Abstract
A key limiting factor impacting the success of cell transplantation for Parkinson’s disease is the survival of the grafted cells, which are often short-lived. The focus of this study was to examine a novel strategy to optimize the survival of exogenous fetal ventromesencephalic (VM) grafts by treatment with the p53 inhibitor, pifithrin-α (PFT-α), to improve the biological outcome of Parkinsonian animals. Adult male Sprague-Dawley rats were given 6-hydroxydopamine into the left medial forebrain bundle to induce a hemi-Parkinsonian state. At 7 weeks after lesioning, animals were grafted with fetal VM or cortical tissue into the lesioned striatum and, thereafter, received daily PFT-α or vehicle injections for 5 days. Apomorphine–induced rotational behavior was examined at 2, 6, 9, and 12 weeks after grafting. Analysis of TUNEL or tyrosine hydroxylase (TH) immunostaining was undertaken at 5 days or 4 months after grafting. The transplantation of fetal VM tissue into the lesioned striatum reduced rotational behavior. A further reduction in rotation was apparent in animals receiving PFT-α and VM transplants. By contrast, no significant reduction in rotation was evident in animals receiving cortical grafts or cortical grafts + PFT-α. PFT-α treatment reduced TUNEL labeling and increased TH (+) cell and fiber density in the VM transplants. In conclusion, our data indicate that early post-grafting treatment with PFT-α enhances the survival of dopamine cell transplants and augments behavioral recovery in Parkinsonian animals.
Keywords: Parkinson.’s disease, transplantation, apoptosis, p53, neuroprotection
INTRODUCTION
Parkinson’s disease (PD) is a progressive neurodegenerative disease which involves the loss of dopaminergic neurons in the A9 (substantia nigra, SN) region of the midbrain. Multiple animal models of PD have shown that the loss of these dopaminergic cells involves apoptosis and involvement of p53-dependent cell death. For example, administration of the dopaminergic neurotoxin 6-hydroxydopamine (6-OHDA) increases p53 mRNA and protein expression (13) and activates PUMA (p53 up-regulated modulator of apoptosis), a target gene for p53 (1,18). Similarly, the dopaminergic toxin 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine (MPTP) enhances the expression of proapoptoic protein BAX, reduces tyrosine hydroxylase (TH) protein levels, and depletes dopamine (DA) (7). These actions were antagonized by treatment with a synthetic p53 inhibitor pifithrin-α (PFT-α) (7). Transgenic mice with a knockout of the p53 gene were resistant to MPTP neurotoxicity (24). Together, these data suggest that activation of p53 is an important mediator for cell death in experimental Parkinsonian animals. Treatment with a p53 inhibitor or suppression of p53 expression limits neuronal dysfunction and cell death induced by exposure to dopaminergic neurotoxins.
Apoptotic (programmed) cell death of midbrain DA neurons is a natural physiological process during prenatal development that permits the selective elimination of cells that have not correctly integrated and made appropriate contacts within the basal ganglion. In rats, for instance, there are two waves of apoptotic DA neuronal death occurring at P2 and P14 (19). It is likely that this apoptotic process is similarly maintained in grafted dopaminergic cells derived from fetal ventromesencephalic (VM) tissue. Although clinical and preclinical studies have shown that transplantation of fetal VM cells integrate and restore dopaminergic functions in Parkinsonian animals (2), over 60 to 90% of grafted dopaminergic cells die after transplantation. The death of these grafted cells likewise involves apoptosis as TUNEL activity within VM grafts was enhanced within the first 4 and 7 days after transplantation to unilaterally 6-OHDA-lesioned rats (20,21).
Treatment with the caspase-3 inhibitor peptide, Ac-YVAD-CMK, increased the survival of embryonic VM cells in culture (20). This protective response to Ac-YVAD-CMK was not consistent in vivo. One report indicates that AC-YVAD-CMK, added to VM cell suspensions prior to grafting, did not augment survival of tyrosine hydroxylase immunoreactive (TH-ir) in the transplant (21). There are several possibilities for this discrepancy. For example, the time course of apoptosis within VM cell suspension grafts can last up to 7 days after grafting (21). Exposure to caspase inhibitors prior to transplantation may not be able to suppress apoptotic cell death several days after grafting, and systemic administration of further peptide is compounded by its limited brain entry. It is additionally possible that apoptosis in the transplant can be mediated through non-caspase pathways. Currently, it remains to be elucidated whether prolonged treatment with anti-apoptotic agents other than caspase inhibitors can improve the survival of VM transplants in vivo.
Previous studies have demonstrated that the p53 inactivator, PFT-α, inhibits the translocation of p53 to mitochondria and the nucleus, prevents the binding of p53 to specific DNA sites, and suppresses the apoptotic cascade. As PFT-α is a small lipophilic compound that readily crosses the blood brain barrier (5,9), systemically administered PFT-α can reach the brain parenchyma and suppress apoptosis of neural cells. We have recently demonstrated that repeated systemic administration of PFT-α optimizes the survival of endogenous neuroprogenitor cells, reduces p53 target gene PUMA expression in the subventricular zone, and provides functional improvement at one week after transient middle cerebral artery occlusion in stroke rats (16). These data suggest that systemic administration of PFT-α may prove valuable to suppress the cell death of precursor cells and, thereby, enhance the survival of DA graft cells and improve functional outcome in Parkinsonian animals.
In this study, we systemically administered PFT-α for 5 days following the transplantation of embryonic VM cells into 6-OHDA lesioned rats. Our data suggest that PFT-α treatment suppressed cell death, increased survival of TH (+) cells, enhanced TH neurite outgrowth from VM grafts, and augmented behavioral recovery.
MATERIALS AND METHODS
Animals
A total of 32 adult male Sprague-Dawley rats (200–250 g) were used in this study. The use of animals was approved (ASP CNRB-91) by the Animal Care and Use Committee, National Institute on Drug abuse, IRP.
6-hydroxydopamine lesioning
Rats were anesthetized with chloral hydrate (400 mg/kg, i.p.) and placed in a stereotaxic frame. 6-hydroxydopamine (2.27 μg/μl × 5 μl in 0.9% NaCl containing 0.2 mg/ml ascorbic acid) was unilaterally injected into the medial forebrain bundle (−4.4 mm AP, 1.2 mm ML relative to bregma and 8.4 mm below skull) over 4 min.
Behavior measurement
Rotational behavior (14,15) was evaluated using a multichannel rotometer system (RotoMax, AccuScan Instruments, Inc). Animals were individually placed in the test cylinder. Four weeks after 6-OHDA lesioning, all animals were challenged with apomorphine (0.05 mg/kg, s.c.). Animals that rotated in excess of 300 turns/hour were selected for subsequent VM or cortical transplantation at 7 weeks after lesioning. Apomorphine–induced rotation was re-examined at 2, 6, 9 and 12 weeks after transplantation.
Grafting Procedures and administration of PFT-α after transplantation
Under chloral hydrate (400mg/kg i.p.) anesthesia, 6-OHDA-lesioned animals were implanted with fetal cortical (from parietal and frontal regions) or VM tissue (2 pieces, approximately 1 mm3 each) directly into the lesioned striatal parenchyma (+0.5 mm AP, 2.5 mm ML relative to bregma and 5.5 mm DV to the dural surface) using a 20-gauge implantation cannula (22,27). VM or cortical tissue samples were harvested from embryos at 15 or 17 days of gestation. Tissues were then placed in Hanks solution containing either PFT-α (1-(4-methylphenyl)-2-(4,5,6,7-tetrahydro-2-imino-3(2H)-benzothiazolyl)ethanone hydrobromidebenzothiazolyl)ethanone hydrobromide, >99.9% purity, synthesized in the Greig NIA laboratory) at concentration of 0.04 mg/ml or vehicle (dimethyl sulfoxide [DMSO]) for about 5 min before transplantation. Thereafter, animals were treated with PFT-α (2 mg/kg/day, i.p.) or 10%DMSO in saline (0.5 ml/100g/day, i.p,) for 5 consecutive days starting from the day of transplantation. Tyrosine hydroxylase (TH) immunoreactivity: Animals were euthanized 4 months after transplantation. TH immunoreactivity was utilized to localize catecholaminergic neurons and fibers. Serial sections of the entire brain were cut at 25 μm thickness in a cryostat. One series from every sixth section was stained using TH antibody. In order to control for staining variability, specimens from all experimental groups were included in every batch and reacted together in a net well tray under the same conditions. Sections were rinsed in 0.1M phosphate buffer, blocked with 4% bovine serum albumin (BSA) and 0.3% Triton x-100 in 0.1M PB. Sections were then incubated in a primary antibody solution mouse monoclonal anti-TH diluted in 4% BSA and 0.3% Triton x-100 in 0.1M PB, concentration 1:100 (Chemicon, Temecula, CA) for 17–19 hours at 4°C. Sections were rinsed in 0.1M PB and incubated in biotinylated horse anti-mouse IgG in the buffer (1:200; Vector Laboratories, Burlingame CA) for 1 hour, followed by incubation for 1 hour with avidin-biotin-horseradish peroxidase complex. Staining was developed with 2,3′ diaminobenzidine tetrahydrochloride (0.5 mg/ml in 50 mM Tris-HCl buffer 7.4). Control sections were incubated without primary antibody. Sections were mounted on slides, and cover slipped.
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) histochemistry: Animals were euthanized at 2 hours after the final injection of either PFT-α or vehicle on day 5 post-transplantation. The brains were removed, frozen in isopentane on dry ice and stored at −80 degrees. For sectioning, frozen brains were cut into 25 μm sections using a cryostat. The sections were mounted onto Superfrost/Plus microscope slides (Cat. 12-550-15; Fisher, PA) and air dried. A standard TUNEL procedure for frozen tissue sections with minor modifications was performed (5). Briefly, slide-mounted sections were rinsed in 0.5% Triton X-100 in 0.01M PB for 20 min at 80 °C to increase permeability of the cells. To label damaged nuclei, 100 μL of the TUNEL reaction mixture was added onto each sample in a humidified chamber followed by a 60 min incubation at 37 °C. Procedures for negative controls were carried out as described in the manufacture’s manual (Roche, IN) and consisted of not adding the label solution (terminal deoxynucleotidyl transferase) to the\ TUNEL reaction mixture. Material was examined by fluorescence microscopy, and quantification of TUNEL activity was performed by blinded observers.
Statistics
ANOVA and post-hoc Newman-Keuls test were used for statistical comparison. Data are presented as mean (+/−s.e.m.). Probability level of less than 5% (p<0.05) was considered to be significant.
RESULTS
Behavior
Behavioral analysis was performed on a total of 24 animals. All possessed unilateral 6-OHDA lesions. Animals were separated (6 rats per group) into 4 groups (specifically, VM+veh, VM+ PFT-α, cortex+veh, and cortex+ PFT-α) to equalize group rotational behavior at 4 weeks after 6-OHDA lesioning. There was thus no difference in apomorphine –induced rotation prior to transplantation among all groups (p=0.968, 1-Way ANOVA). The rotational behavior was reexamined at 2, 6, 9 and 12 weeks after transplantation (Fig. 1). Using a three way ANOVA, we determined that transplantation of fetal VM cells, compared to fetal cortical tissue, significantly reduced apomorphine-mediated contralateral rotation (F1,80=10.735, p<0.002). Treatment with PFT-α by itself did not alter rotation (F1,80=1.562, p=0.215, 3-Way ANOVA). However, there was a significant interaction between PFT-α treatment and VM transplantation (F1,80=6.086, p=0.016, 3-Way ANOVA). Post-hoc Newman-Keuls analysis indicated that treatment with PFT-α, compared to vehicle, significantly reduced rotation in animals receiving VM graft (p=0.010). Such a difference was not found in animals receiving cortical transplant treated with PFT-α or saline (p=0.392).
Figure 1. Pifithrin-α (PFT-α) treatment and transplantation of fetal ventromesencephalic (VM) cells synergistically reduce apomorphine-induced contralateral rotation in hemiparkinsonian rats.
Rotation was induced by administration of 0.05 mg/kg apomorphine at 2, 6, 9, and 12 weeks after transplantation. Grafting of fetal VM cells, but not cortical cells, reduced total number of rotations over 60 min. Treatment with PFT-α, compared to vehicle, significantly reduces rotation in animals receiving VM grafts.
TH immunostaining
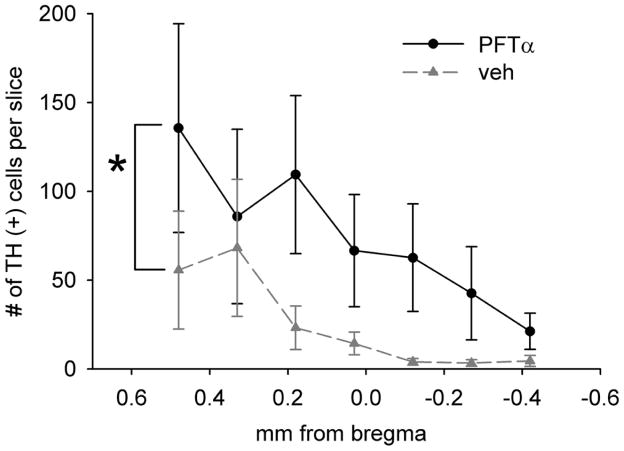
TH immunoreactivity was examined at > 4 months after transplantation in 24 rats. A near complete depletion of TH immunoreactivity was found in the lesioned SN area (Fig. 2A–D). Transplantation of fetal VM (Fig. 2A, B) or cortical tissue (Fig. 2C, D2), as well as treatment with PFT-α (Fig. 2A, C) or vehicle (Fig. 2B, D), did not affect TH immunoreactivity in lesioned (Fig. 2A1–D1) and nonlesioned (Fig. 2A2–D) side SN. Similar to nongrafted lesioned animals from our previous studies (25), a reduction of TH fiber density was also found in the lesioned striatum away from the site of transplant. At the graft site, TH+ dendrites or cell bodies were not found in the lesioned animals that received cortical transplants and given saline (n = 6, Fig. 2E) or PFT-α (n = 6, Fig. 2F) injections. In contrast, TH fibers or cell bodies were found in animals receiving VM transplantation with (n = 6, Fig. 3B, D) or without (n = 6, Fig. 3A, C) PFT-α. Typical TH immunostaining of a VM graft is shown in Figure 3B. TH cell bodies and neurites mainly resided in the graft area. Treatment with PFT-α substantially enhanced the density of TH immunoreactivity in the VM transplants (Fig. 3B vs. A). The number of TH cell bodies in the transplant was also significantly increased by PFT-α [F (1, 63) = 10.319, p = 0.002, two-way ANOVA) (Fig. 4). At higher magnification, treatment with PFT-α not only increased the number of TH cells but also their arborization (Fig. 3D vs. C). TH+ fibers or dendrites were more densely spread amongst TH cell bodies in the graft area after PFT-α treatment (Fig. 3B vs. A). Striatal transplantation of VM cells or administration PFT-α did not alter TH immuno-reactivity in the lesioned nigra (Fig. 2A–D).
Figure 2. Tyrosine hydroxylase (TH) immunoreactivity in cortical transplant and in host nigra.
Unilateral administration of 6-hydroxydopamine (6-OHDA) induced a near complete depletion of TH immunoreactivity in the lesioned substantia nigra (SN) area (A1–D1: lesioned side SN; A2–D2: contralateral side SN). TH immunoreactivity in host SN was not affected by grafting of fetal nigral transplantation or administration of PFT-α (A: nigra graft + PFT-α; B: nigra graft + veh; C: cortical graft + PFT-α; D: cortical graft + veh). (E–F) In animals receiving cortical grafts, TH+ dendrites or cell bodies were not found at the graft site in lesioned striatum (E: cortical graft + saline; F: cortical graft + PFT-α).
Figure 3.
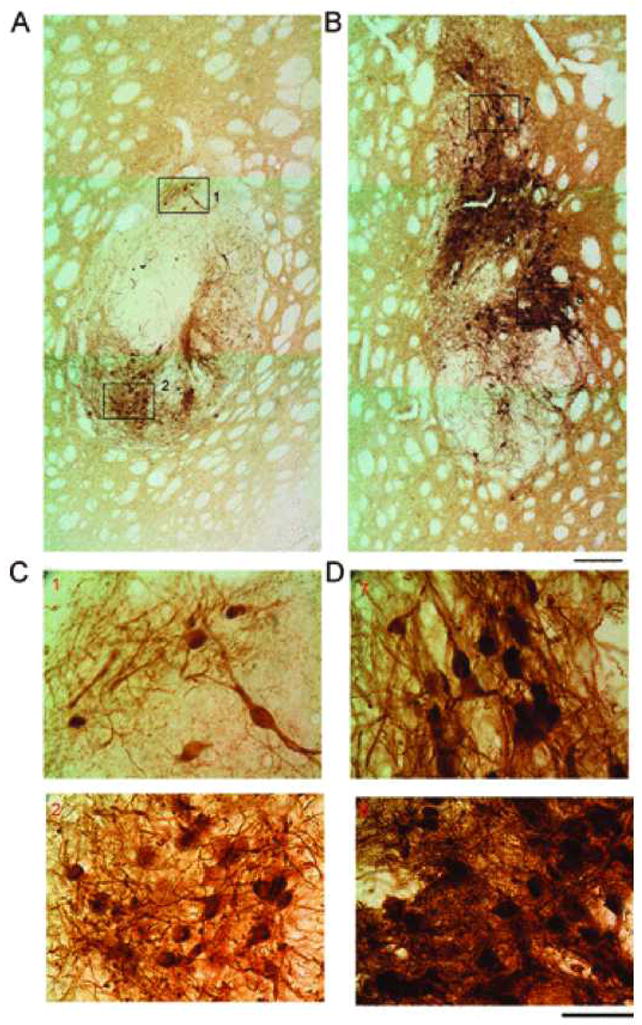
PFT-α increases TH immunoreactivity in VM grafts. Animals were sacrificed at 4 months after transplantation. (A) In an animal receiving VM transplants and saline injection, TH immunoreactivity at the graft site was limited to the graft area. There are less TH+ neurons and fibers. (B) Treatment with PFT-α enhanced the density of TH immunoreactivity in the VM transplants. There are more TH+ cells and fibers near the transplant after PFT-α treatment. Scale bar: 20 μm. (C, D) At higher magnification, treatment with PFT-α increases TH fiber outgrowth and density of TH+ cells in the VM grafts. Numbers in each panel correspond to the blocks in (A) and (B). (D) Administration of PFT-α increased the number of TH cells and fiber arborization in both dorsal (upper panel) and ventral (lower panel) portions of the graft. TH+ fibers or dendrites were much more densely spread among TH cell bodies after PFT-α (D), compared to saline (C) treatment. Scale bar: 10 μm.
Figure 4. PFT-α increases TH+ cell number in the transplant.
TH+ cells in each brain slice were counted every 0.15 mm from 0.48 to −0.42 mm, related to bregma. PFT-α treatment significantly increased TH cell density in graft (*p = 0.002, two way ANOVA).
TUNEL labeling
Eight 6-OHDA lesioned rats were grafted with VM transplants, and then treated with PFT-α (n = 4) or vehicle (n = 4) for 5 days, euthanized, and prepared for TUNEL histochemistry. In all animals, an increase in TUNEL labeling was found in the transplants. Treatment with PFT-α reduced TUNEL activity in the grafts (Fig. 5). The optical density of TUNEL was further analyzed in 0.48, 0.18, and − 0.27 mm sections from bregma in all animals (Fig. 6). PFT-α, compared to vehicle, significantly reduced TUNEL labeling in the graft [F (1, 24) = 7.312, p = 0.012, two-way ANOVA] (Fig. 6).
Figure 5. PFT-α reduced terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) labeling in VM grafts.

6-OHDA-lesioned rats were grafted with VM transplants, and then treated with PFT-α or vehicle for 5 days and perfused. In two animals receiving vehicle (A, C), there is an increase in TUNEL labeling in the transplants. In contrast, less TUNEL activity was found in the other two animals (B, D) treated with PFT-α.
Figure 6. PFT-α significantly reduced TUNEL optical density in VM grafts.
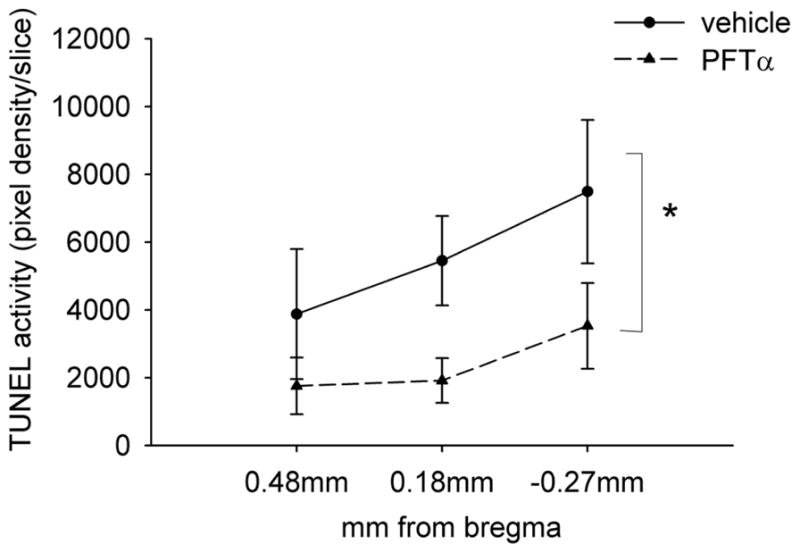
The optical density of TUNEL labeling was examined at 0.48, 0.18, and −0.27 mm sections from bregma in all animals. PFT-α, compared to vehicle, significantly reduced TUNEL labeling in the graft (*p < 0.05, two-way ANOVA).
DISCUSSION
To overcome the extensive apoptosis associated with dopaminergic cell transplantation into brain, a fundamental shortcoming in developing this strategy for Parkinson’s disease treatment, in the current study we used a p53 inhibitor, PFT-α, to increase the survival and viability of VM grafts in hemiparkinsonian animals. As PFT-α is a small molecular weight, lipophilic compound that readily enters the brain (5,9), it is amenable to repeated systemic administration to animals undergoing transplantation. We found that treatment with PFT-α for 5 days significantly improved the survival of grafts, enhanced their viability and potentiated functional recovery of hemiparkinsonian animals. Our results differ from a previous study showing that the peptide caspase inhibitor, Ac-YVADCMK, which has minimal blood-brain barrier permeability, did not augment survival of TH-ir neurons in VM grafts (14). In Marchinoni’s study, Ac-YVAD-CMK was added to the graft cell suspension only prior to transplantation. In contrast, we included PFT-α in the graft media before transplantation and also administered PFT-α for 5 consecutive days after transplantation. It is possible that protection of the transplant requires continuous administration of an apoptotic inhibitor for several days after transplantation, which is achievable with PFT-α since apoptosis in grafts lasts up to a week after transplantation (20,21), analogous to normal developmental programmed cell death.
We, and others, have recently reported that treatment with PFT-α enhanced the survival of nondopaminergic cells after ischemic brain injury (12,16). PFT-α suppressed TUNEL labeling of these cells through the inhibition of p53 target genes, such as PUMA (16). Similarly, in this study, we found that the survival of exogenous VM grafts was also enhanced. At 5 days after grafting, there was an increase in TUNEL labeling at the graft site, which was reduced by PFT-α. Posttransplantation treatment with PFT-α additionally potentiated recovery of motor function in 6-OHDA-lesioned animals receiving VM transplantation for 12 weeks, indicating that the improved cell survival afforded by PFT-α was of physiological relevance. These data suggest that suppressing apoptosis in the early period (specifically, 5 days after grafting) is sufficient to provide enhanced long-term behavioral recovery for up to 12 weeks.
Of note, 5-day PFT-α treatment did not completely abolish TUNEL labeling in transplants, suggesting that the apoptotic reaction is only partially antagonized by PFT-α. Increasing the dose or duration of PFT-α treatment, or combinations with other antiapoptotic agents may maximize the protection, particularly should other antagonists act via non-p53 mechanisms. Previous studies have demonstrated that PFT-α suppresses dopaminergic toxin-mediated neurodegeneration (7). This protective response was found only when PFT-α was given before or shortly after lesion induction. Administration of 6-OHDA activated apoptotic cells in the host SN shortly after injection (11,25,29). Almost no apoptotic cells were found at 1 week after 6-OHDA injection (11). In our study, PFT-α was administered at more than 1 month after 6-OHDA lesioning. The behavioral improvement is hence not due to the suppression of cell death induced by 6-OHDA in host brain because few apoptotic cells were present in host SN at 1 month after lesioning.
In contrast to fetal VM grafting, there was no change in behavioral recovery in animals receiving cortical transplants. Likewise, behavioral improvement was not found in animals receiving cortical transplants in combination with a p53 inhibitor. Our unpublished observations also indicated that treatment with PFT-α alone, compared to vehicle, did not alter apomorphine-induced rotation in unilaterally 6-OHDA-lesioned animals with no grafts (p = 0.934). These data suggest that augmented functional improvement depends on the presence of both VM transplant and PFT-α. Because PFT-α did not increase behavioral recovery in the lesioned animals receiving cortical grafts or no graft but enhanced behavioral recovery in animals receiving VM graft, PFT-α synergistically, but not additively, potentiated the recovery after fetal VM transplantation.
Using TH immunostaining, we found that the transplants in animals receiving PFT-α treatment contained more TH+ neurons and these displayed greater neurite outgrowth. Specifically, TH+ fibers or dendrites were much more densely spread among TH cell bodies in the graft area after PFT-α treatment at 4 months after grafting. These data suggest that suppression of p53 activity not only has protective effects on the DA cell bodies, but results in enhanced the outgrowth of dendrites. Whether this effect resulted from a direct action of p53 on dendrite outgrowth or indirect actions related to improved cell viability remains to be determined. Nevertheless, this outcome is similar to the trophic response to glial cell line-derived neurotrophic factor (GDNF) in dopamine transplants (8,28). In this regard, we previously reported that coadministration of GDNF enhances the survival of VM grafts and TH fiber outgrowth (23,26). Because GDNF can induce protection via antiapoptosis in various injury models (4,10), it is possible that common mechanisms may be involved in GDNF and PFT-α-mediated protection.
In conclusion, our data suggest that early and continuous administration of PFT-α prolongs the survival and function of dopaminergic cell transplants in parkinsonian animals. Posttreatment with PFT-α may therefore be considered a viable therapeutic strategy to optimize cell transplantation.
Acknowledgments
This research was supported by the Intramural Research Program of NIDA and NIA, NIH, DHHS.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Biswas SC, Ryu E, Park C, Malagelada C, Greene LA. Puma and p53 play required roles in death evoked in a cellular model of Parkinson disease. Neurochem Res. 2005;30:839–845. doi: 10.1007/s11064-005-6877-5. [DOI] [PubMed] [Google Scholar]
- 2.Borlongan CV, Su TP, Wang Y. Delta opioid peptide augments functional effects and intrastriatal graft survival of the fetal rat ventral mesencephalic cells. Cell Transplant. 2001;10:53–58. [PubMed] [Google Scholar]
- 3.Chou J, Harvey BK, Ebendal T, Hoffer BJ, Wang Y. Nigrostriatal alterations in bone morphogenetic protein receptor II dominant negative mice. Acta Neurochir Suppl. 2008;101:93–98. doi: 10.1007/978-3-211-78205-7_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarkson ED, Zawada WM, Freed CR. GDNF improves survival and reduces apoptosis in human embryonic dopaminergic neurons in vitro. Cell Tissue Res. 1997;289:207–210. doi: 10.1007/s004410050867. [DOI] [PubMed] [Google Scholar]
- 5.Culmsee C, Zhu X, Yu QS, Chan SL, Camandola S, Guo Z, Greig NH, Mattson MP. A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. J Neurochem. 2001;77:220–228. doi: 10.1046/j.1471-4159.2001.t01-1-00220.x. [DOI] [PubMed] [Google Scholar]
- 6.Deng X, Ladenheim B, Tsao LI, Cadet JL. Null mutation of c-fos causes exacerbation of methamphetamine-induced neurotoxicity. J Neurosci. 1999;19:10107–10115. doi: 10.1523/JNEUROSCI.19-22-10107.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z, Oyler J, Cutler RG, Cadet JL, Greig NH, Mattson MP. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol. 2002;52:597–606. doi: 10.1002/ana.10350. [DOI] [PubMed] [Google Scholar]
- 8.Granholm AC, Mott JL, Bowenkamp K, Eken S, Henry S, Hoffer BJ, Lapchak PA, Palmer MR, van Horne C, Gerhardt GA. Glial cell line-derived neurotrophic factor improves survival of ventral mesencephalic grafts to the 6-hydroxydopamine lesioned striatum. Exp Brain Res. 1997;116:29–38. doi: 10.1007/pl00005741. [DOI] [PubMed] [Google Scholar]
- 9.Greig NH. Drug entry to the brain and its pharmacological manipulation. In: Bradbury MWB, editor. Handbook of experimental pharmacology. Heidelberg, Germany: Springer-Verlag; 1992. pp. 487–523. [Google Scholar]
- 10.Harvey BK, Chang CF, Chiang YH, Bowers WJ, Morales M, Hoffer BJ, Wang Y, Federoff HJ. HSV amplicon delivery of glial cell line-derived neurotrophic factor is neuroprotective against ischemic injury. Exp Neurol. 2003;183:47–55. doi: 10.1016/s0014-4886(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 11.Jeon BS, Jackson-Lewis V, Burke RE. 6-Hydroxydopamine lesion of the rat substantia nigra: Time course and morphology of cell death. Neurodegeneration. 1995;4:131–137. doi: 10.1006/neur.1995.0016. [DOI] [PubMed] [Google Scholar]
- 12.Leker RR, Aharonowiz M, Greig NH, Ovadia H. The role of p53-induced apoptosis in cerebral ischemia: Effects of the p53 inhibitor pifithrin alpha. Exp Neurol. 2004;187:478–486. doi: 10.1016/j.expneurol.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 13.Liang ZQ, Li YL, Zhao XL, Han R, Wang XX, Wang Y, Chase TN, Bennett MC, Qin ZH. NF-kappaB contributes to 6-hydroxydopamine-induced apoptosis of nigral dopaminergic neurons through p53. Brain Res. 2007;1145:190–203. doi: 10.1016/j.brainres.2007.01.130. [DOI] [PubMed] [Google Scholar]
- 14.Liu DM, Lin SZ, Wang SD, Wu MI, Wang Y. Xenografting human T2 sympathetic ganglion from hyperhidrotic patients partially restores catecholaminergic functions in hemi-parkinsonian athymic rats. Cell Transplant. 1999;8:563–591. doi: 10.1177/096368979900800604. [DOI] [PubMed] [Google Scholar]
- 15.Luo Y, Hoffer BJ, Wang Y. Rotation, drug-induced. In: Kompoliti K, Verhagen L, editors. The encyclopedia of movement disorders (MOVE) London: Academic Press; 2010. pp. 49–51. [Google Scholar]
- 16.Luo Y, Kuo CC, Shen H, Chou J, Greig NH, Hoffer BJ, Wang Y. Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Ann Neurol. 2009;65:520–530. doi: 10.1002/ana.21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchionini DM, Collier TJ, Pitzer MR, Sortwell CE. Reassessment of caspase inhibition to augment grafted dopamine neuron survival. Cell Transplant. 2004;13:273–282. doi: 10.3727/000000004783983972. [DOI] [PubMed] [Google Scholar]
- 18.Nair VD. Activation of p53 signaling initiates apoptotic death in a cellular model of Parkinson’s disease. Apoptosis. 2006;11:955–966. doi: 10.1007/s10495-006-6316-3. [DOI] [PubMed] [Google Scholar]
- 19.Oo TF, Burke RE. The time course of developmental cell death in phenotypically defined dopaminergic neurons of the substantia nigra. Brain Res Dev Brain Res. 1997;98:191–196. doi: 10.1016/s0165-3806(96)00173-3. [DOI] [PubMed] [Google Scholar]
- 20.Schierle GS, Hansson O, Leist M, Nicotera P, Widner H, Brundin P. Caspase inhibition reduces apoptosis and increases survival of nigral transplants. Nat Med. 1999;5:97–100. doi: 10.1038/4785. [DOI] [PubMed] [Google Scholar]
- 21.Sortwell CE, Pitzer MR, Collier TJ. Time course of apoptotic cell death within mesencephalic cell suspension grafts: Implications for improving grafted dopamine neuron survival. Exp Neurol. 2000;165:268–277. doi: 10.1006/exnr.2000.7476. [DOI] [PubMed] [Google Scholar]
- 22.Stromberg I, Johnson S, Hoffer B, Olson L. Reinnervation of dopamine-denervated striatum by substantia nigra transplants: Immunohistochemical and electrophysiological correlates. Neuroscience. 1985;14:981–990. doi: 10.1016/0306-4522(85)90270-2. [DOI] [PubMed] [Google Scholar]
- 23.Tang FI, Tien LT, Zhou FC, Hoffer BJ, Wang Y. Intranigral ventromesencephalic grafts and nigrostriatal injection of GDNF restore dopamine release in the striatum of 6-OHDA-lesioned rats. Exp Brain Res. 1998;119:287–296. doi: 10.1007/s002210050344. [DOI] [PubMed] [Google Scholar]
- 24.Trimmer PA, Smith TS, Jung AB, Bennett JP., Jr Dopamine neurons from transgenic mice with a knockout of the p53 gene resist MPTP neurotoxicity. Neurodegeneration. 1996;5:233–239. doi: 10.1006/neur.1996.0031. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Chang CF, Morales M, Chiang YH, Harvey BK, Su TP, Tsao LI, Chen SY, Thiemermann C. Diadenosine tetraphosphate protects against injuries induced by ischemia and 6-hydroxydopamine in rat brain. J Neurosci. 2003;23:7958–7965. doi: 10.1523/JNEUROSCI.23-21-07958.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Tien LT, Lapchak P, Hoffer BJ. GDNF triggers fiber outgrowth of fetal ventral mesencephalic grafts from nigra to striatum in 6-OHDA lesioned rats. Cell Tissue Res. 1996;286:225–234. doi: 10.1007/s004410050691. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Wang SD, Lin SZ, Liu JC. Restoration of dopamine overflow and clearance from the 6-hydroxydopamine lesioned rat striatum reinnervated by fetal mesencephalic grafts. J Pharmacol Exp Ther. 1994;270:814–821. [PubMed] [Google Scholar]
- 28.Yurek DM, Flectcher AM, Kowalczyk TH, Padegimas L, Cooper MJ. Compacted DNA nanoparticle gene transfer of GDNF to the rat striatum enhances the survival of grafted fetal dopamine neurons. Cell Transplant. 2009;18:1183–1196. doi: 10.3727/096368909X12483162196881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zuch CL, Nordstroem VK, Briedrick LA, Hoernig GR, Granholm AC, Bickford PC. Time course of degenerative alterations in nigral dopaminergic neurons following a 6-hydroxydopamine lesion. J Comp Neurol. 2000;427:440–454. doi: 10.1002/1096-9861(20001120)427:3<440::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]