Summary
Mast cells play a critical role in the pathogenesis of allergic diseases. How mast cell function is regulated is still not well understood. Both phosphatidic acid (PA) and diacylglycerol (DAG) are important second messengers involved in mast cell activation. Lipin1 is a phosphatidate phosphatase that hydrolyzes PA to produce DAG. The role of lipin1 in mast cell function has been unknown. In this report, we show that lipin1 is an important and selective inhibitor of mast cell degranulation. Lipin1 deficiency enhanced FcεRI-mediated β-hexosaminidase and prostaglandin D2 release from mast cells in vitro and exacerbated the passive systemic anaphylaxis reaction in vivo. However, Lipin1 deficiency did not exert obvious effects on IL-6 or TNF-α production following FcεRI engagement. FcεRI-induced PKC and SNAP-23 phosphorylation was augmented in the lipin1-deficient mast cells. Moreover, inhibition of PKC activity reduced SNAP-23 phosphorylation and mast cell degranulation in lipin1 deficient mast cells. Together, our findings suggest that lipin1 may negatively control mast cell degranulation and anaphylactic response through inhibiting the PKC-SNAP-23 pathway.
Keywords: Mast cells, lipin1, Phosphatidic acid, PKC, SNAP-23
Introduction
Mast cells express FcεRI, the high-affinity receptor for IgE, on their surface. Cross-linking of IgE-bound FcεRI by antigens or allergens leads to downstream signal transduction and induces the allergic response [1-4]. During the early-phase of the reaction, mast cells degranulate and release active mediators such as histamine and leukotrienes that are critical for type I hypersensitivity. In the late-phase reaction, mast cells secrete cytokines such as IL-6 and TNF-α after transcription and translation of these molecules, contributing to chronic inflammation [2, 3]. The mechanisms by which FcεRI induces degranulation and cytokine production in mast cells remain poorly understood.
FcεRI-mediated signaling initiates phosphorylation of ITAMs within the FcεRI β and γ subunits by the Src-family protein tyrosine kinases (PTKs), such as Lyn, Syk and Fyn [5, 6]. These PTKs induce the phosphorylation and reorganization of downstream molecules, including LAT [7], SLP76 [8], Vav1 [9], Bruton's tyrosine kinase [10], PI3K [11], and phospholipase Cγ (PLCγ).[12, 13] PLCγ hydrolyzes the membrane phospholipid phosphatidylinositol 4,5-bisphosphate (PIP2), generating diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). IP3 induces Ca2+ influx while DAG activates Ras-Erk1/2 and PKC signaling, which consequently results in mast cell activation [14-17].
In addition to these ‘classical’ signaling cascades, evidence has also implicated phosphatidic acid (PA) as an important second messenger for mast cell function. In mast cells, both phospholipase D 1 and 2 (PLD1/2), and DAG kinase ζ (DGKζ) have been found to participate in PA generation following FcεRI stimulation. The PLDs hydrolyze phosphatidic choline to produce PA, while the DGKs phosphorylate DAG to produce PA. Interestingly, decreases in PLD-derived PA or DGKζ-derived PA production both lead to diminished mast cell degranulation [16, 18, 19]. These studies suggest a positive role of PA in mast cell degranulation and raise questions about regulation of the PA concentration in proper mast cell function.
The lpin1 gene encoding the lipin1 protein was cloned from fatty liver dystrophy (fld) mice [20]. Lipin1 deficiency causes lipodystrophy, insulin resistance, fatty liver and hypertriglyceridemia in fld mice [21, 22]. Lipin1 is detected in a wide variety of tissues with the highest levels in adipose tissue, skeletal muscle and testis [23, 24]. The lipin family of enzymes consist of three members, which initiate Mg2+-dependent PAP1 activity by hydrolyzing PA to produce DAG [25]. Lipin1 appears to possess the highest PAP1 activity within the family [26]. In this study, we investigated whether lipin1 regulates mast cell effector functions using fld mice. We demonstrated that lipin1-deficiency does not affect mast cell development or survival in vitro, but increases FcεRI-mediated degranulation in mast cells in vitro and in vivo. However, FcεRI-induced IL-6 and TNF-α production is not affected by lipin1 deficiency. It is suggested that lipin1 inhibits mast cell degranulation by reducing PKC-SNAP-23 signaling.
Results
Normal mast cell development in the absence of Lipin1
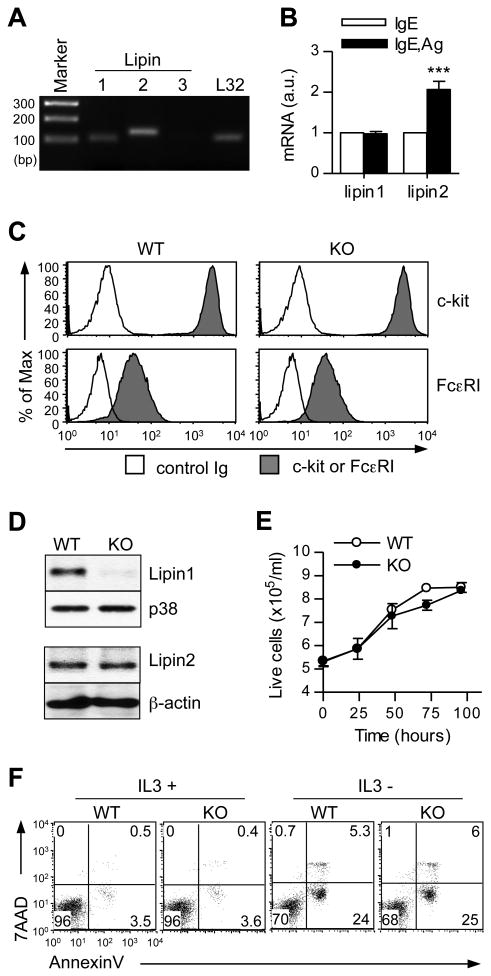
We first assessed the expression of lipin isoforms in mast cells. As shown in Fig. 1A, both lipin1 and lipin2 were detected in wild-type (WT) BMMCs, but lipin3 was undetectable. Following FcεRI stimulation, lipin1 expression was not obviously altered, but lipin2 expression was upregulated approximately 2-fold (Fig. 1B). To investigate the possible roles of lipin1 in mast cells, we generated BMMCs from WT and lipin1 deficient (fld) mice. After culturing bone marrow cells in IL-3 conditioned media for 3 weeks, the levels of FcεRI and c-Kit expression were comparable between the WT and lipin1-deficient BMMCs (Fig. 1C), suggesting that in vitro mast cell development is not affected by the loss of lipin1. Immunoblot analysis following immunoprecipitation demonstrated that both lipin1 and lipin 2 proteins were detected in WT BMMCs. However, only lipin2 but not lipin1 could be detected in lipin1-deficient BMMCs. The Lipin2 protein levels were similar between WT and lipin1-deficient BMMCs (Fig. 1D). In addition, lipin1-deficient BMMCs exhibited normal expansion and survival (Fig. 1E and F).
Figure 1.
Lipin1 deficiency does not affect mast cell development in vitro. (A) RT-PCR detection of mRNA encoding lipin1, 2 and 3 in WT BMMCs. (B) Lipin1 and 2 mRNA levels unstimulated or FcεRI stimulated WT BMMCs. Data are the means ± SE. a.u., arbitrary unit. (C) FACS analysis of FcεRI and c-kit surface expression on BMMCs. WT and lipin1 deficient (KO) BMMCs were loaded with IgE and then detected with an FITC-conjugated anti-IgE secondary antibody. Filled histogram, FcεRI or c-kit; solid line, control. (D) Lipin1 and Lipin2 protein expression in WT and lipin1 deficient BMMCs. WT and lipin1-KO BMMC lysates were subjected to immunoprecipitation (IP) and immunoblotting (IB) analysis using anti-lipin1 antibody. (E) Lipin1 deficiency does not affect mast cell expansion or survival. WT and lipin1-KO BMMCs were cultured in IL-3 conditioned media for the indicated times. Live cells were measured by trypan blue exclusion. Data shown are the mean ± SE from three paired samples. (F) FACS analysis for cell death. Apoptosis of BMMCs was determined by 7AAD and annexinV staining after culture in the presence or absence of IL-3 for 36 h. The data are representative of three experiments.
Nuclear localization of lipin1 in mast cells
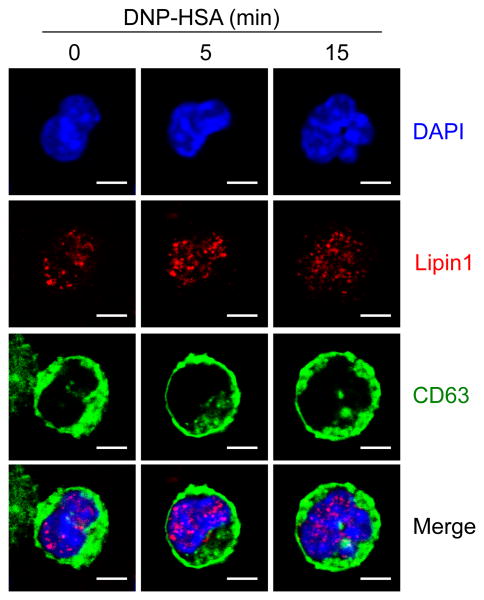
Subcellular localization of lipin1 is regulated by diverse forms of stimulation [27-30]. To investigate the effect of FcεRI stimulation on lipin1 localization, we transduced WT BMMCs with retrovirus expressing CD63-GFP fusion protein and monitored the location of CD63 and lipin1. CD63 is mainly expressed in the granules of mast cells and translocated to the plasma membrane after FcεRI aggregation [31]. Before FcεRI stimulation, the mast cell granules were localized in the cytoplasm as reflected by CD63-GFP and lipin1 was visualized in nucleus (Fig. 2, left columns). After FcεRI stimulation, the granules were translocated to the plasma membrane as previously reported, but lipin1 was retained in the nucleus (Fig. 2, middle and right columns), suggesting that lipin1 is localized in the nuclei in mast cells, and that this nuclear localization is not influenced by FcεRI stimulation.
Figure 2.
Subcellular localization of lipin1 in mast cells. WT BMMCs transduced with GFP-CD63 retrovirus were left unstimulated or stimulated with Ag at the indicated times. Lipin1 was stained using a rabbit anti-lipin1 antibody and detected with a secondary Texas Red-conjugated anti-rabbit antibody, followed by visualization under confocal microscopy. Scale bar represents 3μm. original magnification, 630 ×.
Lipin1 deficiency enhances mast cell degranulation in vitro and passive systemic anaphylaxis in vivo
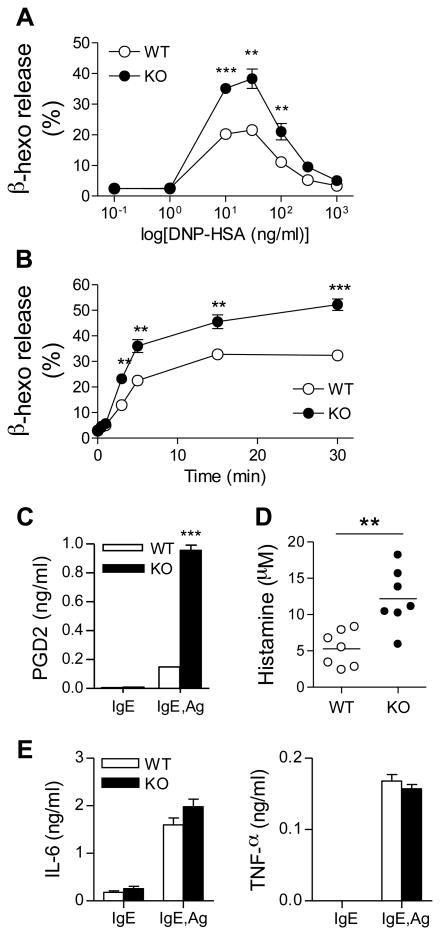
To investigate the roles of lipin1 in mast cell functions, we investigated FcεRI-mediated degranulation in mast cells. IgE-sensitized BMMCs were stimulated with DNP-HSA at the indicated concentrations to induce degranulation. The release of β-hexosaminidase was significantly increased in lipin1-deficient BMMCs, which effect was maximized at 30 ng/ml of DNP-HSA (Fig. 3A). The enhanced degranulation of lipin1-deficient BMMCs was also observed in a time course reaction using the optimal concentration of DNP-HSA (Fig. 3B). In addition, lipin-1 deficiency increased prostaglandin D2 (PGD2) secretion (Fig. 3C). We further assessed the in vivo allergic response by using a passive systemic anaphylaxis assay (PSA). WT and lipin1-deficient mice were injected intravenously with anti-DNP-IgE, followed by a systemic administration of DNP-HSA. Ninety seconds after antigen challenge, blood histamine levels were obviously increased in the lipin-1 deficient mice compared to the WT mice (Fig. 3D). Taken together, these observations indicate that lipin1 negatively controls mast cell degranulation both in vitro and in vivo.
Figure 3.
Increased sensitivity to passive systemic anaphylaxis and degranulation due to lipin1-deficiency. (A) Enhanced IgE-mediated degranulation in lipin1-KO BMMCs. IgE-preloaded BMMCs were stimulated with the indicated concentration of DNP-HSA for 45 min and β-hexosaminidase (β-hexo) activity was determined by colorimetric analysis. (B) Mast cell degranulation was accessed by a time course stimulation with an optimal concentration of Ag (30 ng/ml). (C) Increased PGD2 release by lipin1 deficient BMMCs. PGD2 concentrations in medium of WT and lipin1-KO BMMCs stimulated with 30 ng/ml DNP-HSA for 30 minutes were measured by ELISA. (D) WT and lipin1-KO mice were injected intravenously with IgE. After 24 hr, mice were injected intravenously with DNP-HSA, and plasma histamine levels were quantified with ELISA. (E) IgE-mediated cytokine production. IgE-sensitized BMMCs were left unstimulated or were stimulated with DNP-HSA (30 ng/ml). The amounts of IL-6 and TNF-α in cultural medium were measured by ELISA (means ± SE). Data are representative of three (A, C, E) and two (B, D) experiments. **, p<0.01 as determined by Student t-test.
Effect of lipin1 deficiency on cytokine production by mast cells
FcεRI induced cytokine production by mast cells play important roles in mast cell mediated disease [3]. We investigated FcεRI-mediated cytokine production by lipin1-deficient mast cells. WT and lipin1-deficient BMMCs were sensitized and then left unstimulated or were stimulated with Ag for 6 h. As shown in Fig. 3E, the levels of IL-6 and TNF-α in the cultural supernatant were comparable in these cells. Thus, although lipin1 deficiency enhances FcεRI-mediated degranulation, it has a minimal influence on cytokine production.
Enhanced PKC and SNAP-23 phosphorylation in lipin1 deficient mast cells following FcεRI stimulation
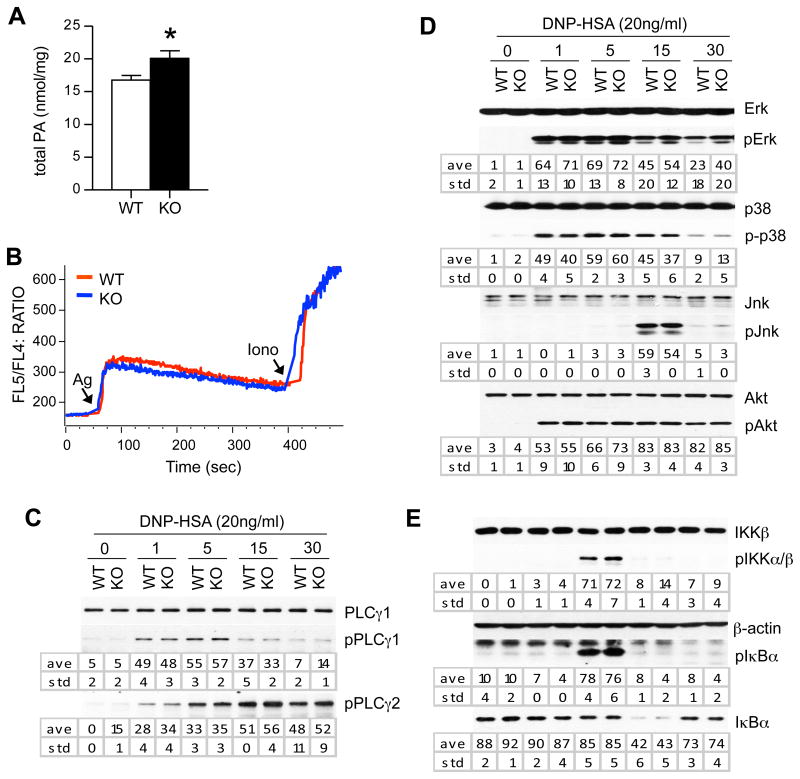
Lipin1 hydrolyzes PA to produce DAG. In lipin1-deficient BMMCs with normal expression of lipin2, PA concentration was 16 % higher than WT BMMCs (Fig. 4A), supporting that lipin1 is involved in PA metabolism in mast cells. The weak increase of PA is likely due to the presence of lipin2 in these cells. To understand the mechanisms by which lipin1 deficiency enhances mast cell degranulation, we examined signaling events downstream of FcεRI. WT and lipin1-deficient BMMCs were sensitized with anti-DNP-IgE and activated with DNP-HSA. The cells were lysed and analyzed by immunoblotting using the indicated antibodies. PLCγ1/2 are crucial for FcεRI-induced DAG and IP3 production and mast cell activation [1, 4, 13]. Ca++ influx and PLCγ1/2 phosphorylation was not obviously affected by lipin1 deficiency in mast cells (Fig. 4B, 4C), suggesting that lipin1 is not essential for PLCγ1/2 activation. MAPKs and PI3K/Akt play critical roles in mast cell activation [6]. There were no obvious differences in the phosphorylation of Erk1/2, Jnk, p38 or Akt between WT and lipin1 deficient mast cells following FcεRI stimulation (Fig. 4D). Furthermore, there were no drastic change in IKKα/β and IκBα phosphorylation and IκBα degradation between these cells, suggesting that FεRI-induced NF-κB signaling was not obviously affected by lipin1 deficiency in BMMCs (Fig. 4E). Thus, lipin1 deficiency does not obviously affect FcεRI-induced MAPK, NFκB, or PI3K/Akt activation.
Figure 4.
FcεRI-induced signaling in lipin1-deficient mast cells. (A) Phosphatidic acid concentraions in WT and lipin1-deficient BMMCs. (B) Calcium responses were visualized by flow cytometry using the FL5/FL4 ratio. iono, ionomycin. (C, D and E) BMMCs were sensitized with IgE for 4 hr and maintained in Tyrode's buffer for 1 hr. After stimulation with 20 ng/ml DNP-HSA for the different times indicated, cell lysates were subjected to immunoblotting analysis using the indicated antibodies. The band intensities were quantified by densitometry. The data shown are representative from three experiments. ave, average; std, standard deviation; *, p<0.05.
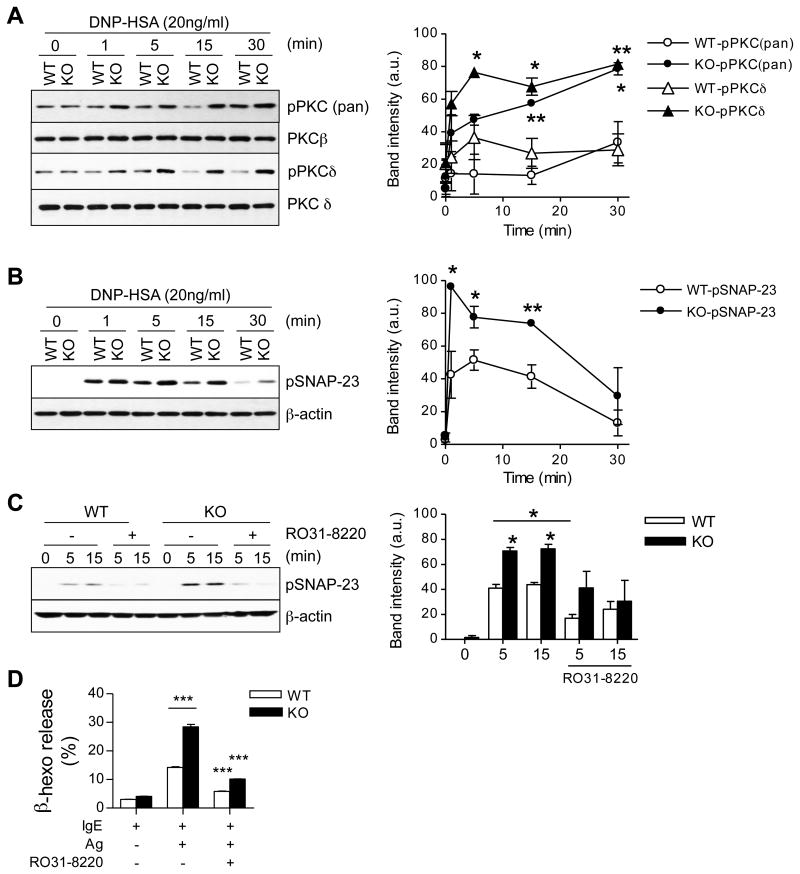
PKC activity plays an important role in mast cell degranulation following FcεRI engagement [6]. The FcεRI-induced phosphorylation of PKCs detected by an anti-phospho-pan-PKC antibody was increased in lipin1-deficient mast cells as compared with WT controls (Fig. 5A). SNAP-23 is an important regulator of granule fusion with the plasma membrane. FcεRI stimulation induces SNAP-23 phosphorylation at Ser95 and Ser120, which event is dependent on PKC activity and is important for mast cell degranulation [32]. As shown in Fig. 5B, FcεRI-induced SNAP-23 phosphorylation in lipin1-deficient BMMCs was both increased and prolonged compared to WT controls. Phospho-SNAP-23 and degranulation were inhibited in WT and lipin1 deficient mast cells in the presence of the PKC inhibitor RO31-8220 (Fig. 5C and D). Together, these data suggest that increased phosphorylation of PKCs and SNAP-23 contributes to an enhanced degranulation of lipin1-deficient mast cells.
Figure 5.
Increased phosphorylation of PKCs and SNAP-23 in lipin1-deficient mast cells. (A, B) Cell lysates were generated and subjected to immunoblotting analysis as described in Fig. 4 by using anti-phospho-pan-PKC, anti-phospho-PKCδ (A) and anti-phospho-SNAP-23 (Ser95) (B) antibodies. Total PKCβ/δ and β-actin were used as the loading controls. Graphs are mean ± SEM presentation of band intensities determined by densitometry. (C) Inhibition of SNAP-23 phosphorylation in WT and lipin1-KO BMMCs is dependent on PKC activity. WT and lipin1-KO BMMCs were incubated with or without 10 μM RO31-8220 for 30 min and then left unstimulated or were stimulated with DNP-HSA, followed by immunoblotting analysis. Bar Graph is mean ± SEM presentation of band intensities determined by densitometry. (D) Enhanced degranulation by lipin1-KO BMMCs is dependent on PKC activity. IgE-sensitized BMMCs were treated with RO31-8220 (10 μM) for 30 min, followed by in vitro mast cell degranulation assay, as indicated in Fig. 2. Data shown are representative from three experiments. * p<0.05, ** p<0.01 and ***, p<0.001 by Student t-test.
Discussion
Both PA and DAG are generated by multiple enzymes and function as second messengers in the immune system [33-35]. DAG has been well documented to play a critical role in the activation of not only mast cells, but also T cells, B cells, macrophages and other cell lineages [16, 35-37]. Recent studies have demonstrated that the DAG concentrations are tightly regulated in immune cells by DGKs. DGKs have been found to control mast cell activation, T cell development and function, and the innate immune response by converting DAG to PA [16, 38-41]. Although less well studied than DAG, PA has been implicated in the signaling of a variety of receptors by associating with and activating multiple signaling molecules, such as Sos, PI5Kα, SHIP1 and mTOR [35, 42-45]. In the immune system, PA is involved in mast cell degranulation, the TLR-induced innate immune response and T cell development [35]. In this study, we demonstrate that lipin1 deficiency selectively enhances mast cell degranulation, suggesting that tight control of the PA concentration is required for proper mast cell function.
In mast cells, both PLD1/2 and DGKζ have been reported to be involved in the production of PA in mast cells. Interestingly, either a reduction of PLD-derived PA or absence of DGKζ activity leads to an impairment of mast cell degranulation. In DGKζ deficient mast cells, DAG-mediated signaling is enhanced [16]. It has been proposed that enhanced DAG-mediated signaling may trigger negative feedback mechanisms so as to inhibit FcεRI signaling and subsequent impairment of Ca2+ influx and degranulation. However, DGKζ-derived PA appears to promote mast cell degranulation, and thus the absence of DGKζ-derived PA may contribute to the impairment of degranulation and the anaphylactic response in DGKζ deficient mast cells. Unlike DGKζ deficiency, a decrease in PLD activity does not cause enhanced DAG signaling in mast cells. However, PKC activation is impaired when PLD-derived PA is reduced [19]. The current data, together with these observations, suggest that lipin1 may mainly inhibit mast cell degranulation by abrogating PA.
Both lipin and PLCγ are involved in the generation of DAG. The differences between PLCγ- and lipin1-derived DAG are not known at present. PLCγ-derived DAG plays a crucial role in mast cell function by activating multiple downstream signaling cascades, such the Ras-Erk1/2 and PKC-NFκB pathways. PLCγ2 deficiency results in impaired degranulation and decreased cytokine production in mast cells [13]. The lack of any effect of lipin1 deficiency on cytokine production and activities of Erk1/2 and NF-κB pathways suggest that lipin1-derived DAG is not essential for these signaling events. However, since lipin2 is also expressed in mast cells, we cannot rule out the possibility that lipin2-derived DAG may compensate for the loss of lipin1. We also do not exclude the possibility that lipin1-derived DAG may perform functions that are different from PLCγ-derived DAG. These two kinds of DAG may differ in their acyl chains and may thus have different kinetics and subcellular locations.
We have shown that Lipin1 is localized in the nucleus in mast cells but its deficiency can affect phosphorylation of PKCs and SNAP-23, which are localized in the cytosol and plasma membrane. Given their different subcellular localizations, it is unlike that lipin1 can directly modulate PKC-SNAP-23 activities. Lipin1 may indirectly affect PKCs via PA accumulation. Furthermore, lipin1 can function as a transactivator or repressor by binding to transcription factors in mammalian cells [46, 47], it is also possible that lipin1 indirectly regulates PKC-SNAP-23 via modulating transcription of yet to be defined genes.
Mast cell degranulation is tightly regulated not only during FcεRI signaling, but also during vesicle transport and membrane fusion. The soluble N-ethylmaleimide-sensitive fusion factor attachment receptor proteins that include VAMP, syntaxin and SNAP-23 are essential for fusion processing [48]. Moreover, addition of PA to syntaxin/SNAP-23 vesicles increases vesicle fusion [49]. SNAP-23 can be directly phosphorylated by both PKC and IκB kinase β, which promotes exocytosis [32, 50, 51]. However, we did not observed enhanced IKKα/β phosphorylation following Ag stimulation in lipin1 deficient BMMCs, whereas PKC-SNAP-23 signaling is hypersensitive to FcεRI stimulation in these cells. The administration of a PKC inhibitor diminished the phosphorylation of SNAP-23 and decreased degranulation in FcεRI-stimulated mast cells, suggesting that SNAP-23 is a downstream signaling molecule of PKCs and that the PKC-SNAP-23 axis may contribute to the enhanced mast cell degranulation which results from lipin1 deficiency.
It is intriguing that lipin1-deficient mast cells display enhanced PKC activity but normal NFκB signaling. At present, the reason leading to the differential effects of enhanced PKC activity on SNAP-23 phosphorylation and on the IKK-NFκB pathway is unclear. One potential explanation is that lipin1 deficiency may also affect signaling events that are downstream of PKCs but are involved in IKKα/β activation. For example, Bcl10/Malt1 complex is important for NFκB activation and TNFα production but is not required for degranulation [52], supporting that there is a branching point downstream of PKCs for the control of NFκB activation and degranulation. Differential control of mast cell degranulation and cytokine production has also been found in Znt5-deficient mice [53]. FcεRI-mediated PKC and NFκB activation are defective in Znt5-defcient BMMCs, where IL-6 and TNF-α production is decreased but degranulation is normal. Our recent studies also show that mast cell degranulation and cytokine production can be differently regulated [16, 54].
Lipin1 deficiency causes fatty liver dystrophy in mice [21, 22]. In humans, mutations in lipin1 result in recurrent muscle pain and myoglobinuria in childhood [55]. Mutations in lipin2 gene causes the rare Majeed syndrome with recurrent osteomyelitis, cutaneous inflammation, and anemia [56-58]. With the increased importance of lipin proteins in human diseases [59], it would be interesting to determine whether mast cell function is similarly affected in human patients and whether deregulated mast cell function may play a roles in disease progression in human patients with lipin mutations or deficiency.
In summary, mast cells play a critical role in allergic diseases. The tight regulation of FcεRI signaling is important for proper mast cell function. This study provides the first genetic evidence that lipin1 selectively inhibits mast cell degranulation by reducing PKC-SNAP-23 signaling.
Materials and Methods
Mice and BMMCs
Lpin1fld/+ mice on a Balb/C background were purchased from the Jackson Laboratory. Bone marrow cells from the femur and tibia of Lpin1fld/fld (KO) and Lpin1+/+ (WT) mice were cultured in IMDM-IL3 media, as previously described [16]. IMDM-IL3 is comprised of Isocove's Modified Dulbecco's media (GIBCO) supplemented with 10% FBS (Hyclone), 100 U/ml penicillin G, 100 U/ml streptomycin, 292 μg/ml of L-glutamine, 25 mM HEPES (pH 7.4), 0.1 mM nonessential amino acids, 1 mM sodium pyruvate and 50 μM 2-ME with 10 % IL3-conditioned medium generated from X63 cells.
Flow cytometry
BMMCs were stained directly with a PE-conjugated c-kit or stained after 4 h sensitization with 1 μg/ml IgE, followed by incubation with FITC-conjugated anti-IgE, and they were then analyzed by flow cytometry using a BD FACSCanto II. To measure cell survival after cytokine withdrawal, the cells were stained with allophycocyanin-conjugated Annexin V and 7-Amino-Actinomycin D (7AAD) for 20 min at RT in buffer A containing 10 mM HEPES (pH 7.4), 140 mM NaCl and 2.5 mM CaCl2. Data were analyzed with FlowJo software (Tree Star).
β-hexosaminidase, prostaglandin D2, and cytokine release
To measure degranulation, BMMCs (1 × 106 cells/ml) were allowed to rest overnight in IMDM-IL3, and were incubated with 1 μg/ml anti-DNP IgE (clone SPE-7, Sigma-Aldrich) for at least 4 h in IMDM media without IL3. The cells were washed once with IMDM and then stimulated with various concentrations of DNP-HSA (Sigma-Aldrich) for 45 min in Tyrode's buffer (130 mM NaCl, 10 mM HEPES (pH 7.4), 1 mM MgCl2, 5 mM KCl, 1.4 mM CaCl2, 5.6 mM glucose and 1 mg/ml bovine serum albumin). Supernatants were incubated with 2 mM p-nitrophenyl-N-acetylß-D-glucosamide (Sigma-Aldrich) dissolved in 0.1 M citrate buffer (pH 4.5) in a final volume of 60 μl for 1 h at 37°C, followed by a termination of the enzymatic reaction with the addition of 60 μl 2M NaOH. Absorbance at 405 nm was read by a plate reader. Total cellular β-hexosaminidase activity was quantified using the supernatant from cells lysed with 0.5 % Triton X-100. PGD2 in supernatants was determined by using PGD2 EIA kit (Cayman Chemical Company). To determine the effect of PKCs on degranulation, IgE-sensitized BMMCs were incubated with 10 μM RO31-8220 (Sigma-Aldrich) for 30 min and then stimulated with DNP-HSA. For cytokine determination, the cells were stimulated for 6 h, and the IL-6 and TNF-α concentrations in the supernatants were determined with Mouse ELISA Max (BioLegend) kits according to the manufacturer's instructions.
Passive systemic anaphylaxis
Mice were injected intravenously with 200 μl of PBS containing 15 μg/ml anti-DNP IgE. After 24 h, mice were anesthetized and injected intravenously with 200 μl PBS of 0.5 mg/ml DNP-HSA. After 90 sec, mice were sacrificed and the plasma was immediately isolated from the blood obtained by cardiac puncture. Histamine levels were determined using a competitive histamine enzyme-linked immunosorbent assay kit (Immunotech).
Phosphatidic acid assay
Total PA concentrations from chloroform-extracted lipids from 1 × 106 BMMCs were determined by ELISA using a total phosphatidic acid kit (Cayman Chemical Company) according to the manufacturer's instruction. PA concentrations were normalized to protein concentrations before lipids extraction.
Immunoblot assay
BMMCs were lysed in RIPA lysis buffer (0.1% SDS, 1% Triton X-100, 0.25% Sodium deoxycolate, 150 mM NaCl, 50 mM Tris, pH 7.4) with a protease inhibitor cocktail and phosphatase inhibitors. Proteins were resolved by SDS-PAGE, transferred to a Trans-Blot Nitrocellulose membrane (Bio-Rad Laboratories) and probed with the appropriate antibodies. The following anti-phosphor-antibodies were used: pPLCγ1 (Tyr783), pPLCγ2 (Tyr1217), pAkt (Ser473), pPKC (pan), pErk1/2 (Thr202/Tyr204), pJnk (Thr183/Tyr185), p-p38 (Thr180/Tyr182), pIκBα (Ser32), and pIKKα/β (Ser176/180) from Cell Signaling Technology. pSNAP-23 (Ser95) was a generous gift from Dr. P. Roche, NCI [32]. Anti-Lipin1 and Lipin2 antibodies were from Santa Cruz Biotechnology. For the loading control, antibodies for total proteins or β-actin were used. In the stimulation assay, IgE-sensitized BMMCs were resuspended in Tyrode's buffer, and then were left unstimulated or stimulated with DNP-HSA (20 ng/ml) for the various times indicated.
Quantitative RT-PCR
RNAs extracted using Trizol Reagent (Invitrogen) were reverse transcribed to cDNA by Superscript III and random primers according to the manufacturer's protocol (Invitrogen). qRT-PCR was performed with Mastercycler realplex and SYBR Green master mix (Eppendorf). The expressed levels of target mRNAs were normalized with L32, calculated using the 2−ΔΔCT method and presented as arbitrary units (a.u.) of fold change.
Calcium flux
BMMCs were sensitized with 1 μg/mL IgE for 6 h, resuspended at 1 × 107 cells/ml in Tyrode's buffer containing 3 μg/ml Indo-1 (Invitrogen) and 4 mM probencid, further incubated for 30 min at 37°C, and washed twice with Tyrode's buffer. 40 μl of cells was added into 460 μl of pre-warmed Tyrode's buffer to determine the calcium response by flow cytometry. After determination of the baseline ratio of FL5 to FL4, cells were stimulated with 10 μl of 5 μg/ml DNP-HSA. Calcium flux was displayed as the ratio of FL5 to FL4 fluorescence.
Immunofluorescence and confocal microscopy
Retrovirus was made using pMX-CD63-GFP, kindly provided by Dr. W. Zhang, Duke University according to the method previously described [60]. Isolated BM cells were grown in IMDM-IL3 medium for 3 days. The cells (3 × 106 cells/ml in 24 wells) were mixed with an equal volume of CD63-GFP retroviral supernatant and polybrene (8 ug/ml), and then centrifuged at 2500 rpm for 90 min at room temperature using a Sorvall Swing Bucket rotor. After spin infection, the cells were further cultured in IMDM-IL3 medium for 4 weeks. The transduced BMMCs were loaded with anti-DNP-IgE and stimulated with DNP-HSA for the indicated times. Cells were immediately fixed with 4 % paraformaldehyde and permeabilized with 0.1% Triton X-100 followed by incubation with a rabbit anti-lipin1 antibody (Cell Signaling Technology) for 1 h. The bound antibody was labeled with Texas Red-conjugated anti-rabbit secondary antibody (Molecular Probes) for 1 h and then visualized under Leica SP5 confocal microscopy.
Statistical analysis
For statistical analysis, two-tail Student t-test was performed. *, p<0.05. **, p<0.01, ***, p<0.001.
Acknowledgments
We thank Dr. Weiguo Zhang for providing reagents and Dr. Paul Roche for providing anti-SNAP-23 antibodies. The authors declare they have no competing financial interests. JS designed and conducted experiments, analyzed data, and wrote the manuscript; PZ performed experiments and provided essential reagents; ZG designed and performed experiments; X-P. Z supervised the project, designed the research and wrote the manuscript. Pacific Edit reviewed the manuscript prior to submission. This study is supported by funding from the National Institute of Health (R01AI076357, R01AI079088, R01AI101206, and R21AI079873), the American Cancer Society (RSG-08-186-01-LIB), the American Heart Association, and the Food Allergy and Anaphylaxis Network to X-P. Z.
Abbreviations
- BMMC
Bone marrow-derived mast cell
- DAG
Diacylglycerol
- DGKζ
DAG kinase ζ
- DNP-HSA
DNP-human serum albumin
- Fld
Fatty liver dystrophy
- IP3
Inositol 1,4,5-trisphosphate
- PSA
Passive systemic anaphylaxis
- PAP
phosphatidate phosphatase
- PA
Phosphatidic acid
- PLCγ
Phospholipase Cγ
- PLD
phospholipase D
- PGD2
prostaglandin D2
Footnotes
Competing Interest Statement: The authors declare that they have no conflict of interests.
References
- 1.Rivera J, Gilfillan AM. Molecular regulation of mast cell activation. J Allergy Clin Immunol. 2006;117:1214–1225. doi: 10.1016/j.jaci.2006.04.015. quiz 1226. [DOI] [PubMed] [Google Scholar]
- 2.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77:1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 3.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 4.Kambayashi T, Koretzky GA. Proximal signaling events in Fc epsilon RI-mediated mast cell activation. J Allergy Clin Immunol. 2007;119:544–552. doi: 10.1016/j.jaci.2007.01.017. quiz 553-544. [DOI] [PubMed] [Google Scholar]
- 5.Kraft S, Kinet JP. New developments in FcεRI regulation, function and inhibition. Nat Rev Immunol. 2007;7:365–378. doi: 10.1038/nri2072. [DOI] [PubMed] [Google Scholar]
- 6.Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. doi: 10.1016/j.coi.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Saitoh S, Arudchandran R, Manetz TS, Zhang W, Sommers CL, Love PE, Rivera J, Samelson LE. LAT is essential for FcεRI-mediated mast cell activation. Immunity. 2000;12:525–535. doi: 10.1016/s1074-7613(00)80204-6. [DOI] [PubMed] [Google Scholar]
- 8.Pivniouk VI, Martin TR, Lu-Kuo JM, Katz HR, Oettgen HC, Geha RS. SLP-76 deficiency impairs signaling via the high-affinity IgE receptor in mast cells. J Clin Invest. 1999;103:1737–1743. [PMC free article] [PubMed] [Google Scholar]
- 9.Manetz TS, Gonzalez-Espinosa C, Arudchandran R, Xirasagar S, Tybulewicz V, Rivera J. Vav1 regulates phospholipase cγ activation and calcium responses in mast cells. Mol Cell Biol. 2001;21:3763–3774. doi: 10.1128/MCB.21.11.3763-3774.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hata D, Kawakami Y, Inagaki N, Lantz CS, Kitamura T, Khan WN, Maeda-Yamamoto M, Miura T, Han W, Hartman SE, Yao L, Nagai H, Goldfeld AE, Alt FW, Galli SJ, Witte ON, Kawakami T. Involvement of Bruton's tyrosine kinase in FcεRI-dependent mast cell degranulation and cytokine production. J Exp Med. 1998;187:1235–1247. doi: 10.1084/jem.187.8.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukao T, Yamada T, Tanabe M, Terauchi Y, Ota T, Takayama T, Asano T, Takeuchi T, Kadowaki T, Hata Ji J, Koyasu S. Selective loss of gastrointestinal mast cells and impaired immunity in PI3K-deficient mice. Nat Immunol. 2002;3:295–304. doi: 10.1038/ni768. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson TP, Kaliner MA, Hohman RJ. Phospholipase C-γ 1 is translocated to the membrane of rat basophilic leukemia cells in response to aggregation of IgE receptors. J Immunol. 1992;148:2194–2200. [PubMed] [Google Scholar]
- 13.Wen R, Jou ST, Chen Y, Hoffmeyer A, Wang D. Phospholipase C γ2 is essential for specific functions of FcεR and FcγR. J Immunol. 2002;169:6743–6752. doi: 10.4049/jimmunol.169.12.6743. [DOI] [PubMed] [Google Scholar]
- 14.Chang EY, Szallasi Z, Acs P, Raizada V, Wolfe PC, Fewtrell C, Blumberg PM, Rivera J. Functional effects of overexpression of protein kinase C-α, -β, -δ, -ε, and -η in the mast cell line RBL-2H3. J Immunol. 1997;159:2624–2632. [PubMed] [Google Scholar]
- 15.Zhang C, Baumgartner RA, Yamada K, Beaven MA. Mitogen-activated protein (MAP) kinase regulates production of tumor necrosis factor-α and release of arachidonic acid in mast cells. Indications of communication between p38 and p42 MAP kinases. J Biol Chem. 1997;272:13397–13402. doi: 10.1074/jbc.272.20.13397. [DOI] [PubMed] [Google Scholar]
- 16.Olenchock BA, Guo R, Silverman MA, Wu JN, Carpenter JH, Koretzky GA, Zhong XP. Impaired degranulation but enhanced cytokine production after FcεRI stimulation of diacylglycerol kinase ζ-deficient mast cells. J Exp Med. 2006;203:1471–1480. doi: 10.1084/jem.20052424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgartner RA, Yamada K, Deramo VA, Beaven MA. Secretion of TNF from a rat mast cell line is a brefeldin A-sensitive and a calcium/protein kinase C-regulated process. J Immunol. 1994;153:2609–2617. [PubMed] [Google Scholar]
- 18.Hitomi T, Zhang J, Nicoletti LM, Grodzki AC, Jamur MC, Oliver C, Siraganian RP. Phospholipase D1 regulates high-affinity IgE receptor-induced mast cell degranulation. Blood. 2004;104:4122–4128. doi: 10.1182/blood-2004-06-2091. [DOI] [PubMed] [Google Scholar]
- 19.Peng Z, Beaven MA. An essential role for phospholipase D in the activation of protein kinase C and degranulation in mast cells. J Immunol. 2005;174:5201–5208. doi: 10.4049/jimmunol.174.9.5201. [DOI] [PubMed] [Google Scholar]
- 20.Peterfy M, Phan J, Xu P, Reue K. Lipodystrophy in the fld mouse results from mutation of a new gene encoding a nuclear protein, lipin. Nat Genet. 2001;27:121–124. doi: 10.1038/83685. [DOI] [PubMed] [Google Scholar]
- 21.Langner CA, Birkenmeier EH, Ben-Zeev O, Schotz MC, Sweet HO, Davisson MT, Gordon JI. The fatty liver dystrophy (fld) mutation. A new mutant mouse with a developmental abnormality in triglyceride metabolism and associated tissue-specific defects in lipoprotein lipase and hepatic lipase activities. J Biol Chem. 1989;264:7994–8003. [PubMed] [Google Scholar]
- 22.Langner CA, Birkenmeier EH, Roth KA, Bronson RT, Gordon JI. Characterization of the peripheral neuropathy in neonatal and adult mice that are homozygous for the fatty liver dystrophy (fld) mutation. J Biol Chem. 1991;266:11955–11964. [PubMed] [Google Scholar]
- 23.Csaki LS, Reue K. Lipins: multifunctional lipid metabolism proteins. Annu Rev Nutr. 2010;30:257–272. doi: 10.1146/annurev.nutr.012809.104729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carman GM, Han GS. Roles of phosphatidate phosphatase enzymes in lipid metabolism. Trends Biochem Sci. 2006;31:694–699. doi: 10.1016/j.tibs.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jamal Z, Martin A, Gomez-Munoz A, Brindley DN. Plasma membrane fractions from rat liver contain a phosphatidate phosphohydrolase distinct from that in the endoplasmic reticulum and cytosol. J Biol Chem 1991. 266:2988–2996. [PubMed] [Google Scholar]
- 26.Donkor J, Sariahmetoglu M, Dewald J, Brindley DN, Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem. 2007;282:3450–3457. doi: 10.1074/jbc.M610745200. [DOI] [PubMed] [Google Scholar]
- 27.Grimsey N, Han GS, O'Hara L, Rochford JJ, Carman GM, Siniossoglou S. Temporal and spatial regulation of the phosphatidate phosphatases lipin 1 and 2. J Biol Chem. 2008;283:29166–29174. doi: 10.1074/jbc.M804278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, Sabatini DM. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valdearcos M, Esquinas E, Meana C, Gil-de-Gomez L, Guijas C, Balsinde J, Balboa MA. Subcellular localization and role of lipin-1 in human macrophages. J Immunol. 2011;186:6004–6013. doi: 10.4049/jimmunol.1003279. [DOI] [PubMed] [Google Scholar]
- 30.Liu GH, Gerace L. Sumoylation regulates nuclear localization of lipin-1α in neuronal cells. PLoS One. 2009;4:e7031. doi: 10.1371/journal.pone.0007031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishida K, Yamasaki S, Ito Y, Kabu K, Hattori K, Tezuka T, Nishizumi H, Kitamura D, Goitsuka R, Geha RS, Yamamoto T, Yagi T, Hirano T. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J Cell Biol. 2005;170:115–126. doi: 10.1083/jcb.200501111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hepp R, Puri N, Hohenstein AC, Crawford GL, Whiteheart SW, Roche PA. Phosphorylation of SNAP-23 regulates exocytosis from mast cells. J Biol Chem. 2005;280:6610–6620. doi: 10.1074/jbc.M412126200. [DOI] [PubMed] [Google Scholar]
- 33.Brindley DN, Pilquil C, Sariahmetoglu M, Reue K. Phosphatidate degradation: phosphatidate phosphatases (lipins) and lipid phosphate phosphatases. Biochim Biophys Acta. 2009;1791:956–961. doi: 10.1016/j.bbalip.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases through inhibition of the diacylglycerol-RasGRP1-Ras-Mek1/2-Erk1/2 pathway. Blood. 2011;117:4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev. 2008;224:249–264. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fu G, Chen Y, Yu M, Podd A, Schuman J, He Y, Di L, Yassai M, Haribhai D, North PE, Gorski J, Williams CB, Wang D, Wen R. Phospholipase Cγ1 is essential for T cell development, activation, and tolerance. J Exp Med. 2010;207:309–318. doi: 10.1084/jem.20090880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hikida M, Casola S, Takahashi N, Kaji T, Takemori T, Rajewsky K, Kurosaki T. PLC-γ2 is essential for formation and maintenance of memory B cells. J Exp Med. 2009;206:681–689. doi: 10.1084/jem.20082100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase ζ deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 39.Guo R, Wan CK, Carpenter JH, Mousallem T, Boustany RM, Kuan CT, Burks AW, Zhong XP. Synergistic control of T cell development and tumor suppression by diacylglycerol kinase α and ζ. Proc Natl Acad Sci U S A. 2008;105:11909–11914. doi: 10.1073/pnas.0711856105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olenchock BA, Guo R, Carpenter JH, Jordan M, Topham MK, Koretzky GA, Zhong XP. Disruption of diacylglycerol metabolism impairs the induction of T cell anergy. Nat Immunol. 2006;7:1174–1181. doi: 10.1038/ni1400. [DOI] [PubMed] [Google Scholar]
- 41.Liu CH, Machado FS, Guo R, Nichols KE, Burks AW, Aliberti JC, Zhong XP. Diacylglycerol kinase ζ regulates microbial recognition and host resistance to Toxoplasma gondii. J Exp Med. 2007;204:781–792. doi: 10.1084/jem.20061856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frank C, Keilhack H, Opitz F, Zschornig O, Bohmer FD. Binding of phosphatidic acid to the protein-tyrosine phosphatase SHP-1 as a basis for activity modulation. Biochemistry. 1999;38:11993–12002. doi: 10.1021/bi982586w. [DOI] [PubMed] [Google Scholar]
- 43.Zhao C, Du G, Skowronek K, Frohman MA, Bar-Sagi D. Phospholipase D2-generated phosphatidic acid couples EGFR stimulation to Ras activation by Sos. Nat Cell Biol. 2007;9:706–712. doi: 10.1038/ncb1594. [DOI] [PubMed] [Google Scholar]
- 44.Jarquin-Pardo M, Fitzpatrick A, Galiano FJ, First EA, Davis JN. Phosphatidic acid regulates the affinity of the murine phosphatidylinositol 4-phosphate 5-kinase-Iβ for phosphatidylinositol-4-phosphate. J Cell Biochem. 2007;100:112–128. doi: 10.1002/jcb.21027. [DOI] [PubMed] [Google Scholar]
- 45.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 46.Kim HB, Kumar A, Wang L, Liu GH, Keller SR, Lawrence JC, Jr, Finck BN, Harris TE. Lipin 1 represses NFATc4 transcriptional activity in adipocytes to inhibit secretion of inflammatory factors. Mol Cell Biol. 2010;30:3126–3139. doi: 10.1128/MCB.01671-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finck BN, Gropler MC, Chen Z, Leone TC, Croce MA, Harris TE, Lawrence JC, Jr, Kelly DP. Lipin 1 is an inducible amplifier of the hepatic PGC-1α/PPARα regulatory pathway. Cell Metab. 2006;4:199–210. doi: 10.1016/j.cmet.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Blank U, Rivera J. The ins and outs of IgE-dependent mast-cell exocytosis. Trends Immunol. 2004;25:266–273. doi: 10.1016/j.it.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Vicogne J, Vollenweider D, Smith JR, Huang P, Frohman MA, Pessin JE. Asymmetric phospholipid distribution drives in vitro reconstituted SNARE-dependent membrane fusion. Proc Natl Acad Sci U S A. 2006;103:14761–14766. doi: 10.1073/pnas.0606881103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suzuki K, Verma IM. Phosphorylation of SNAP-23 by IκB kinase 2 regulates mast cell degranulation. Cell. 2008;134:485–495. doi: 10.1016/j.cell.2008.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polgar J, Lane WS, Chung SH, Houng AK, Reed GL. Phosphorylation of SNAP-23 in activated human platelets. J Biol Chem. 2003;278:44369–44376. doi: 10.1074/jbc.M307864200. [DOI] [PubMed] [Google Scholar]
- 52.Klemm S, Gutermuth J, Hultner L, Sparwasser T, Behrendt H, Peschel C, Mak TW, Jakob T, Ruland J. The Bcl10-Malt1 complex segregates FcεRI-mediated nuclear factor κB activation and cytokine production from mast cell degranulation. J Exp Med. 2006;203:337–347. doi: 10.1084/jem.20051982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nishida K, Hasegawa A, Nakae S, Oboki K, Saito H, Yamasaki S, Hirano T. Zinc transporter Znt5/Slc30a5 is required for the mast cell-mediated delayed-type allergic reaction but not the immediate-type reaction. J Exp Med. 2009;206:1351–1364. doi: 10.1084/jem.20082533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin J, Pan H, Zhong XP. Regulation of mast cell survival and function by tuberous sclerosis complex 1. Blood. 2012;119:3306–3314. doi: 10.1182/blood-2011-05-353342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeharia A, Shaag A, Houtkooper RH, Hindi T, de Lonlay P, Erez G, Hubert L, Saada A, de Keyzer Y, Eshel G, Vaz FM, Pines O, Elpeleg O. Mutations in LPIN1 cause recurrent acute myoglobinuria in childhood. Am J Hum Genet. 2008;83:489–494. doi: 10.1016/j.ajhg.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.El-Shanti HI, Ferguson PJ. Chronic recurrent multifocal osteomyelitis: a concise review and genetic update. Clin Orthop Relat Res. 2007;462:11–19. doi: 10.1097/BLO.0b013e3180986d73. [DOI] [PubMed] [Google Scholar]
- 57.Majeed HA, Al-Tarawna M, El-Shanti H, Kamel B, Al-Khalaileh F. The syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia. Report of a new family and a review. Eur J Pediatr. 2001;160:705–710. doi: 10.1007/s004310100799. [DOI] [PubMed] [Google Scholar]
- 58.Ferguson PJ, Chen S, Tayeh MK, Ochoa L, Leal SM, Pelet A, Munnich A, Lyonnet S, Majeed HA, El-Shanti H. Homozygous mutations in LPIN2 are responsible for the syndrome of chronic recurrent multifocal osteomyelitis and congenital dyserythropoietic anaemia (Majeed syndrome) J Med Genet. 2005;42:551–557. doi: 10.1136/jmg.2005.030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reue K. The lipin family: mutations and metabolism. Curr Opin Lipidol. 2009;20:165–170. doi: 10.1097/MOL.0b013e32832adee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu Y, Zhu M, Nishida K, Hirano T, Zhang W. An essential role for RasGRP1 in mast cell function and IgE-mediated allergic response. J Exp Med. 2007;204:93–103. doi: 10.1084/jem.20061598. [DOI] [PMC free article] [PubMed] [Google Scholar]