Abstract
Background
Clinical monitoring of cerebral blood flow (CBF) autoregulation in patients undergoing liver transplantation may provide a means for optimizing blood pressure to reduce the risk of brain injury. The purpose of this pilot project is to test the feasibility of autoregulation monitoring with transcranial Doppler (TCD) and near infrared spectroscopy (NIRS) in patients undergoing liver transplantation and to assess changes that may occur perioperatively.
Methods
We performed a prospective observational study in 9 consecutive patients undergoing orthotopic liver transplantation. Patients were monitored with TCD and NIRS. A continuous Pearson’s correlation coefficient was calculated between mean arterial pressure (MAP) and CBF velocity and between MAP and NIRS data, rendering the variables mean velocity index (Mx) and cerebral oximetry index (COx), respectively. Both Mx and COx were averaged and compared during the dissection phase, anhepatic phase, first 30 mins of reperfusion, and remaining reperfusion phase. Impaired autoregulation was defined as Mx ≥ 0.4.
Results
Autoregulation was impaired in one patient during all phases of surgery, in two patients during the anhepatic phase, and in one patient during reperfusion. Impaired autoregulation was associated with a MELD score > 15 (p=0.015) and postoperative seizures or stroke (p<0.0001). Analysis of Mx categorized in 5-mmHg bins revealed that MAP at the lower limit of autoregulation (MAP when Mx increased to ≥ 0.4) ranged between 40 and 85 mmHg. Average Mx and average COx were significantly correlated (p=0.0029). The relationship between COx and Mx remained when only patients with bilirubin > 1.2 mg/dL were evaluated (p=0.0419). There was no correlation between COx and baseline bilirubin (p=0.2562) but MELD score and COx were correlated (p=0.0458). Average COx was higher for patients with a MELD score > 15 (p=0.073) and for patients with a neurologic complication than for patients without neurologic complications (p=0.0245).
Conclusions
These results suggest that autoregulation is impaired in patients undergoing liver transplantation, even in the absence of acute, fulminant liver failure. Identification of patients at risk for neurologic complications after surgery may allow for prompt neuroprotective interventions, including directed pressure management.
INTRODUCTION
Autoregulation of cerebral blood flow (CBF) is an intrinsic protective mechanism that ensures a stable supply of oxygenated blood to the brain commensurate with metabolic demands[1]. Impaired autoregulation may predispose the brain to ischemic injury during hypotension or hyperemic encephalopathy with high blood pressure[2]. Impaired autoregulation has been found to be associated with poor neurologic outcome after traumatic brain injury and stroke after cardiac surgery[1, 3]. Conversely, optimizing blood pressure to be within the autoregulatory range is associated with improved outcomes in patients with traumatic brain injury[1, 4, 5].
End-stage liver disease is often associated with subclinical or clinical encephalopathy that increases the risk for adverse outcomes, including mortality, after liver transplantation[6, 7]. The etiology of this condition is poorly understood and likely multifactorial, but its presence may result in reduced intracranial compliance and ultimately increased intracranial pressure (ICP). The presence of elevated ICP can severely compromise cerebral perfusion during the multiple hemodynamic perturbations that occur during and after liver transplantation. Currently, invasive monitoring with an intracranial “bolt” is the only method available to aggressively manage patients with elevated ICP from acute liver failure. The placement of an ICP catheter in patients with liver failure is associated with a risk of brain hemorrhage due the presence of a coagulapathy. Nonetheless, aggressive management of elevated ICP for patients with hepatic encephalopathy from liver failure may improve outcome[6].
Recent studies have shown that it is possible to monitor CBF autoregulation noninvasively with a moving linear regression correlation coefficient between mean arterial pressure (MAP) and middle cerebral artery transcranial Doppler (TCD)-measured blood flow velocity (mean velocity index [Mx])[1]. When autoregulation is lost, flow velocity has a correlation of 1 with perfusion pressure at low frequencies, as CBF is directly dependent on blood pressure. Our group has monitored CBF autoregulation in patients undergoing cardiac surgery[3, 8]. Monitoring autoregulation using TCD has many limitations, though, including inability to obtain a transcranial insonating window in some patients, movement artifact, and interference from electrocautery during surgery. In laboratory studies and in clinical investigations in patients undergoing cardiac surgery, our group has found that regional cerebral oxygen saturation measured with near-infrared spectroscopy (NIRS) a is a clinically suitable surrogate for CBF for autoregulation monitoring[9, 10]. Using NIRS for autoregulation monitoring has many advantages and overcomes limitations of TCD allowing for continuous monitoring in multiple settings despite patient movement. However, in patients with liver failure, an elevated bilirubin level has the potential to confound NIRS measurements because bilirubin is a chromophore that absorbs infrared light thereby reducing NIRS-measured regional cerebral oxygen saturation (rScO2%)[11].
The purpose of this pilot project was to test the feasibility of monitoring autoregulation with TCD and NIRS in patients undergoing liver transplantation and to assess changes that may occur perioperatively.
METHODS
All procedures were approved by the Institutional Review Board at the Johns Hopkins Medical Institutions. Nine consecutive subjects with chronic end-stage liver disease undergoing liver transplantation between October 3, 2011 to January 28, 2012 were enrolled in the study after they provided written informed consent. Inclusion criteria were patients with acute or chronic liver failure undergoing liver transplantation who were able to provide informed consent.
Anesthesia and Monitoring
Patients received a pulmonary artery catheter (Swan-Ganz, Baxter Healthcare Corp., Irvine, CA) with an 8.5 F Arrow introducer (Arrow International, Reading, PA) for measurement of cardiac output, pulmonary arterial mean pressure, pulmonary capillary wedge pressure, and central venous pressure. Routine perioperative values, such as invasive MAP, were measured via a radial artery catheter. Cardiac output was measured with the thermodilution method in triplicate at the following times: (1) immediately before surgery (2) immediately after the incision, (3) 15 mins after portal vein clamping, (4) 30 mins before the end of the anhepatic phase, (5) 10 mins after declamping the portal vein, (6) 30 mins after declamping the portal vein, and then (7) hourly during the late reperfusion phase.
Anesthesia was induced with propofol (1–3 mg/kg), and fentanyl (3–10 mcg/kg) and rocuronium (0.3–0.5 mg/kg) were given for skeletal muscle relaxation. Anesthesia was maintained with isoflurane end-tidal concentration < 1.5%) and intermittent doses of fentanyl and vecuronium. Ventilation was adjusted to maintain end-tidal CO2 between 35 mmHg and 45 mmHg. Before reperfusion, minute ventilation was increased and the fraction of inspired oxygen (FiO2) decreased from 100% to approximately 50%. Body temperature was adjusted with a forced air warmer to maintain a nasal temperature >35°C. Norepinephrine or epinephrine was given as necessary and calcium chloride was given to maintain ionized plasma calcium above 1.1 mmol L−1. Blood was transfused to maintain the hemoglobin level between 8 and 10 g/dL. A 4% solution of human albumin and fresh frozen plasma were infused for volume loading and maintenance of international normalized ratio at 1.5 to 2.0. Normal saline solution was supplied to supplement the volume lost by evaporation and through urine. Platelet transfusion was considered if the platelet count was less than 75 ×109/L.
Surgery
The piggyback technique without venovenous bypass was used in all patients. After dissection of native liver, the hepatic artery and portal vein were cross-clamped in sequence. Suprahepatic and infrahepatic inferior vena cava were not completely clamped. Only the common ostium of the middle and left suprahepatic vein was clamped. The diseased liver was removed. Before the graft was implanted, the vena cava was partially clamped. Then the donor retrohepatic vena cava was anastomosed with the recipient ostium of the suprahepatic vein followed by the anastomoses of portal vein, hepatic artery, and bile duct. To minimize hemodynamic instability at reperfusion, we flushed the graft with approximately 1.0 L of isotonic saline before reperfusion and then with 400 to 500 mL of portal blood by temporarily unclamping the portal vein after completion of portal vein anastomosis. Then we unclamped the portal vein and the suprahepatic common ostium to reperfuse the graft. Total perfusion to the implanted graft was attained after the hepatic artery was anastomosed.
Autoregulation Monitoring
Middle cerebral blood flow velocity was monitored with transcranial Doppler (Doppler Box, DWL; Compumedics USA Inc., Charlotte, NC) after anesthesia was initiated using two 2.5-MHz transducers fitted on a headband; the depth of insonation was varied between 35 mm and 52 mm until representative spectral artery flow was obtained. Regional cerebral oxygen saturation monitoring was performed with 2 self-adhesive sensors placed on the right and left forehead before anesthesia induction that were connected to a NIRS monitor (INVOS™, Somanetics/Covidien, Boulder, CO) Analog arterial blood pressure from the operating room hemodynamic monitor and TCD signals (1 Hz) and the digital signals from the NIRS monitor were processed with ICM+ software (version 6.4, University of Cambridge, Cambridge, UK) via an analog-to-digital converter (60 Hz) as we have described[3, 8]. The output signals were time integrated as 10-sec averaged values to filter high-frequency noise from the respiratory and pulse frequencies and to detect oscillations and transients below 0.05 Hz. Mean velocity index and COx were then calculated by a consecutive, paired, moving Pearson correlation coefficient between 30, 10-sec averages of MAP and TCD blood flow velocity signals and between cerebral oximetry signals and MAP. Accordingly, Mx and COx were calculated from 30 data points in a 5-min time window and with a 60-sec moving window. An Mx and COx that approaches zero signifies intact autoregulation because MAP and CBF velocity are not correlated. When CBF velocity is directly related to MAP, Mx and COx approaches 1, indicating impaired autoregulation.
Data Analysis
The Mx and COx results were analyzed as time-averaged values for the respective surgical periods. Further, Mx values were placed in 5-mmHg bins for analysis of the lower limit of autoregulation. Although the exact Mx value that indicates the lower limit of autoregulation is not known, laboratory experiments and prior clinical studies indicate that it is likely between 0.3 and 0.5[3, 8]. For this analysis, the lower limit of autoregulation was defined as the MAP at which Mx increased from <0.4 to ≥0.4. Right and left TCD recordings and COx results were combined for analysis. Results are expressed as means±SD. Continuous data were analyzed by ANOVA with Bonferroni correction, and dichotomous data were evaluated with Fishers exact test. Analyses were performed with Stata Software (version 9.0, Stata Corp, College Station, TX). For analysis of data, the surgical course was subdivided into four phases through orthotopic liver transplantation: (1) Dissection phase, from beginning of surgery to portal vein clamping, (2) anhepatic phase, from portal vein clamping to donor liver reperfusion, (3) early reperfusion phase, the first 30 mins after the portal vein was unclamped, (4) late reperfusion phase, from the end of early reperfusion to the end of surgery.
RESULTS
Patient medical data are listed in Table 1. Most patients had cirrhosis associated with hepatitis C virus, one patient underwent re-transplantation because of allograft dysfunction 12 days after a prior liver transplantation for primary sclerosing cholangitis, and one patient underwent combined liver and renal transplantation. Four patients had a model for end-stage liver disease (MELD) score > 20 and one had a score > 30. Five patients had baseline total bilirubin levels > 1.2 mg/dL. Two patients had both a generalized seizure and stroke diagnosed after surgery.
Table 1.
Patient medical and surgical data.
| Variable | Result |
|---|---|
| Age (mean±SD; range) | 51±14 (17 to 69) |
| Gender (male/female) | 7/2 |
| Diagnosis (number of patients) | |
| Hepatitis C | 1 |
| Hepatitis C with hepatocellular carcinoma | 4 |
| Alcoholic hepatitis | 1 |
| Nonalcoholic steatohepatitis | 1 |
| Autoimmune hepatitis | 1 |
| Liver graft failure | 1 |
| MELD score (mean±SD, range) | 17.9 ± 3.3 (6 to 33) |
| Preoperative bilirubin (mg/dL; mean ± SD, range) | 5.8 ± 3.4 (0.7 to 31.2) |
| Ammonium (mmol/L; mean ± SD, range) | 66.0 ± 10.4 (41.9 to 90.1) |
| History of encephalopathy (number of patients) | 6 |
| Duration of surgery (min; mean ± SD, range) | 478±132 (320 to 720) |
| Duration of anhepatic stage (min; mean ± SD, range) | 141±37 (87 to 206) |
| Tracheal extubation (mean ± SD) | 1 day in 7 patients, 13 days in 1 patient, and 12 days in 1 patient |
| Postoperative seizure and stroke (number of patients) | 2 |
Arterial blood gases and other laboratory values for each operative period are listed in Table 2. Of the physiologic variables analyzed, only lactate level differed significantly between periods. Hemodynamic, TCD, and autoregulation results are listed in Table 3. There were no differences in MAP between periods. In five of nine patients, CBF velocity was elevated in the early reperfusion (p=0.03) and late reperfuson (p=0.029) phases of surgery compared with baseline measurements. However, for the entire cohort, average CBF velocity did not change between periods. The Mx values during the anhepatic phase, early reperfusion, and late reperfusion tended to be higher than those at baseline, but the difference was not significant. This pattern was similar for patients whose CBF velocity increased in the early and late reperfusion periods. One patient had impaired autoregulation (Mx ≥ 0.4) during all phases of surgery. Two other patients had impaired autoregulation during the anhepatic phase of surgery, and one had impaired autoregulation during reperfusion. Impaired autoregulation was associated with a MELD score > 15 (p=0.015) and postoperative seizures and stroke (p<0.0001). Analysis of Mx categorized in 5-mmHg bins revealed that MAP at the lower limit of autoregulation ranged between 40 and 85 mmHg.
Table 2.
Arterial blood gas results and hemodynamic parameters for the each operative period
| Operative period | pH | PaCO2 (mmHg) | PaO2 (mmHg) | HgB (gm/dL) | Lactate (mmol/L) |
|---|---|---|---|---|---|
| Dissection | 7.41±0.06 | 36±4 | 366±57 | 10.2±2.6 | 1.4±0.3 |
| Anhepatic | 7.40±0.05 | 33±4 | 404±48 | 10.6±1.7 | 3.3±0.7a |
| Early reperfusion | 7.37±0.05 | 35±4 | 393±101 | 9.9±1.6 | 5±1.0a,b |
| Late reperfusion | 7.40±0.05 | 36±3 | 353±94 | 9.8±1.3 | 3.7±1.0a,c |
p<0.0001 vs dissection phase;
p<0.002 vs anhepatic phase;
p=0.029 vs early reperfusion phase
Table 3.
Hemodynamic and autoregulation data for each perioperative perioda
| Dissection | Anhepatic | Early Reperfusion | Reperfusion | |
|---|---|---|---|---|
| Duration (min) | 144±89 (70 to 219) | 143±32 (117 to 170) | 30 | 120±32 (94 to 147) |
| Mean arterial pressure (mmHg) | 86±10 (79 to 94) | 87±9 (80 to 94) | 84±10 (76 to 91) | 83± 8 (76 to 89) |
| Cardiac index (L·min−1·m−2) | 4.3±2.0 (2.7 to 5.9) | 3.3±1.2 (2.4 to 4.3) | 4.6±1.3 (3.6 to 5.5) | 4.0±1.1 (3.2 to 4.9) |
| Systemic vascular resistance (mmHg·m2 min L−1) | 1546.7±786.3 (942.2 to 2151.1) | 2065.2±838.6 (1420.6 to 2709.8) | 1358.1±621.7 (880.2±1836.0) | 1421.7±733.1 (858.1 to 1985.2) |
| CBF velocity (cm/sec) | 26.3±8.0 (20.2 to 32.5) | 30.5±7.4 (24.8 to 36.2) | 33.4±9.6 (26.0 to 40.8) | 34.3±8.1 (28.1 to 40.5) |
| Mx | 0.04±0.09 (−0.03±0.12) | 0.20±0.17 (0.07 to 0.34) | 0.13±0.21 (−0.04 to 0.31) | 0.21±0.10 (0.11±0.30) |
| COx | 0.15±0.09 (0.07 to 0.22) | 0.21±0.13 (0.11 to 0.31) | 0.08±0.13 (−0.02 to 0.18) | 0.17±0.11 (0.09±0.26 |
| rScO2% | 49±20 (34 to 65) | 55±20 (39 to 70) | 55±22 (38±72) | 55±22 (38 to 72) |
CBF, cerebral blood flow; Mx, mean velocity index or the correlation coefficient between CBF velocity and blood pressure; COx, cerebral oximetry index or the correlation between cerebral oximetry and blood pressure; rScO2%, regional cerebral oxygen saturation.
All values are given as means±SD (range). No significant differences were present between periods for any variable.
Neither cerebral oximetry index (COx) nor cerebral oximetry measurements differed between the operative periods. Average Mx and average COx were significantly correlated (p=0.0029). The relationship between average COx and average Mx remained even when only patients with bilirubin > 1.2 mg/dL were evaluated (p=0.0419). No correlation existed between COx and baseline bilirubin (p=0.2562), but MELD score and COx did show a correlation (p=0.0458). Average COx was higher for patients with a MELD score > 15 than for those with a lower MELD score (p=0.073) and for patients with a neurologic complication than for patients without neurologic complications (p=0.0245).
Discussion
In this study we found a large range of blood pressures at the lower limit of autoregulation in patients undergoing liver transplantation. For the most part, autoregulation was functional throughout surgery. However, one patient exhibited impaired autoregulation throughout surgery and two other patients had transient impaired autoregulation during the reperfusion period. Impaired autoregulation detected with TCD-based Mx or NIRS-based COx was associated with subsequent postoperative seizures and stroke. Impaired autoregulation also was associated with more severe hepatic failure based on MELD score.
Mild to severe encephalopathy is commonly present in patients undergoing liver transplantation. A clinical dilemma often arises on how best to monitor adequacy of cerebral perfusion in such patients because in many instances, ICP is only minimally elevated, and the placement of an intracranial catheter poses great risk in a patient with coagulapathy. Nonetheless, in a series of 22 patients who had ICP monitoring for hepatic encephalopathy, survival after liver transplant was 88% and no deaths were caused by cerebral edema[12]. Although the aggressive use of interventions, including hypothermia, may have contributed to these results, the data support recommendations by some groups for aggressive management of elevated ICP in patients with hepatic encephalopathy from liver failure[6].
We have monitored CBF autoregulation in patients undergoing cardiac surgery and found that the MAP at the lower limit of autoregulation varies widely from 40 to 90 mmHg during cardiopulmonary bypass[8]. The results of our current study confirm our previous findings insofar as the MAP at the lower limit of autoregulation in this cohort ranged from 40 to 85 mmHg. Noninvasive monitoring of CBF autoregulation might provide a means for managing blood pressure during and after liver transplantation. Cerebral blood flow becomes pressure-passive when blood pressure is outside the autoregulatory limits or when autoregulation is impaired. During such situations, fluctuations in blood pressure, which often occur perioperatively during liver transplantation, could result in cerebral ischemia and potential injury. In patients with traumatic brain injury, optimizing blood pressure to be within the CBF autoregulation range was found to be associated with improved outcomes[1, 4, 5]. In prior studies of cardiac surgical patients, we have found impaired autoregulation in 20% of patients and that this condition is associated with a high rate of stroke[3, 8]. Similarly, impaired autoregulation was found to be associated with mortality in patients with traumatic brain injury[1, 4, 13]. When autoregulation is impaired, CBF is blood pressure passive. As a consequence, low blood pressure could lead to cerebral hypoperfusion and ischemia and high blood pressure to cerebral hyperemia, both of which may promote brain injur[2].
Impaired autoregulation is a recognized complication of acute liver failure[14, 15]. The mechanism of the latter is not clear but the cause is likely multifactorial and the result of processes that contribute to increased ICP. Several authors have evaluated CBF autoregulation during and after liver transplantation[14, 16]. In a study of six patients with acute liver failure, autoregulation was measured at discrete time points during manipulation of blood pressure with phenylephrine [16]. The authors found that autoregulation was impaired in all patients but that it improved by the end of surgery. In another study of 23 patients with severe liver failure, cerebral vascular resistance was decreased, CBF velocity was increased, and autoregulation was impaired during the first hour after graft implantation compared with values during the anhepatic phase and at the end of surgery. Such changes may expose the patients to cerebral hyperperfusion and neurologic injury.
None of the patients in our study had acute liver failure, yet we did observe impaired autoregulation in three of nine patients during the various stages of surgery. In our study, the use of continuous, rather than intermittent, autoregulation monitoring may have provided more detail on the dynamics of autoregulation during the multiple perturbations during surgery. Further, our methods rely on spontaneous alterations in blood pressure and are not pharmacologically induced. Raising or lowering the blood pressure with the sole intent of measuring autoregulation may not be tolerated in patients with intracranial pathology, thus supporting our method as a more useful clinical tool. In this study we also observed a relationship between impaired autoregulation and postoperative neurologic outcomes. Regardless, our results suggest that subclinical cerebral abnormalities that occur with liver failure may result in impaired maintenance of CBF during hemodynamic fluctuation in some patients. Improved monitoring of patients may allow for more appropriate optimization of hemodynamics perioperatively.
Unlike pulse oximetry, NIRS measurement of cerebral oxygenation does not distinguish between arterial and venous blood. Because most blood in the cranium is venous blood, cerebral oximetry provides a measure of relative oxygen supply versus demand. By focusing on low frequency (0.003 to 0.04 Hz) fluctuations in cerebral oximetry that represent autoregulatory blood pressure compensations, we have previously shown that NIRS monitoring may be a useful clinical surrogate for CBF in autoregulation monitoring[1, 9, 10, 17]. Whether these methods will be useful in patients undergoing liver transplantation is not clear because bilirubin absorbs light in the near-infrared spectrum, resulting in low cerebral oxygen saturation[11]. Our current results suggest that the use of relative changes in cerebral oximetry results in relation to MAP rather than absolute values should enable COx to be measured even in patients with high bilirubin levels. Nissen et al[18] also have proposed that NIRS might be used to monitor autoregulation in patients undergoing liver transplantation. In their study, changes in raw cerebral oximetry data were evaluated in comparison to MAP. Autoregulation data could be derived in only 13 of 33 patients.
Certain limitations to this study must be considered. The patients received isoflurane as part of the anesthetic. Volatile anesthetics cause cerebral vasodilation, and in high doses, can impair autoregulation [19]. In this cohort, isoflurane concentrations were usually < 1 MAC, or in a dose range that would not impair autoregulation. Nonetheless, we did not continuously capture end-tidal isoflurane concentrations and, thus, are unable to adjust for this potentially confounding factor on autoregulation. Cerebral blood flow autoregulation is sensitive to CO2 levels. Although, mechanical lung ventilation was targeted to normocarbia based on end-tidal CO2 monitoring, the average PaCO2 measurement during the anhepatic phase of surgery demonstrated mild hyperventilation. This likely represents clinician preparation for restoration of blood flow to the transplanted liver and attendant potential hypercarbia. Nonetheless, we are unable to control for this factor in our analysis. The small number of patients in this pilot study tempers the conclusions made from our observations. Further, we cannot exclude the possibility that impaired autoregulation might result from a neurologic event rather than the neurologic event occurring as a result of impaired autoregulation. Regardless, our results suggest that impaired autoregulation may occur in patients undergoing liver transplantation even in the absence of acute liver failure. Identification of patients at risk for neurologic complications after surgery may allow for prompt neuroprotective interventions, including optimal blood pressure control and possibly hypothermia[20].
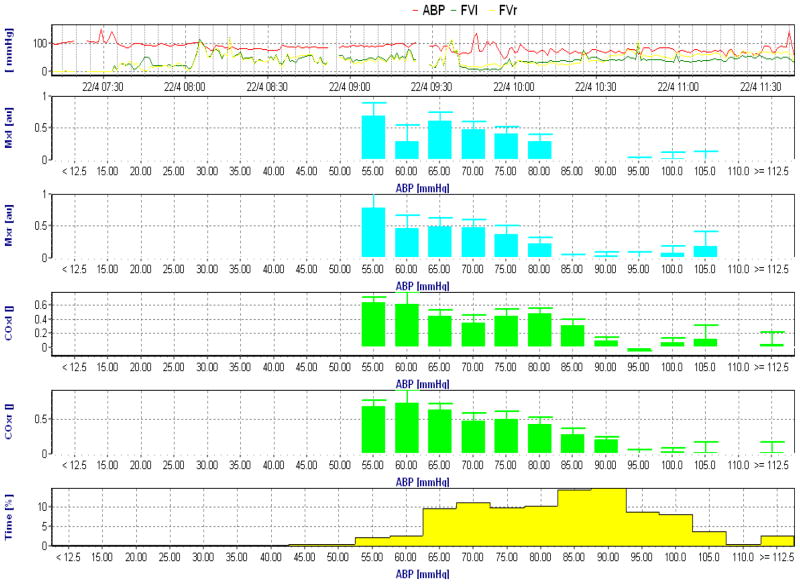
Figure 1.
Intraoperative autoregulation recording with transcranial Doppler based mean velocity index (Mx, blue) and near infrared spectroscopy based cerebral oximetry index (COx, green). The Mx and COx data from the left and right hemisphere are placed into 5 mmHg arterial blood pressure (ABP) bins. The top graph is ABP and bottom graph the percentage of time spent at each blood pressure bin. Increasing Mx and COx indicates increasing correlation between blood pressure and the surrogates for cerebral blood flow indicating increasing passivity of flow to pressure. The lower limit of autoregulation in this patient based on an Mx >0.4 is approximately 80 mmHg.
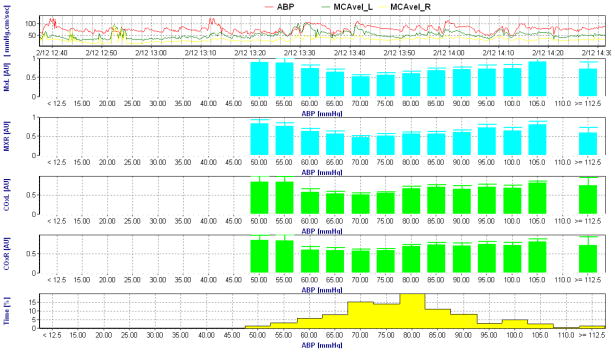
Figure 2.
Intraoperative autoregulation recording with transcranial Doppler based mean velocity index (Mx, blue) and near infrared spectroscopy based cerebral oximetry index (COx, green). The data from the left and right hemisphere are placed into 5 mmHg arterial blood pressure (ABP) bins. The top graph is ABP and bottom graph the percentage of time spent at each blood pressure bin. This patient demonstrates an impaired autoregulation pattern evidence by Mx >0.4 at all ABP bins. A similar pattern is evident on the COx recording. Despite this dysregulated pattern, an optimal blood pressure where autoregulation is most preserved is evident at an ABP of approximately 70 mmHg.
Footnotes
Clinical Trials Registration: NCT01425385 at www.clinicaltrials.gov
References
- 1.Czosnyka M, Brady K, Reinhard M, Smielewski P, Steiner LA. Monitoring of cerebrovascular autoregulation: facts, myths, and missing links. Neurocrit Care. 2009;10(3):373–386. doi: 10.1007/s12028-008-9175-7. [DOI] [PubMed] [Google Scholar]
- 2.van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, de Leeuw PW. Cerebral hyperperfusion syndrome. Lancet Neurol. 2005;4 (12):877–888. doi: 10.1016/S1474-4422(05)70251-9. [DOI] [PubMed] [Google Scholar]
- 3.Joshi B, Brady K, Lee J, Easley B, Panigrahi R, Smielewski P, Czosnyka M, Hogue CW., Jr Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg. 2010;110(2):321–328. doi: 10.1213/ANE.0b013e3181c6fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41(1):11–17. doi: 10.1097/00006123-199707000-00005. discussion 17-19. [DOI] [PubMed] [Google Scholar]
- 5.Steiner LA, Czosnyka M, Piechnik SK, Smielewski P, Chatfield D, Menon DK, Pickard JD. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit Care Med. 2002;30(4):733–738. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Steadman RH, Van Rensburg A, Kramer DJ. Transplantation for acute liver failure: perioperative management. Curr Opin Organ Transplant. 2010;15(3):368–373. doi: 10.1097/MOT.0b013e32833982dd. [DOI] [PubMed] [Google Scholar]
- 7.Dhiman RK, Kurmi R, Thumburu KK, Venkataramarao SH, Agarwal R, Duseja A, Chawla Y. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci. 2010;55(8):2381–2390. doi: 10.1007/s10620-010-1249-7. [DOI] [PubMed] [Google Scholar]
- 8.Joshi B, Ono M, Brown C, Brady K, Easley RB, Yenokyan G, Gottesman RF, Hogue CW. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg. 2012;114(3):503–510. doi: 10.1213/ANE.0b013e31823d292a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady KM, Lee JK, Kibler KK, Smielewski P, Czosnyka M, Easley RB, Koehler RC, Shaffner DH. Continuous time-domain analysis of cerebrovascular autoregulation using near-infrared spectroscopy. Stroke. 2007;38(10):2818–2825. doi: 10.1161/STROKEAHA.107.485706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley RB, Hogue CW., Jr Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke. 2010;41(9):1951–1956. doi: 10.1161/STROKEAHA.109.575159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song JG, Jeong SM, Shin WJ, Jun IG, Shin K, Huh IY, Kim YK, Hwang GS. Laboratory variables associated with low near-infrared cerebral oxygen saturation in icteric patients before liver transplantation surgery. Anesth Analg. 2011;112(6):1347–1352. doi: 10.1213/ANE.0b013e318214b2b0. [DOI] [PubMed] [Google Scholar]
- 12.Raschke RA, Curry SC, Rempe S, Gerkin R, Little E, Manch R, Wong M, Ramos A, Leibowitz AI. Results of a protocol for the management of patients with fulminant liver failure. Crit Care Med. 2008;36(8):2244–2248. doi: 10.1097/CCM.0b013e31818029a3. [DOI] [PubMed] [Google Scholar]
- 13.Steiner LA, Coles JP, Johnston AJ, Chatfield DA, Smielewski P, Fryer TD, Aigbirhio FI, Clark JC, Pickard JD, Menon DK, et al. Assessment of cerebrovascular autoregulation in head-injured patients: a validation study. Stroke. 2003;34 (10):2404–2409. doi: 10.1161/01.STR.0000089014.59668.04. [DOI] [PubMed] [Google Scholar]
- 14.Larsen FS, Ejlersen E, Strauss G, Rasmussen A, Kirkegaard P, Hansen BA, Secher N. Cerebrovascular metabolic autoregulation is impaired during liver transplantation. Transplantation. 1999;68(10):1472–1476. doi: 10.1097/00007890-199911270-00007. [DOI] [PubMed] [Google Scholar]
- 15.Durham S, Yonas H, Aggarwal S, Darby J, Kramer D. Regional cerebral blood flow and CO2 reactivity in fulminant hepatic failure. J Cereb Blood Flow Metab. 1995;15 (2):329–335. doi: 10.1038/jcbfm.1995.38. [DOI] [PubMed] [Google Scholar]
- 16.Ardizzone G, Arrigo A, Panaro F, Ornis S, Colombi R, Distefano S, Jarzembowski TM, Cerruti E. Cerebral hemodynamic and metabolic changes in patients with fulminant hepatic failure during liver transplantation. Transplant Proc. 2004;36(10):3060–3064. doi: 10.1016/j.transproceed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Zweifel C, Castellani G, Czosnyka M, Carrera E, Brady KM, Kirkpatrick PJ, Pickard JD, Smielewski P. Continuous assessment of cerebral autoregulation with near-infrared spectroscopy in adults after subarachnoid hemorrhage. Stroke. 2010;41 (9):1963–1968. doi: 10.1161/STROKEAHA.109.577320. [DOI] [PubMed] [Google Scholar]
- 18.Nissen P, Pacino H, Frederiksen HJ, Novovic S, Secher NH. Near-infrared spectroscopy for evaluation of cerebral autoregulation during orthotopic liver transplantation. Neurocrit Care. 2009;11(2):235–241. doi: 10.1007/s12028-009-9226-8. [DOI] [PubMed] [Google Scholar]
- 19.Patel P, Drummond J. Cerebral physiology and the effects of anesthetics and techniques. In: Miller RD, editor. Anesthesia. 6. Vol. 1. Elsevier Churchill Livinstone; Philadelphia, PA: 2005. [Google Scholar]
- 20.Jalan R, Olde Damink SW, Deutz NE, Hayes PC, Lee A. Restoration of cerebral blood flow autoregulation and reactivity to carbon dioxide in acute liver failure by moderate hypothermia. Hepatology. 2001;34(1):50–54. doi: 10.1053/jhep.2001.25386. [DOI] [PubMed] [Google Scholar]