Abstract
Background
The importance of ectoenzymes CD39 and CD73 in mediating adenosinergic immunosuppression has been recognized, but their roles in human malignant glioma–associated immunosuppression remain largely unknown.
Methods
In this study, the ectoenzyme characteristics of malignant glioma cells and infiltrating CD4+ T lymphocytes isolated from newly diagnosed malignant glioma patients were investigated. The ectoenzyme activities of both cell populations were determined by nucleotide hydrolysis assay. The immunosuppressive property of the CD39-CD73 synergic effect was evaluated via responder T-cell proliferation assay.
Results
We observed that CD39−CD73+ glioma cells and infiltrating CD4+CD39highCD73low T lymphocytes exhibited 2 distinct but complementary ectoenzyme phenotypes, which were further verified by enzyme activity assay. The nucleotide hydrolysis cascade was incomplete unless CD39 derived from T lymphocytes and CD73 collaborated synergistically. We demonstrated that increased suppression of responder CD4+ T-cell proliferation suppression was induced by CD4+CD39+ T cells in the presence of CD73+ glioma cells, which could be alleviated by the CD39 inhibitor ARL67156, the CD73 inhibitor APCP, or the adenosine receptor A2aR antagonist SCH58261. In addition, survival analysis suggested that CD73 downregulation was a positive prognostic factor related to the extended disease-free survival of glioblastoma patients.
Conclusions
Our data indicate that glioma-derived CD73 contributes to local adenosine-mediated immunosuppression in synergy with CD39 from infiltrating CD4+CD39+ T lymphocytes, which could become a potential therapeutic target for treatment of malignant glioma and other immunosuppressive diseases.
Keywords: CD39-CD73-adenosinergic immunosuppression, glioma microenvironment, infiltrating T lymphocytes, malignant glioma, synergic effect
As the most prevalent form of primary brain tumor in humans, malignant gliomas (World Health Organization [WHO] grade III-IV) are characterized by aggressive infiltration and dynamic angiogenesis associated with high morbidity and poor prognosis.1,2 Even with the enormous progress in basic scientific research and clinical practice during the past decades, patients with newly diagnosed glioblastoma multiforme (GBM) have a median survival of 14.6 months, while patients with anaplastic astrocytoma have a median survival of 2–3 years.3 By initiating and amplifying specific antitumor immune responses, immunotherapeutic strategies bring a promising approach in the battle against this devastating neoplasm.4–6 In fact, preliminary immunotherapeutic trials have demonstrated increased disease-free survival and overall survival in glioblastoma patients.7–9 However, malignant gliomas are associated with numerous immunomodulatory properties, such as absent tumor-specific antigen expression, immune checkpoint overexpression, immunosuppressive cytokine secretion, effector lymphocyte anergy, recruitment and induction of regulatory T cells (Tregs) and M2 macrophages, and immune inhibitory factor accumulation.10,11 Thus, antitumor immunity is suppressed profoundly and manipulated to promote tumor progression.
Adenosine has been identified as a universal and potent immune suppressor.12 The surface-expressing ectoenzymes, CD39/ectonucleoside triphosphate diphosphohydrolase–1 (ENTPD1) and CD73/ecto-5′-nucleotidase (NT5E), sequentially convert pro-inflammatory ATP, released from stressed or damaged cells into extracellular space, into AMP and adenosine, respectively. Adenosine deaminase (ADA) is the negative regulator responsible for the rapid deactivation of anti-inflammatory adenosine. Thus, the adenosine balance maintained by the ectoenzymes CD39-CD73-ADA is pivotal for immune homeostasis.13 However, excess adenosine accumulation is associated with certain pathological circumstances, such as chronic inflammations and tumors, which activates Gs-protein-coupled adenosine A2aR receptors expressed by a variety of immune cells and elevates the intracellular cAMP level. Consequently, potent immunosuppression is induced, causing abrogated T-cell proliferation, Th1/Th2 shifting, Treg induction, and inhibition of macrophage activation.14–16 To date, the CD39-CD73-adenosine pathway has been recognized as a critical immunosuppressive mechanism with a promising therapeutic prospect in oncology.17 However, several details have not yet been characterized. For instance, while the importance of tumor-derived CD73 in tumorigenesis, metastasis, and immunosuppression has been demonstrated both in vitro and in vivo,18–20 the CD39 activity in gliomas has not been confirmed.21,22 On the other hand, despite the coexpression of CD73 and CD39 in murine CD4+CD25+Foxp3+ Tregs,23 the expression and activity of CD73 in human CD4+CD39+ T lymphocytes are almost absent.24–26
In this study, the phenotypic and functional characteristics of the ectoenzymes expressed by glioma cells and glioma-infiltrating CD4+ T lymphocytes were evaluated in detail. Based on the distinct but complementary ectoenzyme status of these 2 cell populations, we demonstrate that CD73+ glioma cells contribute to local adenosinergic immunosuppression synergistically with infiltrating CD39+ T lymphocytes in the glioma microenvironment and thus might be of great potential in malignant glioma treatment.
Materials and Methods
Glioma Cell Lines
Human glioma cell lines U-87 MG, T98G, and U-251 were acquired from the Type Culture Collection of the Chinese Academy of Sciences. All glioma cells were cultured in 5% CO2 and humidified air at 37°C in Dulbecco's modified Eagle's medium (Thermo Scientific) supplemented with 10% fetal bovine serum (Gibco) and split when they reached 80% confluency.
Antibodies
The cell surface was stained with fluorescein isothiocyanate–conjugated, phycoerythrin-conjugated, and allophycocyanin-conjugated anti-human monoclonal antibodies against the following antigens: CD3, CD4, CD8, CD14, CD25, CD26, CD39, and CD73 (eBioscience and Biolegend). For intracellular Foxp3 staining, the Foxp3 Fixation/Permeabilization Kit and antibody (eBioscience) were used. Appropriate isotype controls were tested in parallel.
Human Samples and Phenotypic Analysis
Freshly resected malignant glioma specimens (n = 9, including 7 GBM and 2 anaplastic astrocytoma) were obtained from newly diagnosed glioma patients. Tumor grades were assessed by experienced pathologists and classified according to the WHO system (Supplementary Table S1). Matched peripheral blood samples were collected before the surgical procedure. None of the subjects had a history of glucocorticoid use or other immunosuppressive therapies, which might artificially affect their immune function. Peripheral blood samples from healthy donors (n = 10) were included as controls. This study was performed according to the guidelines of the Declaration of Helsinki. The protocol has been fully reviewed and approved by the Medical Ethical Committee, Qilu Hospital of Shandong University (IRB approval number: 1147). Informed consent was obtained from all participating subjects.
All blood and tumor samples were freshly processed within 2 h. Each peripheral blood sample was treated with 1× RBC lysis buffer (Sigma-Aldrich) at room temperature for 10 min to lyse the red blood cells. For glioma samples, the resected specimen was washed twice in phosphate buffered saline, dissociated mechanically into 1- to 2-mm small pieces with sterile scissors, and pipetted mildly and thoroughly. Single cell suspension was obtained after filtering the tissue suspension through a 70-µm mesh size cell strainer. Infiltrating immune cells were further enriched by Ficoll-Paque density gradient centrifugation (Sigma-Aldrich). After antibody labeling, flow cytometry acquisition was done with a FACSCalibur flow cytometer (BD Biosciences). Data analysis was performed using FlowJo software (TreeStar).
PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen) and treated with RNase I (Thermo-Fermentas). Reverse transcription (RT)–PCR was performed with a Moloney murine leukemia virus–RT kit (Thermo-Fermentas) with 1 μg of total RNA according to the manufacturer's protocol. Quantitative RT-PCR was performed using a SYBR Green Master Mix kit (Toyobo) on a LightCycler 2.0 instrument (Roche Applied Science). Relative expression level was calculated using the ΔΔ cycle threshold (Ct) method. All reactions were run in triplicate. Gene-specific amplifications were demonstrated by melting-curve data and electrophoresis. The primer sequences are listed in Supplementary Table S2.
Immunohistochemistry
Formalin-fixed, paraffin-embedded resected specimens of malignant gliomas were obtained from the Department of Pathology, Qilu Hospital of Shandong University (n = 19, including 16 GBM and 3 anaplastic astrocytoma). Deparaffinized and rehydrated slides were treated with 10 mM citrate buffer (pH 6.0) at 98°C for 20 min for antigen retrieval. After endogenous peroxidase inactivation, slides were then incubated with anti-human CD39 (1 : 50; Abcam) and anti-human CD73 (1 : 100; Abcam) overnight at room temperature. Immunoreactivity was visualized using the peroxidase-diaminobenzidine system (Dako). After counterstaining with hematoxylin, samples with >10% positive cancer cells were considered positive. Matched isotype antibodies were used as negative controls.
Cell Sorting
Peripheral blood mononuclear cells were isolated from healthy donors' leukocyte-enriched buffy coats by Ficoll-Paque density gradient centrifugation. CD4+ T lymphocytes were enriched based on the magnetic-activated cell sorting (MACS) cell separation protocol (Miltenyi Biotec). Briefly, CD14+ monocytes, which weakly express CD4, were depleted using anti-human CD14 magnetic beads. Afterward, the monocyte-depleted fraction was incubated with anti-human CD4 magnetic beads, and the positive fraction was selected. The CD4 purity was >98% as determined by flow cytometry. For CD4+CD39+ and CD4+CD39− T-cell sorting, total CD4+ T cells were labeled with anti-human CD39 antibody (eBioscience) and sorted using a Becton Dickinson Influx cell sorter. CD39 purity was >90%–95%.
Ex vivo Adaptive Treg Induction
Freshly MACS-sorted total CD4+ T lymphocytes were stimulated with T-cell activation/expansion microbeads loaded with anti-human CD2/3/28 antibodies (Miltenyi Biotec) in Roswell Park Memorial Institute medium 1640 (Thermo Scientific) plus 10% fetal bovine serum. Recombinant human interleukin (IL)-2 (100 U/mL) and transforming growth factor (TGF)–β1 (10 ng/mL; PeproTech) were supplemented to induce Tregs. After 4 days, cells were harvested and labeled with antibodies for phenotype assay.
Phosphate Production Assay
ENTPDase/5′-nucleotidase enzymatic activity was determined by the release of inorganic phosphate (Pi) as previously described.27 Briefly, 1 × 105 glioma cells or CD4+CD39+/CD39− T cells were washed with phosphate-free buffer (0.5 mM CaCl2, 120 mM NaCl, 5 mM KCl, and 50 mM Tris-HCl, pH 8.0) 3 times and pre-incubated in 100 µL buffer in a 96-well microplate at 37°C for 30 min. To some wells, 250 µM ARL67156, a CD39-specific inhibitor, or 100 µM α,β-methylene adenosine-5′-diphosphate (APCP), a CD73-specific inhibitor, was added. Reactions were started after the addition of 100 µL exogenous ATP or AMP substrate solution (Sigma-Aldrich) at the final nucleotide concentration of 500 µM. To verify enzyme activity, 5′-nucleotidase purified from Crotalus atrox venom (Enzo Life Sciences) was examined in parallel. After 30 min incubation at 37°C, the microplate was transferred to ice for 10 min to stop the reactions. Then, 100 µL supernatant was collected to examine the release of Pi using the Malachite Green Phosphate Detection Kit (R&D Systems) according to the manufacturer's protocol with a microplate reader (Tecan). Non-enzymatic hydrolysis was determined by substrate solution without cells or 5′-nucleotidase. The phosphate release baseline for each condition was obtained by adding blank buffer.
Suppression Assay
The synergistic suppression activity of single cell–sorted CD4+CD39+ T lymphocytes and CD39−CD73+ U-87 MG/T98G glioma cells was confirmed based on a carboxyfluorescein succinimidyl ester (CFSE)–labeled CD4+CD39− responder T-cell proliferation assay. Of note, pretreated with 20 µg/mL mitomycin C (Roche Applied Science) at 37°C for 2 h, U-87 MG/T98G cells were then seeded in the wells of a 48-well plate at a density of 1 × 105 glioma cells per well 1 day ahead of the coculture. On day 0, 2 µM CFSE (Invitrogen)-labeled 1 × 105 CD4+CD39− responder T cells (RC) were incubated in the wells of a 48-well plate with autologous CD4+CD39+ suppressor T cells (S) at the ratio of 1 : 1 in the presence or absence of preseeded glioma cells. Unlabeled CD4+CD39− T cells were used as the control for suppressor T cells. Cell proliferation was stimulated by T-cell activation/expansion microbeads loaded with anti-human CD2/3/28 antibodies in the presence of 100 IU/mL IL-2. Specifically, CD4+CD39+ T cells, U-87 MG/T98G cells, and CD4+CD39− responder T cells were pretreated with 250 µM ARL67156, 100 µM APCP, and 1 µM adenosine A2a receptor–specific antagonist SCH58261 (Tocris Bioscience), respectively, for 30 min before the start of coculture. On day 4, percent cell proliferation was analyzed using a FACSCalibur flow cytometer and FlowJo software. The percent suppression was calculated according to the following equation: [1 − percent proliferation (responder and suppressor)/proliferation (responder only)] × 100%.
TCGA Glioblastoma Data Survival Analysis
Glioblastoma mRNA expression data (Agilent microarray) were obtained from the GBM provisional study set of The Cancer Genome Atlas (TCGA) and analyzed via the cBio Cancer Genomics Portal.28 To define the altered NT5E/CD73 expression subgroups, the z-score threshold was set to ±1, that is, EXP > 1 for upregulation and EXP < −1 for downregulation. The corresponding median overall survival and disease-free survival of different subgroups were provided by the cBio Cancer Genomics Portal and compared. Updated to March 2013, the number of GBM patients with mRNA expression data collected by the GBM provisional study set of TCGA was 500.
Statistical Analysis
Data are presented as mean percents ± SD obtained in at least 3 independent experiments. Student's t-test and ANOVA were used for statistical comparisons. All statistical analyses were conducted using GraphPad Prism 5 software, except for the TCGA GBM patient survival analysis, which is provided by the cBio Portal based on a log-rank test. Statistical significance was considered as P < .05.
Results
Ectoenzyme Phenotype of Glioma Cells
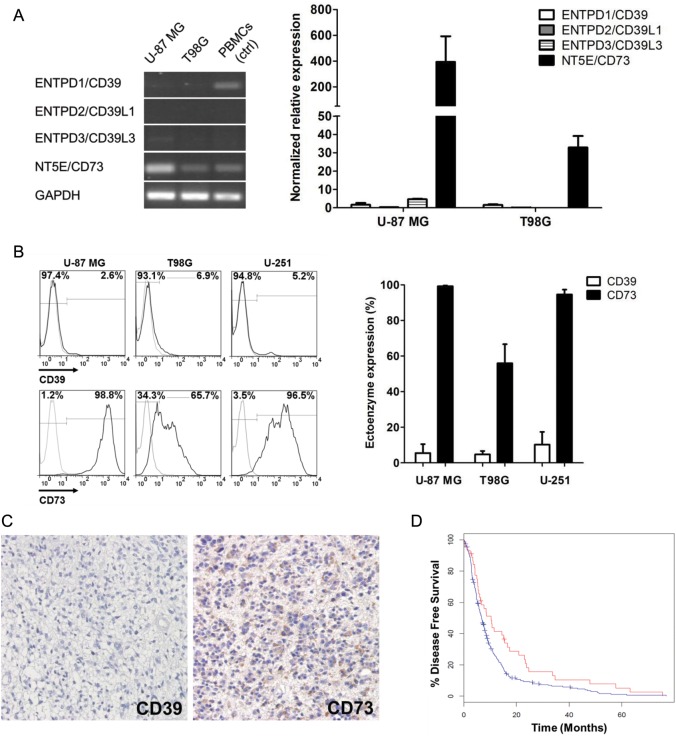
To ascertain the ectoenzyme expression profiles in glioma cells, we extracted total RNA from the glioma cell lines U-87 MG and T98G and assessed the transcriptional levels of ENTPD family members (ie, ENTPD1/CD39, ENTPD2/CD29L1, and ENTPD3/CD39L3) and NT5E/CD73 by RT-PCR. Interestingly, both U-87 MG and T98G exhibited similar preferential expression of CD73. In contrast, expression of CD39 family members was almost negative in both cell lines. Quantitative RT-PCR assays confirmed this expression pattern. Despite the differences in the transcriptional levels between the 2 cell lines, CD73 is the dominant ectoenzyme expressed by glioma cells. Notably, the expression of CD39 was low or even barely detectable (Fig. 1A). These results were consistent with the previous study reporting almost absent CD39 expression in rat C6 glioma cells.29 A similar transcriptional pattern was also found in another glioma cell line, U-251 (Supplementary Fig. S1A). The preferential expression of CD73 over CD39 was further validated by flow cytometry analysis of protein levels in these 3 cell lines (Fig. 1B). Particularly, U-87 MG cells expressed the highest level of CD73, while T98G had the lowest (Supplementary Fig. S1B).
Fig. 1.
Phenotypic characteristics of the glioma cells. (A) Detection of ENTPD/CD39 family members and NT5E/CD73 by RT-PCR (left) and quantitative (q)RT-PCR (right) in glioma cell lines U-87 MG and T98G. Human peripheral blood mononuclear cells (PBMCs) were used as a positive control and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as an internal control. The values of each group are expressed as mean percents ± SD for 3 qRT-PCT assays. (B) Surface expressions of ectoenzymes CD39 and CD73 on U-87 MG, T98G, and U-251 glioma cells were assessed by flow cytometry. Representative results are shown on the left. Numbers in graphs represent the percentages of positively stained cells (black histograms) relative to those labeled with isotype antibodies (gray histograms), which are further summarized as mean percents ± SD on the right. (C) Surgical specimens of malignant gliomas were stained with anti-CD39 (left) and anti-CD73 (right) antibodies for immunohistochemistry. Representative images indicate the preferential expression of CD73 over CD39 in human malignant glioma specimens. Immune detection was revealed by diaminobenzidine and then counterstained with hematoxylin. The images were taken at 400× magnification. (D) Glioblastoma mRNA microarray data were obtained from the TCGA database and analyzed via the cBio Cancer Genomics Portal. The comparison of median disease-free survival between patients with CD73 mRNA z-score < −1 (red curve) and those with CD73 mRNA z-score≥−1 (blue curve) indicated that downregulation of CD73 was a positive prognostic factor. P < .05.
To verify our result with respect to the primary brain tumors, we performed immunohistochemistry to detect expression of ectoenzymes CD39 and CD73 in 19 malignant glioma tissues. Among all the specimens tested (16 GBM and 3 anaplastic astrocytoma), CD73 was expressed in 89.5% (17/19) of cases, but CD39 was verified in merely 21.1% (4/19; Fig. 1C). In addition, large-scale analysis using the TCGA GBM dataset implied a significant correlation between tumor CD73 expression and disease prognosis. Glioblastoma patients with CD73 mRNA downregulation had a prolonged median disease-free survival of 10.4 months, whereas patients without CD73 downregulation had that of 6.7 months (P = .015; Fig. 1D). CD73 mRNA downregulation also benefited median overall survival but was not statistically significant (15.3 mo vs 14.0 mo, P = .132; Supplementary Fig. S1C). The survival evidence therefore indicates that CD73 not only is expressed in gliomas, but is also of considerable therapeutic potential.
The data, taken together, demonstrate that glioma cells preferentially express CD73, whereas the expression of CD39, which is the rate-limiting enzyme in the nucleotide hydrolysis cascade,17 is negligible.
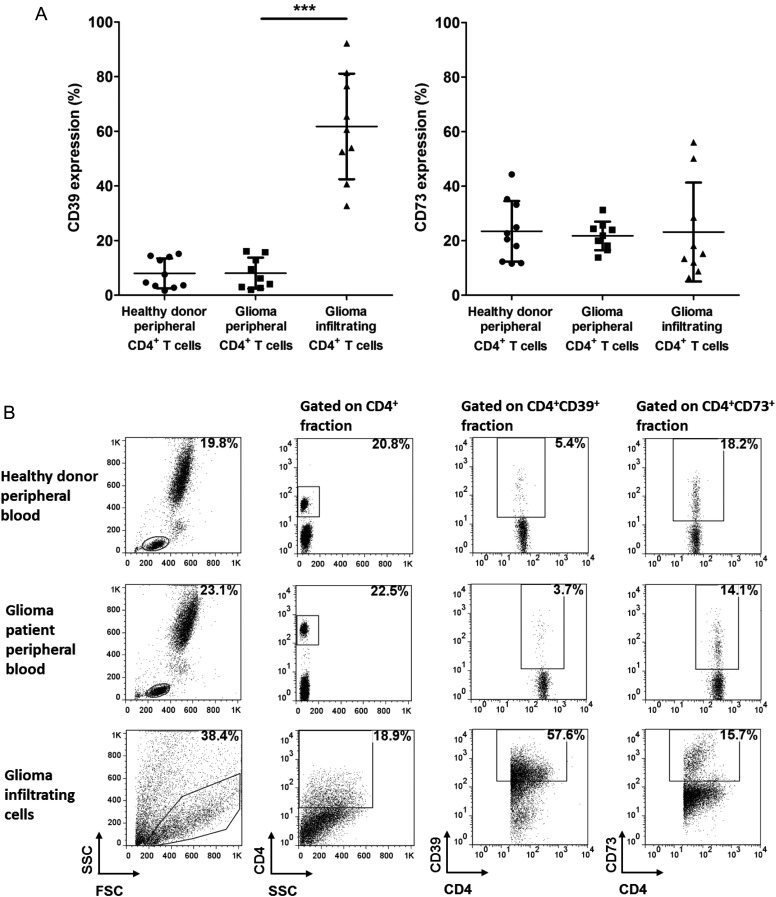
Ectoenzyme Phenotype of Glioma-infiltrating CD4+ T Cells
Despite the low frequency (range ∼0.2%) amongst the numerous cell populations present within malignant gliomas,30 infiltrating CD4+ T cells are known for their notorious role in inducing glioma-associated immunosuppression. To explore the elusive effect they potentially exert on the local adenosinergic pathway, we collected peripheral blood and tumor specimens from newly diagnosed malignant glioma patients, isolated peripheral and tumor-infiltrating CD4+ T lymphocytes, and compared the phenotypic characteristics of these 2 populations (n = 9). Healthy donor peripheral CD4+ T cells were also included as controls (n = 10). As shown in Table 1 and Fig. 2A, malignant glioma patients did not exhibit any ectoenzyme dysregulation in the peripheral CD4+ T lymphocytes compared with those from healthy donors (P > .05). In contrast, robust CD39 expression was observed in the infiltrating CD4+ T lymphocytes, with a prevalence of 61.8 ± 19.3% relative to the 8.0 ± 5.7% from matched peripheral CD4+ T lymphocytes (P < .001). Considering the marked differences among individual specimens, our data also reveal the heterogeneity in the status of the adenosinergic pathway in malignant glioma patients. Meanwhile, CD73 level was not altered in glioma-infiltrating CD4+ T lymphocytes, as was CD39 (P = .827). Representative individuals are presented in Fig. 2B. Together, our data indicate that tumor-infiltrating CD4+ T lymphocytes are associated with dramatically increased CD39 expression, which exhibit distinct but complementary properties compared with the glioma cells.
Table 1.
CD39/CD73 expression frequencies of peripheral and infiltrating CD4+ T lymphocytes
| Healthy Donors (n = 10) | Glioma Patients (n = 9) | ||
|---|---|---|---|
| Peripheral Blood | Peripheral Blood | Glioma Tissue | |
| CD4+CD39+ population/total CD4+ lymphocytes | 8.0 ± 5.5% | 8.0 ± 5.7% | 61.8 ± 19.3% |
| CD4+CD73+ population/total CD4+ lymphocytes | 23.5 ± 11.1% | 21.8 ± 5.3% | 23.2 ± 18.2% |
Data are shown as mean percents ± SD.
Fig. 2.
Ectoenzyme characterization of glioma-infiltrating CD4+ T lymphocytes. (A) Surface expressions of CD39 and CD73 on the peripheral CD4+ T lymphocytes from healthy donors (n = 10) and patients with newly diagnosed malignant glioma (n = 9); the matched tumor-infiltrating CD4+ T lymphocytes were determined by flow cytometry. Scatter plot summarizing flow cytometry data shows percentage of CD4+ T cells expressing CD39 (left) and CD73 (right). Mean percents ± SD indicated by horizontal lines and bars are given. ***P < .001. (B) Dot plots show expression of CD39 and CD73 in a representative individual from each group.
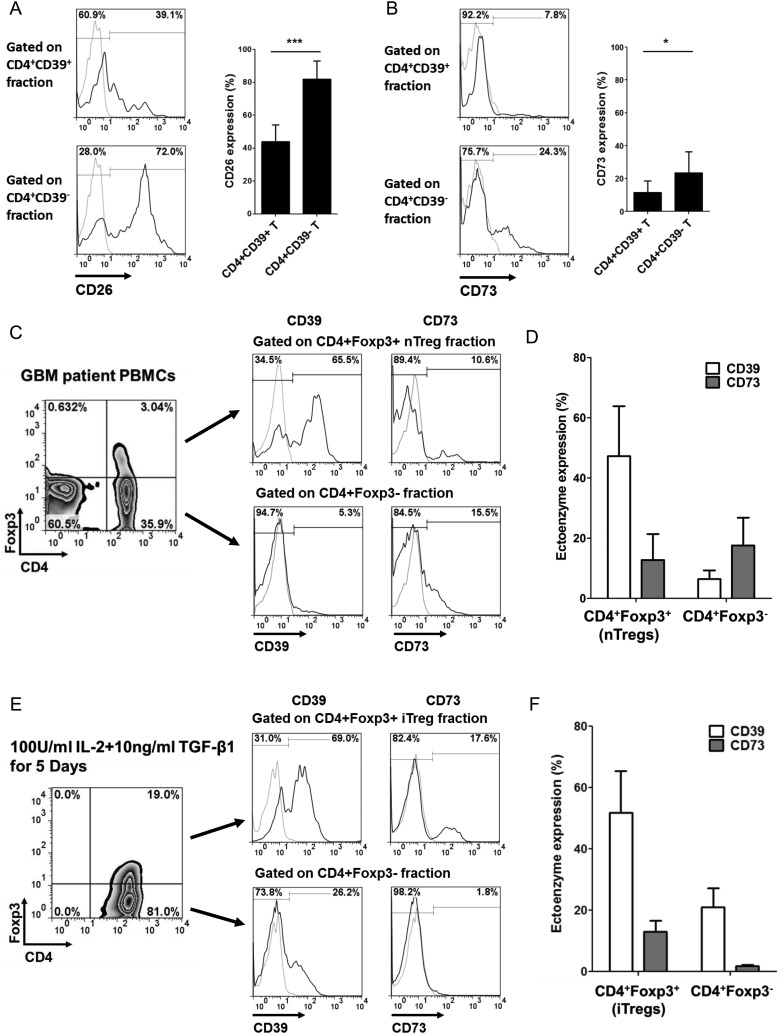
Low CD73 Expression on CD4+CD39+ T Cells
As indicated above, glioma-infiltrating CD4+ T lymphocytes are CD39highCD73low. Unlike the concordant expression in mice, findings of the surface expression and hydrolysis activity of CD73 in human CD4+CD39+ T lymphocytes remain controversial. Therefore, we conducted a detailed ectoenzyme profiling of this particular CD4+CD39+ T-cell subset. ADA is recognized as a pivotal negative regulator of adenosinergic signaling by adenosine deactivation. Detection of the surface-bound glycoprotein CD26, which is the dominant ADA-anchoring protein on human lymphocytes, provides an alternative approach to evaluate ADA expression.31 As shown in Fig. 3A, CD26 was predominantly expressed on CD4+CD39− responder T cells relative to the expression in the CD4+CD39+ population (P < .001), which suggests that CD4+CD39+ T cells favor adenosine accumulation. However, CD73 surface expression in the CD4+CD39+ T-cell population was 11.7 ± 6.99%, even lower than the 23.5 ± 12.8% in the CD4+CD39− responder T cells (P < .05) (Fig. 3B). Since the nucleotide cascade depends on both CD39 and CD73, this particular phenotype suggests that CD4+CD39+CD73low T cells might be defective in generating adequate adenosine to exert its suppressive effect.
Fig. 3.
Phenotypic characterization of CD4+CD39+ T lymphocytes. (A and B) Surface expressions of CD26 (A) and CD73 (B) in peripheral CD4+CD39+ and CD4+CD39− T-cell subsets were determined by flow cytometry. Left panel, ectoenzyme expression histograms gated on each subset from a representative sample. Right panel, bar graphs summarizing data obtained. (C) Surface expressions of CD39 and CD73 in natural CD4+Foxp3+ Tregs (nTregs) from GBM patient peripheral blood mononuclear cells (PBMCs) were verified. (D) Bar graphs summarizing the nTreg CD39/CD73 surface data (n = 7). (E) After in vitro induction of adaptive CD4+Foxp3+ Tregs (iTregs), CD39/CD73 surface expressions were determined by flow cytometry. (F) Bar graphs summarizing the iTreg CD39/CD73 expression from 3 independent experiments. Numbers in histogram graphs represent the percentages of positively stained cells (black histograms) compared with isotype controls (gray histograms). Bars in bar graphs represent mean percents + SD. *P < .05, ***P < .001.
It has been reported that the prevalence of Foxp3+ Treg within gliomas is correlated with tumor pathology and WHO grade.32 Although the concordant surface expression of the ectoenzymes CD39 and CD73 had been validated in murine Tregs, our in situ data highly suggest that tumor-infiltrating T lymphocytes, many of which were Tregs, lacked CD73 expression. Therefore, we verified the surface expression of CD73 on the natural Tregs (nTregs) from glioblastoma patients as well as the adaptive Tregs (iTregs) induced by suppressive TGF-β (Supplementary Fig. S2). Compared with conventional CD4+Foxp3− T cells, peripheral CD4+Foxp3+ nTregs from patients with glioblastoma exhibited higher expression of the Treg marker CD39, but not of CD73 (n = 7, Fig. 3C and D). Similarly, CD4+Foxp3+ iTregs also expressed low levels of CD73 (Fig. 3E and F). In summary, we observed that human CD39+ T cells lack CD73 surface expression, which suggests that probably certain compensatory mechanisms exist to induce local adenosinergic immunosuppression within the glioma microenvironment.
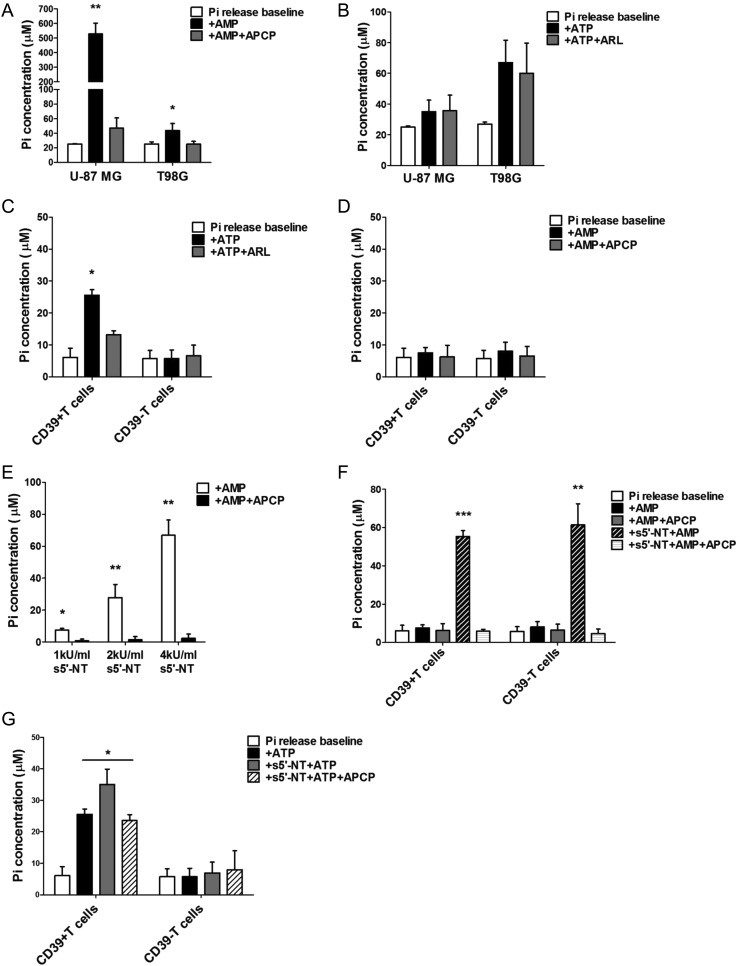
Synergy Between CD39 and CD73 Is Essential for Nucleotide Hydrolysis Cascade
Despite the defective phenotypic characteristics of both glioma cells and CD4+CD39+ T cells indicated above, the associated nucleotide hydrolyzing activities of these cells remain undefined. To understand the functional status of these cells better, we determined the Pi generated during ectoenzyme-mediated nucleotide dephosphorylation by using a malachite green-phosphate assay. In particular, U-87 MG and T98G glioma cells were tested here because they express the highest and lowest levels of CD73, respectively (Supplementary Fig. S1B). As expected, both glioma cells hydrolyzed exogenous AMP robustly, which could be abrogated by a specific CD73 inhibitor, APCP (P < .01; Fig. 4A). Consistent with the higher CD73 level, U-87 MG cells exhibited much higher 5′-nucleotidase activity (∼12-fold of T98G). Interestingly, neither CD39-deficent U-87 MG nor T98G displayed significant ATP hydrolysis (Fig. 4B). On the contrary, single cell–sorted CD4+CD39+ T lymphocytes exhibited significant ENTPDase activity, which could be blocked by a CD39 inhibitor, ARL 67156 (P < .05; Fig. 4C). However, 5′-nucleotidase activity was not observed (Fig. 4D). CD4+CD39− T lymphocytes were deficient in either ENTPDase or 5′-nucleotidase activities, which was not surprising because they were categorized as conventional/responder T lymphocytes without abundant ectoenzyme expressions.31
Fig. 4.
Ectoenzyme activity measurement by determining the Pi generated during nucleotide hydrolysis. (A and B) 105 U-87 MG and T98G glioma cells were assessed for 5′-nucleotidase (A) or ENTPDase (B) activity by adding exogenous AMP or ATP in the presence or absence of 100 µM APCP or 250 µM ARL67156, respectively. After 30 min incubation, cell supernatants were collected, and corresponding concentrations of Pi were determined. Baseline phosphate release was determined by the supernatant harvested from cells incubated without nucleotide substrate. (C–D) 105 CD4+CD39+/CD4+CD39− T cells sorted by flow cytometry were assessed for ENTPDase (C) or 5′-nucleotidase (D) activity. (E) 5′-nucleotidase activity of soluble 5′-nucleotidase (s5′-NT) purified from Crotalus atrox venom was verified. (F) Exogenous AMP was added to freshly sorted CD4+CD39+/CD4+CD39− T cells in the presence of 2 kU/mL s5′-NT. After 30 min incubation, Pi concentration was determined. (G) Exogenous ATP was added to freshly sorted CD4+CD39+/CD4+CD39− T cells in the presence of 2 kU/mL s5′-NT. After 30 min incubation, Pi concentration was determined. All the data are presented as mean percents ± SD acquired from 3 independent experiments. *P < .05, **P < .01, ***P < .001.
Thus, the data demonstrated that the glioma cells and infiltrating CD4+CD39+ T lymphocytes possess distinct but complementary defects in ectoenzyme phenotypic and functional status. Hence, we evaluated whether they could work synergistically to induce local adenosine generation. To mimic CD73 activity, we utilized the soluble 5′-nucleotidase purified from Crotalus atrox venom,33 which allowed us to focus on the synergy between CD39 and CD73 and avoid the complexity of using entire glioma cells (Fig. 4E). More phosphate was generated from AMP by CD39+ T cells in the presence of soluble 5′-nucleotidase than by the cells alone (P < .001; Fig. 4F). This synergistic effect was specifically blocked by a CD73 inhibitor, APCP. We also measured more phosphate generated from ATP by CD4+CD39+ T cells in the presence of soluble 5′-nucleotidase compared with that generated by CD4+CD39+ T cells alone (P < .05). Because soluble 5′-nucleotidase cannot hydrolyze ATP directly, this particular evidence indicates that synergy between CD39 and CD73 does exist, which can be abrogated by APCP (Fig. 4G).
Significant Immunosuppression Induced by Synergy Between CD39 and CD73
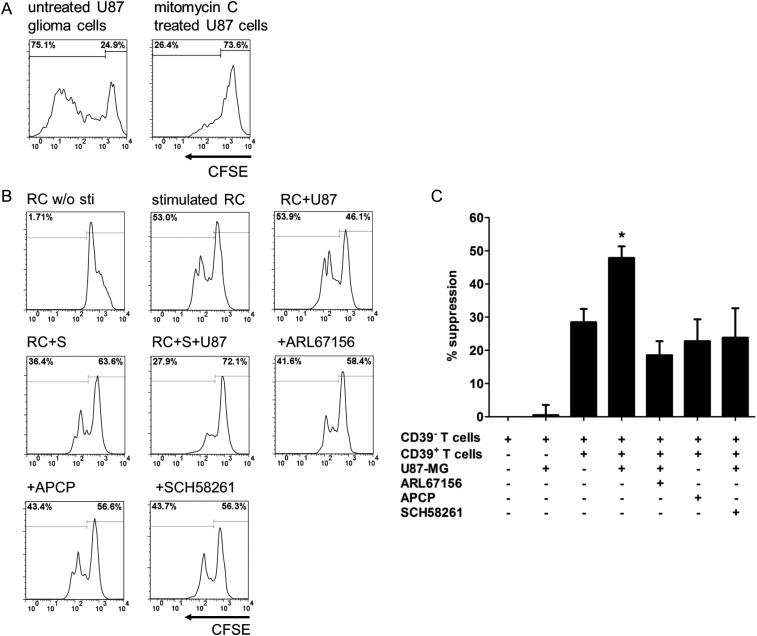
To ascertain our hypothesis that immunoregulatory CD4+CD39+ T lymphocytes could induce a more significant immunosuppressive effect in synergy with glioma cells, CFSE-labeled single-sorted CD4+CD39− responder T lymphocytes were cocultured with autologous CD4+CD39+ T lymphocytes in the absence or presence of glioma cells for 4 days. The percent suppression of responder cell proliferation was then calculated. Before coculture, glioma cells were pretreated with mitomycin C, an antimitotic agent to arrest cell division, to minimize potential nutrition deprivation for responder cells (Fig. 5A).
Fig. 5.
CD4+CD39+ T cells induce more significant proliferation suppression in the presence of CD73+ glioma cells. (A) CFSE-labeled U-87 MG glioma cells were treated with 20 µg/mL mitomycin C (Mito C) for 2 h. After 3 days, the proliferation percent of U-87 MG cells was evaluated by flow cytometry. (B) CFSE-labeled CD4+CD39− responder T cells (RC) were cocultured with autologous CD4+CD39+ suppressor T cells (S) at the ratio of 1 : 1 in the presence or absence of preseeded U-87 MG glioma cells (U87). Microbeads coated with anti-human CD2/3/28 antibodies were used to stimulate T-cell proliferation. The effects of ARL67156, APCP, and the adenosine receptor A2aR antagonist SCH58261 were tested as described in Materials and Methods. After 4 days, cells were harvested and analyzed via flow cytometry. Representative plots are shown. The numbers represent the percentages of proliferating CFSE-labeled responder T cells. (C) The suppression percents obtained in 3 independent experiments were summarized and presented as mean percents ± SD. *P < .05.
Consistent with previous reports, autologous CD4+CD39+ T lymphocytes inhibited the proliferation of CD4+CD39− responder T lymphocytes, with percent suppression of 28.5 ± 4.0% (P < .05). In contrast, U-87 MG glioma cells alone did not affect the proliferation of CD4+CD39− responder T lymphocytes (% suppression: 0.49 ± 2.2%, P > .05). Interestingly, more significant proliferation suppression of responder T lymphocytes was induced by CD4+CD39+ T lymphocytes in the presence of U-87 MG glioma cells (47.8 ± 3.5% vs 28.5 ± 4.0%, P < .05; Fig. 5B and C). Both the CD39 inhibitor ARL67156 and the CD73 inhibitor APCP could alleviate this synergistic suppression, suggesting that CD39+ lymphocytes and CD73+ glioma cells interacted with each other and suppressed proliferation. Likewise, the T98G glioma cells induced a similar but less significant suppressive effect on proliferation, consistent with lower CD73 expression (P > .05; Supplementary Fig. S3). The inhibition of proliferation was also arrested by the adenosine receptor A2aR antagonist SCH58261, indicating the participation of this Gs protein-coupled receptor in adenosinergic signaling.
Discussion
Glioma-associated immunosuppression is common and involves multiple mechanisms, including the adenosinergic signaling pathway. It has been generally believed that tumor cells and infiltrating immune cells exert their effects on tumor immunity independently. Specifically, it has been reported that tumor-derived CD73 mediates tumoral immune escape and metastasis in breast cancer.19 Hepatic metastatic tumor growth can also be promoted by CD39 expression in regulatory T cells.34 In this study, however, we provide evidence that neither CD39−CD73+ glioma cells nor infiltrating CD4+CD39highCD73low T lymphocytes by themselves are sufficient to induce glioma-associated adenosinergic immune suppression. Instead, this cascade works like a “jigsaw puzzle”. More potent immunosuppression is induced by CD4+CD39+ T cells in synergy with CD73+ glioma cells, which can be abrogated by either CD39 or CD73 inhibition (Fig. 6).
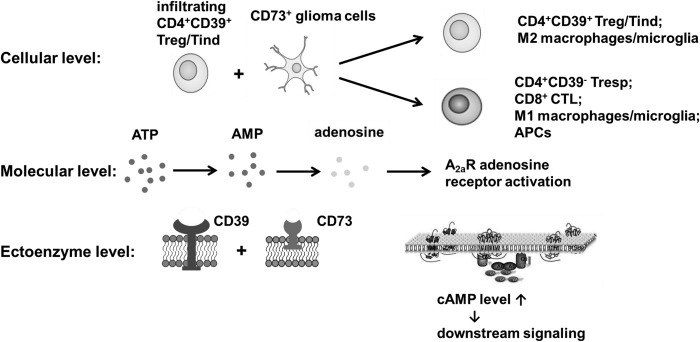
Fig. 6.
Schematic diagram showing the synergy between ectoenzymes CD39 and CD73 demonstrated in the human malignant glioma microenvironment. Extracellular ATP is hydrolyzed by CD39 on infiltrating T lymphocytes (including Foxp3+CD25+ Tregs and Foxp3−CD25− Tinds) to AMP and then further converted rapidly to anti-inflammatory adenosine by glioma-derived CD73. Thus, ectoenzymes CD39 and CD73 derived from different cell populations contribute to the generation and accumulation of extracellular adenosine, which activates adenosine receptor A2aR expressed by immune cells, elevates the intracellular cAMP level, and initiates downstream signaling of protein kinase A/cAMP response element binding protein and Epac/phospholipase C. CD39/CD73-mediated adenosinergic immunosuppression not only inhibits the activation and function of pro-inflammatory immune cells, such as infiltrating CD4+CD39−/CD8+ effector T cells, M1 macrophages/microglia, and dendritic cells, but also promotes the induction of CD39+ Treg cells and tumor-supportive M2 macrophages/microglia.
Convergent evidence indicates that CD4+CD39+ T cells can be subdivided into at least 2 distinguishable heterogeneous subsets, CD39+Foxp3+CD25+ Tregs and CD39+Foxp3−CD25− “inducer” T cells (Tinds).35–37 Although CD39+Foxp3−CD25− Tinds possess high ATP hydrolysis activity similar to Tregs, they are a unique immunostimulating population.25 CD39+Foxp3−CD25− Tinds have been reported to augment the proliferation and cytokine production of responder T cells significantly with a distinct repertoire of cytokines.35 These studies revealed that CD39 itself was not an immunosuppressive regulator. Our study revealed that CD39 expression was significantly upregulated in glioma-infiltrating CD4+ T lymphocytes, which has also been observed in patients with head and neck squamous cell carcinoma.26 However, we indicated that these “defective” infiltrating CD4+ T cells are incompetent to hydrolyze AMP efficiently. The importance of CD73 lies in adenosine generation and accumulation, and the participation of glioma-derived CD73 completes the CD39-CD73 ectoenzyme cascade, as we demonstrated. Glioma-derived CD73 may collaborate with both CD39+Foxp3+CD25+ Tregs and CD39+Foxp3−CD25− Tinds, because this mechanism depends on the synergy between ectoenzymes CD39 and CD73 rather than the regulatory properties or the cytokine repertoire of T lymphocytes. The role of adenosine A2aR receptors, which are expressed by a variety of immune cell populations, including CD4+/CD8+ T lymphocytes, macrophages/microglia, and dendritic cells, should also be highlighted. A2aR receptors exert potent immunoregulatory effects via intracellular cAMP elevation and downstream signaling of protein kinase A/cAMP response element binding protein and Epac/phospholipase C. Therefore, we assume that the immunosuppressive effect of the CD39-CD73 synergic pathway includes but is not limited to the responder T cells used in this study. For instance, the activation and function of macrophages/microglia are modified by adenosine signaling, which triggers the shift of classical macrophage (M1)/alternative macrophage (M2) balance and induces vascular endothelial growth factor–mediated angiogenesis.15 Thus, this CD39-CD73 pathway can also affect infiltrating macrophages/microglia, since they are present as the predominant immune cell population that infiltrate gliomas.30 It has also been reported that genetic elimination of the A2aR receptor abrogates the inhibition of antitumor T cells and improves tumor rejection by cytotoxic CD8+ T cells.38 Intriguingly, this synergic effect might induce a positive feedback loop of adenosinergic immunosuppression, since A2aR receptor activation promotes the generation of adaptive CD39+ Tregs.16 Thus, we suppose that crosstalk between cancer cells and infiltrating immune cells in the tumor environment is extensive and dynamic and that together they regulate tumor immunity.
Concordant expression of CD39 and CD73 in murine Tregs has been demonstrated; however, CD73 expression pattern in human CD39+ T cells is not evident. Dwyer et al.39 first described that CD39, independently of CD73, was expressed by a subset of blood-derived human CD4+CD25+CD127low Tregs with a robust expression of Foxp3. Lower CD73 expression in nodal tissue CD4+ T cells has also been observed, while CD39 expression was significantly upregulated.40 Moncrieffe et al. further demonstrated that an increased fraction of CD39+ T cells was present at the inflammatory site of juvenile idiopathic arthritis with the downregulation of CD73 expression and activity. They hypothesized that in the absence of adequate CD73 activity, the breakdown of ATP at inflamed sites would be inadequate to completely ease joint inflammation, which leads to chronic autoimmunity.25 Similarly, we observed that CD73 expression level was not altered despite dramatic CD39 upregulation in glioma-infiltrating CD4+ T cells. To the best of our knowledge, there are no prior studies that reported such a “defective” phenotype of infiltrating immune cells in human solid tumors. CD39 and CD73 expressions are under the regulation of hypoxia-inducible transcription factor–1.41,42 Within the hypoxic-ischemic microenvironment of malignant gliomas, CD39 upregulation in infiltrating CD4+ T lymphocytes is quite logical. Further studies are required to explain the reason that CD73 expression is stable or downregulated. On the other hand, Mandapathil et al.31 reported that intracellular CD73 could be detected in the CD4+CD25high Tregs lacking surface expression of CD73. However, the release and uptake of lipophobic adenosine require nucleoside membrane transport, which is controlled by multiple factors including transporter proteins and transmembrane concentration gradient.43 Moreover, compared with the typical ectoenzyme form, the function of cytosolic CD73 remains to be elucidated.
CD73, as a potent suppressor for antitumor immune responses, is expressed by tumor cells, endothelial cells, and suppressive immune cell subsets such as Tregs and myeloid-derived suppressor cells.44,45 In particular, CD73 has been shown to be relevant in cell migration,46 adhesion,47 cell apoptosis,48 exosome immunosuppressive activity,49 cancer metastasis,50 and drug resistance.51 Several recent studies have revealed that targeted CD73 inhibition reduced tumorigenesis and metastasis and enhanced the potency of T-cell–directed therapies.18–20,52,53 For instance, the knockdown of CD73 in ovarian cancer cells increased tumor-specific T-cell activation and expansion and decreased T-cell apoptosis in a series of in vitro and in vivo experiments. Consistent with previous reports, we observed that tumor CD73 downregulation is associated with better prognosis of glioblastoma patients as per TCGA mRNA survival analysis, which indicates that CD73 can be of great clinical potential.
In summary, we demonstrate that glioma-derived CD73 contributes to local adenosinergic immune suppression by collaboration with CD39 present on infiltrating CD4+CD39+ T lymphocytes, thus emphasizing the necessity of recognizing the tumor environment as an integrated system. Furthermore, our data give insight into the potential of blocking this synergic pathway to therapeutically initiate/increase host immune responses against malignant glioma and other immune-related diseases.
Supplementary Material
Funding
This work was supported by grants from the National Natural Science Foundation of China (81172404/81072062 to X.-G.L., 81072406/31270971 to X.Q.), the Shandong Provincial Outstanding Medical Academic Professional Program (to X.-G.L.), and the Special Foundation for Taishan Scholars (to X.-G.L.). Additional support was from the China Scholarship Council (to S.X.) and the Shandong Provincial Foundation for Distinguished Young Scholars (BS2012YY016 to B.H.).
Supplementary Material
Acknowledgments
We thank Dr Gang Li and Dr Qing-Lin Liu for kindly providing the U-87 MG cell line. We also thank Ali Alam for manuscript proofreading.
Conflict of interest statement. None declared.
References
- 1.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heimberger AB, Sampson JH. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro Oncol. 2011;13(1):3–13. doi: 10.1093/neuonc/noq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin. 2012;62(5):309–335. doi: 10.3322/caac.20132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu JS, Wheeler CJ, Zeltzer PM, et al. Vaccination of malignant glioma patients with peptide-pulsed dendritic cells elicits systemic cytotoxicity and intracranial T-cell infiltration. Cancer Res. 2001;61(3):842–847. [PubMed] [Google Scholar]
- 8.De Vleeschouwer S, Fieuws S, Rutkowski S, et al. Postoperative adjuvant dendritic cell-based immunotherapy in patients with relapsed glioblastoma multiforme. Clin Cancer Res. 2008;14(10):3098–3104. doi: 10.1158/1078-0432.CCR-07-4875. [DOI] [PubMed] [Google Scholar]
- 9.Sampson JH, Heimberger AB, Archer GE, et al. Immunologic escape after prolongd progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–4729. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albesiano E, Han JE, Lim M. Mechanisms of local immunoresistance in glioma. Neurosurg Clin N Am. 2010;21(1):17–29. doi: 10.1016/j.nec.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 12.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene. 2010;29(39):5346–5358. doi: 10.1038/onc.2010.292. [DOI] [PubMed] [Google Scholar]
- 13.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783(5):673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Ernst PB, Garrison JC, Thompson LF. Much ado about adenosine: adenosine synthesis and function in regulatory T cell biology. J Immunol. 2010;185(4):1993–1998. doi: 10.4049/jimmunol.1000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasko G, Pacher P. Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):865–869. doi: 10.1161/ATVBAHA.111.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zarek PE, Huang CT, Lutz ER, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111(1):251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene. 2012;32(14):1743–1751. doi: 10.1038/onc.2012.269. [DOI] [PubMed] [Google Scholar]
- 18.Jin D, Fan J, Wang L, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70(6):2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stagg J, Divisekera U, McLaughlin N, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107(4):1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stagg J, Divisekera U, Duret H, et al. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71(8):2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 21.Wink MR, Lenz G, Braganhol E, et al. Altered extracellular ATP, ADP and AMP catabolism in glioma cell lines. Cancer Lett. 2003;198(2):211–218. doi: 10.1016/s0304-3835(03)00308-2. [DOI] [PubMed] [Google Scholar]
- 22.Morrone FB, Oliveira DL, Gamermann P, et al. In vivo glioblastoma growth is reduced by apyrase activity in a rat glioma model. BMC Cancer. 2006;6:226. doi: 10.1186/1471-2407-6-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110(4):1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 25.Moncrieffe H, Nistala K, Kamhieh Y, et al. High expression of the ectonucleotidase CD39 on T cells from the inflamed site identifies two distinct populations, one regulatory and one memory T cell population. J Immunol. 2010;185(1):134–143. doi: 10.4049/jimmunol.0803474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuler PJ, Schilling B, Harasymczuk M, et al. Phenotypic and functional characteristics of CD4+ CD39+ FOXP3+ and CD4+ CD39+ FOXP3neg T-cell subsets in cancer patients. Eur J Immunol. 2012;42(7):1876–1885. doi: 10.1002/eji.201142347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Hernandez MH, Portales-Cervantes L, Cortez-Espinosa N, et al. Expression and function of P2X(7) receptor and CD39/Entpd1 in patients with type 2 diabetes and their association with biochemical parameters. Cell Immunol. 2011;269(2):135–143. doi: 10.1016/j.cellimm.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Braganhol E, Huppes D, Bernardi A, Wink MR, Lenz G, Battastini AM. A comparative study of ectonucleotidase and P2 receptor mRNA profiles in C6 cell line cultures and C6 ex vivo glioma model. Cell Tissue Res. 2009;335(2):331–340. doi: 10.1007/s00441-008-0723-4. [DOI] [PubMed] [Google Scholar]
- 30.Hussain SF, Yang D, Suki D, Aldape K, Grimm E, Heimberger AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006;8(3):261–279. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandapathil M, Hilldorfer B, Szczepanski MJ, et al. Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem. 2010;285(10):7176–7186. doi: 10.1074/jbc.M109.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14(16):5166–5172. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 33.Hart ML, Kohler D, Eckle T, Kloor D, Stahl GL, Eltzschig HK. Direct treatment of mouse or human blood with soluble 5′-nucleotidase inhibits platelet aggregation. Arterioscler Thromb Vasc Biol. 2008;28(8):1477–1483. doi: 10.1161/ATVBAHA.108.169219. [DOI] [PubMed] [Google Scholar]
- 34.Sun X, Wu Y, Gao W, et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139(3):1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ndhlovu LC, Leal FE, Eccles-James IG, et al. A novel human CD4+ T-cell inducer subset with potent immunostimulatory properties. Eur J Immunol. 2010;40(1):134–141. doi: 10.1002/eji.200939258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuler PJ, Harasymczuk M, Schilling B, Lang S, Whiteside TL. Separation of human CD4+CD39+ T cells by magnetic beads reveals two phenotypically and functionally different subsets. J Immunol Methods. 2011;369(1–2):59–68. doi: 10.1016/j.jim.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Q, Yan J, Putheti P, et al. Isolated CD39 expression on CD4+ T cells denotes both regulatory and memory populations. Am J Transplant. 2009;9(10):2303–2311. doi: 10.1111/j.1600-6143.2009.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sitkovsky MV, Kjaergaard J, Lukashev D, Ohta A. Hypoxia-adenosinergic immunosuppression: tumor protection by T regulatory cells and cancerous tissue hypoxia. Clin Cancer Res. 2008;14(19):5947–5952. doi: 10.1158/1078-0432.CCR-08-0229. [DOI] [PubMed] [Google Scholar]
- 39.Dwyer KM, Hanidziar D, Putheti P, et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am J Transplant. 2010;10(11):2410–2420. doi: 10.1111/j.1600-6143.2010.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilchey SP, Kobie JJ, Cochran MR, et al. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. 2009;183(10):6157–6166. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eltzschig HK, Kohler D, Eckle T, Kong T, Robson SC, Colgan SP. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood. 2009;113(1):224–232. doi: 10.1182/blood-2008-06-165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Synnestvedt K, Furuta GT, Comerford KM, et al. Ecto-5′-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110(7):993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gorlach A. Control of adenosine transport by hypoxia. Circ Res. 2005;97(1):1–3. doi: 10.1161/01.RES.0000174112.36064.77. [DOI] [PubMed] [Google Scholar]
- 44.Beavis PA, Stagg J, Darcy PK, Smyth MJ. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33(5):231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 45.Quezada C, Garrido W, Oyarzun C, et al. 5′-ectonucleotidase mediates multiple-drug resistance in glioblastoma multiforme cells. J Cell Physiol. 2013;228(3):602–608. doi: 10.1002/jcp.24168. [DOI] [PubMed] [Google Scholar]
- 46.Mills JH, Thompson LF, Mueller C, et al. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105(27):9325–9330. doi: 10.1073/pnas.0711175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cappellari AR, Vasques GJ, Bavaresco L, Braganhol E, Battastini AM. Involvement of ecto-5′-nucleotidase/CD73 in U138MG glioma cell adhesion. Mol Cell Biochem. 2012;359(1–2):315–322. doi: 10.1007/s11010-011-1025-9. [DOI] [PubMed] [Google Scholar]
- 48.Zhi X, Wang Y, Zhou X, et al. RNAi-mediated CD73 suppression induces apoptosis and cell-cycle arrest in human breast cancer cells. Cancer Sci. 2010;101(12):2561–2569. doi: 10.1111/j.1349-7006.2010.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clayton A, Al-Taei S, Webber J, Mason MD, Tabi Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187(2):676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 50.Wang H, Lee S, Nigro CL, et al. NT5E (CD73) is epigenetically regulated in malignant melanoma and associated with metastatic site specificity. Br J Cancer. 2012;106(8):1446–1452. doi: 10.1038/bjc.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Serra S, Horenstein AL, Vaisitti T, et al. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood. 2011;118(23):6141–6152. doi: 10.1182/blood-2011-08-374728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stagg J, Beavis PA, Divisekera U, et al. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72(9):2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 53.Zhang B. CD73: a novel target for cancer immunotherapy. Cancer Res. 2010;70(16):6407–6411. doi: 10.1158/0008-5472.CAN-10-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.