Abstract
Objective
This article addresses considerations for using a posterior (popliteal) instead of anterior (para-patellar) approach for experimental insult to the rabbit knee medial femoral condyle (MFC) surface in vivo. The posterior approach is particularly advantageous when intending to address the pathomechanisms of OA associated with habitual cartilage loading, or the efficacy of a cartilage repair method, in a clinically relevant experimental setting.
Design
Studies using anterior versus posterior approaches for such purposes in survival rabbit models of the MFC articular surface insults were systematically surveyed. The anterior-posterior span of the primary weight-bearing region of that surface was demonstrated cadaverically.
Results
Of a total of 31 papers identified in 2007-2012, an anterior approach was utilized in 28 studies (> 90%). More than half (17/28) explicitly regarded the cranial half (inferior aspect) of the MFC surface as being a “weight-bearing” region. The insult site through anterior approach (identified in figures) was located in the cranial half region in all cases. Cadaverically, however, the center of habitual tibio-femoral contact locations on the MFC surface was located in the caudal half region (posterior aspect) of the MFC surface. The majority of the habitual contact region was accessible only by a posterior surgical approach.
Conclusion
For the above-noted purposes, use of a posterior (popliteal) approach, rather than an anterior approach, is highly recommended.
Keywords: animal models, rabbit knee, medial femoral condyle, cartilage, weight-bearing region
Introduction
The rabbit knee is one of the most commonly utilized joints in survival animal studies of osteoarthritis (OA) and cartilage repair1. The relatively large physical size of the rabbit knee is well suited for creating chondral or osteochondral defects or for acute cartilage injury (blunt impactions). The articular surface of the medial femoral condyle (MFC) is often chosen as a site of interest, because it is presumed to be a high weight-bearing surface. Given that osteochondral lesions in corresponding regions in the human knee present a substantial challenge for cartilage repair2-4, testing new treatment methods in a clinically relevant experimental setting in vivo is crucial for obtaining valid pre-clinical information.
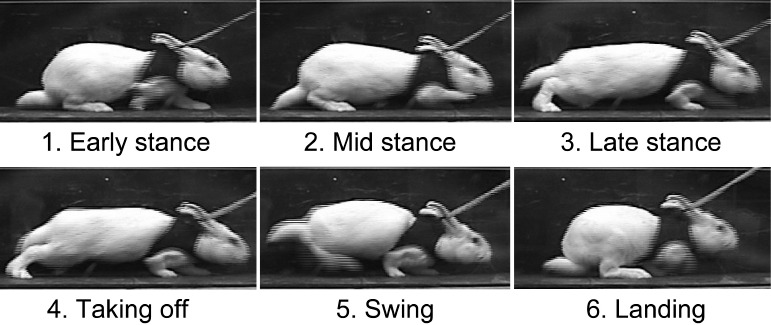
Among the many survival rabbit model studies in the literature, anterior (typically medial para-patellar) approaches are commonly utilized to apply experimental insults to the MFC surface. From a functional and anatomical perspective, however, it is questionable whether or not the habitual weight-bearing region in this surface is truly accessible anteriorly. The rabbit’s habitual posture is one of squatting, in which the knees are deeply flexed. Even during hopping (Figure 1), the rabbit knee appears to remain flexed throughout the majority of the motion event. Based on this consideration, Hurtig et al. reasoned that the posterior region of the rabbit knee MFC surface was responsible for habitual weight-bearing, leading them to adopt a posterior (popliteal) approach to create osteochondral defects there. Nevertheless, most investigators before and since have used an anterior approach for such purposes. There appears to be insufficient awareness about the relatively predominant posterior location of weight-bearing in this species, and about accessibility of this location when using an anterior surgical approach. The purpose of the present article is to draw attention to this issue, which will improve the human clinical relevance of rabbit knee models addressing the pathogenesis of OA, and hopefully improve the efficacy of treatment methods in high weight-bearing articular surfaces.
Figure 1. Successive instances during rabbit hopping. Note that the knee is kept in flexion except for a very short duration at the instant of “taking off.”.
Methods
Literature Survey
A systematic survey was performed of the English language literature regarding survival rabbit model studies, in which articular surface defects or blunt impaction cartilage injuries were created in the MFC surface. The PubMed database from 2007 was searched using a combination of keywords [“rabbit” AND “knee” AND “cartilage” AND (“defect” OR “impact”)]. Next, the searched articles were screened by the journal’s impact factor (1.0 or higher). These candidates (a total of 110 papers) were subjected to quick review of the abstract (or main text as needed) to select the papers that satisfied the above-noted (underlined) criteria. Finally, these selected papers were carefully reviewed to assess the methodological details. The points of interest included: 1) research interest, 2) insult modality, 3) surgical approach, 4) statement that regarded the insult site as a weight-bearing region, and 5) the insult site location identified in figures.
Cadaver Demonstration
The anterior-posterior span of the primary weightbearing region in the rabbit knee MFC surface was explored in a normal whole-leg cadaver specimen harvested from an adult New Zealand While rabbit. The specimen was dissected free from soft tissue from the femoral head through the ankle, except for the major passive knee stabilizing structures (including the cruciate and collateral ligaments and the menisci). The femur was secured horizontally to a plastic-foam baseplate using two perpendicular Kirshner wires (diameter: 1.6 mm), one proximally through the femoral head, and the other distally across the medial and lateral distal femoral epi-condyles. Another perpendicular K-wire was placed at the ankle (through the talar dome), so that the leg could be stabilized at predetermined knee flexion positions. The K-wire insertion points on the baseplate were regarded as reference points for the sagittal-plane positions of the hip, knee, and ankle, respectively. The angle between the hip-knee and knee-ankle axes (Figure 2A) was measured using an analog goniometer, and then defined as the knee flexion angle (where 0° indicated full extension).
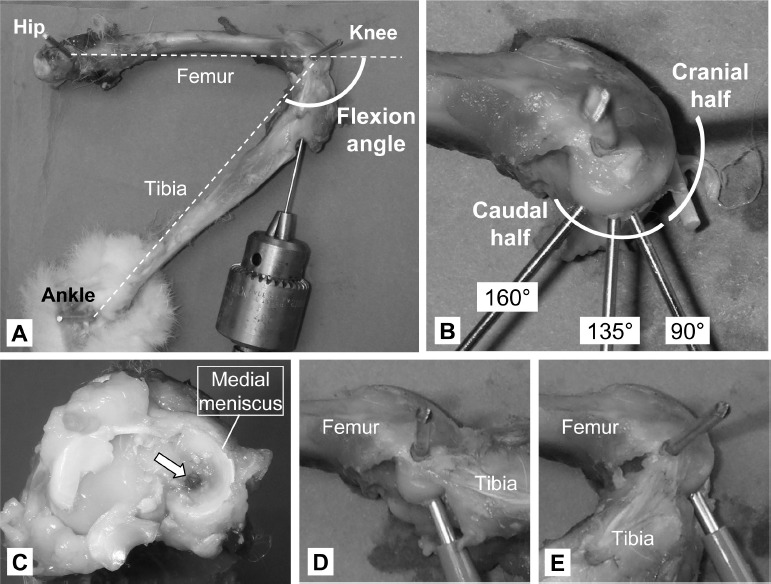
Figure 2. A) Definition of knee flexion angle in the present cadaver demonstration setting. B) Mediolateral view of the rabbit medial femoral condyle, on which the center-of-contact locations at 160°, 135°, and at 90° of knee flexion were indicated using metallic pins. c) superior view of the proximal tibia after disarticulation. The opening of the tibial tunnel (white arrow) is accurately positioned at the center of the medial tibial plateau (at which articular cartilage is uncovered by the meniscus). D) Anterior limit of posterior perpendicular access. E) Posterior limit of posterior perpendicular access.
Information regarding the physiologic range of motion of the rabbit knee was searched in the literature. Beloozerova et al.5 reported that the rabbit knee’s flexion angle at rest ranged from 90° to 120°, while Mansour et al.6 reported that the range of motion during hopping was from 120° to 160°. (Note: In the original reference papers, knee flexion positions were expressed as “extension angles,” where 0° indicated full flexion. Therefore, the above-noted ranges were reported as 60° to 90° and 20° to 60°, respectively.) Accordingly, flexion angles of 90°, 135°, and 160° were selected as representative habitual weight-bearing positions for the rabbit knee.
For each of these three positions, the center of the tibio-femoral contact location in the MFC was marked using a 1.25-mm K-wires, which was pierced in a retrograde fashion through the proximal tibia (Figure 2A). For accurate pin positioning, a double trocar-tipped K-wire was pierced from proximal to distal into the center of the medial plateau (at which the tibial surface was uncovered by the meniscus, Figure 2C) while visualizing the insertion point by internally rotating the tibia. In addition, perpendicular access to the medial femoral condyle surface, both anteriorly and posteriorly to the proximal tibia, was simulated using a 2-mm dermal biopsy punch (Figures 2D and 2E), and medial-to-lateral digital photographs were taken at the most posterior position accessible from anteriorly and the most anterior position accessible from posteriorly. The joint was then disarticulated by transecting all knee ligaments, and the tibia was removed. Finally, the anterior-posterior distribution of the three above-noted bone holes (marked by inserting K-wires) was recorded, again by means of digital photographs (Figure 2B).
Results
In the literature review (Table 1), a total of 31 papers7-37 were found to meet the above-noted criteria. Of these, the vast majority (28 of 31, > 90%)7-11,13,15-28,30-37 utilized anterior approaches to access the MFC surface. More than half (17 of 28) of these anterior approach studies7,8,10,11,16-19,21,23,25,27,32-34,36,37 regarded the site of the experimental insult as being in the weight-bearing region. The insult site through anterior approach (when identified in figures) was located in the cranial half region of the MFC surface (Figure 2B) in all cases7-11,13,15-24,26-28,30,32-36. Posterior approaches were utilized in only three studies12,14,29, one of which was from our group.
In the cadaver demonstration, the tibio-femoral contact locations on the MFC surface at the representative habitual weight-bearing knee flexion positions (indicated by the pins in Figure 2B) were distributed only within the caudal half region. When using a dermal punch to access the MFC surface from posteriorly to the tibia (Figure 2D), perpendicular apposition was feasible across almost the entire habitual contact region. By contrast, using anterior access, the dermal punch could only reach the boundary between the cranial and caudal halves (Figure 2E).
Discussion
The literature review documents that the cranial half region (inferior aspect) of the rabbit MFC surface has most commonly been regarded as a high weight-bearing region. However, cadaverically, it is evident that the primary (habitual) weight-bearing region lies mostly within the condyle’s caudal half region (posterior aspect). Presumably, investigators tend to regard the cranial half region as the “primary weight-bearing” because this is the corresponding region in the human knee MFC during bipedal gait. However, given the substantial difference in posture during gait, it is reasonable that the tibio-femoral contact characteristics in the rabbit knee are very different from those in the human knee. It is also evident that the center of contact in the rabbit MFC surface is accessible only from posteriorly. These facts need to be recognized when designing studies that involve perpendicular-access survival insults to weight-bearing cartilage in the rabbit knee. And, these same factors should be considered when interpreting results from studies which used anterior access.
The above cadaveric demonstration is intended only for illustrative purposes, rather than to provide formal quantitative information as to whether or not a specific insult technique would permit reproducible experimental insult to the primary weight-bearing region of the rabbit MFC surface. However, it is obvious that the posterior approach provides better accessibility to the center of primary weight-bearing region14,38, while leaving all major joint stabilizing structures (including the extensor mechanisms) uninjured. For future survival rabbit knee model studies that involve surgical insult to the MFC surface to study the pathomechanisms of OA associated with habitual cartilage loading, or to address the efficacy of cartilage repair methods in a clinically relevant experimental setting, using a posterior approach rather than an anterior approach is highly recommended.
Acknowledgments
This research was supported by NIH Grant P50 AR055533, and by US Department of Defense CDM-RP-PRORP Technology Development Award W81X-WH-10-1-0864.
References
- 1.Oegema TR, Visco D. Animal models of osteoarthritis. In: An YH, Friedman RJ., editors. Animal Models in Orthopaedic Research. CRC Press; 1999. pp. 349–67. [Google Scholar]
- 2.Gomoll AH, Farr J, Gillogly SD, Kercher J, Minas T. Surgical management of articular cartilage defects of the knee. J Bone Joint Surg Am. 2010;92:2470–90. [PubMed] [Google Scholar]
- 3.Bedi A, Feeley BT, Williams RJ. Management of articular cartilage defects of the knee. J Bone Joint Surg Am. (3rd.) 2010;92:994–1009. doi: 10.2106/JBJS.I.00895. [DOI] [PubMed] [Google Scholar]
- 4.Cole BJ, Pascual-Garrido C, Grumet RC. Surgical management of articular cartilage defects in the knee. J Bone Joint Surg Am. 2009;91:1778–90. [PubMed] [Google Scholar]
- 5.Beloozerova In, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG. Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol. 2003;90:3783–93. doi: 10.1152/jn.00590.2003. [DOI] [PubMed] [Google Scholar]
- 6.Mansour JM, Wentorf FA, Degoede KM. In vivo kinematics of the rabbit knee in unstable models of osteoarthrosis. Ann Biomed Eng. 1998;26:353–60. doi: 10.1114/1.133. [DOI] [PubMed] [Google Scholar]
- 7.Tay LX, Ahmad RE, Dashtdar H, Tay KW, Mas-juddin T, Ab-Rahim S, et al. Treatment outcomes of alginate-embedded allogenic mesenchymal stem cells versus autologous chondrocytes for the repair of focal articular cartilage defects in a rabbit model. Am J Sports Med. 2012;40:83–90. doi: 10.1177/0363546511420819. [DOI] [PubMed] [Google Scholar]
- 8.Lyon R, Liu XC, Kubin M, Schwab J. Does ex-tracorporeal shock wave therapy enhance healing of osteochondritis dissecans of the rabbit knee?: A pilot study. Clin Orthop Relat Res. 2012 doi: 10.1007/s11999-012-2410-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heir S, Aroen A, Loken S, Holme I, Engebret-sen L, Reinholt FP. Cartilage repair in the rabbit knee: mosaic plasty resulted in higher degree of tissue filling but affected subchondral bone more than microfracture technique: a blinded, randomized, controlled, long-term follow-up trial in 88 knees. Knee Surg Sports Traumatol Arthrosc. 2012;20:197–209. doi: 10.1007/s00167-011-1596-8. [DOI] [PubMed] [Google Scholar]
- 10.Dormer NH, Singh M, Zhao L, Mohan N, Berk-land CJ, Detamore MS. Osteochondral interface regeneration of the rabbit knee with macroscopic gradients of bioactive signals. J Biomed Mater Res A. 2012;100:162–70. doi: 10.1002/jbm.a.33225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang NJ, Lin CC, Li CF, Wang DA, Issariyaku N, Yeh ML. The combined effects of continuous passive motion treatment and acellular PLGA implants on osteochondral regeneration in the rabbit. Biomaterials. 2012;33:3153–63. doi: 10.1016/j.biomaterials.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 12.Brophy RH, Martinez M, Borrelli J,Jr., Silva MJ. Effect of combined traumatic impact and radial transection of medial meniscus on knee articular cartilage in a rabbit in vivo model. Arthroscopy. 2012;28:1490–6. doi: 10.1016/j.arthro.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yokota M, Yasuda K, Kitamura N, Arakaki K, Onodera S, Kurokawa T, et al. Spontaneous hyaline cartilage regeneration can be induced in an osteochondral defect created in the femoral condyle using a novel double-network hydrogel. BMC Mus-culoskelet Disord. 2011;12:49. doi: 10.1186/1471-2474-12-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaseenon T, Tochigi Y, Heiner AD, Goetz JE, Baer TE, Fredericks DC, et al. Organ-level histological and biomechanical responses from localized osteoarticular injury in the rabbit knee. J Orthop Res. 2011;29:340–6. doi: 10.1002/jor.21259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue Eng Part A. 2011;17:2845–55. doi: 10.1089/ten.tea.2011.0135. [DOI] [PubMed] [Google Scholar]
- 16.Kamarul T, Ab-Rahim S, Tumin M, Selvaratnam L, Ahmad TS. A preliminary study of the effects of glucosamine sulphate and chondroitin sulphate on surgically treated and untreated focal cartilage damage. Eur Cell Mater. 2011;21:259–71. doi: 10.22203/ecm.v021a20. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- 17.Jaberi FM, Keshtgar S, Tavakkoli A, Pishva E, Geramizadeh B, Tanideh N, et al. A moderate-intensity static magnetic field enhances repair of cartilage damage in rabbits. Arch Med Res. 2011;42:268–73. doi: 10.1016/j.arcmed.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Dashtdar H, Rothan HA, Tay T, Ahmad RE, Ali R, Tay LX, et al. A preliminary study comparing the use of allogenic chondrogenic pre-differentiated and undifferentiated mesenchymal stem cells for the repair of full thickness articular cartilage defects in rabbits. J Orthop Res. 2011;29:1336–42. doi: 10.1002/jor.21413. [DOI] [PubMed] [Google Scholar]
- 19.Chen H, Yang X, Liao Y, Zeng X, Liang P, Kang N, et al. MRI and histologic analysis of collagen type II sponge on repairing the cartilage defects of rabbit knee joints. J Biomed Mater Res B Appl Biomater. 2011;96:267–75. doi: 10.1002/jbm.b.31762. [DOI] [PubMed] [Google Scholar]
- 20.Aulin C, Bergman K, Jensen-Waern M, Heden-qvist P, Hilborn J, Engstrand T. In situ cross-linkable hyaluronan hydrogel enhances chondrogenesis. J Tissue Eng Regen Med. 2011;5:e188–96. doi: 10.1002/term.415. [DOI] [PubMed] [Google Scholar]
- 21.Xie J, Han Z, Naito M, Maeyama A, Kim SH, Kim YH, et al. Articular cartilage tissue engineering based on a mechano-active scaffold made of poly(L-lactide-co-epsilon-caprolactone): In vivo performance in adult rabbits. J Biomed Mater Res B Appl Biomater. 2010;94:80–8. doi: 10.1002/jbm.b.31627. [DOI] [PubMed] [Google Scholar]
- 22.Pei M, Yan Z, Shoukry M, Boyce BM. Failure of xenoimplantation using porcine synovium-derived stem cell-based cartilage tissue constructs for the repair of rabbit osteochondral defects. J Orthop Res. 2010;28:1064–70. doi: 10.1002/jor.21096. [DOI] [PubMed] [Google Scholar]
- 23.Mrosek EH, Schagemann JC, Chung HW, Fitzsimmons JS, Yaszemski MJ, Mardones RM, et al. Porous tantalum and poly-epsilon-caprolactone biocomposites for osteochondral defect repair: preliminary studies in rabbits. J Orthop Res. 2010;28:141–8. doi: 10.1002/jor.20983. [DOI] [PubMed] [Google Scholar]
- 24.Heir S, Aroen A, Loken S, Sulheim S, Engebret-sen L, Reinholt FP. Intraarticular location predicts cartilage filling and subchondral bone changes in a chondral defect. Acta Orthop. 2010;81:619–27. doi: 10.3109/17453674.2010.524593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss E, Schachter A, Frenkel S, Rosen J. The efficacy of intra-articular hyaluronan injection after the microfracture technique for the treatment of articular cartilage lesions. Am J Sports Med. 2009;37:720–6. doi: 10.1177/0363546508328415. [DOI] [PubMed] [Google Scholar]
- 26.Pei M, He F, Boyce BM, Kish VL. Repair of fullthickness femoral condyle cartilage defects using allogeneic synovial cell-engineered tissue constructs. Osteoarthritis Cartilage. 2009;17:714–22. doi: 10.1016/j.joca.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Ozsoy MH, Aydogdu S, Taskiran D, Sezak M, Hayran M, Oztop F, et al. The effects of early or late treatment of osteochondral defects on joint ho-moeostasis: an experimental study in rabbits. Knee Surg Sports Traumatol Arthrosc. 2009;17:578–89. doi: 10.1007/s00167-008-0675-y. [DOI] [PubMed] [Google Scholar]
- 28.Custers RJ, Creemers LB, van Rijen MH, Ver-bout AJ, Saris DB, Dhert WJ. Cartilage damage caused by metal implants applied for the treatment of established localized cartilage defects in a rabbit model. J Orthop Res. 2009;27:84–90. doi: 10.1002/jor.20709. [DOI] [PubMed] [Google Scholar]
- 29.Borrelli J,Jr., Silva MJ, Zaegel MA, Franz C, Sandell LJ. Single high-energy impact load causes posttraumatic OA in young rabbits via a decrease in cellular metabolism. J Orthop Res. 2009;27:347–52. doi: 10.1002/jor.20760. [DOI] [PubMed] [Google Scholar]
- 30.Loken S, Jakobsen RB, Aroen A, Heir S, Shah-dadfar A, Brinchmann JE, et al. Bone marrow mesenchymal stem cells in a hyaluronan scaffold for treatment of an osteochondral defect in a rabbit model. Knee Surg Sports Traumatol Arthrosc. 2008;16:896–903. doi: 10.1007/s00167-008-0566-2. [DOI] [PubMed] [Google Scholar]
- 31.Lin L, Zhou C, Wei X, Hou Y, Zhao L, Fu X, et al. Articular cartilage repair using dedifferentiated articular chondrocytes and bone morphogenetic protein 4 in a rabbit model of articular cartilage defects. Arthritis Rheum. 2008;58:1067–75. doi: 10.1002/art.23380. [DOI] [PubMed] [Google Scholar]
- 32.Leone G, Fini M, Torricelli P, Giardino R, Bar-bucci R. An amidated carboxymethylcellulose hydrogel for cartilage regeneration. J Mater Sci Mater Med. 2008;19:2873–80. doi: 10.1007/s10856-008-3412-7. [DOI] [PubMed] [Google Scholar]
- 33.Jansen EJ, Emans PJ, Van Rhijn LW, Bulstra SK, Kuijer R. Development of partial-thickness articular cartilage injury in a rabbit model. Clin Orthop Relat Res. 2008;466:487–94. doi: 10.1007/s11999-007-0050-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jansen EJ, Emans PJ, Douw CM, Guldemond NA, Van Rhijn Lw, Bulstra SK, et al. One intra-articular injection of hyaluronan prevents cell death and improves cell metabolism in a model of injured articular cartilage in the rabbit. J Orthop Res. 2008;26:624–30. doi: 10.1002/jor.20569. [DOI] [PubMed] [Google Scholar]
- 35.garcia-Alvarez F, Castiella T, Guallar E, Grasa JM, Gomez-Barrena E, Lacleriga A. Influence of platelet time activation on articular cartilage growth in the rabbit knee: preliminary study. Knee. 2008;15:314–7. doi: 10.1016/j.knee.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 36.custers RJ, Dhert WJ, Van Rijen MH, Verbout AJ, creemers Lb, Saris DB. Articular damage caused by metal plugs in a rabbit model for treatment of localized cartilage defects. Osteoarthritis Cartilage. 2007;15:937–45. doi: 10.1016/j.joca.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Costouros JG, Kim HT. Preventing chondrocyte programmed cell death caused by iatrogenic injury. Knee. 2007;14:107–11. doi: 10.1016/j.knee.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Borrelli J,Jr., Burns ME, Ricci WM, Silva MJ. A method for delivering variable impact stresses to the articular cartilage of rabbit knees. J Orthop Trauma. 2002;16:182–8. doi: 10.1097/00005131-200203000-00008. [DOI] [PubMed] [Google Scholar]


