Abstract
Background
The medial patellofemoral ligament (MPFL) is the most frequently injured soft tissue structure following acute lateral patellar dislocation. MPFL reconstruction has become a popular option to restore patellar stability following lateral patellar dislocation due to the high incidence of recurrent instability following conservative management. Anatomic reconstruction of the MPFL minimizes graft length changes during full knee range of motion and restores patellar stability.
Materials & Methods
Four fresh frozen cadaver specimens underwent biomechanical testing in a materials testing machine. With the knee fixed in 30° of flexion, the patella was translated laterally a distance of 10 mm and continuous force-displace- ment data was collected with the intact MPFL and again following a newly described MPFL reconstruction technique. Lateral force-displacement and stiffness data were calculated, allowing comparison between the intact and reconstructed MPFL.
Results
The average lateral restraining force provided by the intact MPFL was 10.6 ± 5.7, 36.6 ± 2.7, and 69.0 ± 5.9 N while the lateral restraining force following MPFL reconstruction was 0.4 ± 4.3, 50.3 ± 16.3, and 110.2 ± 17.5 N at 1, 5, and 10 mm of lateral displacement, respectively.
Conclusion
Anatomic MPFL reconstruction displays similar lateral restraining force compared to the intact MPFL at low levels of lateral displacement. At higher levels of displacement, the reconstructed MPFL provides increased lateral restraining force compared to the intact MPFL, improving patellar stability in pathologic knees.
Introduction
Bony anatomy, soft tissue restraints, and the dynamic action of the quadriceps all play a role in maintaining patellar stability throughout knee motion. The medial patellofemoral ligament (MPFL) serves as the primary soft tissue restraint to lateral patellar displacement during low degrees of flexion when the patella has yet to engage the femoral trochlea1-4. Following acute lateral patellar dislocation, the MPFL is the most frequently injured soft tissue structure, disrupting one of the key components required to maintain patellar stability5,6. Acute lateral patellar dislocations occur most frequently in the second decade, with recurrent episodes of instability reported in 15-44% of patients treated nonopera- tively7,8. Due to the high rate of recurrent episodes of instability following conservative management of acute lateral patellar dislocation, numerous bony and soft tissue procedures have been described to restore patellar stability including MPFL repair and reconstruction9-12.
Disruption of the MPFL has been identified as an essential lesion following acute lateral patellar dislocation, occurring in up to 96% of individuals following the injury13. Early MPFL repair fails to achieve the same load to failure characteristics as the native MPFL14, and clinical studies have not shown a difference between early MPFL repair and conservative management of acute lateral patellar dislocations15-17. MPFL reconstruction aims to restore the form and function of the native MPFL, making it a popular option following acute injury. In vivo, the native MPFL originates between the medial epicondyle and adductor tubercle of the femur and courses extracapsularly just distal to the vastus medialis obliquus (VMO) before inserting on the proximal one- third of the patella5,9,18-21. The MPFL plays an important role in guiding the patella into the femoral trochlea during the first 20-30° of knee flexion by providing a passive checkrein to lateral patellar translation before the bony architecture of the femoral trochlea directs patellar motion in higher degrees of flexion9,12.
During MPFL reconstruction, numerous biomechanical studies have noted the importance of anatomic graft placement, particularly on the femoral side, in order to minimize graft length changes throughout full knee range of motion5,21-25. Failure to achieve anatomic graft fixation may over-constrain the patella and lead to premature osteoarthritis due to increased medial patellofemoral contact pressures or result in recurrent instability due to graft failure23,25. Additionally, excessive tensioning with more robust auto- and allograft materials has been shown to alter normal patellar motion26,27. In order to replicate the actions of the native MPFL while avoiding over-constraint, tensioning with as little as 2 N of force has been suggested26,27.
While the role the native MPFL plays in maintaining patellar stability has been extensively investigated, relatively few studies have investigated patellar stability following anatomic MPFL reconstruction. The purpose of this study is to describe our preferred anatomic MPFL reconstruction technique, while also comparing patellar stability following anatomic MPFL reconstruction with patellar stability in the native knee using a cadaver model. While popular autograft and allograft tendon options undoubtedly display different biomechanical properties compared to the native MPFL11,12,14,28,29, we believe that anatomic reconstruction of the MPFL can restore patellar stability by recreating the checkrein function of the native MPFL.
Materials and Methods
Specimens and Specimen Preparation
Four fresh frozen cadaver specimens from two subjects aged 57 and 95 years (mean 76 years) were obtained for this study. The specimens included 20 cm of femur and 15 cm of tibia as well as overlying soft tissue structures. Visual inspection of all specimens failed to reveal evidence of prior surgery. Specimens were stored at -20° C prior to thawing at room temperature for a period of 24 hours. Magnetic resonance images (3.0-T MRI, Siemens Medical Solutions, Erlangen, Germany) were acquired for all specimens, and all investigators independently reviewed the images to confirm the continuity of the MPFL prior to testing. Preparation of the specimens included careful removal of the fibula and all soft tissue structures with the exception of the medial patellofemoral ligament and distal quadriceps extensor mechanism. Prior to testing, the MPFL was isolated from the surrounding medial retinacular structures which were subsequently excised. The distal quadriceps was then separated into three components including the vastus lateralis (VL), vastus medialis (VM), and combined rectus femoris/vastus intermedius (RF/VI) muscle bellies. Cloth strips were looped around the free ends of the isolated quadriceps muscle bellies and attached using sutures in standard Krakow fashion through the muscle bulk in order to allow loading of the quadriceps extensor mechanism through a pulley mechanism as described by Farahmand et al30. The ends of the tibia and femur were potted in polymer resin (BondoTM, 3M Corporation, St. Paul, MN) with wood screws placed through the femoral and tibial diaphysis to enhance interdigitation.
Experimental Setup
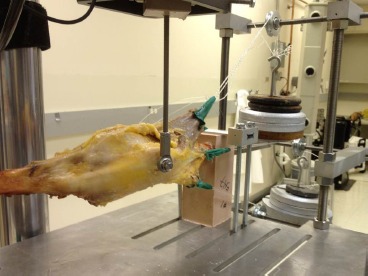
Lateral displacement of the patella was achieved using a biaxial servo-hydraulic 858 Bionix II materials testing machine (MTS Corporation, Eden Prairie, MN). All four specimens were tested with the native MPFL intact and again following MPFL reconstruction. Specimens were mounted in the materials testing machine using a custom fixture that allowed the specimens to be fixed at varying degrees of knee flexion. For the purpose of this study, all knees were mounted and tested in 30° of flexion where lateral restraining forces are lowest and optimal graft fixation is achieved18,22,30,31. The isolated quadriceps components were loaded with a total of 178 N of force taking into consideration loading direction and physiologic cross-sectional area of the muscles using previously established patellofemoral models26,30,32. Briefly, the VL, VM, and RF/VI were loaded with 35, 25, and 40% of the total force, respectively. The VL was oriented 20° lateral and 0° anterior, the VM 35° medial and 0° anterior, and the RF/VI 0° lateral and 5° anterior relative to the axis of the femoral diaphysis using a custom pulley system. The loading cell was connected to the patella using a screw and a ball joint at the end of the load cell which allowed for rotation of the patella about the anterior-posterior and proximal-distal axes. The screw was placed in the center of the patella perpendicular to the coronal plane without disrupting the underlying cartilaginous surface. The experimental setup can be seen in Figure 1. Using displacement control, the patella was cyclically translated 10 mm laterally from a neutral position at a rate of 100mm/minute30. The patella underwent four cycles of lateral displacement, with force-displacement data collected on the fourth cycle.
Figure 1. Experimental setup with the knee mounted in the Bionix II material testing machine with the quadriceps loaded using a custom pulley system through the cloth loops. The load cell is connected to the patella using a rod with a ball joint at the end to allow out of plane patellar motion. the patella was cyclically translated 10 mm while collecting continuous force and displacement data.
Description of MPFL Reconstruction
After testing of the intact MPFL, the MPFL was sectioned and an anatomic MPFL reconstruction was performed. A split tibialis anterior allograft was prepared with Krakow suturing of the terminal ends.
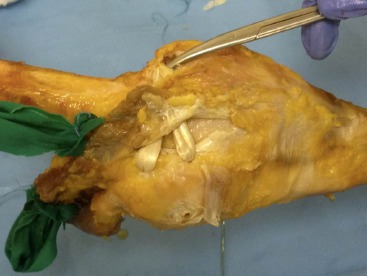
A two incision MPFL reconstruction was then performed, with the first incision centered along the medial border of the patella. The apex of the prepared tibialis anterior allograft was secured along the proximal one- third of the patella using two SutureTak® suture anchors (Arthrex, Inc., Naples, FL) in order to recreate the footprint of the MPFL on the patella. A second incision was centered over the medial femoral epicondyle. In the absence of superficial soft tissues in this cadaver study, the saddle between the medial femoral epicon- dyle and the adductor tubercle was directly visualized and palpated. This portion of the procedure is typically performed under fluoroscopic guidance as palpation and visualization of the osseous landmarks is often difficult intraoperatively33. The two free limbs of the tibialis anterior allograft were then tunneled extracapsularly between layers two and three of the medial knee. A 2.4 mm Beath pin was then placed in the previously identified saddle between the medial femoral epicondyle and adductor tubercle and driven through the femur. An 8 mm cannulated reamer was then used to create the femoral tunnel. The two free limbs of the tibialis anterior allograft were then docked into the femoral tunnel. [With the knee placed in 30° of flexion and the patella centered in the femoral trochlea, a 9 mm Bio-Tenodesis ScrewTM (Arthrex, Inc., Naples, FL) was used to secure the graft in the femoral tunnel, while ensuring that the graft was not loose nor tensioned by ensuring that the patella remained centered in the trochlear groove during gentle passive range of motion.] An example of the completed procedure can be seen in Figure 2. In all specimens, a good checkrein to lateral translation was established with less than 1 cm of lateral patellar translation with the knee in 30° of flexion.
Figure 2. Anatomic medial patellofemoral ligament reconstruction using a split tibialis anterior allograft. The graft was fixed to the superior one-third of the medial patellar border and the two free ends tunneled extracapsularly between layers two and three of the medial side of the knee before being fixed in the femur in the saddle between the medial femoral epicondyle and adductor tubercle.
Graft Positioning
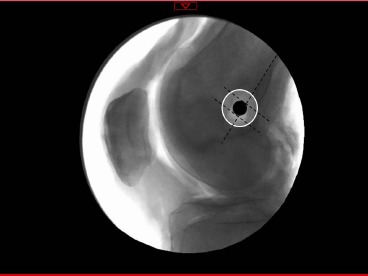
Following testing in the materials testing machine, fluoroscopy was used to obtain true lateral images of the specimens in order to confirm anatomic femoral tunnel positioning. Anatomic femoral tunnel positioning was confirmed according to the method described by Ser- vien et al., using Schottle’s point as the anatomic center of the femoral tunnel which was expanded by 7 mm to account for the diameter of the femoral tunnel20,33. The 7 mm diameter was expanded to 8 mm for this study as this is the senior author’s preferred tunnel size for MPFL reconstruction. A tunnel was considered anatomically positioned when the center of the tunnel fell within the enhanced anatomic zone as seen in Figure 3.
Figure 3. Femoral tunnel positioning using the landmarks described by Schottle et al20. The dashed lines represent the posterior cortical extension and perpendicular lines intersecting the most posterior aspect of Blumensaat’s line and tangent to the posterior femoral condyle. The black circle represents Schottle’s point, the anatomic femoral insertion of the MPFL. The white circle represents an 8 mm expansion of Schottle’s point considering reamer width as described by Servien et al.33 A tunnel was considered anatomic when the center of the tunnel fell within the 8 mm expansion.
Data Analysis
Continuous force-displacement data was collected during biomechanical testing using the software provided with the 858 Bionix II materials testing machine (MTS Corporation, Eden Prairie, MN). (Raw data was organized using Microsoft Excel 2007 (Microsoft Corporation, Redmond, WA).) The mean lateral restraining force (N) with associated standard deviations was calculated in both the intact and reconstructed specimens. The slope of the force-displacement graph was calculated to determine the stiffness (N/mm) of the patella in response to lateral force displacement for both the intact and reconstructed MPFL.
Results
The center of the MPFL femoral tunnel was anatomically placed in all four specimens according to the enhanced anatomic zone described by Servien et al. and displayed in Figure 333. The average lateral restraining force provided by the intact MPFL with the knee fixed in 30° of flexion was 10.6 ± 5.7, 36.6 ± 2.7, and 69.0 ± 5.9 N while the lateral restraining force following MPFL reconstruction was 0.4 ± 4.3, 50.3 ± 16.3, and 110.2 ± 17.5 N at 1, 5, and 10 mm of lateral displacement, respectively. The average lateral restraining force is displayed in one millimeter increments in Table 1 for both the intact and reconstructed specimens.
Table 1.
Comparison of Lateral Restraining Force in the Intact and Reconstructed MPFL
| Displacement (mm) | Lateral Restraining Force (N) | |
|---|---|---|
| MPFL Intact | MPFL Reconstruction | |
| 0 | -16.8 ± 3.6 | -28.1 ± 6.5 |
| 1 | 10.6 ± 5.7 | 0.4 ± 4.3 |
| 2 | 24.6 ± 7.1 | 16.5 ± 7.4 |
| 3 | 29.6 ± 5.4 | 28.1 ± 11.2 |
| 4 | 31.6 ± 6.7 | 40.8 ± 14.6 |
| 5 | 36.6 ± 2.7 | 50.3 ± 16.3 |
| 6 | 43.2 ± 8.7 | 61.2 ± 18.7 |
| 7 | 45.7 ± 3.6 | 72.5 ± 20.6 |
| 8 | 50.9 ± 4.3 | 83.1 ± 19.3 |
| 9 | 57.9 ± 8.1 | 94.6 ± 17.5 |
| 10 | 69.0 ± 5.9 | 110.2 ± 17.5 |
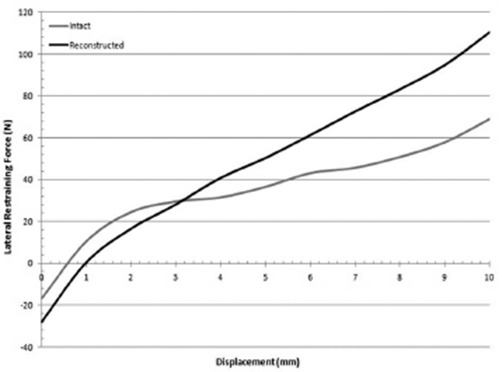
The average lateral force-displacement curves for the intact and reconstructed MPFL can be seen in Figure 4. Two linear regions were observed in both curves from 0-1.5 mm displacement and 1.5-10 mm displacement. From 0-1.5 mm of lateral displacement, the average stiffness was 24.5 ± 5.0 N/mm in the intact MPFL and 23.1 ± 4.1 N/mm following MPFL reconstruction. From 1.5-10 mm of lateral displacement, the average stiffness was 5.00 ± 1.6 N/mm in the intact MPFL and 11.2 ± 1.8 N/mm following MPFL reconstruction.
Figure 4. Force versus displacement graph for the intact and reconstructed MPFL. The slope of the lines corresponds to the stiffness of the construct.
Discussion
Anatomic reconstruction of the MPFL is important to restore patellar stability and prevent proposed complications including increased patellofemoral contact pressures and early graft failure5,21-25. Less is known about patella contact pressures, forces, and graft biomechanics following anatomic MPFL reconstruction. Additionally, other factors, including the material properties of the graft and tensioning of the graft intraoperatively, may affect patellar biomechanics. Numerous techniques and graft options have been described for MPFL reconstruc- tion12,27,33. This study used an anatomic MPFL reconstruction technique similar to the technique described by Farr and Schepsis, with the exception that the apex of the graft was placed on the patellar side, allowing the broad- based insertion of the MPFL on the proximal one-third of the patella to be recreated12,21. We typically prefer to use fluoroscopy intraoperatively to guide femoral tunnel placement, as we agree with Servien et al. that the sole use of palpation and visualization does not allow consistent anatomic tunnel placement. Interestingly, Servien et al. did not find any subjective differences between anatomic and nonanatomic femoral tunnel placement at minimum 2-year follow-up<33. Additionally, we take great care to set the appropriate graft tension by placing the knee in 30° of flexion and making sure the patella is centered in the femoral trochlea when the graft tension and length are secured26,27.
In this study, the MPFL reconstruction technique with fluoroscopic confirmation of anatomic femoral tunnel placement yielded similar biomechanical properties compared to the intact MPFL. The MPFL reconstruction produced similar lateral restraining force compared to the intact MPFL from 0-3 mm of displacement, suggesting that the reconstruction did not over constrain the patella as has been cited as a concern previously (Figure 4)26. At higher values of displacement, the MPFL reconstruction resulted in increased lateral restraining force compared to the intact MPFL. This could potentially be accounted for by the material properties of the tibialis anterior allograft used in this study, which has a load to failure strength of 1553 N and stiffness of 236 N/mm compared to the load to failure strength of 208 N and stiffness of 12 N/mm exhibited by the native MPFL12,14,28,29. Furthermore, in pathologic knees that exhibit trochlear dysplasia and vastus medialis obliquus (VMO) deficiency, which both reduce the amount of force required to displace the patella laterally34. increased lateral restraining force at higher values of displacement may be beneficial.
Our results are similar to those previously reported using intact MPFL specimens. The shape of the force- displacement curve (Figure 4) is similar to the curves presented by Fahramand et al. and Senavongse et al30,32. The average lateral restraining force of the intact MPFL in this study was 69.0 N at 10 mm of displacement, which is similar to the 75 N value reported by Senavongse et al32. The stiffness of the intact MPFL in the present study, 5.0 N/mm, was lower than the 17.6 N/mm value reported by Senavongse et al32. who tested knees in full extension as opposed to 30° of flexion in the present study, limiting comparison of these values.
Limitations of the present study include the small number of specimens, which does not allow determination of statistically significant differences between the intact MPFL and MPFL reconstruction. Additionally, the mean age of the specimens in this study, 76 years, is older than when patients typically present with acute patellar dislocation, which may affect the soft tissue properties of the specimens. The specimens in this study also failed to exhibit pathologic characteristics that often predispose to patellar instability including rotational abnormalities, VMO deficiency, and trochlear dysplasia34. Specimens in this study were only tested in 30° of flexion, which has been cited as the angle at which the MPFL is longest and is therefore optimal for fixation12,21,22. Other studies have extensively explored the way knee flexion affects lateral restraining force, with the general consensus that knees are least stable from 20-30° of flexion30,32.
Despite the assumption that anatomic reconstruction is essential to restore normal patellar motion, relatively little has been done to determine whether current reconstruction techniques actually restore the native function of the MPFL. This study describes a new technical description of MPFL reconstruction that not only restores the femoral anatomic footprint, but also restores the wide footprint of the MPFL on the proximal portion of the medial patella.
Conclusion
The MPFL reconstruction technique presented in this study produced similar patellar stability compared to the intact MPFL tested in the present study as well as previously reported in the literature. The similar lateral restraining forces between the intact MPFL and MPFL reconstruction during low levels of displacement suggests that the described technique also avoids the most frequent concerns associated with MPFL reconstruction, namely medial patellar overload and excessive tensioning of the graft leading to graft failure. While this study is limited by sample size, it provides a basis for future studies to explore MPFL reconstruction biomechanics while confirming that meticulous attention to detail with regard to graft position and tensioning can produce biomechanically satisfactory results.
References
- 1.Conlan T, Garth WP, Jr, Lemons JE. Evaluation of the medial soft-tissue restraints of the extensor mechanism of the knee. J Bone Joint Surg Am. 1993;75(5):682–693. doi: 10.2106/00004623-199305000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Desio SM, Burks RT, Bachus KN. Soft tissue restraints to lateral patellar translation in the human knee. Am J Sports Med. 1998;26(1):59–65. doi: 10.1177/03635465980260012701. [DOI] [PubMed] [Google Scholar]
- 3.Panagiotopoulos E, Strzelczyk P, Herrmann M, scuderi G. Cadaveric study on static medial patellar stabilizers: the dynamizing role of the vastus medialis obliquus on medial patellofemoral ligament. Knee Surg Sports Traumatol Arthrosc. 2006;14(1):7–12. doi: 10.1007/s00167-005-0631-z. [DOI] [PubMed] [Google Scholar]
- 4.Warren LF, Marshall JL, Girgis F. The prime static stabilizer of the medial side of the knee. J Bone Joint Surg Am. 1974;56(4):665–674. [PubMed] [Google Scholar]
- 5.Amis AA, Firer P, Mountney J, senavongse W, Thomas NP. Anatomy and biomechanics of the medial patellofemoral ligament. The Knee. 2003;10:215–220. doi: 10.1016/s0968-0160(03)00006-1. [DOI] [PubMed] [Google Scholar]
- 6.Burks RT, desio SM, Bachus KN, Tyson L, springer K. Biomechanical evaluation of lateral patellar dislocations. Am J Knee Surg. 1998;11:24–31. [PubMed] [Google Scholar]
- 7.Fithian Dc, Paxton EW, Stone ML, silva P, Davis DK, Elias Da, White LM. Epidemiology and natural history of acute patellar dislocation. Am J Sports Med. 2004;32:1114–21. doi: 10.1177/0363546503260788. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins RJ, Bell RH, Anisette G. Acute patellar dislocations: the natural history. Am J Sports Med. 1986;14:117–20. doi: 10.1177/036354658601400204. [DOI] [PubMed] [Google Scholar]
- 9.Bicos J, Fulkerson JP, Amis A. Current Concepts Review: The Medial Patellofemoral Ligament. Am J Sports Med. 2007;35:484–492. doi: 10.1177/0363546507299237. [DOI] [PubMed] [Google Scholar]
- 10.Camp CL, Krych AJ, Dahm DL, Levy BA, Stuart MJ. Medial patellofemoral ligament repair for recurrent patellar dislocation. Am J Sports Med. 2010;38:2248–2254. doi: 10.1177/0363546510376230. [DOI] [PubMed] [Google Scholar]
- 11.Colvin AC, West RV. Current concepts review: patellar instability. J Bone Joint Surg Am. 2008;90:2751–2762. doi: 10.2106/JBJS.H.00211. [DOI] [PubMed] [Google Scholar]
- 12.Farr J, Schepsis AA. Reconstruction of the medial patellofemoral ligament for recurrent patellar instability. J Knee Surg. 2006;19:307–16. doi: 10.1055/s-0030-1248123. [DOI] [PubMed] [Google Scholar]
- 13.Nomura E, Horiuchi Y, Inoue M. Correlation of MR imaging findings and open exploration of medial patellofemoral ligament injuries in acute patellar dislocations. The Knee. 2002;9:139–143. doi: 10.1016/s0968-0160(02)00002-9. [DOI] [PubMed] [Google Scholar]
- 14.Mountney J, senavongse W, Amis AA, Thomas NP. Tensile strength of the medial patellofemoral ligament before and after repair or reconstruction. J Bone Joint Surg [Br]. 2005;87-B:36–40. [PubMed] [Google Scholar]
- 15.Sillanpää PJ, Maenpaa HM. First-time patellar dislocation: surgery or conservative treatment? Sports Med Arthrosc Rev. 2012;20:128–135. doi: 10.1097/JSA.0b013e318256bbe5. [DOI] [PubMed] [Google Scholar]
- 16.Smith TO, Song F, Donell ST, Hing CB. Operative versus nonoperative management of patellar dislocation. A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2011;19:988–998. doi: 10.1007/s00167-010-1355-2. [DOI] [PubMed] [Google Scholar]
- 17.Hing CB, Smith TO, Donell S, et al. Surgical versus nonsurgical interventions for treating patellar dislocation. Cochrane Database Syst Rev. 2011 doi: 10.1002/14651858.CD008106.pub2. 11CD008106. [DOI] [PubMed] [Google Scholar]
- 18.LaPrade RG, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the Knee. J Bone Joint Surg Am. 2007;89:2000–2010. doi: 10.2106/JBJS.F.01176. [DOI] [PubMed] [Google Scholar]
- 19.Redfern J, Kamath G, Burks R. Anatomical confirmation of the use of radiographic landmarks in medial patellofemoral ligament reconstruction. Am J Sports Med. 2010;38(2):293–297. doi: 10.1177/0363546509347602. [DOI] [PubMed] [Google Scholar]
- 20.Schottle PB, Schmeling A, Rosenstiel N, Weiler A. Radiographic landmarks for femoral tunnel placement in medial patellofemoral ligament reconstruction. Am J Sports Med. 2007;35:801–804. doi: 10.1177/0363546506296415. [DOI] [PubMed] [Google Scholar]
- 21.Steensen RN, Dopirak RM, McDonald WG. The anatomy and isometry of the medial patellofemoral ligament. implications for reconstruction. Am J Sports Med. 2004;32(6):1509–13. doi: 10.1177/0363546503261505. [DOI] [PubMed] [Google Scholar]
- 22.Yoo YS, Chang HG, Seo YJ, Byuen JC, Lee GK, Im H, Song SY. Changes in the length of the medial patellofemoral ligament. an in vivo analysis using 3-dimensional computed tomography. Am J Sports Med. 2012;40(9):2142–48. doi: 10.1177/0363546512453301. [DOI] [PubMed] [Google Scholar]
- 23.Elias JJ, Cosgarea AJ. Technical errors during medial patellofemoral ligament reconstruction could overload medial patellofemoral cartilage – a computational analysis. Am J Sports Med. 2006;34:1478–1485. doi: 10.1177/0363546506287486. [DOI] [PubMed] [Google Scholar]
- 24.Stephen JM, Lumpaopong P, Deehan DJ, Kader D, Amis AA. The medial patellofemoral ligament – location of femoral attachment and length change patterns resulting from anatomic and nonanatomic attachements. Am J Sports Med. 2012;40:1871–1879. doi: 10.1177/0363546512449998. [DOI] [PubMed] [Google Scholar]
- 25.Bollier M, Fulkerson J, Cosgarea A, Tanaka M. Case report: technical failure of medial patellofemoral ligament reconstruction. Arthroscopy. 2011;27:1153–1159. doi: 10.1016/j.arthro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Beck P, Brown NA, Greis PE, Burks RT. Patel- lofemoral contact pressures and lateral patellar translation after medial patellofemoral ligament reconstruction. . Am J Sports Med. 2007;35:1557–1563. doi: 10.1177/0363546507300872. [DOI] [PubMed] [Google Scholar]
- 27.Phillopot R, Boyer B, Testa R, Farizon F, Moyen B. Study of patellar kinematics after reconstruction of the medial patellofemoral ligament. Clin Biomech. 2012;27:22–26. doi: 10.1016/j.clinbiomech.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Almqvist KF, Jan H, Vercruysse C, Verbeeck R, Verdonk R. The tibialis tendon as a valuable anterior cruciate ligament allograft substitute: biomechanical properties. Knee Surg Sports Traumatol Arthrosc. 2007;15:1326–1330. doi: 10.1007/s00167-007-0396-7. [DOI] [PubMed] [Google Scholar]
- 29.Sherman SL, Chalmers PN, Yanke AB, Bush-Joseph CA, Verma NN, Cole BJ, Bach BR. Graft tensioning during knee ligament reconstruction: principles and practice. J Am Acad Orthop Surg. 2012;20:633–645. doi: 10.5435/JAAOS-20-10-633. [DOI] [PubMed] [Google Scholar]
- 30.Farahmand F, Tahmasbi MN, Amis AA. Lateral force-displacement behavior of the human patella and its variation with knee flexion – a biomechanical study in vitro. J Biomech. 1998;31:1147–1152. doi: 10.1016/s0021-9290(98)00125-0. [DOI] [PubMed] [Google Scholar]
- 31.Bicos J, Fulkerson JP, Amis A. Current concepts review: the medial patellofemoral ligament. Am J Sports Med. 2007;35:484–492. doi: 10.1177/0363546507299237. [DOI] [PubMed] [Google Scholar]
- 32.Senavongse W, Farahmand F, Jones J, Andersen H, Bull A, Amis AA. Quantitative measurement of patellofemoral joint stability force-displacement behavior of the human patella in vitro. J Orthop Res. 2003;21:780–786. doi: 10.1016/S0736-0266(03)00061-5. [DOI] [PubMed] [Google Scholar]
- 33.Servien E, Fritsch B, Lustig S, Demey G, Debarge R, Lapra C, Neyret P. In vivo positioning analysis of medial patellofemoral ligament reconstruction. Am J Sports Med. 2011;39:134–139. doi: 10.1177/0363546510381362. [DOI] [PubMed] [Google Scholar]
- 34.Senavongse W, Amis AA. The effects of articular, retinacular, or muscular deficiencies on patellofemoral joint stability – a biomechanical study In Vitro. J Bone Joint Surg [Br]. 2005;87-B:577–582. doi: 10.1302/0301-620X.87B4.14768. [DOI] [PubMed] [Google Scholar]


