Abstract
Background
Cerebral radiation necrosis (RN) is a difficult to treat complication of stereotactic radiosurgery (SRS) that can result in progressive neurologic decline. Currently, steroids are the standard of care treatment for brain RN despite their adverse effect profile and limited efficacy. The purpose of this study was to evaluate the treatment efficacy of cerebral RN to bevacizumab in patients with brain metastases previously treated with SRS.
Methods
We retrospectively reviewed 14 lesions in 11 patients treated with bevacizumab for brain RN secondary to SRS for their brain metastases. Steroid dosing, RN-associated symptoms, and magnetic resonance imaging (MRI) scans were examined before, during, and after bevacizumab administration.
Results
Of the 11 patients included, 6 had metastatic non–small cell lung cancer, and 5 had metastatic breast cancer. The mean percentage decrease in RN volume seen on T1 post-Gadolinium and fluid-attenuated inversion recovery (FLAIR) MRI at first follow-up, at a mean of 26 days (range, 15–43 days), was 64.4% and 64.3%, respectively. MRI changes were sustained on follow-up MRI scans, obtained at a mean of 33 days (range, 7–58 days) after bevacizumab discontinuation. After bevacizumab treatment, all patients initially receiving steroids had a reduction in steroid requirement, and all but one had an improvement in or stability of RN-associated symptoms. No patients experienced intratumoral bleeds or other adverse effects related to their bevacizumab treatment.
Conclusions
Bevacizumab is effective and safe for the treatment of RN after SRS for brain metastasis. In this context, bevacizumab offers symptomatic relief, a reduction in steroid requirement, and a dramatic radiographic response.
Keywords: bevicizumab, radiation, radionecrosis, stereotactic
Cerebral metastases are the most common tumors found in the central nervous system.1 Once diagnosed, treatment usually requires surgery, radiation therapy, or both. Radiation therapy provides a noninvasive treatment of brain metastases for palliation and, in some cases, survival prolongation.2 Stereotactic radiosurgery (SRS) is a common approach for patients with limited brain metastasis and is an effective, efficient, and generally well-tolerated form of radiation for these patients. Unfortunately, SRS leads to radionecrosis (RN) in ∼10% of patients, potentially resulting in progressive neurologic decline.3 Currently, steroids are the standard of care treatment for brain RN despite their adverse effect profile.4 Antiplatelet, anticoagulation, and hyperbaric regimens have been evaluated, although definitive evidence of their efficacy has yet to be shown.5 Surgery can provide symptomatic relief but may be associated with a high risk of complications and/or neurological worsening.6
The etiology of RN is still under investigation. The long-standing explanation points to an underlying endothelial cell dysfunction that, in turn, results in increased capillary permeability and extracellular edema.7 However, there is recent evidence that points to the role of a chronic inflammatory process.8 Affected neurons also experience demyelination and, ultimately, necrosis secondary to small-vessel occlusive disease.3 The resulting neurologic deficits seen clinically can be the direct result of the necrosis or the indirect result of increased intracranial pressure.9
Evidence has recently emerged that supports the role of vascular endothelial growth factor (VEGF) as a contributor to RN pathogenesis. Although classically characterized as an angiogenic factor, VEGF also potentiates capillary permeability.10 Further studies have revealed overexpression of VEGF in resected RN lesions.11 In addition, the degree of radiation injury has been correlated with the amount of VEGF expression.12 Such studies have suggested that VEGF blocking agents, such as bevacizumab, may have a role in treating RN.4
Recent retrospective and prospective studies have shown significant decreases in volume on T2-weighted fluid-attenuated inversion recovery (FLAIR) and T1-weighted gadolinium-enhanced magnetic resonance imaging (MRI) in patients treated with bevacizumab.4,13,14 However, these studies have focused primarily on necrosis after radiation therapy for primary brain tumors. In this study, our aim is to describe the imaging and clinical effects of bevacizumab treatment of brain RN secondary to SRS for brain metastases.
Materials and Methods
Patients
A retrospective search of a departmental database was conducted to identify patients treated by SRS for brain metastases and who subsequently received bevacizumab for RN over a 3-year period. After patients were selected, clinical and pathologic data were extracted from the electronic medical record. This study was approved by the institutional review board with use of a waiver of informed consent and was compliant with Health Insurance Portability and Accountability Act regulations.
Diagnosis
We determined RN to be present on the basis of the following clinical and radiographic features. First, the patient must have received high-dose, single-fraction or hypofractionated SRS. Second, the patient must have developed an enlarging contrast-enhancing mass lesion within the radiation fields on MRI after receiving SRS. Third, the imaging features of the lesions must be consistent with the presence of RN rather than recurrent tumor. This was based on conventional imaging features, such as a “Swiss cheese” or “soap bubble” appearance, and advanced imaging features, such as low-perfusion on dynamic susceptibility contrast MRI perfusion.15–17 Low uptake on 18F-fludeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) scan was also used as a diagnostic factor.18–20 All patients originally received a diagnosis by both MRI and PET/CT imaging. When available, the diagnosis was confirmed by surgical pathology.
Radiology
To assess the volume of the RN, FLAIR and T1 post-gadolinium contrast MRI images were analyzed. Each slice was 5 mm thick, and there was no interslice gap. T1 post-contrast images were used to measure enhancement and FLAIR images to measure perilesional edema. Collected images were placed into 1 of 4 groups: before bevacizumab treatment, at first follow-up, at second follow-up, and after bevacizumab treatment. All pre-bevacizumab images were collected within 21 days before starting bevacizumab treatment. The first follow-up images were collected within the first 45 days of treatment, and the second follow-up images were collected within 90 days of the first follow-up. The post-bevacizumab images were analyzed as they were made available, without a defined time frame. Volumes were segmented using semi-automated techniques with use of Volume Viewer 11.3 on an Advantage Workstation 4.6 (GE Healthcare, Milwaukee, WI) for T1 post-contrast MRI volume and in-house contouring software for FLAIR images. To reduce operator variance, the T1 post-contrast and FLAIR images were each evaluated by a single trained operator (both with 1 year experience in MRI) under the direct supervision of a board-certified radiologist holding a Certificate of Added Qualification in neuroradiology (with 13 years of experience in MRI).
Clinical Response
Clinical symptoms related to brain RN were identified and documented over time. Because of the clinical complexity of the patients involved, defining clinical response was difficult. Many of our patients before, during, and after treatment with bevacizumab had neurological symptoms that were multifactorial in origin. Therefore, we defined symptomatic response as a decrease in severity of symptoms thought to be secondary to RN only. Statistical analysis was performed using Wilcoxon signed-rank tests with use of Stata, version 11.1 (StataCorp LP, College Station, TX). Significance was set to P = .05.
Results
Clinical Data
We identified 14 lesions in 11 patients with metastatic brain tumor who were treated with bevacizumab for RN that was attributable to treatment for metastatic non–small cell lung cancer (n = 6) or invasive ductal breast cancer (n = 5) (Table 1). The median number of metastases before radiation was 1.3 (range, 1–4). Of the 9 patients who received SRS, 5 had received whole brain radiation (WBRT) either prior or subsequent SRS (Table 1). Two patients received 5-fraction SRS only. The mean time from last radiation therapy to diagnosis of RN was 12.4 months and from RN to initiation of bevacizumab treatment was 59.6 days. Only 2 patients had biopsy-confirmed RN before bevacizumab administration. The majority of patients received doses of 10 mg/kg every 2 weeks. The mean duration of bevacizumab administration was 96.2 days, (range, 2–62). The difference in regimen duration represents our institution's movement from an extended (≥10 weeks) regimen to a more abbreviated course (≤6 weeks). The one patient who received 62 weeks of bevacizumab therapy was being treated concurrently for systemic metastases.
Table 1.
Patient characteristics
| Patient | Lesion | Sex | Age | Primary tumor | WBRT (Gy) | SRS (Gy) | Radionecrosis site | Method of diagnosis | Symptoms | Bevacizumab dosage | Bevacizumab treatment duration (weeks) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | M | 58 | NSCLC | 37.5 | 25 | Right frontal | Imaging | Noneb | 10 mg/kg q 2 weeks | 10 |
| 2 | 25 | Left temporal | |||||||||
| 2 | 3 | F | 50 | Breast | 30a | Right occipital | Imaging | Visual field disturbance, headaches | 10 mg/kg q 2 weeks | 10 | |
| 3 | 4 | F | 27 | Breast | 37.5 | 18 | Left Frontal | Imaging | Seizures | 10 mg/kg q 2 weeks | 6 |
| 5 | 21 | Left temporal | |||||||||
| 6 | 21 | Right parietal | |||||||||
| 4 | 7 | F | 79 | NSCLC | 18 | Right Parietal | Imaging | Lower left leg weakness | 10 mg/kg q 2 weeks | 2 | |
| 5 | 8 | F | 67 | Breast | 30 | 18 | Cerebellum | Imaging | Headaches, lower leg weakness | 10 mg/kg q 2 weeks | 62b |
| 6 | 9 | F | 54 | Breast | 37.5 | 15 | Right Frontal | Surgery | Left arm weakness | 10 mg/kg q 2 weeks | 16 |
| 7 | 10 | M | 67 | NSCLC | 30a | Right Frontal | Imaging | Seizures, left sided weakness | 15 mg/kg q 6 weeks | 12 | |
| 8 | 11 | F | 50 | Breast | 35 | 21 | Right Frontal | Imaging | Fatigue, lethargy, facial asymmetry | 7.5 mg/kg q 3 weeks | 6 |
| 9 | 12 | F | 67 | NSCLC | 21 | Left Occipital | Imaging | Confusion, visual hallucinations | 10 mg/kg q 2 weeks | 4 | |
| 10 | 13 | M | 73 | NSCLC | 21 | Left Parietal | Imaging | Seizure, right-sided hemiparesis | 10 mg/kg q 2 weeks | - | |
| 11 | 14 | M | 63 | NSCLC | 21 | Left Occipital | Surgery | Imbalance, right sided tinnitis | 15 mg/kg q 4 weeks | 8 |
Abbreviations: NSCLC, non-small cell lung cancer; SRS, stereotactic radiosurgery; WBRT, whole brain radiation therapy.
aPatient received 5-fraction stereotactic radiosurgery.
bBevacizumab use for systemic metastatic disease in addition to radiation necrosis.
Imaging Results
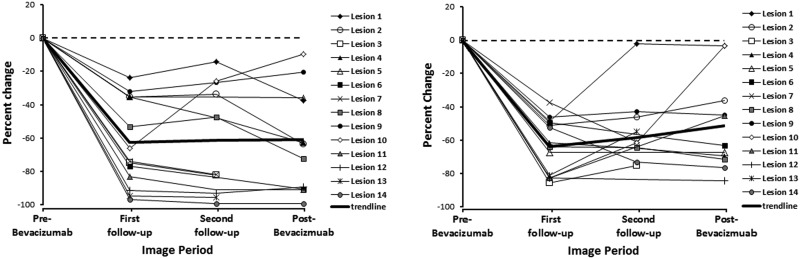
The pre-bevacizumab MRI was obtained a mean of 10 days (range, 4–21 days) before bevacizumab treatment initiation. The mean initial volumes of the enhancing lesions on T1 post-contrast images and hyperintense nonenhancing lesions on FLAIR images were 69.9 cm3 (range, 21.2–168.5 cm3) and 8.3 cm3 (range, 2.5–16.1 cm3), respectively. The first follow-up MRI was obtained a mean of 26 days (range, 15–43 days) after bevacizumab treatment initiation, and the second follow-up MRI was obtained a mean of 49 days (range, 21–84 days) after the initial follow-up MRI. From the pre-bevacizumab to first follow-up scans (n = 14), the enhancing RN volume decreased by a mean of 64.4% (range, 23.8%–96.8%), and the nonenhancing RN volume decreased by 64.3% (range, 47.0%–87.3%; P = .001). The original volume decreases in enhancing and nonehancing images were maintained in the second follow-up scans, with a 66.6% (range, 14.4%–99.4%) and 62.7% (range, 2.1%–76.4%), respectively (P = .005) decrease from baseline (Figs. 1–2).
Fig. 1.
Linear plot of percentage change in necrosis volumes on (A) T1-weighted post-gadolinium contrast magnetic resonance imaging and (B) T2-weighted fluid-attenuated inversion recovery magnetic resonance imaging, before bevacizumab treatment, at first follow-up, at second follow-up, and after bevacizumab treatment.
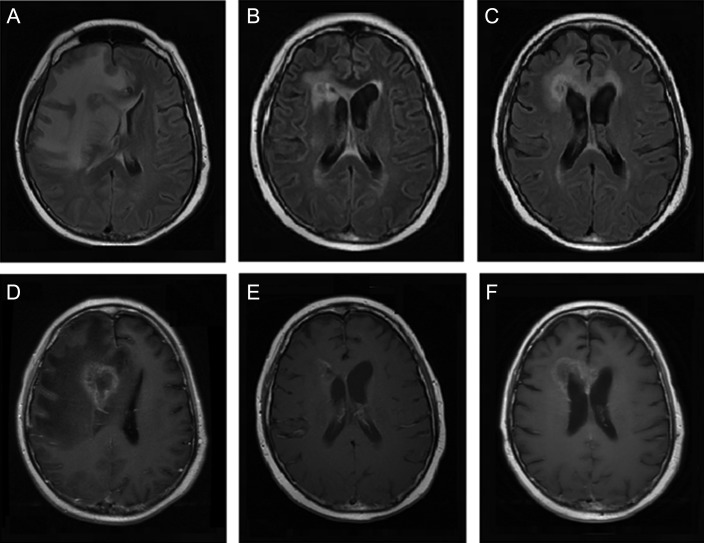
Fig. 2.
Axial (A–C) T2-weighted fluid-attenuated inversion recovery magnetic resonance imaging and (D–F) T1-weighted post-gadolinium contrast magnetic resonance imaging of patient 8 before bevacizumab treatment (A,D), at first follow-up (B,E), and after bevacizumab treatment (C,F).
For those lesions with post-bevacizumab imaging available (n = 12), the first follow-up scans were completed 33 days (range, 7–58 days) after bevacizumab treatment discontinuation. The mean percentage change in volume from pre-bevacizumab imaging was 67.1% (range, 9.7%–99.3%) for enhancing RN volume and 61.3% (range, 3.3%–86.0%) for nonenhancing RN volume (P = .018). The maximum decrease in T1 post-contrast and FLAIR RN volume was seen at first follow-up (n = 12). Lesions with long-term follow-up available (n = 7), were imaged 101 days (range, 75–121 days) after bevacuzimab treatment discontinuation. When compared with pre-bevacizumab RN volumes, there was a mean decrease of 52.2% (range, −99.2% to 42.1%) in enhancing and a mean increase of 56.7% (range, −76.2% to 316.2%) in nonenhancing RN volumes. When compared with pre-bevacizumab discontinuation volumes, there was a significant increase noted in both enhancing and nonenhancing RN volumes, with a 50.4% (range, −89.5 to 236%) and 289.4% (range, −7.3 to 1014.3%) mean increase noted, respectively (Table 2).
Table 2.
Long-term post-bevacizumab radiographic response
| Patient | Days after discontinuation | Volume change from pre-bevacizumab (%) |
Volume change from pre-discontinuation (%) |
||
|---|---|---|---|---|---|
| FLAIR | Post-contrast | FLAIR | Post-contrast | ||
| 3a | 75 | −60.5 | −38.4 | −38.6 | 64.6 |
| 3b | −89.5 | −2.5 | −89.5 | 209.5 | |
| 3c | −93.3 | 20.9 | 1.6 | 144.3 | |
| 6 | 103 | 17.2 | 247.7 | 196.4 | 514.5 |
| 7 | 101 | 49.1 | 316.2 | 236.1 | 1014.3 |
| 9 | 121 | −89.2 | −70.5 | 26.2 | 86 |
| 11 | 106 | −99.2 | −76.2 | 20.8 | −7.3 |
| Mean | 101.2 | −52.2 | 56.7 | 50.4 | 289.4 |
Abbreviations: FLAIR, fluid-attenuated inversion recovery MRI.
Clinical Results
Nine patients were receiving steroids for control of the RN-associated symptoms before treatment with bevacizumab. The 2 patients who were not initially treated with steroids had extensive metastatic disease, an additional indication for bevacizumab treatment. The pre-bevacizumab steroid requirement was a median of 7.4 mg/day (range, 0.5–24 mg/day). By the first follow-up, all patients receiving steroids experienced a decrease in their daily requirement, and 7 patients (77.8%) were weaned off steroids entirely (Table 2). All but one patient experienced either stable or improved symptoms during bevacizumab treatment (Table 3). Of the 11 patients, 7 were living at the time of study end, and 3 died due to progression of systemic disease. Patient 3 ultimately had a rapidly progressive and treatment refractory course after initial treatment with bevacizumab. The patient showed little to no response to hyperbaric oxygen treatment and an additional round of bevacizumab treatment. Because autopsy was not done to establish cause of death, the patient's death was thought to be secondary to either progression of brain metastases or RN.
Table 3.
Clinical response to bevacizumab treatment
| Patient | Change in daily steroid dose (mg) | Symptomatic response | Reason for discontinuation |
|---|---|---|---|
| 1 | 8–> 0 | Worsened | No symptomatic response |
| 2 | 2–> 0.3 | Improved | Good response |
| 3 | 4–> 0 | Improved* | Good response |
| 4 | 0.2–> 0 | Stable | No symptomatic response |
| 5 | – | Improved | Good response |
| 6 | – | Improved | Current |
| 7 | 24–> 0 | Stable | No symptomatic response |
| 8 | 20–> 0 | Improved | Good response |
| 9 | 6–> 0 | Improved | Good response |
| 10 | 8–> 2 | Improved | Current |
| 11 | 8–> 0 | Stable | Current |
*Patient also treated with anti-epileptic, potentially contributing to response.
Discussion
Brain RN is a type of radiation injury that can result in dramatic radiographic and clinical changes.3 Although radiation injury can occur at any point during or after radiation treatment, RN is thought to occur months to years after treatment.21 The resultant lesions are often identified during routine follow-up imaging or when new neurologic symptoms are investigated.3 Common symptoms include psychomotor slowing, seizures, sensorimotor deficits, and language impairment.22 of interest, imaging can be suggestive of RN without clinical symptoms.23 The incidence of brain RN is dependent on dose, fractionation, and volume and, perhaps, may be related to other factors, such as chemotherapy exposure, anatomic location of tumor/treatment, and factors inherent to patients.24 Evidence of radiographic RN has been estimated to occur in up to 46% of patients, and symptomatic RN has been shown to occur in up to 14%.25
Although a variety of treatments for RN have been proposed, few have shown definitive efficacy.3 The current standard of care is corticosteroids. However, because of the associated adverse effect profile, there has been a significant need for an alternative.4 Antiplatelet and anticoagulant regimens have been proposed, targeting the proposed underlying vascular changes associated with RN. Specifically, pentoxyfylline, ticlopidine, and aspirin have been examined, although effects were minimal, and to date, there are no prospective trials that support any clinical or radiographic benefit.5 Anticoagulation with warfarin and heparin has shown a modest benefit, although these studies were small, and the risk of bleeding and adverse reactions with chemotherapeutics remains significant.26 Hyperbaric oxygen treatment is an additional treatment option typically reserved for progressive and refractory cases. Unfortunately, because of the expense and minimal supporting evidence, routine treatment with this technique is not justified.27
Bevacizumab, an anti-VEGF antibody, has been proposed as treatment for RN. Gonzalez et al. conducted a retrospective study of 8 patients with primary brain cancer treated with bevacizumab for RN diagnosed by imaging. After a mean of 8.1 weeks from the start of bevacizumab treatment, there was a reported 48% and 60% decrease in maximum bidirectional measurements for post-contrast T1 and FLAIR images, respectively.4 Torcuator et al. conducted a similar study evaluating the use of bevacizumab in 6 patients with biopsy-proven brain RN. Percentage change in volume was found to be 79% for post-contrast studies and 49% for FLAIR images.13 More recently, Levin et al. conducted a prospective randomized trial of patients with primary brain tumors and RN evaluating bevacizumab's effect on radiographic and clinical activity. They showed a mean percentage change in RN volume on T1 post-contrast and FLAIR studies as 59% and 63%, respectively.14 Our results confirm that bevacizumab treatment can result in dramatic decreases in enhancement and edema.
Our results support bevacizumab's ability to diminish the radiographic and clinical manifestations of brain RN. The change in FLAIR and T1 post-contrast imaging seen among our patients was similar to studies done by Levin et al., Gonzalez et al., and Torcuator et al. The benefit of our study is that it shows bevacizumab's effect on RN caused by SRS for brain metastases, the most common cause of RN. Our patient cohort represents only 2 types of primary cancers: breast and non–squamus cell lung, which are the most common type of metastases treated with SRS at our center. Of importance, we did not observe any adverse effects of bevacizumab in our patient group who had active systemic cancer. In addition, we showed that bevacizumab can affect large-volume and multifocal RN disease, because several of our patients had multifocal RN. Although RN lesion volumes increased slightly after initial bevacizumab treatment discontinuation, post-bevacizumab enhancement is markedly decreased, compared with pre-bevacizumab imaging. The long-term radiographic response was limited in number and yielded a mixed response with a large variation. Two of the 5 patients had clear RN progression on long-term follow-up, a significantly increased proportion, compared with results reported by Levin et al. This diminishing response can be explained by the decreasing concentrations of the drug, with the half-life of bevacizumab being 20 days (range, 11–50 days).28 However, 3 of the 5 patients with long-term follow-up had enduring responses, with RN volumes smaller than pre-bevacizumab volumes.
The radiographic and clinical changes seen in this study are most likely explained by bevacizumab's anti-VEGF properties. Because VEGF is known to increase vessel permeability, a blockage of VEGF would lead to decreased vessel permeability and resultant edema.7 Of consequence, a decrease in abnormality on T1 post-contrast and FLAIR MRI would be expected.4 However, this conclusion is limited by the diagnostic methods available. The majority of our patients received a diagnosis of RN by clinical and radiographic—not pathologic—evidence. Therefore, it is possible that some of the lesions identified could represent either active metastatic disease or a mix of RN and active metastatic disease. Indeed, small studies have documented the ability of bevacizumab to decrease the size of non-small-cell lung cancer and breast metastases.29,30 It is also possible the coadministration of dexamethasone could contribute to the percentage change in signal abnormality and diminution of clinical symptoms. However, the prompt dexamethasone tapering in each patient and the prolonged changes seen make this explanation unlikely. This study is also limited by a retrospective design. Radiation necrosis can have a relapsing and remitting course. Therefore, because measurements were made retrospectively, a proportion of diminishing volumes could be secondary to the natural course of the pathology. A retrospective review also limits the ability to attribute clinical response to bevacizumab alone rather than alternative variables. In addition, it also limits the ability to detect adverse effects related to therapy.
In the future, further studies are needed to establish diagnostic and treatment regimens. The results of our study support the use of bevacizumab as treatment for brain RN secondary to treatment of brain metastases. However, pure RN remains a difficult diagnosis with clinical and current radiographic methods. Although our study supports the use of an abbreviated regimen of bevacizumab treatment, studies comparing dose schedules are also needed. Despite our study's small numbers and the lack of an adequate control group, the dramatic decrease in the clinical and radiographic manifestations of brain RN should prompt further studies evaluating bevacizumab treatment for patients with brain RN after treatment for brain metastases.
Funding
None declared.
Conflict of interest statement. None declared.
Acknowledgments
We thank the Physics Department at Memorial Sloan-Kettering, for their excellent technical support, and Eve Ferdman, for her expert editorial assistance.
References
- 1.Patel TR, Knisely JP, Chiang VL. Management of brain metastases: surgery, radiation, or both? Hematol Oncol Clin North Am. 2012;26:933–947. doi: 10.1016/j.hoc.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665–1672. doi: 10.1016/S0140-6736(04)16250-8. [DOI] [PubMed] [Google Scholar]
- 3.Giglio P, Gilbert MR. Cerebral RN. Neurologist. 2003;9:180–188. doi: 10.1097/01.nrl.0000080951.78533.c4. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 5.Glantz MJ, Burger PC, Friedman AH, Radtke RA, Massey EW, Schold SC., Jr. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994;44:2020–2027. doi: 10.1212/wnl.44.11.2020. [DOI] [PubMed] [Google Scholar]
- 6.McPherson CM, Warnick RE. Results of contemporary surgical management of RN using frameless stereotaxis and intraoperative magnetic resonance imaging. J Neurooncol. 2004;68:41–47. doi: 10.1023/b:neon.0000024744.16031.e9. [DOI] [PubMed] [Google Scholar]
- 7.Cheng KM, Chan CM, Fu YT, Ho LC, Cheung FC, Law CK. Acute hemorrhage in late RN of the temporal lobe: report of five cases and review of the literature. J Neurooncol. 2001;51:143–150. doi: 10.1023/a:1010631112015. [DOI] [PubMed] [Google Scholar]
- 8.Yoshii Y. Pathological review of late cerebral radionecrosis. Brain Tumor Pathol. 2008;25:51–58. doi: 10.1007/s10014-008-0233-9. [DOI] [PubMed] [Google Scholar]
- 9.Cross NE, Glantz MJ. Neurologic complications of radiation therapy. Neurol Clin. 2003;21:249–277. doi: 10.1016/s0733-8619(02)00031-2. [DOI] [PubMed] [Google Scholar]
- 10.Connolly DT, Heuvelman DM, Nelson R, et al. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nonoguchi N, Miyatake S, Fukumoto M, et al. The distribution of vascular endothelial growth factor-producing cells in clinical RN of the brain: pathological consideration of their potential roles. J Neurooncol. 2011;105:423–431. doi: 10.1007/s11060-011-0610-9. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Chung YG, Kim CY, Kim HK, Lee HK. Upregulation of VEGF and FGF2 in normal rat brain after experimental intraoperative radiation therapy. J Korean Med Sci. 2004;19:879–886. doi: 10.3346/jkms.2004.19.6.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torcuator R, Zuniga R, Mohan YS, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral RN. J Neurooncol. 2009;94:63–68. doi: 10.1007/s11060-009-9801-z. [DOI] [PubMed] [Google Scholar]
- 14.Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for RN of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–1495. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar AJ, Leeds NE, Fuller GN, et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217:377–384. doi: 10.1148/radiology.217.2.r00nv36377. [DOI] [PubMed] [Google Scholar]
- 16.Mullins ME, Barest GD, Schaefer PW, Hochberg FH, Gonzalez RG, Lev MH. Radiation necrosis versus glioma recurrence: conventional MR imaging clues to diagnosis. Am J Neuroradiol. 2005;26:1967–1972. [PMC free article] [PubMed] [Google Scholar]
- 17.Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Am J Neuroradiol. 2009;30:367–372. doi: 10.3174/ajnr.A1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatzoglou V, Ulaner GA, Zhang Z, Beal K, Holodny AI, Young RJ. Comparison of the effectiveness of MRI perfusion and fluorine-18 FDG PET-CT for differentiating radiation injury from viable brain tumor: a preliminary retrospective analysis with pathologic correlation in all patients. Clin Imaging. 2012;12(suppl.):0899–7071. doi: 10.1016/j.clinimag.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ricci PE, Karis JP, Heiserman JE, Fram EK, Bice AN, Drayer BP. Differentiating recurrent tumor from radiation necrosis: time for re-evaluation of positron emission tomography? Am J Neuroradiol. 1998;19:407–413. [PMC free article] [PubMed] [Google Scholar]
- 20.Di Chiro G, Oldfield E, Wright DC, et al. Cerebral necrosis after radiotherapy and/or intraarterial chemotherapy for brain tumors: PET and neuropathologic studies. Am J Roentgenol. 1988;150:189–197. doi: 10.2214/ajr.150.1.189. [DOI] [PubMed] [Google Scholar]
- 21.Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: A review. Front Oncol. 2012;2:73. doi: 10.3389/fonc.2012.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler JM, Rapp SR, Shaw EG. Managing the cognitive effects of brain tumor radiation therapy. Curr Treat Options Oncol. 2006;7:517–523. doi: 10.1007/s11864-006-0026-5. [DOI] [PubMed] [Google Scholar]
- 23.Stockham AL, Tievsky AL, Koyfman SA, et al. Conventional MRI does not reliably distinguish RN from tumor recurrence after stereotactic radiosurgery. J Neurooncol. 2012;109:149–158. doi: 10.1007/s11060-012-0881-9. [DOI] [PubMed] [Google Scholar]
- 24.Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;15:46–48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 26.De Braganca KC, Janjigian YY, Azzoli CG, et al. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J Neurooncol. 2010;100:443–447. doi: 10.1007/s11060-010-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mathews MS, Linskey ME, Hasso AN, Fruehauf JP. The effect of bevacizumab(Avastin) on neuroimaging of brain metastases. Surg Neurol. 2008;70:649–652. doi: 10.1016/j.surneu.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 28.Reidy DL, Chung KY, Timoney JP, et al. Bevacizumab 5 mg/kg can be infused safely over 10 minutes. J Clin Oncol. 2007;25:2691–5. doi: 10.1200/JCO.2006.09.3351. [DOI] [PubMed] [Google Scholar]
- 29.Happold C, Ernemann U, Roth P, Wick W, Weller M, Schmidt F. Anticoagulation for radiation-induced neurotoxicity revisited. J Neurooncology. 2008;90:357–362. doi: 10.1007/s11060-008-9674-6. [DOI] [PubMed] [Google Scholar]
- 30.Chuba PJ, Aronin P, Bhambhani K, et al. Hyperbaric oxygen therapy for radiation-induced brain injury in children. Cancer. 1997;80:2005–2012. doi: 10.1002/(sici)1097-0142(19971115)80:10<2005::aid-cncr19>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]