to the associated risks involved and the reported recurrence of the leiomyomas.
Uterine leiomyomas are ideal candidates for direct delivery of therapeutic gene-based vectors because of their localized nature and their accessibility through imaging and endoscopic methods. Gene therapy involves various strategies to deliver genetic material to target cells to achieve therapeutic benefits (Hassan et al., 2009a,b). Suicide gene therapy is a frequently applied method that involves delivery of the herpes simplex virus 1 thymidine kinase gene (HSV1TK) followed by delivery of a non-toxic guanosine analog, ganciclovir (GCV). GCV is phosphorylated by HSV1TK and mammalian cellular kinases to form a toxic, triphosphorylated form (GCVTP) (Tasciotti et al., 2003). This toxic product, in turn, inhibits DNA synthesis and blocks the cell cycle, ultimately leading to cell death via apoptosis (Reid et al., 1988; Robe et al., 2000). Our previous in vitro and in vivo (Eker rat model) studies have demonstrated that adenoviral vectors are able to infect uterine leiomyoma cells and severely inhibit cell proliferation, resulting in an increased number of apoptotic cells and the regression of uterine leiomyoma tumors (Hassan et al., 2008, 2009b). To optimize this approach of treating uterine leiomyomas by targeting therapeutic genes, we also tested several modified adeno vectors in the uterine leiomyoma cell line to identify the most selective and efficient virus for targeting human leiomyoma cells. Our data showed that Ad5-RGD-luc, compared with the Ad5-luc viruses, had enhanced transduction efficiency in leiomyoma cells (Hassan et al., 2008). The fiber-modified Ad5-RGD-Luc vector is constructed through the insertion of a short peptide (21 amino acids) composed of arginine, glycine and aspartate (RGD) into the H1 loop of the wild fiber knob domain (Dmitriev et al., 1998; Cripe et al., 2001).
Phase 1 trials for cancers other than uterine leiomyomas using the adenovirus TK/GCV protocol have demonstrated the safety of this therapy (Hemminki et al., 2002). The adenovirus serotype 5 is usually used for gene therapies that bind to the coxsackie-adenovirus receptor (CAR). Several clinical trials for tumor treatment using adenovirus gene therapy showed unimpressive results, which may be due to poor or absent expression of the coxsackie receptor in primary tumor cells as a consequence of increased aggressiveness of the tumor cells or of higher activity of the MAPK pathway (Anders et al., 2001). Microarray studies of uterine leiomyoma tissues have demonstrated that CAR is down-regulated in leiomyomas in comparison with the myometrium, where it might have a role in myometrial contractions (Tsibris et al., 2002). A promising approach that has been suggested to circumvent this dependence on CAR and to enhance transduction efficiency is the genetic modification of the adenovirus fiber with an arginine–glycine–aspartic acid (RGD-4C) motif (Dmitriev et al., 1998).
Previous reports on gene therapy for ovarian and other similar cancer cell lines have shown an increase in infectivity through CAR-independent transduction, achieving higher reporter gene expression by several orders of magnitude in the primary tumor cells (Dmitriev et al., 1998; Kasono et al., 1999; Vanderkwaak et al., 1999). Addition of this peptide modification in the H1 loop of the fiber domain allows virus entry via cellular integrins rather than through the CAR (Dmitriev et al., 1998). Moreover, another potential reason for the low transduction of Ad HSV-TK is the presence of antivirus-neutralizing antibodies. This potential limitation has also been reported to be partially overcome by modification of the adenovirus using RGD-4C (Blackwell et al., 2000; Hemminki et al., 2001). Evaluation of the safety and efficacy of a gene therapy candidate is demonstrated by the level, persistence and location of the transgene expression. Our earlier work reported the construction of an RGD-4C infectivity-enhanced bicistronic type 5 adenoviral vector, Ad-RGD-TK-SSTR, which encodes two transgenes, a herpes simplex virus thymidine kinase (TK) and the human somatostatin receptor subtype 2 (SSTR), expressed from a cytomegalovirus (CMV) early promoter (Hemminki et al., 2001). SSTR is an imaging cassette that permits assessment of transduction in vivo. A phase I clinical trial in women diagnosed with ovarian cancer has been successfully completed using 109–1012 pfu/day doses to determine the safety and clinical outcome and the biological effects of Ad-RGD-TK-SSTR (Kimball et al., 2010; Kim et al., 2012).
The purpose of this study was to assess the in vitro efficacy of the infectivity-enhanced Ad-SSTR-RGD-TK vector compared with unmodified Ad-TK in uterine leiomyoma cells versus normal uterine myometrial cells.
Materials and Methods
Recombinant adenovirus
We used an Ad vector encoding the HSV1TK gene under transcriptional control of the Rous sarcoma virus (Ad-HSV1TK), which has been previously described (Chen et al., 1994). Ad-LacZ is an adenovirus vector expressing a marker gene coding for bacterial β-galactosidase; we used this vector as a negative control. Both these viruses were kind gifts from Dr Savio Woo (Mount Sinai School of Medicine, NY, USA). The Ad-RGD-SSTR-TK vector is a modified adenovirus vector that contains the herpes simplex virus thymidine kinase gene and the human somatostatin receptor subtype 2 for non-invasive imaging and was prepared in Dr Curiel's lab. A genetic incorporation of the RGD-4C motif into the H1 loop of the fiber to enhance the infectivity of the vector by permitting binding to the αvβ3 and αvβ5 integrins is another modification we have described previously (Hemminki et al., 2001).
Cell culture
Human immortalized leiomyoma cells and uterine smooth muscle cells were kind gifts from Dr Darlene Dixon, (National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA). The cells were cultured and maintained in smooth muscle cell basal medium (SmBM) containing 10% fetal bovine serum (FBS), 0.1% insulin, 0.2% human fibroblast growth factor–basic (hFGF-B), 0.1% gentamycin sulfate and amphotericin-B (GA-1000) and 0.1% human epidermal growth factor (hEGF) (Lonza, Walkersville, MD, USA). For the cell proliferation assay, cells were grown in a 96-well culture plate and transduced with adenoviral vectors (0–100 pfu/cell) and GCV (Sigma Co, St. Louis, MO, USA) at a concentration of 10 µg/ml for 5 days. Cell growth was measured with the CyQuant kit (Invitrogen, Carlsbad, CA, USA) per the manufacturer's instructions. Briefly, at the end of Day 5 of culture, the medium was aspirated from the wells, and the wells were then washed carefully with PBS. The plates were immediately frozen at −70°C for an hour. The plates were then thawed at room temperature, and 200 µl of CyQuant GR dye/cell lysis buffer was added to each well and mixed gently. After 5-min incubation at room temperature while the samples were protected from light, the sample fluorescence was measured using a fluorescence microplate reader with filters set at 480 nm for excitation and 520 nm for emission.
Transduction
HuLM and UtSM cells were cultured in 60-mm plates at 4.5 × 105 cells/plate and were fed regular medium for myometrial cells (SmBM medium with 10% FBS) for 24 h. Cells were then incubated with the adenoviral vectors at various multiplicities of infection (5, 10 and 50 pfu/cell) in transduction medium containing 2% FBS with continuous gentle shaking. After 5 h, the infection medium was removed and replaced with regular maintenance medium. After 12 h of incubation, the medium was replaced with medium containing GCV at 10 µg/ml, which was replaced every 48 h.
Western blot
After treatment with the adenovirus vector/GCV, the HuLM and UtSM cells were harvested and lysed with a lysis buffer (Cellytic-M, Sigma) containing a protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN, USA). Protein concentrations were determined with bicinchoninic acid (BCA) protein assay reagent (Thermo Scientific, Inc., Rockford, IL, USA). The samples were diluted with 4× SDS (sodium dodecyl sulfate) loading buffer containing β-mercaptoethanol. Equal amounts of protein (10 μg) for each sample were separated by SDS-polyacrylamide gel electrophoresis and electrotransferred to a polyvinylidene difluoride membrane (Immobilon-P; Millipore Corporation, Bedford, MA, USA). Proteins were detected by immunoblotting followed by enhanced chemiluminescence detection (Amersham Biosciences, GE Healthcare, Piscataway, NJ, USA). Chemiluminescence signals were detected by a luminoimage analyzer, SRX-101A (Konica Minolta, Ramsey, NJ, USA). Membranes were immunoblotted with primary antibodies against PCNA (1:500), BCL-2 (1:500), BAX (1:500) and Cyclin D1 (1:500). Anti-β-actin (1:5000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used as loading control. The membranes were washed and then incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. The intensity of each protein band was determined using a scanning densitometer (FluorChem FC2 Imager, Alpha Innotech Imager, Santa Clara, CA, USA) and normalized against the values obtained for β-actin.
Statistical analyses
All data are presented as the means ± standard error (SE) of the values obtained from three replicates. Differences between groups were analyzed using Student's t-test and were considered to be significant if P < 0.05.
Results
Effects of Ad-TK/GCV and Ad-SSTR-RGD-TK/GCV on proliferation of immortalized human leiomyoma cells
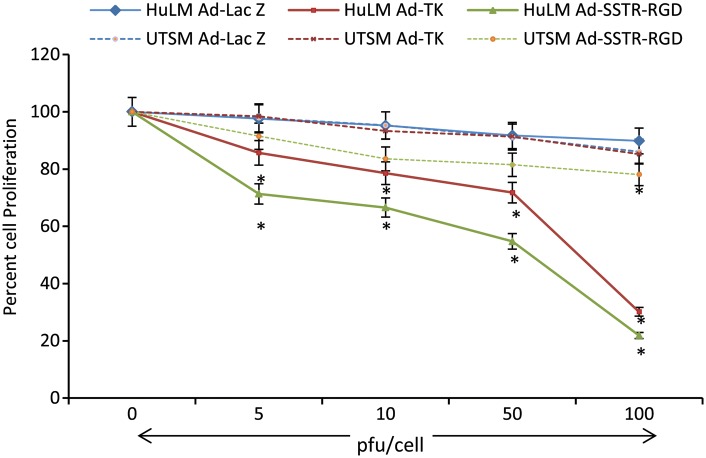
We tested the effects of adenoviral vector systems Ad-TK and Ad-SSTR-RGD-TK/GCV at different MOIs (5, 10, 50 and 100 pfu/cell) and GCV at 10 μg/ml on immortalized human leiomyoma cells. We had previously reported the ability of adenoviruses to transduce leiomyoma cells (Al-Hendy et al., 2004). Both vectors showed a significant (P < 0.05) and MOI-dependent reduction in cell proliferation. Compared with the non-transduced negative control, the percent reduction in cell proliferation with the Ad-TK/GCV system was 17, 21, 28 and 70% at MOIs of 5, 10, 50 and 100 pfu/cell, respectively (P < 0.05) (Fig. 1). Compared with the negative control, the percent reduction in cell proliferation with the Ad-SSTR-RGD-TK/GCV system was 28, 33, 45 and 84% at MOIs of 5, 10, 50 and 100 pfu/cell, respectively (P < 0.05). The reductions in cell proliferation that were achieved using Ad-SSTR-RGD-TK were significantly higher than those obtained using the Ad-TK vector, suggesting that the Ad-SSTR-RGD-TK vector had a greater efficacy (Fig. 1).
Figure 1.
Cell proliferation in human leiomyoma cells (HuLM) and human uterine smooth muscle cells (UtSM) transduced with the adenovirus vectors Ad-Lac Z, Ad-TK and Ad-SSTR-RGD-TK at 0–100 plaque-forming units (pfu)/cell. HuLM and UtSM cells were cultured in 96-well plates, and then transfected with respective vectors at 0, 5, 10, 50 and 100 pfu/cell for 5 h. The media was then replaced containing ganciclovir (GCV) at 10 µg/ml. Cells were subjected to CyQuant assay for cell proliferation after 5 days of culture. Individual data points are the mean ± SE of triplicate determinations. *A significant difference from non-transduced control. P < 0.05 assessed by Student's t-test was considered significant.
Effect of Ad-SSTR-RGD-TK/GCV on human uterine smooth myometrial cells
To determine whether Ad-SSTR-RGD-TK is capable of transducing UtSM cells, we infected the cells with Ad-SSTR-RGD-TK, Ad-TK and Ad-LacZ. Both the Ad-SSTR-RGD-TK and Ad-TK vectors yielded a significant MOI-dependent reduction in cell proliferation compared with the respective negative control at 0 pfu (P < 0.05) (Fig. 1). Compared with the non-transduced negative control, the percent reduction in cell proliferation with the Ad-TK/GCV system was 1.57, 6.7, 8.6 and 14.7% at MOIs of 5, 10, 50 and 100 pfu/cell, respectively (P < 0.05). Compared with the negative control, the percent reduction in cell proliferation with the Ad-SSTR-RGD-TK/GCV system was 8.5, 16.4, 18.4 and 21.8% at MOIs of 5, 10, 50 and 100 pfu/cell, respectively (P < 0.01). Overall, the transduction observed in myometrial cells was reduced compared with the transduction in leiomyoma cells (Fig. 1).
Ad-SSTR-RGD-TK/GCV inhibits the expression of proliferation- and cell cycle-related genes in human leiomyoma cells
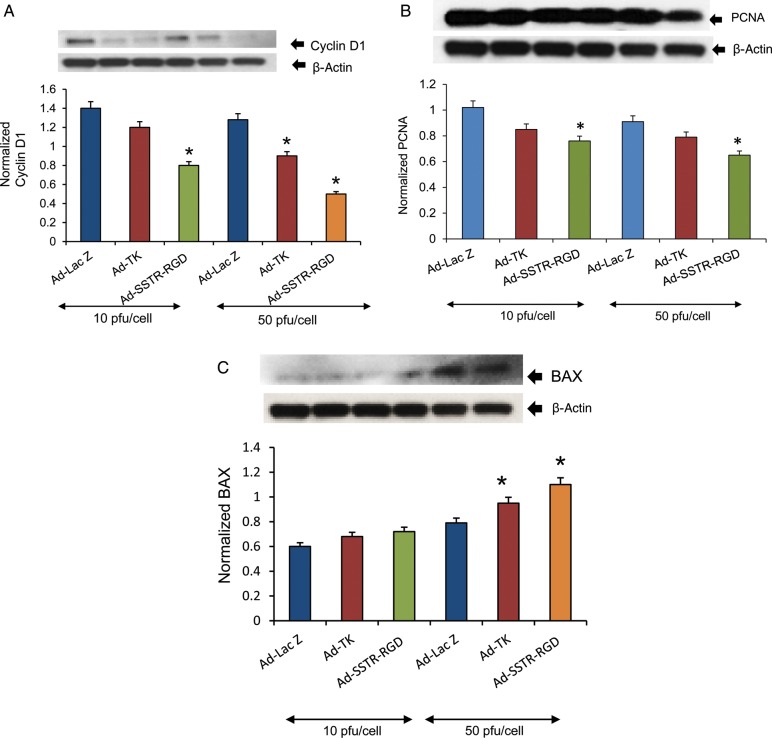
To test whether Ad-SSTR-RGD-TK/GCV induced any changes in the expression of proteins that regulate cell proliferation and the cell cycle, we performed western blot analysis using lysates from HuLM cells transduced with Ad-LacZ, Ad-TK or Ad-SSTR-RGD-TK at 10 and 50 pfu/cell combined with 10 µg/ml of GCV.
As shown in Fig. 2A, transduction of leiomyoma cells with Ad-SSTR-RGD-TK/GCV at 10 and 50 pfu/cell, compared with transduction of Ad-TK and Ad-LacZ, yielded a significant decrease (P < 0.05) in the expression of a cell cycle protein (Cyclin D1). A similar significant decrease was observed in the expression of a cell proliferation protein (PCNA) at 50 pfu/cell in cells transduced with Ad-SSTR-RGD-TK/GCV compared with those treated with Ad-TK or Ad-LacZ (Fig. 2B). Therefore, these results suggest that the targeted transduction-enhanced Ad-SSTR-RGD-TK/GCV vector has superior capabilities, compared with the untargeted Ad-TK vector, for reducing cell cycling and cell proliferation in HuLM cells.
Figure 2.
Adenovirus vectors induce significant decreases in expression of proliferation-related Cyclin D1 (A) and proliferating cell nuclear antigen (PCNA; B) and an increase in expression of apoptosis-related BAX (C) in human uterine leiomyoma cells (HuLM). HuLM cells were transduced with Ad-LacZ, Ad-TK and Ad-SSTR-RGD-TK at 10 and 50 plaque-forming units (pfu)/cell and ganciclovir (GCV) at 10 µg/ml for 48 h. Lysates were analyzed by western blotting. The intensity of each protein signal was quantified and normalized with corresponding beta-actin. Data are mean ± SE, n = 3. *A significant difference from Ad-lacZ (P < 0.05, Student's t-test). The results are indicative of two independent experiments.
Ad-SSTR-RGD-TK/GCV regulates apoptosis-related genes in human leiomyoma cells
To test whether Ad-SSTR-RGD-TK affected expression of proapoptotic BAX in HuLM cells, we performed western blot analyses as described in the Materials and Methods section. HuLM cells were transduced with Ad-LacZ, Ad-TK or Ad-SSTR-RGD-TK and subsequently treated with 10 µg/ml of GCV at 10 and 50 pfu/cell. Cell lysates were analyzed by western blotting using anti-BAX antibody. We found that Ad-SSTR-RGD-TK/GCV yielded a significant increase (P < 0.05) in proapoptotic BAX protein (Fig. 2C) at 50 pfu/cell. These results suggest that Ad-SSTR-RGD-TK/GCV has a greater ability than Ad-TK or Ad-LacZ to induce apoptosis in HuLM cells.
Ad-SSTR-RGD-TK/GCV and Ad-TK demonstrate no effect on proliferation- and cell cycle-related genes in human myometrial cells
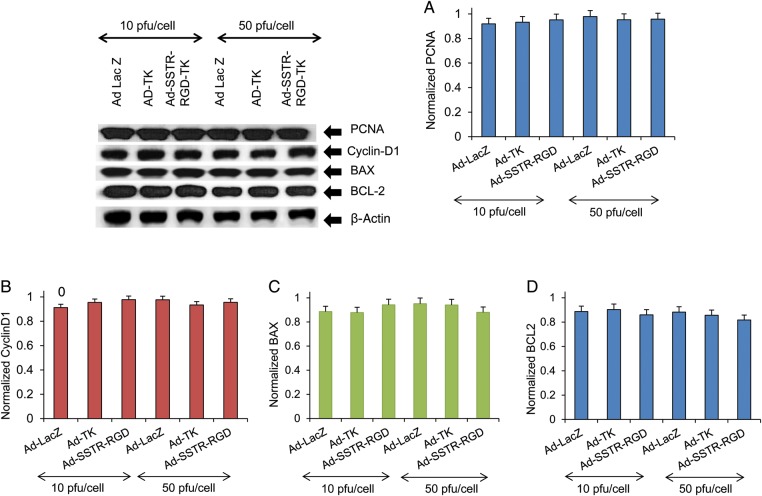
To test the effect of Ad-SSTR-RGD-TK/GCV on the expression of proteins regulating cell proliferation and cell cycle in human myometrial cells, we performed western blot analysis using lysates from UtSM cells transduced with Ad-LacZ, Ad-TK or Ad-SSTR-RGD-TK and subsequently treated with 10 µg/ml GCV at 10 and 50 pfu/cell. As shown in Fig. 3, transduction of myometrial cells with Ad-SSTR-RGD-TK/GCV, compared with Ad-TK and Ad-LacZ, showed no significant alteration in expression of proteins related to cell proliferation (PCNA, Fig. 3A) or cell cycling (Cyclin D1, Fig. 3B) at 10 and 50 pfu/cell (P > 0.05). Therefore, these results suggest that Ad-SSTR-RGD-TK/GCV and Ad-TK have no effect on cell proliferation and cell cycling in human myometrial cells.
Figure 3.
The adenovirus vectors Ad-TK and Ad-SSTR-RGD-TK do not induce any significant changes compared with Ad-LacZ in either proliferation—proliferating cell nuclear antigen (PCNA) (A) and Cyclin D1 (B) or apoptosis— BAX (C) and antiapoptosis BCL-2 (D) related gene expression in human uterine smooth muscle cells (UtSM). UtSM cells were transduced with Ad-LacZ, Ad-TK and Ad-SSTR-RGD-TK at 10 and 50 plaque-forming units (pfu)/cell and GCV at 10 µg/ml for 48 h. Lysates were analyzed by western blotting with anti-PCNA, anti-Cyclin D1, anti-BAX and anti-BCL-2 antibodies. The intensity of each protein signal was quantified and normalized with corresponding beta-actin. Data are mean ± SE, n = 3. The results are indicative of three independent experiments.
Ad-SSTR-RGD-TK/GCV and Ad-TK demonstrate similar expression of antiapoptotic BCL-2 and proapoptotic BAX proteins in UtSM cells
To test whether Ad-SSTR-RGD-TK affected the expression of the antiapoptotic protein BCL-2 and the proapoptotic protein BAX in UtSM cells, we performed western blot analyses similar to those described earlier. UtSM cells were transduced with Ad-LacZ, Ad-TK or Ad-SSTR-RGD-TK and then treated with 10 µg/ml of GCV at 10 and 50 pfu/cell. Cell lysates were analyzed by western blotting using anti-BCL-2 antibody and anti-BAX antibody. We found that Ad-SSTR-RGD-TK/GCV, compared with Ad-TK, yielded similar expression of BAX and BCL-2 proteins at both 10 and 50 pfu/cell (P > 0.05). These results suggest that Ad-SSTR-RGD-TK/GCV has a potential similar to that of Ad-TK and Ad-LacZ to induce apoptosis in UtSM cells (Fig. 3C and D).
Discussion
Uterine leiomyomas continue to pose a major challenge due to the lack of effective, non-surgical, localized therapeutic options. The radical surgical option of total hysterectomy continues to be the mainstay of management for this very common premenopausal disorder. Gene therapy appears to be a potentially safe, effective, localized and non-surgical method of treatment for women with symptomatic uterine leiomyomas. We and others have reported on the efficacy of the Ad-TK/GCV system in several malignant and non-malignant disorders (Ketola et al., 2004; Salama et al., 2007). We have previously shown that such a gene therapy approach can effectively reduce leiomyoma cell proliferation in vitro as well as reduce tumor volume in vivo in the Eker rat model of uterine leiomyomas (Salama et al., 2007; Hassan et al., 2009a,b). Additionally, we have demonstrated an overexpression of gap junction proteins in leiomyoma cells obtained from human fibroid explants compared with cells from adjacent normal myometrium as well as in the fibroid capsule. This differential expression of gap junction proteins and connexin 43 in leiomyoma cells in comparison with surrounding normal myometrium cells greatly contributes to the targeting ability of adenovirus vectors (Salama et al., 2007).
In spite of these encouraging results, we realize that one of the limitations of this vector is its dependence on CAR for effective gene transfer, as CAR expression is generally reduced in tumor cells, including fibroids (Anders et al., 2001). Consequently, the non-target cells, which express high levels of CAR, will potentially sequester a large number of recombinant virions, leaving the target tumor cells, which express low levels of CAR, poorly transduced (Dmitriev et al., 1998; Nakayama et al., 2006). An obvious solution would be to administer a higher dose of the vector; however, this approach could ultimately lead to an increased risk of toxicity and the initiation of adverse immune responses against the vector. Therefore, to enhance the targeting ability of the vector, we needed to modify it by reducing its dependency on the CAR for transduction. Addition of an arginine-glycine-aspartic acid motif has been shown to have enhanced in vivo targeting abilities (Pasqualini et al., 1997; Arap et al., 1998). Furthermore, Dmitriev et al. have shown that a recombinant adenoviral vector containing fibers with the RGD motif in the H1 loop demonstrates superior transduction via a CAR-independent mechanism of target cell entry (Dmitriev et al., 1998). Microarray studies have shown that, among other prominent genes, the Coxsackie virus receptor is down-regulated in leiomyomas relative to the myometrium in women (Tsibris et al., 2002). Therefore, a modified adenovirus vector that can circumvent the CAR to enter cells will have greater utility in the gene therapy of leiomyomas. Previously, we had shown that fiber-modified Ad5-RGD-Luc yielded higher reporter gene activity in HuLM cells and lower activity in both normal uterine smooth muscle cells (HM9) and immortalized liver cells (THLE3) compared with that induced by the unmodified Ad5-luc vector. This result indicated that modified Ad5-RGD is a promising candidate for use as a vector in targeted gene therapy for uterine leiomyomas (Hassan et al., 2008).
In the present study, we have demonstrated the enhanced efficacy of a modified adenovirus vector in transducing targeted human leiomyoma cells compared with normal myometrial cells. As a pertinent step, we compared Ad-SSTR-RGD-TK with Ad-TK to demonstrate the effect of the modified vector on cell proliferation, and our results indicate that the reduction in cell number was greater following transduction of Ad-SSTR-RGD-TK compared with other vectors. Consistent with other published studies (Bakker et al., 2001; Volk et al., 2003; Wu et al., 2004) our findings indicate that the incorporation of the RGD motif contributes immensely to the transduction of the low-CAR leiomyoma cells through an alternate path. Additionally, the transduction in leiomyoma cells is greater than that in normal myometrial cells as determined by the lack of inhibition of cell proliferation in the latter, indicating that the RGD motif enables Ad-SSTR-RGD-TK to selectively target human leiomyoma cells. The limited cytotoxic effect of Ad-SSTR-RGD-TK on human myometrial cells is an additional safety feature for fibroid-targeted gene therapy. Future application of this approach dictates direct injection of the therapeutic adenovirus into the fibroid lesions. The inability of potential minimal leakage into adjacent myometrium to affect cell proliferation is an encouraging biological observation.
PCNA is a 36-kD nuclear protein that is synthesized in dividing cells and is a well-established proliferation marker. We observed a significant reduction of PCNA expression in HuLM cell lysates transduced with AD-SSTR-RGD-TK, and this reduction was significantly more remarkable than that observed in lysates from cells treated with Ad-TK. This observation confirms that the RGD-fiber modification enhanced the transduction characteristics of this vector and increased its targeting efficiency toward human leiomyoma cells. Moreover, transduction of human myometrial cells with the same set of adenoviral vectors did not yield any changes in the expression of the PCNA protein. This finding supports our conclusion that Ad-SSTR-RGD-TK possesses a selective targeting ability, caused by the presence of the RGD motif in its construct that makes the vector leiomyoma/ON and normal myometrium/OFF.
Cyclin D1 is a regulator of the cell cycle and is essential for progression of the G1 phase of the cell cycle (Baldin et al., 1993). We observed that AD-SSTR-RGD-TK significantly down-regulated the expression of Cyclin D1 in HuLM cell lysates compared with Ad-TK or the control vector at all doses. This finding indicates that HuLM cells transduced with AD-SSTR-RGD-TK do not progress in their cell cycle. Therefore, our finding supports the notion that transduction of HuLM cells with AD-SSTR-RGD-TK will prevent cell cycle progression and that AD-SSTR-RGD-TK is a strong candidate as an effective vector for gene therapy to control fibroid tumor growth when used in vivo.
BAX, a member of the BCL-2 family, is an inducer of apoptosis in various cell types. Overexpression of BAX accelerates cell death or apoptosis in cells through the activation of caspase pathways (Tsunemitsu et al., 2004). In vivo studies in lung and prostate cancer models have demonstrated that adenovirus-mediated expression of BAX protein effectively induced apoptosis in tumor lesions (Kagawa et al., 2000; Li et al., 2001; Honda et al., 2002). BAX has also been shown as a novel apoptotic gene for efficacious treatment of ovarian cancer (Xiang et al., 2000; Huh et al., 2001). Intratumor administration of Ad-TK/GCV in the Eker rat has shown effective induction of capase-3 activity and BAX expression with shrinkage in tumor size (Hassan et al., 2009b).
We observed significant induction of BAX in HuLM cells transduced with Ad-SSTR-RGD-TK compared with Ad-TK or Ad-LacZ. In UtSM cells, minimal induction of BAX protein expression was observed, with no significant difference in BAX expression among the vectors or among the various doses that were used. This finding further indicates that the modified Ad-SSTR-RGD-TK vector is selective in its targeting of HuLM cells, a phenomenon that is likely caused by the insertion of the RGD motif in this construct.
BCL-2 is a 24-KD cytosolic protein localized in the mitochondria and perinuclear membrane (Misao et al., 1996). The BCL-2 protein has been well described for its ability to extend the lifespan of cells and to promote cell replication (Yang et al., 1997; Danial and Korsmeyer, 2004). Elevation of the BCL-2 gene in vivo or in vitro indicates a prevention of apoptosis in cells (Korsmeyer, 1992; Reed, 1994). BCL-2 is abundantly expressed in leiomyomas compared with the normal myometrium (Matsuo et al., 1997). Our results demonstrate that transduction of normal uterine smooth muscle cells with modified vectors did not induce any changes in the expression of the BCL-2 protein. This finding indicates that the modified adenovirus vector Ad-SSTR-RGD-TK has selective targeting ability toward human leiomyoma cells and does not transduce normal human myometrial cells sufficiently to bring about effective down-regulation of antiapoptotic proteins such as BCL-2; this finding adds an additional safety margin for future in vivo utilization of such a vector for localized treatment of uterine fibroids.
In conclusion, the Ad-SSTR-RGD-TK/GCV system induced superior inhibition of cell proliferation in human leiomyoma cells, and it down-regulated cell proliferation-regulating protein, PCNA and the cell cycling protein Cyclin D-1 while up-regulating BAX, which is reflective of apoptosis induction. These changes were significantly more prominent in HuLM cells transduced with Ad-SSTR-RGD-TK compared with Ad-TK or Ad-LacZ. On the other hand, when Ad-SSTR-RGD-TK was tested against human uterine smooth muscle cells, it exerted a minimal effect on cell proliferation and on all associated markers of cell cycle progression and apoptosis. These findings highlight the enhanced selectivity of this targeted vector, which indeed exhibits a leiomyoma/ON and normal myometrium/OFF profile. Therefore, our results strongly suggest that Ad-SSTR-RGD-TK with the RGD motif has an improved transduction ability compared with Ad-TK in HuLM cells but not in UtSM cells. Our in vitro results also demonstrate that Ad-SSTR-RGD-TK has enhanced selective targeting ability compared with other adenoviral vectors in HuLM cells and is a suitable candidate for further testing in vivo for its efficacy in reducing tumor volume and burden in animal models (e.g. nude mouse) through a CAR-independent mechanism. This study has generated important preclinical data for the development of leiomyoma-targeted gene therapy as a potential therapeutic approach for a safe, non-surgical treatment for uterine leiomyomas.
Authors' roles
S.N. made substantial contributions toward the conception and design of the study including data acquisition, analysis and interpretation. She also participated in the drafting and critical revision of the manuscript contents. She contributed toward the physical work of the study and was actively involved in the final approval of the version to be published. D.T.C. made a substantial contribution in providing the vectors and in critical review of the manuscript by providing intellectual contributions. V.R. made substantial contribution in the review of the manuscript. C.T. made substantial contribution in providing the human myometrial cells and in critical review of the manuscript. A.A.-H. made substantial contributions in the conception, design and interpretation of the data. He was involved in the drafting of the manuscript and in the critical review of its intellectual content as well as final approval of the version to be published. He also provided the laboratory space and all facilities, including funding for conducting the study.
Funding
This work was supported by a grant from National Institute of Child Health and Human Development, National Institutes of Health (R01 HD046228).
Conflict of interest
None declared.
References
- Al-Hendy A, Lee EJ, Wang HQ, Copland JA. Gene therapy of uterine leiomyomas: adenovirus-mediated expression of dominant negative estrogen receptor inhibits tumor growth in nude mice. Am J Obstet Gynecol. 2004;191:1621–1631. doi: 10.1016/j.ajog.2004.04.022. doi:10.1016/j.ajog.2004.04.022. [DOI] [PubMed] [Google Scholar]
- Anders M, Ding RX, Lipner EM, Balmain A, McCormick F, Korn WM. Inhibition of the MAPK pathway up-regulates the human coxsackie and adenovirus receptor (CAR) and increases the infectivity of cancer cells with adenovirus. Proc Am Assoc Cancer Res. 2001;42:703. [Google Scholar]
- Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. doi:10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- Bakker AC, Van de Loo FA, Joosten LA, Bennink MB, Arntz OJ, Dmitriev IP, Kashentsera EA, Curiel DT, van den Berg WB. A tropism-modified adenoviral vector increased the effectiveness of gene therapy for arthritis. Gene Ther. 2001;8:1785–1793. doi: 10.1038/sj.gt.3301612. doi:10.1038/sj.gt.3301612. [DOI] [PubMed] [Google Scholar]
- Baldin V, Lukas J, Marcote MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. doi:10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Blackwell JL, Li H, Gomez-Navarro J, Dmitriev I, Krasnykh V, Richter CA, Shaw DR, Alvarez RD, Curiel DT, Strong TV. Using a tropism-modified adenoviral vector to circumvent inhibitory factors in ascites fluid. Hum Gene Ther. 2000;11:1657–1669. doi: 10.1089/10430340050111313. doi:10.1089/10430340050111313. [DOI] [PubMed] [Google Scholar]
- Chen SH, Shine HD, Goodman JC, Grossman RG, Woo SL. Gene therapy for brain tumors: regression of experimental gliomas by adenovirus-mediated gene transfer in vivo. Proc Natl Acad Sci USA. 1994;91:3054–3057. doi: 10.1073/pnas.91.8.3054. doi:10.1073/pnas.91.8.3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripe TP, Dunphy EJ, Holub AD, Saini A, Vasi NH, Mahller YY, Collins MH, Snyder JD, Krasnykh V, Curiel DT, et al. Fiber knob modifications overcome low, heterogeneous expression of the coxsackievirus-adenovirus receptor that limits adenovirus gene transfer and oncolysis for human rhabdomyosarcoma cells. Cancer Res. 2001;61:2953–2960. [PubMed] [Google Scholar]
- Danial N, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. doi:10.1016/S0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MH, Khatoon N, Curiel DT, Hamada FM, Arafa HM, Al-Hendy A. Toward gene therapy of uterine fibroids: targeting modified adenovirus to human leiomyoma cells. Hum Reprod. 2008;23:514–524. doi: 10.1093/humrep/dem410. doi:10.1093/humrep/dem410. [DOI] [PubMed] [Google Scholar]
- Hassan MH, Othman EE, Hornung D, Al-Hendy A. Gene therapy of benign gynecological diseases. Adv Drug Deliv Rev. 2009a;61:822–835. doi: 10.1016/j.addr.2009.04.023. doi:10.1016/j.addr.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MH, Zhang D, Salama S, Hammada F, Arafa H, Foud H, Walker C, Al-Hendy A. Towards fibroid gene therapy: adenovirus-mediated delivery of herpes simplex virus 1 thymidine kinase gene/ganciclovir shrinks uterine leiomyoma in the Eker rat model. Gynecol Obstet Invest. 2009b;68:19–32. doi: 10.1159/000209675. doi:10.1159/000209675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healy DL, Lawson SR, Abbott M, Baird DT, Fraser HM. Toward removing uterine fibroids without surgery: subcutaneous infusion of a luteinizing hormone-releasing hormone agonist commencing in the luteal phase. J Clin Endocrinol Metab. 1986;63:619–625. doi: 10.1210/jcem-63-3-619. doi:10.1210/jcem-63-3-619. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Belousova N, Zinn KR, Liu B, Wang M, Chaudhuri TR, Rogers BE, Buchsbaum DJ, Siegal GP, Barnes MN, et al. An adenovirus with enhanced infectivity mediates molecular chemotherapy of ovarian cancer cells and allows imaging of gene expression. Mol Ther. 2001;4:223–231. doi: 10.1006/mthe.2001.0446. doi:10.1006/mthe.2001.0446. [DOI] [PubMed] [Google Scholar]
- Hemminki A, Zinn KR, Liu B, Chaudhuri TR, Desmond RA, Rogers BE, Barnes MN, Alvarez RD, Curiel DT. In vivo molecular chemotherapy and noninvasive imaging with an infectivity-enhanced adenovirus. J Natl Cancer Inst. 2002;94:741–749. doi: 10.1093/jnci/94.10.741. doi:10.1093/jnci/94.10.741. [DOI] [PubMed] [Google Scholar]
- Honda T, Kagawa S, Spurgers KB, Gjertsen BT, Roth JA, Fang B, Lowe SL, Norris JS, Meyn RE, McDonnell TJ. A recombinant adenovirus expressing wild-type Bax induces apoptosis in prostate cancer cells independently of their Bcl-2 status and androgen sensitivity. Cancer Biol Ther. 2002;1:163–167. doi: 10.4161/cbt.63. [DOI] [PubMed] [Google Scholar]
- Huh WK, Gomez-Navarro J, Arafat WO, Xiang J, Mahasreshti PJ, Alvarez RD, Barnes MN, Curiel DT. Bax-induced apoptosis as a novel gene therapy approach for carcinoma of the cervix. Gynecol Oncol. 2001;83:370–377. doi: 10.1006/gyno.2001.6403. doi:10.1006/gyno.2001.6403. [DOI] [PubMed] [Google Scholar]
- Kagawa S, Gu J, Swisher SG, Ji L, Roth JA, Lai D, Stephens LC, Fang B. Antitumor effect of adenovirus-mediated Bax gene transfer on p53-sensitive and p53-resistant cancer lines. Cancer Res. 2000;60:1157–1161. [PubMed] [Google Scholar]
- Kasono K, Blackwell JL, Douglas JT, Dmitriev I, Strong TV, Reynolds P, Kropf DA, Carroll WR, Peters GE, Bucy RP, et al. Selective gene delivery to head and neck cancer cells via an integrin targeted adenoviral vector. Clin Cancer Res. 1999;5:2571–2579. [PubMed] [Google Scholar]
- Ketola A, Määttä AM, Pasanen T, Tulimäki K, Wahlfors J. Osteosarcoma and chondrosarcoma as targets for virus vectors and herpes simplex virus thymidine kinase/ganciclovir gene therapy. Int J Mol Med. 2004;13:705–710. [PubMed] [Google Scholar]
- Kim KH, Dmitriev I, O'Malley JP, Wang M, Saddekni S, You Z, Preuss MA, Harris RD, Aurigemma R, Siegal GP, et al. A phase I clinical trial of Ad5.SSTR/TK.RGD, a novel infectivity-enhanced bicistronic adenovirus, in patients with recurrent gynecologic cancer. Clin Cancer Res. 2012;18:3440–3451. doi: 10.1158/1078-0432.CCR-11-2852. doi:10.1158/1078-0432.CCR-11-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball KJ, Preuss MA, Barnes MN, Wang M, Siegal GP, Wan W, Kuo H, Saddekni S, Stockard CR, Grizzle WE, et al. A phase I study of a tropism-modified conditionally replicative adenovirus for recurrent malignant gynecologic diseases. Clin Cancer Res. 2010;16:5277–5287. doi: 10.1158/1078-0432.CCR-10-0791. doi:10.1158/1078-0432.CCR-10-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsmeyer SJ. BCL-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992;80:879–886. [PubMed] [Google Scholar]
- Li X, Marani M, Yu J, Nan B, Roth JA, Kagawa S, Fang B, Denner L, Marcelli M. Adenovirus-mediated Bax overexpression for the induction of therapeutic apoptosis in prostate cancer. Cancer Res. 2001;61:186–191. [PubMed] [Google Scholar]
- Matsuo H, Maruo T, Samoto T. Increased expression of Bcl-2 protein in human uterine leiomyoma and its up-regulation by progesterone. J Clin Endocrinol Metab. 1997;82:293–299. doi: 10.1210/jcem.82.1.3650. doi:10.1210/jc.82.1.293. [DOI] [PubMed] [Google Scholar]
- Misao J, Hayakawa Y, Ohno M, Kato S, Fujiwara T, Fujiwara H. Expression of bcl-2 protein, an inhibitor of apoptosis, and Bax, an accelerator of apoptosis, in ventricular myocytes of human hearts With myocardial infarction. Circulation. 1996;94:1506–1512. doi: 10.1161/01.cir.94.7.1506. doi:10.1161/01.CIR.94.7.1506. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Both GW, Banizs B, Tsuruta Y, Yamamoto S, Kawakami Y, Douglas JT, Tani K, Curiel DT, Glasgow JN. An adenovirus serotype 5 vector with fibers derived from ovine atadenovirus demonstrates CAR-independent tropism and unique biodistribution in mice. Virology. 2006;350:103–115. doi: 10.1016/j.virol.2006.01.037. doi:10.1016/j.virol.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Pasqualini R, Koivunen E, Ruoslahti E. Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol. 1997;15:542–546. doi: 10.1038/nbt0697-542. doi:10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- Reed JC. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. doi:10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid R, Mar EC, Huang ES, Topal MD. Insertion and extension of acyclic, dideoxy and Ara nucleotides by herpesviridae, human alpha and human beta polymerase; a unique inhibition mechanism for 9-(1, 3-dihydroxy-2-propoxymethyl) guanine triphosphate. J Biol Chem. 1988;263:3898–3904. [PubMed] [Google Scholar]
- Robe PA, Princen F, Martin D, Malgrange B, Stevenaert A, Moonen G, Gielen J, Merville MP, Bours V. Pharmacological modulation of the bystander effect in the herpes simplex virus thymidine kinase/ganciclovir gene therapy system; effects of dibutyryl adenosine 3′, 5′-cyclic monophosphate, alpha-glycrrhetinic acid, and cystosine arabinoside. Biochem Pharmacol. 2000;60:241–249. doi: 10.1016/s0006-2952(00)00315-4. doi:10.1016/S0006-2952(00)00315-4. [DOI] [PubMed] [Google Scholar]
- Salama SA, Kamel M, Christman G, Wang HQ, Fouad HM, Al-Hendy A. Gene therapy of uterine leiomyoma: adenovirus-mediated herpes simplex virus thymidine kinase/ganciclovir treatment inhibits growth of human and rat leiomyoma cells in vitro and in a nude mouse model. Gynecol Obstet Invest. 2007;63:61–70. doi: 10.1159/000095627. doi:10.1159/000095627. [DOI] [PubMed] [Google Scholar]
- Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. doi:10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- Tasciotti E, Zoppe M, Giacca M. Transcellular transfer of active HSV-1 thymidine kinase mediated by an 11-amino-acid peptide from HIV-1 TAT. Cancer Gene Ther. 2003;10:64–74. doi: 10.1038/sj.cgt.7700526. doi:10.1038/sj.cgt.7700526. [DOI] [PubMed] [Google Scholar]
- Tsibris JC, Segars J, Coppola D, Mane S, Wilbanks GD, O'Brien WF, Spellacy WN. Insights from gene arrays on the development and growth regulation of uterine leiomyomata. Fertil Steril. 2002;78:114–121. doi: 10.1016/s0015-0282(02)03191-6. doi:10.1016/S0015-0282(02)03191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemitsu Y, Kagawa S, Tokunaga N, Otani S, Umeoka T, Roth JA, Fang B, Tanaka N, Fujiwara T. Molecular therapy for peritoneal dissemination of xenotransplanted human MKN-45 gastric cancer cells with adenovirus mediated Bax gene transfer. Gut. 2004;53:554–560. doi: 10.1136/gut.2003.021683. doi:10.1136/gut.2003.021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkwaak TJ, Wang M, Gómez-Navarro J, Rancourt C, Dmitriev I, Krasnykh V, Barnes M, Siegal GP, Alvarez R, Curiel DT. An advanced generation of adenoviral vectors selectively enhances gene transfer for ovarian cancer gene therapy approaches. Gynecol Oncol. 1999;74:227–234. doi: 10.1006/gyno.1999.5432. doi:10.1006/gyno.1999.5432. [DOI] [PubMed] [Google Scholar]
- Volk AL, Rivera AA, Kanerva A, Bauerschmitz G, Dmitriev I, Nettelbeck DM, Curiel DT. Enhanced adenovirus infection of melanoma cells by fiber-modification: incorporation of RGD peptide or Ad5/3 chimerism. Cancer Biol Ther. 2003;2:511–515. doi: 10.4161/cbt.2.5.440. [DOI] [PubMed] [Google Scholar]
- Walker CL, Burroughs KD, Davis B, Sowell K, Everitt JI, Fuchs-Young R. Preclinical evidence for therapeutic efficacy of selective estrogen receptor modulators for uterine leiomyoma. J Soc Gynecol Investig. 2000;7:249–256. doi:10.1016/S1071-5576(00)00056-3. [PubMed] [Google Scholar]
- Wu H, Han T, Lam JT, Leath CA, Dmitriev I, Kashentseva E, Barnes MN, Alvarez RD, Curiel DT. Preclinical evaluation of a class of infectivity-enhanced adenoviral vectors in ovarian cancer gene therapy. Gene Ther. 2004;11:874–878. doi: 10.1038/sj.gt.3302249. doi:10.1038/sj.gt.3302249. [DOI] [PubMed] [Google Scholar]
- Xiang J, Gómez-Navarro J, Arafat W, Liu B, Barker SD, Alvarez RD, Siegal GP, Curiel DT. Pro-apoptotic treatment with an adenovirus encoding Bax enhances the effect of chemotherapy in ovarian cancer. J Gene Med. 2000;2:97–106. doi: 10.1002/(SICI)1521-2254(200003/04)2:2<97::AID-JGM99>3.0.CO;2-S. doi:10.1002/(SICI)1521-2254(200003/04)2:2<97::AID-JGM99>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. doi:10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]