Abstract
Objective
Intraspinal microstimulation (ISMS) is a promising method for activating the spinal cord distal to an injury. The objectives of this study were to examine the ability of chronically implanted stimulating wires within the cervical spinal cord to (1) directly produce forelimb movements, and (2) assess whether ISMS stimulation improved subsequent volitional control of paretic extremities following injury.
Approach
We developed a technique for implanting intraspinal stimulating electrodes within the cervical spinal cord segments C6-T1 of Long-Evans rats. Beginning 4 weeks after a severe cervical contusion injury at C4–C5, animals in the treatment condition received therapeutic ISMS 7 hours/day, 5 days/week for the following 12 weeks.
Main Results
Over 12 weeks of therapeutic ISMS, stimulus-evoked forelimb movements were relatively stable. We also explored whether therapeutic ISMS promotes recovery of forelimb reaching movements. Animals receiving daily therapeutic ISMS performed significantly better than unstimulated animals during behavioral tests conducted without stimulation. Quantitative video analysis of forelimb movements showed that stimulated animals performed better in the movements reinforced by stimulation, including extending the elbow to advance the forelimb and opening the digits. While threshold current to elicit forelimb movement gradually increased over time, no differences were observed between chronically stimulated and unstimulated electrodes suggesting that no additional tissue damage was produced by the electrical stimulation.
Significance
The results indicate that therapeutic intraspinal stimulation delivered via chronic microwire implants within the cervical spinal cord confers benefits extending beyond the period of stimulation, suggesting future strategies for neural devices to promote sustained recovery after injury.
Keywords: spinal cord injury, ISMS, regenerative stimulation, neuroprosthesis
1. Introduction
Among individuals with spinal cord injury, incomplete injury to the cervical spinal cord is the most common diagnosis (NSCISC, (2012). Based on a survey of individuals with cervical spinal cord injuries, restoration of hand and arm function would have the greatest impact on their quality of life (Anderson, 2004). Despite this frequent occurrence and high priority, few studies have demonstrated improved forelimb function in models of cervical spinal cord injury (Houle et al., 2006; Wang et al., 2011). Here we explored intraspinal microstimulation (ISMS) as a method to promote long-term recovery of forelimb function after cervical contusion injury.
By implanting wires within the spinal cord, ISMS is capable of directly activating spinal motor neurons and interneuron circuits, often in highly functional patterns. Several groups have utilized ISMS of the lumbosacral spinal cord to reanimate the hind limbs, evoking functional and complex movements in the rat, cat and frog (Bamford et al., 2005; Giszter et al., 1993; Mushahwar et al., 2002; Tresch and Bizzi, 1999). ISMS applied to the cervical spinal cord via acute electrodes is capable of evoking forelimb movements in spinally-intact monkeys (Moritz et al., 2007; Zimmermann et al., 2011). Our group has recently found that a cervical spinal cord contusion injury only briefly interrupts the ability of ISMS to elicit a wide range of functional movements (Sunshine et al., 2013). While ISMS generates functional forelimb movement, the stability of a chronic electrode implant caudal to a mid-cervical contusion injury, and the subsequent ability of ISMS to promote long-term recovery of forelimb function after injury, have not previously been explored.
Recent studies have demonstrated functional improvements in movement during tonic epidural stimulation applied to the dorsal surface of the lumbar spinal cord (Harkema et al., 2011a; Ichiyama et al., 2005; van den Brand et al., 2012). Epidural stimulation of the lumbar spinal cord combined with serotonergic agonism evoked hind limb stepping in partially (van den Brand et al., 2012) and completely transected rats (Gerasimenko et al., 2008; Ichiyama et al., 2005). A case study demonstrated that epidural stimulation of the lumbar spinal cord permitted volitional control of leg movements in a patient with a largely motor complete T1 subluxation injury (Harkema et al., 2011a). In these studies, epidural stimulation is believed to enhance lower extremity function by bringing spinal circuits closer to threshold such that residual descending input from the brain or peripheral sensation is sufficient to trigger volitional movements (Edgerton and Harkema, 2011).
Electrical stimulation may also have long-term benefits for the injured brain and spinal cord. Stimulation applied to the forelimb area of contralateral motor cortex (Carmel et al., 2010) or contralateral medullary pyramid (Brus-Ramer et al., 2007) after a unilateral pyramidotomy promotes axon sprouting and improves motor function persisting beyond the period of stimulation. Furthermore, electrical stimulation of the descending tracts promotes sprouting and maintenance of spinal connections that are otherwise pruned during development (Salimi and Martin, 2004). New connections are formed spontaneously after midthoracic partial dorsal hemisection but subsequently lost if they do not connect with intact neurons such as long propriospinal neurons that bridge the lesion site after injury (Bareyre et al., 2004). Thus, promoting electrical activity in physiologically relevant circuits caudal to a lesion may be critical to creating and maintaining connections that bypass an incomplete spinal injury.
The goals of the present study were both to develop a method for delivering chronic ISMS within the rodent cervical spinal cord, and to determine whether therapeutic ISMS could improve forelimb motor function beyond the period of stimulation. We found that electrodes implanted within the cervical spinal cord caudal to a contusion injury evoked relatively stable forelimb responses, and that therapeutic ISMS resulted in modest but sustained improvement in forelimb function. These results demonstrate a new application for ISMS in producing durable improvements in motor function after neurological injury.
2. Materials and Methods
Experiments to quantify the effect of therapeutic intraspinal microstimulation (ISMS) on recovery of forelimb function were performed on 22 adult female Long-Evans rats (250 g). All procedures were approved by the University of Washington Institutional Animal Care and Use Committee (IACUC).
2.1 Methods Overview
All animals were trained to proficiency on the precision forelimb reaching task (McKenna and Whishaw, 1999; Schrimsher and Reier, 1992) before receiving a severe, lateralized C4–C5 contusion injury (see experimental timeline in Figure 1). Chronic intraspinal stimulating electrodes were implanted ipsilateral and caudal to the injury three weeks later. Animals were randomly assigned to the stimulated or unstimulated condition (n = 11 animals per group), with stimulation beginning a total of four weeks after injury. Animals in the stimulated group subsequently received therapeutic ISMS for 7 hours/day, 5 days/week for 12 weeks. Both groups of animals received reach training 5 days/week in the absence of stimulation.
Figure 1.
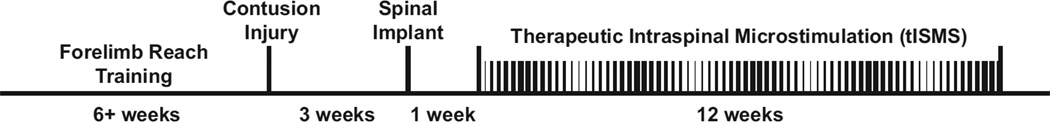
Experimental timeline. Following six or more weeks of reach training, rats received a severe lateralized contusion injury to the cervical spinal cord. Chronic intraspinal stimulating electrodes were implanted caudal to the injury three weeks later. Beginning 4 weeks after injury, therapeutic intraspinal microstimulation (tISMS) was delivered for 7 hours/day, 5 days/week for the following 12 weeks. Control animals received identical injuries, spinal stimulation implants, and reach training, but stimulation was only applied very briefly in order to test motor thresholds of intraspinal microwires.
2.2 Cervical Injury
Animals were deeply anesthetized via intraperitoneal injection of ketamine (80mg/kg) and xylazine (12mg/kg). Animals then received a severe, lateralized contusion injury (0.8 mm displacement, 14 ms dwell time) to spinal segments C4–C5 using a modified Ohio State injury device (McTigue et al., 1998; Stokes et al., 1992). Buprenorphine (0.05 mg/kg) was given twice daily for three days for analgesia. These injuries result in substantial grey matter cavitation with radiating demyelination of the surrounding white matter fiber tracks at the level of the injury (Sunshine et al., 2013).
2.3 Microwire Implant
To study the therapeutic benefits of ISMS following contusion injury, a method for chronic stimulation of the cervical spinal cord was developed. Stimulating electrode arrays were adapted for the cervical spinal cord from those developed by the Mushahwar group for the lumbar spinal cord of rodents (Bamford et al., 2005). Arrays consisted of six 30 µm polyimide-coated platinum-iridium wires (California Fine Wire). Wire tips were cut at an acute angle to minimize electrical impedance and facilitate insertion, with insulation near the tips otherwise undisturbed. Due to the high density of separate motor pools, insulation was not stripped back from wire tips to isolate individual motor pools (Sunshine et al., 2013). Wires were threaded through a 19 gauge epidural catheter (Arrow International) to a skull-mounted connector (Plastics One).
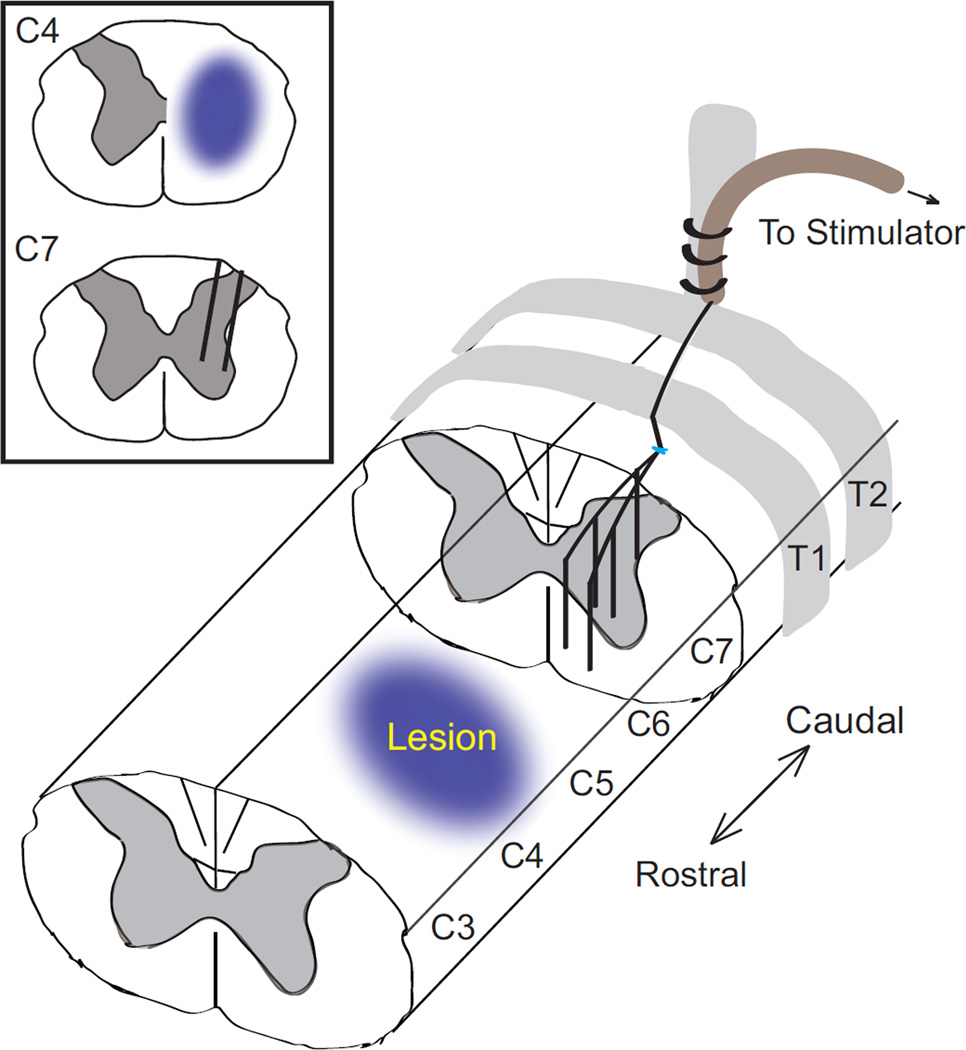
Three weeks after injury, animals were implanted with intraspinal stimulating electrodes caudal and ipsilateral to injury in the intermediate and ventral lamina of spinal segments C6-T1. Animals were anesthetized by inhalation of 1–3% isoflurane in 100% oxygen. An incision was made from C4-T2, muscle layers retracted, and hemilaminectomies of C6–C7 were performed to expose the dorsal surface of the cord. The catheter containing the wires was secured to the T2 dorsal spinous process with silk sutures; T2 was chosen as an anchor because of its substantial size relative to adjacent processes and its proximity to the cervical segments targeted by ISMS. A longitudinal slit was made in the dura and five stimulating wires were inserted into the spinal cord targeting the forelimb motor pools in the ventral horn of the grey matter (Figure 2). Wire tips targeted ventral gray matter (depth of 1.2–1.6 mm) and were placed between the center of C7 and the rostral end of C6 (spread over a rostral/caudal distance of 1.5–2.5 mm) ipsilateral and caudal to the injury. A reference wire, with insulation removed, was placed above the surface of the spinal cord. The dura was sewn over the top of the wire bundle using 8-0 silk suture and a microdrop of cyanoacrylate glue delivered via a 30 gauge needle was used to seal the surface of the dura. The catheter was routed under the skin to a connector fixed to the skull via stainless steel screws and dental acrylic. Muscle and skin were closed in layers, and buprenorphine (0.05 mg/kg) was given twice daily for three days for analgesia.
Figure 2.
Diagram showing cervical contusion injury and placement of intraspinal stimulating electrodes. The five 30 µm platinum-iridium electrodes are routed along the surface of the spinal cord before passing through a small incision in the dura and penetrating to a depth of 1.2–1.8 mm within the spinal cord. The dura is then sutured closed and sealed with a microdrop of cyanoacrylate, and the implant is secured to T2 with sutures. The return electrode is placed above the spinal cord (not shown).
2.4 Therapeutic intraspinal stimulation
Beginning four weeks after injury, animals in the stimulated group received therapeutic ISMS for 7 hours/day, 5 days/week for 12 weeks. Treatment began four weeks after injury to model the sub-chronic injury (Jin et al., 2002; Lu et al., 2002) at a potentially realistic time point for future clinical intervention. The stimulation regimen was chosen to maximize the duration of daily stimulation delivered to the sub-chronically injured spinal cord, while still permitting animals to be observed during stimulation and forelimb testing to occur shortly after the completion of daily stimulation. The 12-week treatment duration was selected to model a standard clinical intervention (Dobkin et al., 2007; Field-Fote and Tepavac, 2002), and because forelimb function plateaued after this duration of therapeutic stimulation in preliminary studies. Stimulation was delivered during the animals’ active (dark) cycle. Symmetric, biphasic pulses with duration of 300 µs/phase were chosen as the shortest pulse duration still capable of eliciting a maximal response at low stimulus currents (based on (Grill et al., 1999; Stoney et al., 1968). Stimulus current was set at threshold to just evoke forelimb movement when delivered via a single electrode with current returning to the reference. Stimuli were delivered at a rate of 4 ± 1.5 Hz in a Gaussian distribution designed to approximate the average activity of rodent cortical neurons (Freire et al., 2011). Stimulation duty cycle was set to 75%, with stimulation delivered for periods of 15 minutes followed by 5 minutes without ISMS. This paradigm was chosen to maximize therapeutic electrical stimulation, while still providing rest periods to avoid fatigue of muscles. This duty cycle represents a compromise between the nearly 100% duty cycles used in spinal epidural stimulation and closed-loop cortical stimulation (Gerasimenko et al., 2008; Jackson et al., 2006), and the 30–70% duty cycles used clinically for peripheral nerve and muscle stimulation (Baker et al., 2000; Doucet et al., 2012). Alternating periods of stimulation with rest may also allow time for uninterrupted nervous system consolidation of connections that were potentially enhanced by the stimulation therapy as is observed in the induction of long-term potentiation (Abraham et al., 2002). Spinal stimulation thresholds were re-measured weekly on all electrodes. The electrode used to deliver therapeutic ISMS for the subsequent week was changed only if another electrode exhibited a much lower threshold, and/or evoked a more complex forelimb movement.
2.5 Precision forelimb reaching task
Rats were trained to perform a precision forelimb reaching task to greater than 70% success prior to injury (McKenna and Whishaw, 1999; Schrimsher and Reier, 1992). Animals reached across a 1 cm gap to retrieve a 45 mg chocolate-flavored food pellet (BioServ) from a 3 cm tall block at a total distance of 2 cm from the inside of an acrylic arena. A score of 1 was given for a successful retrieval of the pellet and a score of 0 was given for any unsuccessful attempt in which the animal extended its paw outside the acrylic arena. Each animal was allowed 20 individual attempts to retrieve a food pellet using the injured forelimb, and total scores were calculated for each animal (modified from (Gharbawie et al., 2005).
Following injury, all animals were tested 5 days/week at the forelimb reaching task by a blinded trainer. Stimulation was not applied during the reaching task, as animals were detached from stimulation cables 10–60 minutes prior to testing. Daily reaching scores were averaged across two week periods for each rat, and then normalized to a percentage of each animal’s pre-injury skill level.
To precisely quantify graded recovery of function, rats were recorded while reaching for food pellets using a high frame rate video camera (Toshiba Camileo H30) and an established 12-point scoring system was used to capture the details of forelimb function (Alaverdashvili et al., 2008). Rats were recorded in the sagittal plane while reaching and three representative reaches were selected for detailed scoring. An observer blind to experimental condition assigned a score of 0 for normal movements, a 0.5 for movements present but abnormal, and a 1 for absent movements in each of the 12 standardized categories of Alaverdashvili et al. (2008).
2.6 Statistics
Data from each outcome were tested for normality using Lilliefors test (Matlab 2007; The Mathworks) to determine whether parametric or non-parametric tests were appropriate. Nearly all data sets failed the test for normality, so non-parametric tests were used except where noted below. Movements evoked from chronic spinal stimulation electrodes were analyzed using a Wilcoxon signed-rank test to identify changes across the treatment period (SPSS vs. 11; IBM). Precision forelimb reaching scores were compared between treatment groups using the General Estimator Equation (SPSS vs. 11). Data from both the 12-point video analysis and forelimb asymmetry were compared among treatments using the Wilcoxon rank-sum test (Matlab 2007). A Bonferroni correction for multiple comparisons was further applied to the 12-point video analysis. Allodynia testing results were compared between treatment groups using a T-Test, as these data were normally distributed. An alpha level of p < 0.05 was used for significance of all tests.
2.7 Untrained behavioral test
Animals were also tested using a forelimb asymmetry task (Schallert et al., 2000). When animals reared to explore an acrylic cylinder, the number of contacts using their forepaw ipsilateral to injury was compared to the total number of forepaw contacts on the wall of the cylinder. Behavior was video recorded and scored at a later time point by a blinded rater. Forelimb asymmetry did not differ between the stimulated and unstimulated animals (rank-sum p > 0.29), suggesting the benefits of ISMS may be specific to goal-directed movements.
2.8 Allodynia testing
At the conclusion of the study, the injured forelimb of all animals was assessed for mechanical allodynia and sensory function using both rigid and flexible von Frey hairs (IITC Life Science Inc.). No difference was observed between the stimulated and unstimulated animals in either mechanical allodynia or sensation (T-Test p = 0.47 and p = 0.32, respectively), confirming that ISMS did not promote excessive sprouting of sensory fibers distal to the lesion.
3. Results
3.1 Chronic cervical spinal stimulation evokes functional and stable movements
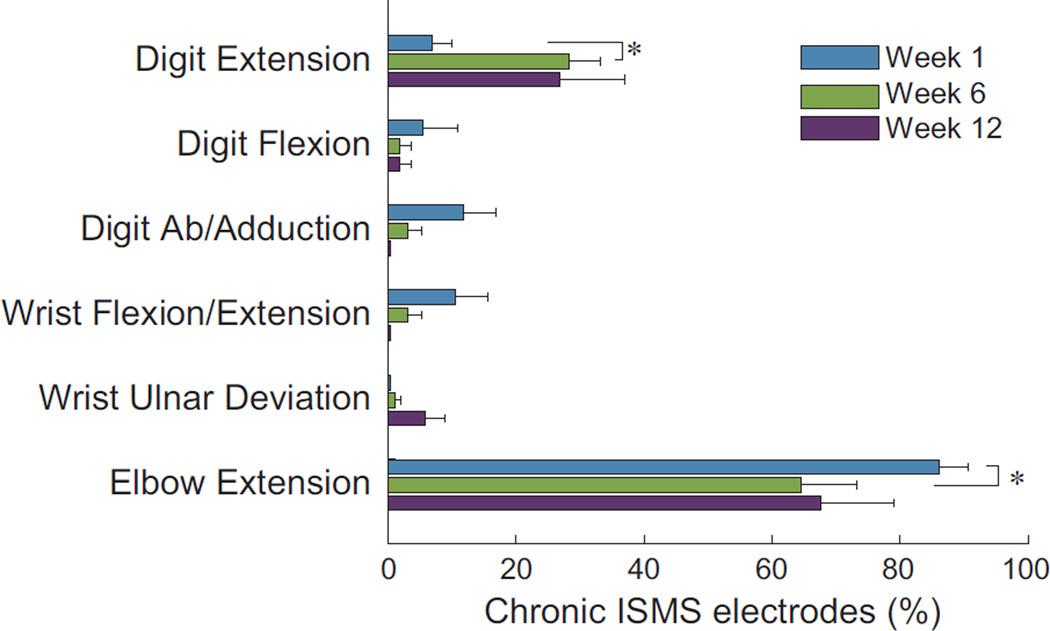
Cervical intraspinal microstimulation via chronically-implanted electrodes elicited a range of forelimb motor responses (Figure 3). Extension of the elbow was the most commonly evoked movement, decreasing slightly between the first and sixth week of treatment (Wilcoxon signed-rank p = 0.03). Notably, extension of the digits was more often evoked after six weeks of stimulation compared to immediately after implantation (Wilcoxon signed-rank p = 0.01). Both extension of the elbow and digits are highly-functional movements that counter the typically flexed posture of the forelimb after injury.
Figure 3.
ISMS delivered through chronically implanted electrodes elicits generally similar effects at 1-, 6- and 12- weeks of therapeutic stimulation (* Wilcoxon signed-rank p < 0.05; mean + SEM). Stimulation most commonly evoked highly-functional movements such as elbow and digit extension, with the later increasing during treatment. Evoked movements at each time-point total more than 100% as synergistic movement were evoked from ~30% of electrodes.
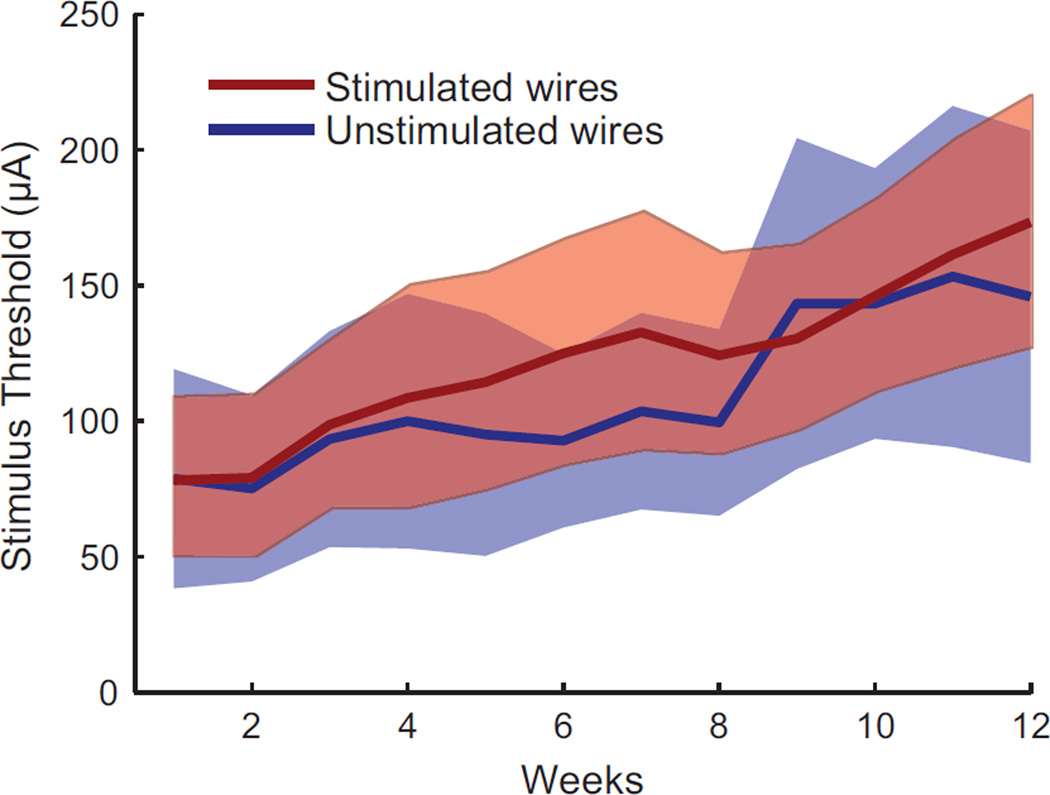
Although the threshold current needed to evoke a forelimb movement gradually increased over the 12 week study, there was no difference between wires that delivered therapeutic stimulation and wires that were not chronically stimulated (Figure 4). Single pulse stimulus thresholds increased by an average of 7.9 ± 4.6 µA/week for the stimulated wires, compared to 7.2 ± 8.3 µA/week for the unstimulated wires (mean ± SD; T-test p = 0.78). Further, there was no significant correlation between the number of weeks a given wire was stimulated (mean 7.8, range: 1–12 weeks) and its change in threshold over the study (linear regression p = 0.18), suggesting that therapeutic stimulation did not negatively affect the ability to evoke movements by damaging nearby neural tissue.
Figure 4.
A gradual increase in threshold to evoke a movement was observed over the 12 weeks of therapeutic ISMS treatment. Thresholds increased equally on wires that delivered therapeutic stimulation and wires that did not (mean ± 95% confidence intervals).
3.2 Improvement in forelimb function after prolonged intraspinal stimulation
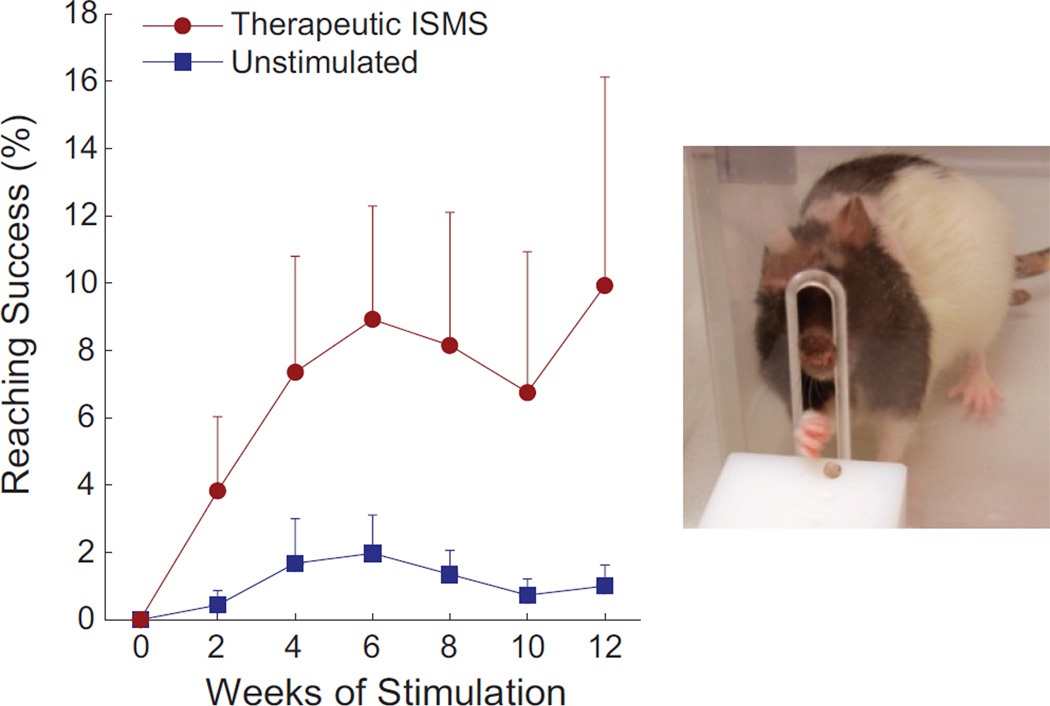
Animals receiving therapeutic intraspinal microstimulation demonstrated modest but durable improvement in forelimb reaching function, with benefits persisting beyond the period of stimulation. The stimulated group performed the forelimb reaching task significantly better than the unstimulated group (GEE p < 0.05, n = 11 animals/group). Improvements in skilled forelimb function began within the first two weeks of stimulation and continued for the duration of the 12 weeks of treatment (Figure 5).
Figure 5.
Animals receiving therapeutic ISMS rapidly improved forelimb function, retrieving food pellets significantly better than the unstimulated animals (GEE p < 0.05, n = 11 animals/group, Mean + SEM). All animals were tested when the stimulator was inactive, demonstrating sustained improvements beyond the period of stimulation.
3.3 Improved forelimb tone and coordination following intraspinal stimulation
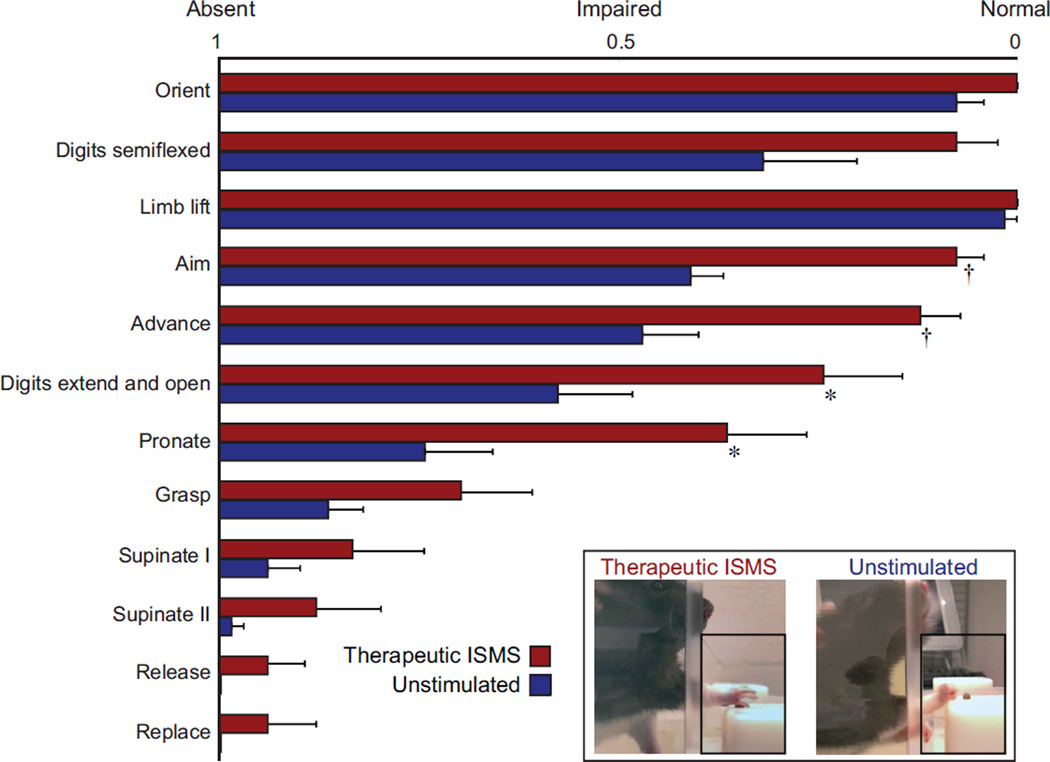
In addition to overall improvements in skilled forelimb reaching, therapeutic ISMS led to reduced forelimb flexor tone and increased volitional movement coordination. We utilized high-speed video recordings to quantify 12 components of each reach (Alaverdashvili et al., 2008). In all twelve components, stimulated rats performed better than unstimulated rats (Figure 6). Performance was significantly better in the categories of aim, advance, digit extension and opening, and pronation (rank sum p < 0.05). Even when controlling for multiple comparisons in the most conservative manner, the stimulated animals’ ability to aim and advance were much better than unstimulated animals (p < 0.004; Bonferroni correction). Some of the movements most different between groups were those involved in advancing/extending the arm and extending the digits in preparation for grasping the pellet. Interestingly, the muscles responsible for these functional movements were also the most commonly activated by ISMS (see Figure 3). These correlations between stimulation-evoked extension and parallel functional improvement suggests that ISMS may successfully combat the excessive flexor tone observed in the forelimbs after injury, which is a substantial clinical problem (Skold et al., 1999).
Figure 6.
Detailed analysis of forelimb reaching demonstrates marked improvement after 12 weeks of therapeutic ISMS treatment. The forelimb reaching task was segmented into 12 components, presented in order of occurrence from top to bottom. In all 12 components, stimulated rats performed better than unstimulated animals, and these differences were significant between treatment groups in 4 of the 12 measures (* rank sum p < 0.05; † p < 0.004 after Bonferroni correction; mean + SEM). Inset: Images of a stimulated (left) and unstimulated (right) animal performing the precision forelimb reaching task. Note that the stimulated animal has reduced flexor tone of the forelimb, manifested as improved ability to extend the arm and open the digits. These changes are evident in the group scores for animals receiving therapeutic stimulation (main figure).
4. Discussion
Here we demonstrate the development and implementation of a chronic electrode array for stimulation of the cervical spinal cord following contusion injury. Forelimb movements evoked are relatively stable over 12 weeks of stimulation, and threshold currents to evoke a movement increase similarly on chronically stimulated compared to unstimulated electrodes. Using these implanted electrodes to deliver therapeutic intraspinal microstimulation caudal to a cervical contusion injury leads to modest but sustained improvements in forelimb motor function.
The chronic cervical ISMS electrode was adapted from similar arrays implanted in the lumbar spinal cord of cats and rodents by the Mushahwar group (Bamford et al., 2005; Mushahwar et al., 2000). Key modifications for the cervical spinal cord included the caudal approach and anchoring procedure to the second thoracic vertebra, performance of hemilaminaectomies in order to preserve stability of the cervical vertebra, and the subdural placement of the electrode leads combined with dural sutures to secure the implant within the spinal cord parenchyma. Although the exact location of the stimulation electrodes within the spinal lamina was not determined, the chronic electrode array evoked movements that were well aligned with the location of spinal motor neuron locations (compare Figure 3 to McKenna et al., 2000; Tosolini and Morris, 2012). The limited rostro-caudal extent of the implant likely restricted the range of forelimb movements observed compared to our recent study exploring ISMS-evoked movements from a much larger portion of the cervical enlargement both before and after injury (Sunshine et al., 2013). In the present study, the implanted electrodes spanned segments C6-T1, nearly perfectly overlapping with the triceps motor neuron pools, and likely explaining the predominance of elbow extension movements observed. A key goal of this implant is to activate motor neurons innervating muscles that are otherwise disconnected from cortical input after injury. The additional observation of a range of wrist, and digit movements evoked via stimulation throughout the duration of chronic implant suggest this is possible (see Figure 3).
The results also demonstrate that therapeutic intraspinal stimulation caudal to a cervical contusion injury leads to modest but sustained improvements in forelimb motor function. This effect occurs within the first several weeks of stimulation, with continued improvements over the 12-week duration of the study. With the exception of one case report using epidural stimulation in a human patient (Herman et al., 2002), this is the first demonstration of durable functional improvement induced by spinal cord activation extending beyond the period of stimulation.
The improved function observed in animals treated with therapeutic intraspinal stimulation may occur via similar mechanisms as stimulation of the brainstem or cortex. Axonal sprouting and locomotor recovery are observed after electrical stimulation applied to the contralateral motor cortex or pyramidal tract following unilateral pyramidotomy (Brus-Ramer et al., 2007; Carmel et al., 2010). The cervical intraspinal stimulation employed here may similarly activate spared, descending fibers as well as local spinal circuits below the injury. Intraspinal stimulation may also act to re-regulate neural circuits deprived of natural descending drive after spinal cord injury (Edgerton and Harkema, 2011). In addition, improvements following therapeutic ISMS may result from activating muscles that are partially or completely paralyzed after injury. Functional Electrical Stimulation (FES) applied directly to muscles after incomplete spinal cord injury improves locomotor coordination and tone lasting beyond the period of stimulation (Mirbagheri et al., 2002; Jung et al., 2009).
Although difficult to quantify in a small animal model, flexor tone and spasticity appear substantially reduced in animals treated with therapeutic ISMS. We observed significant improvements in forelimb aim, extension and opening of the digits during a forelimb reaching task. All of these movements are compromised by the excessive flexor tone typical after cervical contusion injury. Flexor tone, hyperreflexia and spasticity may result from excessive sprouting of sensory fibers and additional synapse formation on motor neurons deprived of cortical input after injury to the descending tracts (Tan et al., 2012). It is possible that therapeutic ISMS provides sufficient activation of motor neurons, either directly or via intraspinal circuits, to discourage the formation of additional afferent synapses after injury. Spasticity is a complex and very significant clinical problem, effecting 40–60 % of patients with spinal cord injury (Roy and Edgerton, 2012; Skold et al., 1999).
Therapeutic ISMS may also augment and enhance the limited spontaneous recovery observed following incomplete injuries. Damaged corticospinal tract axons sprout above the injury to form new synaptic connections with propriospinal neurons, and maintain connections with those spinal relay neurons that bypass the lesion (Bareyre et al., 2004). These results are confirmed by both electrophysiological and neuroanatomical evidence, as well as improved locomotor function when coupled with motor retraining and treatment with brain-derived neurotrophic factor (BDNF;(Vavrek et al., 2007). Indeed, humans with moderate incomplete spinal cord injury demonstrate substantial recovery of function with intensive motor retraining even years after injury (Fox et al., 2010; Harkema et al., 2011b). These studies emphasize the potential for rewiring of spinal circuits to bypass an incomplete lesion, a rewiring that may be enhanced by therapeutic ISMS.
Conclusions
Intraspinal microstimulation has shown promise for directly re-animating the limbs when applied to the lumbar spinal cord (e.g., (Mushahwar et al., 2002). Here we demonstrate for the first time a long-term therapeutic benefit of ISMS applied to the cervical spinal cord, where functional recovery persists beyond the period of stimulation. This provides exciting evidence for the therapeutic or perhaps even regenerative capacity of neuroprosthetic devices. Studies are underway to elucidate the mechanisms by which ISMS promotes lasting improvements in motor function, and explore the potential for stimulation to positively combine with stem cell transplants and pharmacological interventions to promote regeneration after central nervous system injury.
Acknowledgments
The authors thank Tia Secasiu, Anand Kaul, Nathaniel Cook, and Molly Cooper for animal training, Sam Nutt and Laura Schlosser for assistance with injuries, Larry Shupe for technical support, Behnum Habibi and David Boe for data analysis, Steve Perlmutter for methodological insight, and Vivian Mushahwar and Jeremy Bamford for expertise in electrode manufacture and implantation. Research reported in this publication was supported by a EUREKA award from the National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health under award number (1R01NS066357), an American Heart and Stroke Association Scientist Development Grant (NCRP 09SDG2230091), a DARPA Young Faculty Award (D12AP00251) and the Center for Sensorimotor Neural Engineering (CNSE), a National Science Foundation Engineering Research Center (EEC-1028725). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, National Science Foundation, or other funding agencies.
Footnotes
Conflict of interest: NA
References
- Abraham WC, Logan B, Greenwohod JM, Dragunow M. Induction and experience-dependent consolidation of stable long-term potentiation lasting months in the hippocampus. J Neurosci. 2002;22:9626–9634. doi: 10.1523/JNEUROSCI.22-21-09626.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaverdashvili M, Leblond H, Rossignol S, Whishaw IQ. Cineradiographic (video X-ray) analysis of skilled reaching in a single pellet reaching task provides insight into relative contribution of body, head, oral, and forelimb movement in rats. Behav Brain Res. 2008;192:232–247. doi: 10.1016/j.bbr.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Baker C, Wederich D, McNael C, Newsam R, Walters R. Neuromuscular Electrical Stimulation: A Practical Guide. 4th edition. Downey, CA: Los Amigos Research & Education Institute; 2000. [Google Scholar]
- Bamford JA, Putman CT, Mushahwar VK. Intraspinal microstimulation preferentially recruits fatigue-resistant muscle fibres and generates gradual force in rat. J Physiol. 2005;569:873–884. doi: 10.1113/jphysiol.2005.094516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Chakrabarty S, Martin JH. Electrical stimulation of spared corticospinal axons augments connections with ipsilateral spinal motor circuits after injury. J Neurosci. 2007;27:13793–13801. doi: 10.1523/JNEUROSCI.3489-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel JB, Berrol LJ, Brus-Ramer M, Martin JH. Chronic electrical stimulation of the intact corticospinal system after unilateral injury restores skilled locomotor control and promotes spinal axon outgrowth. J Neurosci. 2010;30:10918–10926. doi: 10.1523/JNEUROSCI.1435-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin B, Barbeau H, Deforge D, Ditunno J, Elashoff R, Apple D, Basso M, Behrman A, Harkema S, Saulino M, Scott M. The evolution of walking-related outcomes over the first 12 weeks of rehabilitation for incomplete traumatic spinal cord injury: the multicenter randomized Spinal Cord Injury Locomotor Trial. Neurorehabil Neural Repair. 2007;21:25–35. doi: 10.1177/1545968306295556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet BM, Lam A, Griffin L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J Biol Med. 2012;85:201–215. [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Harkema S. Epidural stimulation of the spinal cord in spinal cord injury: current status and future challenges. Expert Rev Neurother. 2011;11:1351–1353. doi: 10.1586/ern.11.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field-Fote EC, Tepavac D. Improved intralimb coordination in people with incomplete spinal cord injury following training with body weight support and electrical stimulation. Phys Ther. 2002;82:707–715. [PubMed] [Google Scholar]
- Fox EJ, Tester NJ, Phadke CP, Nair PM, Senesac CR, Howland DR, Behrman AL. Ongoing walking recovery 2 years after locomotor training in a child with severe incomplete spinal cord injury. Phys Ther. 2010;90:793–802. doi: 10.2522/ptj.20090171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freire MA, Morya E, Faber J, Santos JR, Guimaraes JS, Lemos NA, Sameshima K, Pereira A, Ribeiro S, Nicolelis MA. Comprehensive analysis of tissue preservation and recording quality from chronic multielectrode implants. PLoS One. 2011;6:e27554. doi: 10.1371/journal.pone.0027554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimenko Y, Roy RR, Edgerton VR. Epidural stimulation: Comparison of the spinal circuits that generate and control locomotion in rats, cats and humans. Exp Neurol. 2008;209:417–425. doi: 10.1016/j.expneurol.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharbawie OA, Gonzalez CL, Whishaw IQ. Skilled reaching impairments from the lateral frontal cortex component of middle cerebral artery stroke: a qualitative and quantitative comparison to focal motor cortex lesions in rats. Behav Brain Res. 2005;156:125–137. doi: 10.1016/j.bbr.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Giszter SF, Mussa-Ivaldi FA, Bizzi E. Convergent force fields organized in the frog's spinal cord. J Neurosci. 1993;13:467–491. doi: 10.1523/JNEUROSCI.13-02-00467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill WM, Bhadra N, Wang B. Bladder and urethral pressures evoked by microstimulation of the sacral spinal cord in cats. Brain Res. 1999;836:19–30. doi: 10.1016/s0006-8993(99)01581-4. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011a;377:1938–1947. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil. 2011b;93:1508–1517. doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- Herman R, He J, D'Luzansky S, Willis W, Dilli S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord. 2002;40:65–68. doi: 10.1038/sj.sc.3101263. [DOI] [PubMed] [Google Scholar]
- Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system "bridge" and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR. Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neurosci Lett. 2005;383:339–344. doi: 10.1016/j.neulet.2005.04.049. [DOI] [PubMed] [Google Scholar]
- Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- Jin Y, Fischer I, Tessler A, Houle JD. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177:265–275. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- Jung R, Belanger A, Kanchiku T, Fairchild M, Abbas JJ. Neuromuscular stimulation therapy after incomplete spinal cord injury promotes recovery of interlimb coordination during locomotion. J Neural Eng. 2009;6:055010. doi: 10.1088/1741-2560/6/5/055010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Feron F, Mackay-Sim A, Waite PM. Olfactory ensheathing cells promote locomotor recovery after delayed transplantation into transected spinal cord. Brain. 2002;125:14–21. doi: 10.1093/brain/awf014. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ. Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: a retrograde carbocyanine dye analysis. J Comp Neurol. 2000;419:286–296. doi: 10.1002/(sici)1096-9861(20000410)419:3<286::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- McKenna JE, Whishaw IQ. Complete compensation in skilled reaching success with associated impairments in limb synergies, after dorsal column lesion in the rat. J Neurosci. 1999;19:1885–1894. doi: 10.1523/JNEUROSCI.19-05-01885.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirbagheri MM, Ladouceur M, Barbeau H, Kearney RE. The effects of long-term FES-assisted walking on intrinsic and reflex dynamic stiffness in spastic spinal-cord-injured subjects. IEEE Trans Neural Syst Rehabil Eng. 2002;10:280–289. doi: 10.1109/TNSRE.2002.806838. [DOI] [PubMed] [Google Scholar]
- Moritz CT, Lucas TH, Perlmutter SI, Fetz EE. Forelimb movements and muscle responses evoked by microstimulation of cervical spinal cord in sedated monkeys. J Neurophysiol. 2007;97:110–120. doi: 10.1152/jn.00414.2006. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Collins DF, Prochazka A. Spinal cord microstimulation generates functional limb movements in chronically implanted cats. Exp Neurol. 2000;163:422–429. doi: 10.1006/exnr.2000.7381. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Gillard DM, Gauthier MJ, Prochazka A. Intraspinal micro stimulation generates locomotor-like and feedback-controlled movements. IEEE Trans Neural Syst Rehabil Eng. 2002;10:68–81. doi: 10.1109/TNSRE.2002.1021588. [DOI] [PubMed] [Google Scholar]
- NSCISC. Spinal Cord Injury Facts and Figures at a Glance. Birmingham, Alabama: The National Spinal Cord Injury Statistical Center; 2012. [Google Scholar]
- Roy RR, Edgerton VR. Neurobiological perspective of spasticity as occurs after a spinal cord injury. Exp Neurol. 2012;235:116–122. doi: 10.1016/j.expneurol.2012.01.017. [DOI] [PubMed] [Google Scholar]
- Salimi I, Martin JH. Rescuing transient corticospinal terminations and promoting growth with corticospinal stimulation in kittens. J Neurosci. 2004;24:4952–4961. doi: 10.1523/JNEUROSCI.0004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Schrimsher GW, Reier PJ. Forelimb motor performance following cervical spinal cord contusion injury in the rat. Exp Neurol. 1992;117:287–298. doi: 10.1016/0014-4886(92)90138-g. [DOI] [PubMed] [Google Scholar]
- Skold C, Levi R, Seiger A. Spasticity after traumatic spinal cord injury: nature, severity, and location. Arch Phys Med Rehabil. 1999;80:1548–1557. doi: 10.1016/s0003-9993(99)90329-5. [DOI] [PubMed] [Google Scholar]
- Stokes BT, Noyes DH, Behrmann DL. An electromechanical spinal injury technique with dynamic sensitivity. J Neurotrauma. 1992;9:187–195. doi: 10.1089/neu.1992.9.187. [DOI] [PubMed] [Google Scholar]
- Stoney SD, Jr, Thompson WD, Asanuma H. Excitation of pyramidal tract cells by intracortical microstimulation: effective extent of stimulating current. J Neurophysiol. 1968;31:659–669. doi: 10.1152/jn.1968.31.5.659. [DOI] [PubMed] [Google Scholar]
- Sunshine MD, Cho FS, Lockwood DR, Fechko AS, Kasten MR, Moritz CT. Cervical intraspinal microstimulation evokes robust forelimb movements before and after injury. J Neural Eng. 2013;10:036001. doi: 10.1088/1741-2560/10/3/036001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan AM, Chakrabarty S, Kimura H, Martin JH. Selective corticospinal tract injury in the rat induces primary afferent fiber sprouting in the spinal cord and hyperreflexia. J Neurosci. 2012;32:12896–12908. doi: 10.1523/JNEUROSCI.6451-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosolini AP, Morris R. Spatial characterization of the motor neuron columns supplying the rat forelimb. Neuroscience. 2012;200:19–30. doi: 10.1016/j.neuroscience.2011.10.054. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Bizzi E. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Exp Brain Res. 1999;129:401–416. doi: 10.1007/s002210050908. [DOI] [PubMed] [Google Scholar]
- van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- Vavrek R, Pearse DD, Fouad K. Neuronal populations capable of regeneration following a combined treatment in rats with spinal cord transection. J Neurotrauma. 2007;24:1667–1673. doi: 10.1089/neu.2007.0290. [DOI] [PubMed] [Google Scholar]
- Wang D, Ichiyama RM, Zhao R, Andrews MR, Fawcett JW. Chondroitinase combined with rehabilitation promotes recovery of forelimb function in rats with chronic spinal cord injury. J Neurosci. 2011;31:9332–9344. doi: 10.1523/JNEUROSCI.0983-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann JB, Seki K, Jackson A. Reanimating the arm and hand with intraspinal microstimulation. J Neural Eng. 2011;8:054001. doi: 10.1088/1741-2560/8/5/054001. [DOI] [PMC free article] [PubMed] [Google Scholar]