Abstract
Objectives
To examine the relationship between N-terminal pro-brain natriuretic peptide (NT-proBNP) and exercise capacity in a large contemporary cohort of patients with chronic heart failure.
Background
Natriuretic peptides such as NT-proBNP are important biomarkers in heart failure. The relationship between NT-proBNP and exercise capacity has not been well studied.
Methods
We analyzed the relationship between baseline NT-proBNP and peak VO2 or distance in the 6 minute walk test in 1383 subjects enrolled in the HF-ACTION study. Linear regression models were used to analyze the relationship between NT-proBNP and peak VO2 or distance in the 6 minute walk test in the context of other clinical variables. Receiver operator curve (ROC) analysis was used to evaluate the ability of NT-proBNP to accurately predict a peak VO2 < 12 mL/kg/min.
Results
NT-proBNP was the most powerful predictor of peak VO2 (partial R2=0.13, p<0.0001) out of 35 candidate variables. Although NT-proBNP was also a predictor of distance in the 6 minute walk test, this relationship was weaker than that for peak VO2 (partial R2 = 0.02, p<0.0001). For both peak VO2 and distance in the 6 minute walk test, much of the variability in exercise capacity remained unexplained by the variables tested. ROC analysis suggested NT-proBNP had moderate ability to identify patients with peak VO2 < 12 mL/kg/min (c-index=0.69).
Conclusions
In this analysis of baseline data from HF-ACTION, NT-proBNP was the strongest predictor of peak VO2 and a significant predictor of distance in the 6 minute walk test. Despite these associations, NT-proBNP demonstrated only modest performance in identifying patients with a low peak VO2 who might be considered for cardiac transplantation. These data suggest that, while hemodynamic factors are important determinants of exercise capacity, much of the variability in exercise performance in heart failure remains unexplained by traditional clinical and demographic variables.
Keywords: Heart failure, exercise, biomarker, clinical trials
Circulating biomarkers play an increasingly critical role in the diagnosis and management of patients with chronic heart failure1. Natriuretic peptides such as brain natriuretic peptide (BNP) and its N-terminal pro-brain natriuretic peptide (NT-proBNP) have been demonstrated to be powerful tools for the diagnosis, risk stratification, and management of patients with heart failure2. In addition to being useful for clinical management, biomarkers may suggest insights into the mechanisms underlying important physiologic relationships.
Exercise intolerance, typically manifest as exertional dyspnea, is the major morbidity of chronic heart failure. Both maximal (as measured by peak oxygen uptake (peak VO2))3, 4 and sub-maximal (as measured by distance in the 6 minute walk test)5 exercise capacity have been demonstrated to be of substantial prognostic importance in chronic heart failure. Assessment of peak VO2 plays a central role in risk stratification of patients with advanced heart failure being considered for cardiac transplantation6. Despite the clinical importance of exercise capacity, substantial controversy persists about the primary determinants of exercise intolerance in heart failure, and the relative contribution of cardiac performance, hemodynamic factors, pulmonary function, and peripheral skeletal muscle remain incompletely understood7–11. The purpose of this study was to evaluate the association of NT-proBNP with peak VO2 and distance in the 6 minute walk test in a large cohort of patients enrolled in a randomized trial of exercise training, the Heart Failure and a Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) study.
Methods
Details of design, rationale, and primary results of HF-ACTION have been published elsewhere12, 13. Briefly, HF-ACTION (clinicaltrials.gov, NCT00047437) was a randomized clinical trial evaluating the effect of exercise training on long term morbidity and mortality in patients with chronic heart failure due to left ventricular systolic dysfunction. Enrolled patients were randomized to exercise training in addition to usual care versus usual care alone. HF-ACTION was approved by local Institutional Review Boards, and all enrolled patients provided written informed consent.
Exercise Testing
2329 of 2331 patients enrolled in the HF-ACTION study underwent baseline exercise testing as has been previously described12, 14. Patients underwent symptom limited cardiopulmonary exercise testing with gas exchange analysis to evaluate peak VO2, utilizing either a motor driven treadmill or exercise cycle ergometer protocol. For treadmill exercise, a modified extended Naughton protocol was used; for cycle ergometer exercise, a ramped protocol was used with 10 watt/minute increases starting at 0 watts. A six minute walk test was to be performed as previously described5 at least 2 hours before or after baseline cardiopulmonary exercise (CPX) testing. CPX testing data were interpreted by a central core laboratory (Duke University).
NT- proBNP Assay
Patients enrolled in the HF-ACTION study and in the biomarker substudy underwent plasma collection at the baseline, 3-month, and 12-month visits. Baseline blood samples were obtained on the same day as baseline exercise testing, but were to be obtained prior to exercise. Samples were collected via peripheral vein into EDTA containing tubes, and then centrifuged immediately and stored at −70° C for subsequent analysis. Assays for NT-proBNP were performed using a commercially available assay platform (Roche Diagnostics, Inc.) at a central core laboratory (Duke University).
Statistical Analysis
Continuous variables were presented as medians with the 25th and 75th percentiles. Categorical data were presented as percentages. Patient characteristics were compared between patients above and below the median value of Peak VO2 using the Chi square test for categorical, and the t-test for continuous variables. Where specific distributional assumptions for these tests are were violated, the Fisher's Exact and Wilcoxon signed rank tests, respectively, were used instead. Because data on NT-proBNP were not normally distributed, we log transformed NT-proBNP values (ln[NT-proBNP]) for all statistical analyses.
The aim of this analysis was to evaluate the relationship between NT proBNP and measures of exercise capacity, specifically the ability of NT-proBNP to predict peak VO2 and distance in the 6 minute walk test alone and in combination with other clinical predictors. We used Pearson correlation coefficients to assess the univariable relationship between NT-proBNP and each of these primary endpoints, as well as other exercise parameters of interest (CPX test duration and Ve/VCO2 slope).
In order to understand the relationship between NT-proBNP and exercise capacity in the context of other clinical variables, we created multivariable linear regression models for both peak VO2 and distance in the 6 minute walk test, with the measure of exercise capacity as the dependent outcome variable. For each of these endpoints, the best predictive model was constructed using baseline clinical data from the overall trial population (n=2331) in a backwards variable selection process. Variables were sequentially eliminated from an initial set of candidate predictors, the variable with the highest P-value eliminated at each step. This stage ended when all remaining variables had a P-value < 0.05. In order to further isolate factors most significant in determining functional capacity measures, all variables which remained in the model derived by this process, but which possessed a partial R2 <0.01 after eliminating non-significant predictors, were also removed. Candidate variables considered for each model were 35 demographic and clinical variables thought to be potential predictors of exercise capacity based on review of the literature and clinical judgment (see Appendix for list of candidate variables). Once a best model was generated from the overall study population, this model was then applied to the subset of patients (n=1383) for whom NT-proBNP data were available. Finally, NT-proBNP was added to the model to evaluate the relative contribution of NT-proBNP in the context of other predictors of exercise capacity. We also evaluated interaction terms for NT-proBNP with age, gender, and body mass index based on previous data showing that these were important potential determinants of both NT-proBNP levels and exercise capacity. The relative strength of association between each predictor variable and exercise capacity was based on the partial R2 in the final multivariable model. A p value <0.05 was considered statistically significant for all analyses.
Because peak VO2 ≤ 12 mL/kg/min has been recommended as a clinically important cut point for cardiac transplant listing in patients treated with beta-blocker therapy6, we used receiver-operator curve (ROC) analysis to assess the ability of NT-proBNP to predict a peak VO2 ≤ 12 mL/kg/min in the study population. The area under the curve for the ROC was used to assess the accuracy of NT-proBNP levels in predicting a peak VO2 ≤ 12 mL/kg/min.
Results
Study Cohort
Baseline characteristics for the study cohort are shown in Table 1. Generally, the cohort in whom NT-proBNP data were available (n=1383) was similar to the overall study population (n=2331). The study cohort was diverse with regard to gender (29% women), race (34% African-American), and age (20% with age ≥ 70). The mean ejection fraction was 25% and most patients (66%) had NYHA class II heart failure symptoms. Notably, patients enrolled in HF-ACTION were exceptionally well-treated in terms of evidence-based therapies, with extremely high rates of utilization for beta-blockers (95%), angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) (95%), and implantable cardioverter-defibrillators (ICDs) (39%).
Table 1.
Baseline Characteristics of Study Cohort
| Variable | Total Cohort | Peak VO2 ≤ 14.4 | Peak VO2 > 14.4 | P value | |
|---|---|---|---|---|---|
| N= 1383 | N = 694 | N = 675 | |||
| Age (years) Median (Q1, Q3) |
59 (50, 68) | 66 (53, 71) | 56 (48, 64) | <0.001 | |
| Female sex | 29% | 35% | 23% | <0.001 | |
| Race (%) | |||||
| White | 60% | 55% | 65% | 0.001 | |
| Black or African American |
34% | 38% | 29% | ||
| Other | 7% | 7% | 6% | ||
| LVEF (%) | 25 (20, 30)% | 24 (19, 29)% | 26 (21, 31)% | <0.001 | |
| NYHA Class | <0.001 | ||||
| II | 66% | 52% | 80% | ||
| III/IV | 34% | 48% | 20% | ||
| Ischemic etiology of heart failure |
49% | 53% | 45% | 0.003 | |
| Diabetes mellitus | 32% | 39% | 25% | <0.001 | |
| Hospitalizations (last 6 months) | 0.025 | ||||
| 0 | 59% | 59% | 58% | ||
| 1 | 29% | 26% | 32% | ||
| ≥ 2 | 12% | 15% | 10% | ||
| Hypertension | 62% | 65% | 59% | 0.014 | |
| Stroke | 10% | 12% | 8% | 0.018 | |
| Active smoking | 16% | 16% | 16% | 0.006 | |
| Resting heart rate (beats/min) |
70 (63, 78) | 70 (64, 78) | 70 (62, 78) | 0.204 | |
| SBP (mmHg) | 112 (101, 128) | 110 (100, 126) | 114 (104, 128) | 0.001 | |
| DBP | 70 (62, 80) | 70 (60, 78) | 70 (62, 80) | <0.001 | |
| Weight (kg) | 91 (78, 107) | 91 (78, 110) | 91 (78, 105) | 0.225 | |
| Height (cm) | 173 (166, 180) | 173 (165, 178) | 175 (168, 180) | <0.001 | |
| BMI (kg/m2) | 30 (26, 36) | 31 (26, 37) | 30 (26, 34) | <0.001 | |
| Peak VO2 (mL/kg/min) |
14.4 (11.5, 17.5) | 11.6 (9.8, 13.1) | 17.5 (16.0, 20.0) | <0.001 | |
| Distance in the 6 minute walk test (m) |
372 (300, 431) | 329 (259, 385) | 414 (357, 472) | <0.001 | |
| KCCQ overall summary score |
68 (51, 83) | 64 (48, 80) | 73 (56, 87) | <0.001 | |
| Beck Depression Inventory II score |
8 (4, 15) | 9 (5, 16) | 8 (4, 13) | 0.016 | |
| ACE or ARB | 95% | 95% | 95% | 0.999 | |
| Beta blocker | 95% | 95% | 95% | 0.809 | |
| ICD | 39% | 43% | 36% | 0.005 | |
| NT-proBNP (pg/mL) | 815 (341, 1805) | 1210 (503, 2921) | 524 (241, 1171) | <0.001 | |
Exercise Capacity and NT-proBNP
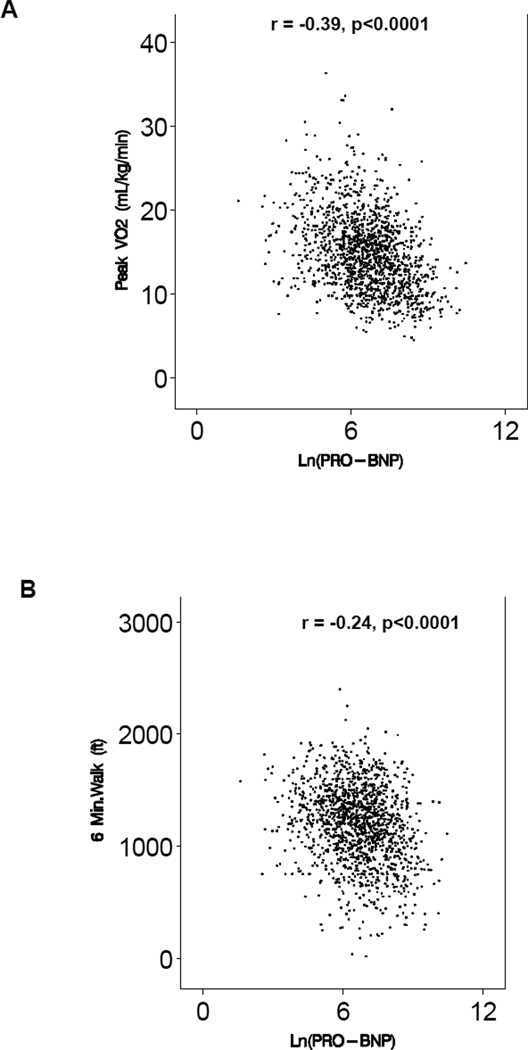
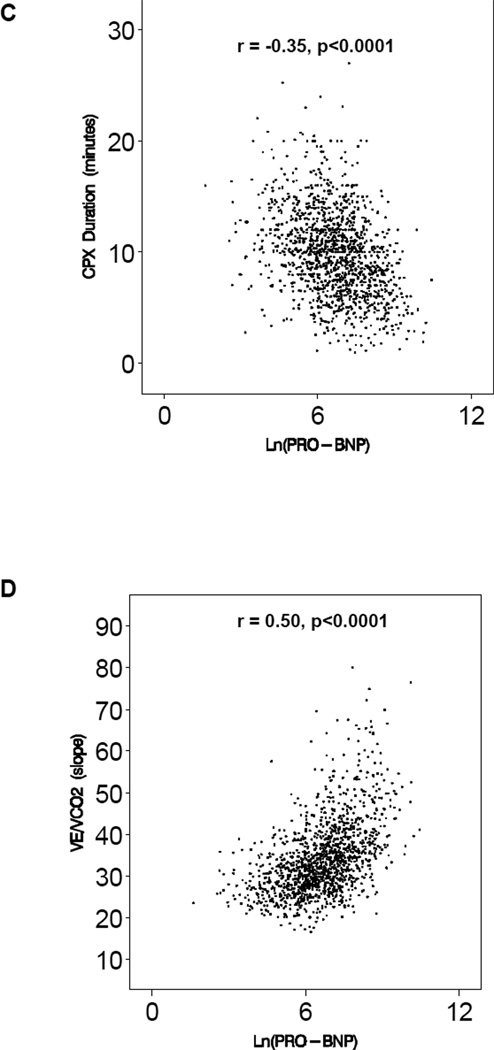
The median peak VO2 in the study cohort was 14.4 mL/kg/min (inter-quartile range 11.5 – 17.5 mL/kg/min), consistent with moderate impairment of exercise capacity. The median distance in the 6 minute walk test was 372 meters (interquartile range 300 – 431 meters). The median NT-proBNP was 815 pg/mL (interquartile range 341 – 1805 pg/mL). In univariable analysis, there was a significant correlation between the logarithm of NT-proBNP and all measures of exercise capacity: peak VO2 (r = −0.38, p<0.0001), distance in the 6 minute walk test (r = −0.23, p<0.0001), CPX duration (r = - 0.35, p<0.0001), and Ve/VCO2 slope (r=0.50, p<0.0001). Scatter plots for each of the relationships are shown in Figure 1.
Figure 1.
Association between ln (NT-proBNP) and Measures of Exercise Capacity; A. peak V02, B. distance in the 6 minute walk test, C. CPX duration, and D. Ve/VCO2 slope.
NT-proBNP in the Context of Other Predictors: Multivariable Modeling
In order to understand the relationship between exercise capacity and NT-proBNP in the context of other demographic and clinical variables, we evaluated the added value of NT-proBNP in the context of those clinical variables most predictive of exercise capacity in the overall HF-ACTION cohort. In the HF-ACTION study as a whole, the most significant predictors of peak VO2 were age, race, body mass index, NYHA class, and gender. When NT-proBNP was entered into the multivariable model for peak VO2, ln(NT-proBNP) was strongly associated with peak VO2 even after adjustment for other determinants (p<0.0001, partial R2 = 0.13). In the final model that included ln(NT-proBNP), ln(NT-proBNP) was the strongest overall predictor of peak VO2 (partial R2 = 0.13), followed by body mass index (partial R2 =0.13) and age (partial R2 = 0.09). The addition of NT-proBNP to the best clinical model raised the R2 for the overall model from 0.38 (clinical model only) to 0.46 (clinical model + NT-proBNP). The final multivariable regression model for predicting peak VO2 is shown in Table 2.
Table 2.
Final Multivariable model for prediction of peak V02 (listed in order of R2)
| Variable | Parameter Estimate |
P-value | Partial R-square |
|---|---|---|---|
| ln NT-proBNP | -1.15 | <0.0001 | 0.129 |
| BMI | −0.02 | <0.0001 | 0.125 |
| Age | −0.10 | <0.0001 | 0.086 |
| Sex | −2.17 | <0.0001 | 0.075 |
| Race | -- | <0.0001 | 0.058 |
| NYHA class (II vs. III/IV) |
−1.61 | <0.0001 | 0.048 |
| CPX mode | 2.35 | <0.0001 | 0.029 |
| PVD | −1.86 | <0.0001 | 0.020 |
| ECG ventricular conduction |
-- | 0.0002 | 0.017 |
| Diabetes mellitus | −0.64 | 0.0020 | 0.007 |
| Region | -- | 0.0017 | 0.002 |
| LVEF | 0.02 | 0.15 | 0.0016 |
Reference categories: sex = male, NYHA Class = Class II, CPX mode = bicycle
Using the same approach, we investigated the added value of NT-proBNP in predicting distance in the 6 minute walk test. The strongest clinical predictors for distance in the 6 minute walk test were age, NYHA class, height, weight, race, and the presence of peripheral vascular disease. As for peak VO2, the NT-proBNP remained a highly significant predictor of distance in the 6 minute walk test (p=<0.0001) even after adjustment for other clinical predictors. Unlike peak VO2, however, the relative contribution of NT-proBNP to prediction of distance in the 6 minute walk test was modest (partial R2 = 0.020). NT-proBNP was the 5th strongest overall predictor of performance in the 6 minute walk test, after NYHA class, age, height, and weight (Table 3). The addition of NT-proBNP to the best clinical model resulted in a modest improvement in overall model performance (R2 changed from 0.28 to 0.30).
Table 3.
Final Multivariable model for prediction of distance in the 6 minute walk test (listed in order of R2)
| Variable | Parameter Estimate |
P-value | Partial R-square |
|---|---|---|---|
| NYHA class (II vs. III/IV) |
−59.19 | <0.0001 | 0.091 |
| Age | −2.41 | <0.0001 | 0.086 |
| Height | −2.41 | <0.0001 | 0.057 |
| Weight | −1.10 | <0.0001 | 0.056 |
| ln NT-proBNP | -10.49 | <0.0001 | 0.020 |
| Race | -- | <0.0001 | 0.019 |
| Heart Failure hospitalizations | -- | <0.0001 | 0.018 |
| PVD | −35.95 | 0.0002 | 0.010 |
| Region | -- | 0.0233 | 0.009 |
Reference categories: NYHA Class = Class II
Impact of Age, Gender, and Body Mass Index
Because age, gender, and body mass index may affect both NT-proBNP levels and exercise capacity, we explored the possibility of differential relationship between NT-proBNP and peak VO2 and distance in the 6 minute walk test for each of these variables using interaction terms in the overall models. For each of gender and obesity (defined as body mass index > 30 kg/m2), we found evidence for a quantitative interaction between NT-proBNP and peak VO2. There was a stronger correlation between NT-proBNP and peak VO2 in men compared to women (R = - 0.44 for men vs. −0.33 for women, p <0.0001 for interaction) and in non-obese patients compared to obese patients (R = - 0.48 for BMI ≤ 30 vs. −0.36 for BMI < 30, p = 0.04 for interaction). There was also evidence for a quantitative interaction between age and the correlation of NT-proBNP and distance in the 6 minute walk test, with older patients (above the median age of 58.5 years) having a stronger correlation between NTproBNP and distance in the 6 minute walk test than younger patients (R = −0.24 for age > median vs. −0.13 for age < median, p < 0.001 for interaction).
NT-proBNP as a Surrogate for Peak VO2
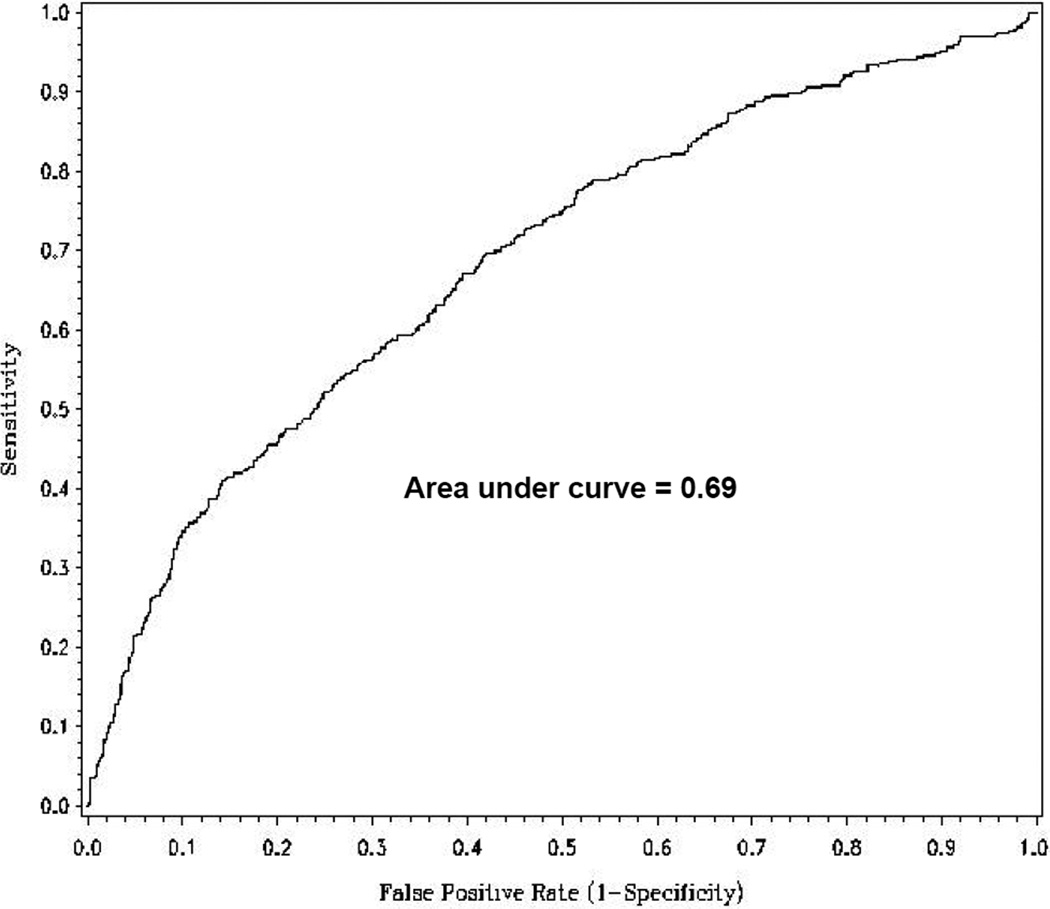
Since assessment of peak VO2 is central to the selection of patients for cardiac transplantation, we examined the sensitivity and specificity of NT-proBNP levels for predicting peak VO2 < 12 mL/kg/min. This cut-point was chosen based on recent guidelines for transplant evaluation in patients treated with beta-blockers (95% of patients in HF-ACTION were taking beta-blockers at enrollment)6. Three hundred ninety three patients (29%) in the study cohort had peak VO2 < 12 mL/kg/min. The optimal NT-proBNP cut-off for predicting a peak V02 < 12 mL/kg/min was 876 pg/mL, which had a sensitivity of 0.67, a specificity of 0.61, positive predictive value of 0.63, and negative predictive value of 0.65. The area under the curve for the ROC curve was 0.69, suggesting modest discriminatory ability for NT-proBNP in predicting a low peak VO2 (Figure 2)
Figure 2.
Receiver operator curve for NT-proBNP predicting peak V02 < 12 mL/kg/min. Optimal cut point for maximizing performance was NTproBNP of 876 (sensitivity = 0.67, specificity = 0.61).
Discussion
The primary finding of this analysis was that NT-proBNP levels were associated with both peak VO2 and distance in the 6 minute walk test in a large well-treated contemporary cohort of patients with chronic heart failure. NT-proBNP was the most significant overall predictor of peak VO2 even after adjustment for 35 demographic and clinical candidate variables. For distance in the 6 minute walk test, NT-proBNP was also a significant independent predictor of exercise capacity, although the relationship was weaker than that for peak VO2. These data represent the largest analysis to date evaluating the relationship between natriuretic peptide levels and exercise capacity in heart failure.
Previous data on the relationship between exercise capacity and natriuretic peptide levels are primarily from small single center studies of patients referred for exercise testing. Smaller studies by Kruger et al, Kallistratos et al, and Passino et al have demonstrated significant correlations (r= −0.56, −0.77, and −0.53 respectively) between natriuretic peptide levels and peak VO2 in patients with heart failure referred for exercise testing15–18. Similarly, other studies have found higher BNP or NT-proBNP levels to be associated with shorter distance in the 6 minute walk test19, 20. Our findings regarding the relationship between NT-proBNP and exercise capacity are broadly consistent with these prior results, but extend them in several important areas. First, the detailed ascertainment of baseline characteristics, large sample size, and broadly representative study cohort of the HF-ACTION study allowed us to perform detailed adjustment for other demographic and clinical characteristics that might impact exercise capacity. Additionally, the size of our cohort also allowed us to evaluate the relationship in relevant subgroups. We found the relationship between peak VO2 and NT-proBNP was stronger for men and for non-obese patients, potentially due to the known effects of gender and obesity on NT-proBNP levels21, 22. Finally, ours is the first study to examine the differences between peak VO2 and distance in the 6 minute walk test in relation to the NT-proBNP levels in the same patient population. The correlation between NT-proBNP and exercise capacity, especially distance in the 6 minute walk test, seen in our study was somewhat weaker in absolute terms than that seen in previous studies, possibly due to variability introduced by a multi-center trial as compared to a single center study.
Although correlation coefficients and linear regression models are useful for quantifying relationships between continuous variables, they are not readily applicable to individual patients for clinical use. Notably, despite the strong association between NT-proBNP levels and peak VO2 in multivariable models, the performance of NT-proBNP in predicting a peak VO2< 12 mL/kg/min was modest, with a c-index of 0.69, a sensitivity of 0.67, and a specificity of 0.61 at an optimal NT-proBNP cut point of 876 pg/mL. These data suggest that NT-proBNP is not a sufficient predictor of peak VO2 to act as a clinically useful surrogate for identifying those patients with a low peak VO2 that might be considered for transplant listing. Notably, of the exercise variables evaluated, NT-proBNP was most strongly correlated with Ve/VCO2 slope, which has been suggested to be a more powerful predictor of prognosis than peak VO2 in several studies23, 24. Previous data have shown that natriuretic peptides and cardiopulmonary exercise testing provide independent and complementary information in predicting prognosis in patients with heart failure.25
Mechanistic Considerations
The physiologic determinants of exercise capacity in heart failure have been the subject of substantial interest. Measures of cardiac systolic function such as ejection fraction have not been shown to correlate well with exercise capacity26. Although the relationship between hemodynamic measures (such as cardiac output or ventricular filling pressures) and maximal exercise capacity is stronger than that of ejection fraction, the invasive assessment of hemodynamics and exercise is technically challenging and has been limited to small studies of highly selected populations27, 28. A substantial component of exercise intolerance in heart failure remains unexplained, and is posited to be due to non-cardiac factors such as changes in peripheral skeletal muscle29. Although natriuretic peptide levels may reflect a variety of cardiac processes including ischemia, inflammation, and oxidative stress, they primarily reflect central hemodynamics30, and therefore serve as a non-invasive means to estimate the relative contribution of hemodynamic factors to exercise capacity in heart failure. The finding that NT-proBNP was the strongest overall predictor of peak VO2 out of 35 candidate variables suggests that hemodynamic factors play a significant role in mediating exercise performance in heart failure. Importantly, however, even with NT-proBNP included in the best clinical model, the overall model performance (R2 = 0.46) suggest that over half of the variability in exercise capacity among patients with heart failure is not explained by the factors analyzed in our models. This finding argues for the importance of other unmeasured variables (such as skeletal muscle abnormalities or genetic factors) as important determinants of exercise capacity in heart failure, and suggests the need for ongoing research into the mechanisms of exercise intolerance in these patients.
Maximal vs. Sub-maximal Capacity
Although NT-proBNP was also a significant predictor of distance in the 6 minute walk test, this relationship was substantially less strong than that seen for peak VO2. Previous data suggest that distance in the 6 minute walk test may be more influenced by non-physiologic factors (such as patient motivation and investigator prompting) than is peak VO2.31 In comparing the best clinical models with NT-proBNP for peak VO2 and distance in the 6 minute walk test, there was substantially more variability in distance in the 6 minute walk test that was not explained by our models, suggesting that unmeasured factors and possibly non-physiologic aspects may play a greater role in determining sub-maximal as compared to maximal exercise capacity.
Limitations
As with any cross-sectional analysis, our study was able to demonstrate associations but is unable to establish causation. The study population was from a multi-center randomized trial, and patients enrolled in clinical trials are known to differ from the broader heart failure population in potentially important ways32. As in previous large heart failure trials, the population enrolled in HF-ACTION was somewhat younger and had a greater proportion of non-ischemic heart failure etiology than the general heart failure population. Specific strengths of this analysis include the use of a large, multi-center cohort with careful phenotyping of baseline characteristics, extremely high utilization of contemporary evidence based therapy, and the use of centralized core laboratories to standardize analysis of both exercise data and biomarker assays.
CONCLUSIONS
NT-proBNP was strongly associated with peak VO2 in a large well-treated cohort of patients with heart failure due to left ventricular systolic dysfunction. NT-proBNP was the strongest predictor of peak VO2, even after adjustment for multiple other demographic and clinical characteristics. NT-proBNP was also significantly associated with distance in the 6 minute walk test, although this relationship was substantially weaker than for peak VO2. These data support the concept that hemodynamic factors play an important role in determining exercise tolerance in heart failure, but also confirm that much of the variability in exercise capacity in heart failure remains unaccounted for by currently available clinical measures. Given that exertional limitation is the primary morbidity of heart failure, these findings highlight the need for ongoing research in the determinants of exercise capacity in patients with heart failure. Future analyses of data from the HF-ACTION trial will provide further insights into the relationships between changes in circulating biomarkers, exercise training, changes in exercise performance measures, and clinical outcomes over time.
ACKNOWLEDGEMENTS
A complete list of the HF-ACTION investigators is available as the last item in this supplement. This research was supported by National Institutes of Health grants: 5U01HL063747, 5U01HL068973, 5U01HL066501, 5U01HL066482, 5U01HL064250, 5U01HL066494,5U01HL064257, 5U01HL066497, 5U01HL068980, 5U01HL064265, 5U01HL066491, 5U01HL064264, 5U01HL066461, R37AG18915, P60AG10484. Additional support for the HF-ACTION Biomarkers Core Laboratory was provided by a grant from Roche Diagnostics.
Abbreviations
- BNP
brain-natriuretic peptide
- NT-proBNP
N-terminal pro-brain natriuretic peptide
- Peak VO2
maximal oxygen uptake
- BMI
body mass index
- ROC
Receiver operator characteristic
APPENDIX
Candidate Variables for Linear Regression Models for Predicting Peak V02 and distance in the 6 minute walk test
sex
diabetes (history of)
stroke (history of)
hypertension (history of)
prior CABG
prior valve surgery
prior percutaneous coronary intervention
prior myocardial infarction
peripheral vascular disease (history of)
chronic obstructive pulmonary disease (history of)
depression (history of)
atrial fibrillation/atrial flutter (history of)
pacemaker
bi-ventricular pacemaker
on an ACE inhibitor at baseline
on a beta blocker at baseline
etiology of heart failure
CPX mode (treadmill or bicycle)
heart failure hospitalizations in the last 6 months (0, 1, 2, or 3+)
region (4 regions of US, Canada, or France)
race (Black or African American, White, or Other)
NYHA class (II vs. III/IV) at baseline
CCS angina class at baseline
rest ECG ventricular conduction prior to baseline CPX test (normal, LBBB, RBBB, IVCD, or paced)
rest ECG rhythm prior to baseline CPX test (sinus, atrial fibrillation, or other)
smoking status (never, current, or past)
diastolic BP
systolic BP
height
weight
BMI
resting HR (clinic visit)
resting HR (CPX test)
LVEF
age
REFERENCES
- 1.Braunwald E. Biomarkers in heart failure. New England Journal of Medicine. 2008;358(20):2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 2.Felker GM, Petersen JW, Mark DB. Natriuretic peptides in the diagnosis and management of heart failure. CMAJ. 2006;175(6):611–617. doi: 10.1503/cmaj.060236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. 1997;95(12):2660–2667. doi: 10.1161/01.cir.95.12.2660. [DOI] [PubMed] [Google Scholar]
- 4.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 5.Bittner V, Weiner DH, Yusuf S, Rogers WJ, McIntyre KM, Bangdiwala SI, et al. Prediction of mortality and morbidity with a 6-minute walk test in patients with left ventricular dysfunction SOLVD Investigators. Journal of the American Medical Association. 1993;270(14):1702–1707. [PubMed] [Google Scholar]
- 6.Mehra MR, Kobashigawa J, Starling R, Russell S, Uber PA, Parameshwar J, et al. Listing Criteria for Heart Transplantation: International Society for Heart and Lung Transplantation Guidelines for the Care of Cardiac Transplant Candidates--2006. The Journal of Heart and Lung Transplantation. 2006;25(9):1024–1042. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Sullivan MJ, Higginbotham MB, Cobb FR. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation. 1988;77(3):552–559. doi: 10.1161/01.cir.77.3.552. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80(4):769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- 9.Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85(6):2119–2131. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- 10.Duscha BD, Annex BH, Green HJ, Pippen AM, Kraus WE. Deconditioning fails to explain peripheral skeletal muscle alterations in men with chronic heart failure. J Am Coll Cardiol. 2002;39(7):1170–1174. doi: 10.1016/s0735-1097(02)01740-0. [DOI] [PubMed] [Google Scholar]
- 11.Hambrecht R, Niebauer J, Fiehn E, Kalberer B, Offner B, Hauer K, et al. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll.Cardiol. 1995;25(6):1239–1249. doi: 10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- 12.Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing (HF-ACTION): Design and rationale. Am Heart J. 2007;153(2):201–211. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 13.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and Safety of Exercise Training in Patients With Chronic Heart Failure: HF-ACTION Randomized Controlled Trial. Jama. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bensimhon DR, Leifer ES, Ellis SJ, Fleg JL, Keteyian SJ, Piña IL, et al. Reproducibility of Peak Oxygen Uptake and Other Cardiopulmonary Exercise Testing Parameters in Patients With Heart Failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing) The American Journal of Cardiology. 2008;102(6):712–717. doi: 10.1016/j.amjcard.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruger S, Graf J, Kunz D, Stickel T, Hanrath P, Janssens U. brain natriuretic peptide levels predict functional capacity in patients with chronic heart failure. J Am Coll Cardiol. 2002;40(4):718–722. doi: 10.1016/s0735-1097(02)02032-6. [DOI] [PubMed] [Google Scholar]
- 16.Kruger S, Graf J, Merx MW, Stickel T, Kunz D, Hanrath P, et al. Brain natriuretic peptide kinetics during dynamic exercise in patients with chronic heart failure. Int.J Cardiol. 2004;95(1):49–54. doi: 10.1016/j.ijcard.2003.04.021. [DOI] [PubMed] [Google Scholar]
- 17.Kallistratos MS, Dritsas A, Laoutaris ID, Cokkinos DV. N-terminal prohormone brain natriuretic peptide as a marker for detecting low functional class patients and candidates for cardiac transplantation: linear correlation with exercise tolerance. J Heart Lung Transplant. 2007;26(5):516–521. doi: 10.1016/j.healun.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 18.Passino C, Poletti R, Bramanti F, Prontera C, Clerico A, Emdin M. Neuro-hormonal activation predicts ventilatory response to exercise and functional capacity in patients with heart failure. Eur J Heart Fail. 2006;8(1):46–53. doi: 10.1016/j.ejheart.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Ingle L, Rigby AS, Nabb S, Jones PK, Clark AL, Cleland JGF. Clinical determinants of poor six-minute walk test performance in patients with left ventricular systolic dysfunction and no major structural heart disease. European Journal of Heart Failure. 2006;8(3):321–325. doi: 10.1016/j.ejheart.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Jourdain P, Funck F, Bellorini M, Guillard N, Loiret J, Thebault B, et al. Bedside B-type natriuretic peptide and functional capacity in chronic heart failure. European Journal of Heart Failure. 2003;5(2):155–160. doi: 10.1016/s1388-9842(02)00247-7. [DOI] [PubMed] [Google Scholar]
- 21.Das SR, Drazner MH, Dries DL, Vega GL, Stanek HG, Abdullah SM, et al. Impact of body mass and body composition on circulating levels of natriuretic peptides: results from the Dallas Heart Study. Circulation. 2005;112(14):2163–2168. doi: 10.1161/CIRCULATIONAHA.105.555573. [DOI] [PubMed] [Google Scholar]
- 22.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40(5):976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 23.Francis DP, Shamim W, Davies LC, Piepoli MF, Ponikowski P, Anker SD, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO2slope and peak VO2. Eur Heart J. 2000;21(2):154–161. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 24.Arena R, Myers J, Aslam SS, Varughese EB, Peberdy MA. Peak VO2 and VE/VCO2 slope in patients with heart failure: a prognostic comparison. American Heart Journal. 2004;147(2):354–360. doi: 10.1016/j.ahj.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 25.deGroote P, Dagorn J, Soudan B, Lamblin N, McFadden E, Bauters C. B-type natriuretic peptide and peak exercise oxygen consumption provide independent information for risk stratification in patients with stable congestive heart failure. J Am Coll Cardiol. 2004;43(9):1584–1589. doi: 10.1016/j.jacc.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 26.Marantz PR, Tobin JN, Wassertheil-Smoller S, Steingart RM, Wexler JP, Budner N, et al. The relationship between left ventricular systolic function and congestive heart failure diagnosed by clinical criteria. Circulation. 1988;77(3):607–612. doi: 10.1161/01.cir.77.3.607. [DOI] [PubMed] [Google Scholar]
- 27.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58(2):281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with chronic heart failure delays ventilatory anaerobic threshold and improves submaximal exercise performance. Circulation. 1989;79(2):324–329. doi: 10.1161/01.cir.79.2.324. [DOI] [PubMed] [Google Scholar]
- 29.Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, et al. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II-III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33(7):1956–1963. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 30.Kazanegra R, Cheng V, Garcia A, Krishnaswamy P, Gardetto N, Clopton P, et al. A rapid test for B-type natriuretic peptide correlates with falling wedge pressures in patients treated for decompensated heart failure: A pilot study. J Card Fail. 2001;7(1):21–29. doi: 10.1054/jcaf.2001.23355. [DOI] [PubMed] [Google Scholar]
- 31.Myers J, Zaheer N, Quaglietti S, Madhavan R, Froelicher V, Heidenreich P. Association of Functional and Health Status Measures in Heart Failure. Journal of Cardiac Failure. 2006;12(6):439–445. doi: 10.1016/j.cardfail.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Heiat A, Gross CP, Krumholz HM. Representation of the Elderly, Women, and Minorities in Heart Failure Clinical Trials. Arch Intern Med. 2002;162(15):1682–1688. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]