INTRODUCTION
Filamentous fungi are important aetiological agents of keratitis globally.1 Hyaline hyphomycetes including Fusarium and Aspergillus spp. are most common, but dematiaceous fungi such as Curvularia and Bipolaris spp. constitute approximately 20% of cases.2,3 Commonly reported signs of fungal keratitis include feathery edges, raised lesions, hypopyon, stromal infiltrates and, less frequently, satellite lesions and ring infiltrates.1 In dematiaceous cases, reported characteristics include raised lesions and macroscopic pigmentation.4,5 In this report, we analyse clinical signs of fungal keratitis, comparing hyaline and dematiaceous fungi, and the hyaline fungi Fusarium and Aspergillus spp.
METHODS
Clinical examination and microbiological reports were collected prospectively in a clinical trial setting. Methods for the trial have been described previously.6 In brief, eligible cases had evidence of filamentous fungus on Gram stain and/or potassium hydroxide. Scrapings were inoculated onto sheep's blood agar, chocolate agar and potato dextrose agar. Fungal cultures were deemed positive with growth on two media or moderate to heavy growth on one media.
After a positive smear, but prior to any fungal identification, clinical examination was performed and ulcers were assessed for feathery edges, raised lesions, ring infiltrates, keratic precipitates, satellite lesions, endothelial plaque, pigment and absence of corneal sensation. Dated, auditable exams ensure that the observers were masked to fungal identification. We performed backwards stepwise regression in the R statistical package (http://www.R-project.org; Vienna, Austria). Briefly, all covariates were included in the initial model, including age and gender. The covariate whose removal resulted in the lowest Akaike's information criterion (AIC) was deleted from the model until the removal of any remaining covariates no longer improved AIC. Contrasts included dematiaceous versus hyaline fungus, Fusarium versus non-Fusarium spp. isolates, Aspergillus versus non-Aspergillus spp. isolates and Aspergillus flavus isolates versus non-Aspergillus flavus. Mixed infections (N=2) were excluded from the analysis. Ethical approval was obtained from the University of California, San Francisco, and Aravind Eye Care System Institutional Review Boards.
RESULTS
Of the 120 cases enrolled into the trial, 101 had positive cultures. Of these, 37 (37%) were dematiaceous and 64 (63%) were hyaline. Of the hyaline fungal isolates, 44 (69%) were Fusarium spp. and 17 (27%) were Aspergillus spp., of which 11 isolates were A flavus. Seventy-four cases (73%) had feathery edges: 28 dematiaceous (76%) and 46 hyaline (72%). Forty-three cases (43%) had a hypopyon at presentation: 16 dematiaceous (43%) and 27 hyaline (42%). Forty cases (40%) had a raised lesion: 20 dematiaceous (71%) and 20 hyaline (31%). Six cases (6%) were pigmented, all of which were dematiaceous (figure 1).
Figure 1.
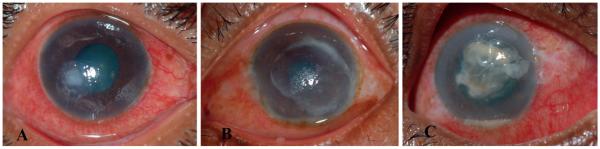
(A) Fusarium spp. keratitis showing neither a ring infiltrate nor a raised lesion. (B) Aspergillus flavus keratitis showing a ring infiltrate and feathery borders. (C) Dematiaceous fungal keratitis showing macroscopic pigmentation and a raised lesion.
Table 1 lists the optimal multivariate models. Dematiaceous fungi were more likely to have a raised lesion than hyaline fungi (p=0.007). Aspergillus spp. were more likely to have a ring infiltrate (p=0.042), and Fusarium spp. were less likely to have a raised lesion or an endothelial plaque.
Table 1.
Backwards stepwise regression model, using Akaike's information criterion for clinical signs predicting hyaline fungus (vs dematiaceous); Fusarium spp., Aspergillus spp. and Aspergillus flavus (vs all other fungi)
| Hyaline vs dematiaceous |
Fusarium spp. |
Aspergillus spp. |
A flavus
|
|||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Feathery edges | – | – | 0.40 (0.13 to 1.24) | 0.11 | – | – | – | – |
| Raised lesions | 0.28 (0.11 to 0.70) | 0.0065 | 0.20 (0.072 to 0.55) | 0.002 | 2.64 (0.74 to 9.35) | 0.13 | 3.27 (0.77 to 13.57) | 0.10 |
| Ring infiltrates | – | – | – | – | 14.03 (1.10 to 178.66) | 0.042 | 23.96 (1.67 to 340.58) | 0.019 |
| Endothelial plaque | – | – | 0.17 (0.031 to 0.97) | 0.046 | 4.73 (0.88 to 25.55) | 0.071 | – | – |
| Age | – | – | – | – | 0.97 (0.93 to 1.01) | 0.14 | 0.95 (0.90 to 0.99) | 0.041 |
DISCUSSION
Clinical signs have been previously reported as characteristic of fungal keratitis. Here, feathery edges and hypopyon were commonly found in both dematiaceous and hyaline fungal keratitis, with no difference between the two groups. Raised lesions were predictive of dematiaceous fungus, and as expected, all pigmented cases were dematiaceous. No dematiaceous case had a ring infiltrate, consistent with previous reports where this was a rare sign.4 Ring infiltrate was predictive of Aspergillus spp. and A flavus in particular. Within hyaline cases, raised lesions and endothelial plaques were more common in Aspergillus spp. and less common in Fusarium spp.
This study suggests that clinical signs can distinguish between hyaline and dematiaceous, and even the genus and species of fungi. Because of the delay in confirming species identification by culture, these clinical signs may help with guiding initial diagnosis and treatment decisions.
Acknowledgments
Funding None of the authors has any financial disclosures related to this manuscript. This research was funded by That Man May See and the South Asia Research Fund. The Department of Ophthalmology at UCSF is supported by a core grant from the National Eye Institute, EY02162. Dr Acharya is supported by a National Eye Institute K23EY017897 grant and a Research to Prevent Blindness Career Development Award. Dr Lietman is supported by a National Eye Institute grant U10-EY015114 and a Research to Prevent Blindness award. The sponsors did not have a role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript.
Footnotes
Competing interests None.
Ethics approval This study was conducted with the approval of the University of California, San Francisco; Aravind Eye Hospital, Madurai, India; Dartmouth-Hitchcock Medical Center, Hanover, NH.
Provenance and peer review Not commissioned; not externally peer reviewed.
REFERENCES
- 1.Srinivasan M. Fungal keratitis. Curr Opin Ophthalmol. 2004;15:321–7. doi: 10.1097/00055735-200408000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Gopinathan U, Garg P, Fernandes M, et al. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center. Cornea. 2002;21:555–9. doi: 10.1097/00003226-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bharathi M, Ramakrishnan R, Vasu S, et al. Epidemiological characteristics and laboratory diagnosis of fungal keratitis: a three-year study. Indian J Ophthalmol. 2003;51:315–21. [PubMed] [Google Scholar]
- 4.Garg P, Gopinathan U, Choudhary K, et al. Keratomycosis: clinical and microbiologic experience with dematiaceous fungi. Ophthalmology. 2000;107:574–80. doi: 10.1016/s0161-6420(99)00079-2. [DOI] [PubMed] [Google Scholar]
- 5.Wilhelmus K, Jones D. Curvularia keratitis. Tr Am Ophth Soc. 2001;99:111–32. [PMC free article] [PubMed] [Google Scholar]
- 6.Prajna N, Mascarenhas J, Krishnan T, et al. Comparison of natamycin and voriconazole for the treatment of fungal keratitis. Arch Ophthalmol. 2010;128:672–8. doi: 10.1001/archophthalmol.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]


