Abstract
Melatonin, a close derivative of serotonin, is involved in physiological regulation of circadian rhythms. In the gastrointestinal (GI) system, melatonin exhibits endocrine, paracrine and autocrine actions and is implicated in the regulation of GI motility. However, it is not known whether melatonin can also act directly on GI smooth muscle cells. The aim of the present study was to determine the expression of melatonin receptors in smooth muscle and identify their signaling pathways. MT1, but not MT2 receptors are expressed in freshly dispersed and cultured gastric smooth muscle cells. Melatonin selectively activated Gq and stimulated phosphoinositide (PI) hydrolysis in freshly dispersed and cultured muscle cells. PI hydrolysis was blocked by the expression of Gq, but not Gi minigene in cultured muscle cells. Melatonin also caused rapid increase in cytosolic Ca2+ as determined by epifluorescence microscopy in fura-2 loaded single smooth muscle cells, and induced rapid contraction. Melatonin-induced PI hydrolysis and contraction were blocked by a non-selective MT1/MT2 antagonist luzindole (1 μM), but not by a selective MT2 antagonist 4P-PDOT (100 nM), and by the PLC inhibitor U73122. MT2 selective agonist IIK7 (100 nM) had no effect on PI hydrolysis and contraction. We conclude that rabbit gastric smooth muscle cells express melatonin MT1 receptors coupled to Gq. Activation of these receptors causes stimulation of PI hydrolysis and increase in cytosolic Ca2+, and elicits muscle contraction.
1. Introduction
Melatonin (N-acetyl-5-methoxytrypatmine), a derivative of 5-hydroxytryptomin (serotonin) and an endogenous signal of darkness, is secreted by the pineal gland following a circadian rhythm and regulates diverse physiological processes [1]. Enterochromaffin (EC) cells of the gastrointestinal (GI) tract are the main source of extra-pineal melatonin and substantially contribute to the peripheral blood concentrations of melatonin [2-5]. Melatonin concentration in the GI tract is 10-100 times more than the circulating levels and nearly 400 times more than in the pineal gland [6]. The melatonin synthesizing enzyme is reportedly present in enterochromaffin cells of the intestinal mucosa where it synthesizes melatonin from its precursor, serotonin [6,7]. While pineal-produced melatonin acts mostly as an endocrine substance, extrapineal-derived melatonin functions not only as endocrine, but also as autocrine or paracrine substance and regulates many GI functions such as water and ion transport, proliferation of epithelium, secretion of acid, immune system, and motility [5-10].
Two mammalian subtypes of G-protein coupled melatonin receptors have been cloned and identified, MT1, and MT2 [11-13]. While both share generally similar binding characteristics for 125I-melatonin, the human MT2 receptor has a lower affinity (Kd = 160 pM) compared to the human MT1 receptor (Kd = 20-40 pM) [14]. MT1 and MT2 receptors are expressed both singly and together in various tissues of the body [11, 14, 15]. Melatonin MT2 receptors are more restrictively expressed, being found mainly in the brain, although their presence has also been detected in the lung, cardiac, aortic and coronary tissue, myometrium and granulosa cells, immune cells, duodenum and adipocytes [14].
Administered intraperitoneally, melatonin increased intestinal myoelectrical activity. This effect was reversed by the non-selective MT1/MT2 receptor antagonist, luzindole, and seems to be mediated by peripheral receptors [16]. Binding to selective receptors expressed on the smooth muscles and myenteric neurons of gastrointestinal tract allows melatonin to have a significant influence on gastrointestinal motility. A study by Kasimay et al.[17] indicated that melatonin inhibit gastric motility by interacting with serotonin receptors present on the vagal afferent fibers via vago-vagal inhibitory reflexes. Storr et al. [18,19] has demonstrated the expression of MT1, but not MT2 receptors using RNA isolated from the muscle layers of rat stomach and intestine. Addition of melatonin to isolated muscle strips inhibited non-aderenergic and non-cholinergic mediated relaxations, and exogenous nitric oxide (NO)-mediated relaxation [19]. These results suggest that melatonin actions are mediated by inhibition of nNOS activity via MT1 receptors.
The effect of melatonin may vary in different regions of the gut depending on whether the activated receptor is present predominantly on smooth muscle cells or enteric neurons. Transmitters released from the enteric neurons, in turn, modulate the intrinsic electrical and mechanical activity of the gastrointestinal smooth muscle [7, 20, 21]. These studies indicate the difficulties in identifying the signaling pathways activated by melatonin in vivo and in innervated in vitro preparations. To avoid the confounding effects of neural activation by melatonin, the present study has characterized the receptors for melatonin and the signaling pathways to which these receptors are coupled in freshly dispersed and cultured smooth muscle cells of the gut. Our studies demonstrate that gastric smooth muscle cells express receptors (MT1) for melatonin preferentially coupled to Gq The receptors mediate stimulation of phosphoinositide-specific phospholipase-C β (PI-PLC-β) activity and intracellular Ca2+ levels and cause muscle contraction.
2. Materials and methods
Melatonin was purchased from Bachem (Torrance, CA). [3H]myo-inositol were obtained from PerkinElmer Life Sciences, (Boston, MA). Polyclonal antibodies to Gαi1, Gαi2, Gαi3, Gαs, and Gαq were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal antibodies to MT1 and MT2 were obtained from Abcam (Cambridge, MA). Western blotting, Dowex AG-1 × 8 resin (100-200 mesh in formate form), chromatography material and protein assay kit, 15% Tris-HCl Ready Gels were obtained from Bio-Rad Laboratories (Hercules, CA); collagenase and soybean trypsin inhibitor were obtained from Worthington Biochemical (Freehold, NJ). 4P-PDOT was obtained from Tocris (Minneapolis, MN). IIK7 was obtained from Sigma-Aldrich (St. Louis, MO). RT- PCR primers were obtained from Integrated DNA technologies, Inc (Coralville, IA). Fura-2/AM was obtained from Molecular Probes (Carlsbad, CA). Effectene Transfection Reagent, QIAEX®II Gel extraction Kit and QIAprep®Spin Miniprep Kit were obtained from QIAGEN Sciences,(Valencia, CA); PCR reagents were obtained from Applied Biosystems, Roche (Carlsbad, CA); SuperScript™ II Reverse Transcriptese and TOPO TA Cloning® Kit Dual Promoter were obtained from Invitrogen (Carlsbad, CA); EcoR I was obtained from New England Bio Labs(Ipswich, MA); Dulbecco's modified Eagle's medium (DMEM) was obtained from Fisher Scientific (Pittsburgh, PA). All other chemicals were obtained from Sigma, (St. Louis, MO).
Animals were housed in the animal facility administered by the Division of Animal Resources, Virginia Commonwealth University. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University and all procedures were conducted in accordance with the guidelines set by this committee.
2.1. Preparation of dispersed gastric smooth muscle cells
Smooth muscle cells from the circular muscle layer of the rabbit stomach were isolated by sequential enzymatic digestion of muscle strips, filtration, and centrifugation as described previously [22, 23]. The antrum was cut into thin slices using a Stadie-Riggs tissue slicer and then the slices were incubated for 30 min in a smooth muscle buffer at 31°C containing 0.1% collagenase (300 U/ml) and 0.01% soybean trypsin inhibitor (w/v). The tissue was continuously gassed with 100% oxygen during the entire isolation procedure. The partly digested tissues were washed twice with 50-ml of collagenase-free smooth muscle buffer and the muscle cells were allowed to disperse spontaneously for 30 min in collagenase-free medium. Cells were harvested by filtration through 500 μm Nitex and centrifuged twice at 350 × g for 10 min to eliminate broken cells and organelles. The cells were counted in a hemocytometer and it is estimated that 95% of the cells excluded trypan blue. The experiments were done within 2-3 h of cell dispersion.
For some experiments, dispersed muscle cells were resuspended in DMEM containing penicillin (200 U/ml), streptomycin (200 μg/ml), gentamycin (100 μg/ml), amphotericin B (2.5 μg/ml) and 10% fetal bovine serum (DMEM-10). The muscle cells were plated at a concentration of 5 × 105 cells/ml and incubated at 37°C in a CO2 incubator. DMEM-10 medium was replaced every three days for 2-3 weeks until confluence was attained. The muscle cells in confluent primary cultures were trypsinized (0.5 mg trypsin/ml), re-plated at a concentration of 2.5 × 105 cells/ml and cultured under the same conditions. All experiments were done on cells in the first passage. Previous studies have determined the purity of cultured muscle cells with smooth muscle-specific γ-actin [24]. Cultured muscle cells were starved in serum-free medium for 24 hours before each use.
2.2. Expression of Gαq and Gαi minigenes
The cDNA sequences encoding the last COOH-terminal 11 amino acids of mouse Gαq (MGLQLNLKEYNLV) and human Gαi (MGIKNNLKDCGLF) were amplified by PCR and verified by DNA sequencing as previously described [25-30]. The 5′-end of sense primers contained a BamHI site followed by the ribosome binding consensus sequence (5′-GCCGCCACC-3′), a methionine (ATG) start code, and a glycine (GGA) to protect the ribosome binding site during translation and the nascent peptide against proteolytic degradation. An EcoRI site was synthesized at the 5′-end of the antisense primers immediately after the stop codon (TGA). The purified PCR products were subcloned into the mammalian expression vector pcDNA3.1(+). The oligonucleotide sequence corresponding to the COOH-terminal 11 amino acid residuals of Gαi in random order was synthesized and ligated into pcDNA3.1(+) as a control minigene. All Gα minigene constructs used for transfection experiments were purified with an endotoxin-free maxiprep kit (Qiagen) following the manufacturing protocol.
2.3. Expression of MT1 in smooth muscle cells measured by RT-PCR and western blot
Total RNA was isolated from smooth muscle cells with TRIzol® reagent (Invitrogen) and treated with TURBO DNase (Ambion). RNA from each preparation was reversely transcribed using the SuperScript™ II system containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 10 mM dithiothreitol (DTT), 0.5 mM deoxynucleoside triphosphates (dNTP), 2.5 μM random hexamers and 200 units of reverse transcriptase in a 20 μl reaction volume. The reactions were carried out at room temperature for 10 min and at 42°C for 50 min, and terminated by heating at 70°C for 15 min. Three μl of the reversely transcribed cDNA was amplified in a final volume of 50 μl by PCR in standard conditions (2 mM MgCl2, 200 μM dNTP, 2.5 units Taq polymerase) with specific primers for MT1 designed based on sequence in rabbit and MT2 based on the conserved sequence in human, rat and mouse cDNAs:
- MT1:
- Forward: 5′GATCCAAGGGTCTATTCCTG-3′
- Reverse: 5′CCTGAAGTCCTGTGGTTTC-3′
- MT2:
- Forward: 5′GTGCTCAGGAACCGCAAGC-3′
- Reverse: 5′GTCTGGATGAAGGTGCAGGAA-3′
PCR for MT1 and MT2 receptors was performed for 30 cycles. For each experiment, a parallel control without reverse transcriptase was processed. The amplified PCR products were analyzed on 1.5% agarose gel containing 0.1 μg/ml ethidium bromide [24].
Muscle cells were solubilized in Triton X-100-based lysis buffer plus protease and phosphatase inhibitors (100 μg/ml PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 30 mM sodium fluoride and 3 mM sodium vanadate). After centrifugation of the lysates at 20000 × g for 10 min at 4 °C, the protein concentrations of the supernatant were determined with a Dc protein assay kit from Bio-Rad. Equal amounts of protein were fractionated by SDS/PAGE, and transferred onto nitrocellulose membrane. Blots were blocked in 5% (w/v) non-fat dried milk/TBS-T [trisbuffered saline (pH 7.6) plus 0.1% Tween-20] for 1 h and then incubated overnight at 4 °C with MT1 or MT2 receptor primary antibodies (1:1000) in TBS-T plus 1% (w/v) non-fat dried milk. After incubation for 1 h with horseradish-peroxidase-conjugated corresponding secondary antibody (1:2000; 10 μg/ml, Pierce) in TBS-T plus 1% (w/v) non-fat dried milk, immunoreactive proteins were visualized using SuperSignal Femto maximum sensitivity substrate kit (Pierce). All washing steps were performed with TBS-T. The protein bands were identified by enhanced chemiluminescence reagent [28-30].
2.4. Identification of G proteins activated by melatonin
G proteins selectively activated by melatonin was identified from the increase in Gα binding to the [35S]GTPγS (5′-O-3-thiotriphosphate) as described previously [28-30]. Ten ml of muscle cell suspension (3 × 106 cells/ml) were homogenized in 20 mM HEPES medium (pH 7.4) containing 2 mM MgCl2, 1 mM EDTA and 2 mM DTT. After centrifugation at 30,000 g for 15 min, the crude membranes were solubilized for 60 min at 4 °C in 20 mM HEPES medium (pH 7.4) containing 2 mM EDTA, 240 mM NaCl, 0.5% CHAPS (3-[(3-cholamidopropyl) dimethylammonio]-1-pro-panesulfonate), 2 mM PMSF, 20 μg/ml aprotinin, and 20 μM leupetin. The membrane were incubated for 20 min at 37°C with 60 nM [35S]GTPγS in the presence or absence of melatonin (1 μM) in a solution containing 10 mM HEPES (pH 7.4), 100 μM EDTA and 10 mM MgCl2. The reaction was terminated with 10 volumes of 100 mM of Tris-HCl medium (pH 8.0) containing 10 mM MgCl2, 10 mM NaCl and 10 μM GTP, and the mixture was placed in wells precoated with specific antibodies to Gαq, Gαi1, Gαi2, Gαi3, and Gαs. Coating with G protein antibodies (1:1000) was done after the wells were first coated with anti-rabbit IgG (1:1000) for 2 h on ice. After incubation for 2 h on ice, the wells were washed three times with phosphate buffer saline solution (PBS) containing 0.05% Tween-20 and the radioactivity from each well was counted by liquid scintillation. The amount of [35S]GTPγS bound to the activated Gα subunit was expressed as counts per minute (cpm) per milligram of protein.
2.5. Phosphoinositide(PI)-specific phospholipase C (PLC-β) activity
PI hydrolysis (PLC-β activity) was determined in freshly dispersed or cultured smooth muscle cells by measuring the formation of inositol phosphates using ion-exchange chromatography as previously described [22, 23]. Ten ml of cell suspension (2 × 106 cells/ml) were labeled with myo-[3H] inositol (15 μCi/ml) for 90 min at 31 °C. Then cells were centrifuged at 350 × g for 10 min to remove excess [3H] inositol and resuspended in 10 ml of fresh medium. Lithium was added to a final concentration of 10 mM and the suspension was incubated for 10 min followed by melatonin (1 pM to 10 μM) or MT2 receptor selective agonist IIK7 (100 nM) for 60 s. In some experiments cells were treated with melatonin in the presence and absence of a non-selective MT1/MT2 receptor antagonist luzindole (100 mM), a selective MT2 receptor antagonist 4P-PDOT, PLC β inhibitor U73122 (10 μM) or MLCK inhibitor ML-9 (1 μM) for 60 s [31-33]. Cultured smooth muscle cells were labeled with [3H]myo-inositol (0.5 μCi/ml) for 24 h in inositol-free DMEM medium. The cultures were washed with phosphate-buffered saline (PBS) and treated with melatonin (1 μM) for 1 min in HEPES medium (pH 7.4). The reaction was terminated by the addition of chloroform methanol:HCl (50:100:1 v/v/v). After chloroform (310 μl) and water (310 μl) were added, the samples were vortexed and the phases were separated by centrifugation at 1000 g for 15 min. The upper aqueous phase was applied to a column containing 1 ml of 1:1 slurry of Dowex AG-1 ×8 resin (100-200 mesh in formate form) and distilled water. The column was washed with 10 ml of water followed by 10 ml of 5 mM sodium tetraborate-60 mM ammonium formate to remove [3H]glycerophosphoinositol. Total inositol phosphates were eluted with 6 ml of 0.8 M ammonium formate-0.1 M formic acid. The eluates were collected into scintillation vials and counted in gel phase after addition of 10 ml of scintillant. The results were expressed as counts per minute per mg protein.
2.6. Measurement of Ca2+ release
Melatonin-induced increase in [Ca2+]i was measured by fluorescence in single smooth muscle cell loaded with fluorescent Ca2+ dye fura 2 [30]. Dispersed muscle cells were plated on coverslips for 12 h in DMEM. After being washed with PBS, the cells were loaded with 5 μM fura 2-AM for 1 h at room temperature. The cells were visualized through a 40× objective (ZEISS; 0.9 NA) with a Zeiss Axioskop 2 plus upright fluorescence microscope and imaged with a setup consisting of a charge coupled device camera (Imago, TILL Photonics, Applied Scientific Instrumentation, Eugene, OR) attached to an image intensifier. The cells were alternately excited at 380 and 340 nm. The background and autofluorescence were corrected from images of a cell without the fura 2. Results are expressed as increase in 340/380 ratio.
2.7. Measurement of contraction in dispersed smooth muscle cells
Contraction in freshly dispersed gastric circular smooth muscle cells was determined by scanning micrometry as previously described [28-30]. An aliquot (0.4 ml) of cells containing approximately 104 cells/ml was treated with melatonin (1 pM to 10 μM) or MT2 receptor selective agonist IIK7 (100 nM) for 30 s and the reaction was terminated with 1% acrolein at a final concentration of 0.1%. In some experiments cells were treated with melatonin in the presence and absence of the non-selective MT1/MT2 receptor antagonist luzindole (100 mM), a selective MT2 receptor antagonist 4P-PDOT, PLC β inhibitor U73122 (10 μM) or MLCK inhibitor ML-9 (1 μM) for 30 s. The resting cell length was determined in control experiments in which muscle cells were incubated with 100 μl of 0.1% bovine serum albumin without the agonists. The mean length of 50 muscle cells treated with various agonists was measured by scanning micrometry and compared with the mean length of untreated cells. The contractile response was expressed as the percent decrease in mean cell length from control cell length.
2.8. Statistical analysis
The results were expressed as means ± S.E. of n experiments and analyzed for statistical significance using Student's t-test for paired and unpaired values. Each experiment was done on cells obtained from different animals. Differences among multiple groups were tested by using ANOVA and checked for significance using Fisher's protected least significant difference test. A probability of P< 0.05 was considered significant.
3. Results
3.1.Expression of MT1 in gastric smooth muscle
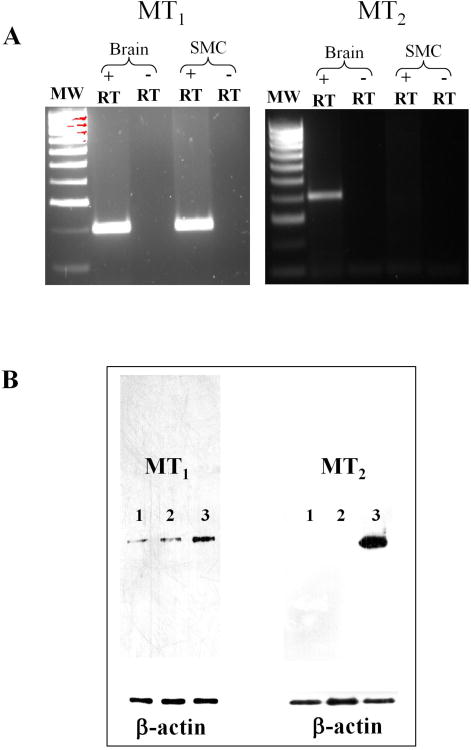
Specific primers for MT1 and MT2 were designed based on the conserved sequences in human and rat cDNAs. MT1, but not MT2 receptors were detected by RT-PCR on RNA extracted from cultures of gastric smooth muscle cells in first passage. PCR product of the expected size (194 bp) was obtained for MT1 (Fig. 1A). The isolated partial nucleotide sequence of rabbit MT1 was similar to the corresponding amino acid sequences of human (86%) and rat (83%). mRNA expression of MT1 (194 bp) and MT2 (392 bp) receptors was identified on RNA extracted from rabbit brain (Fig. 1). As shown previously, the use of confluent cultures of smooth muscle in first passage ensured the absence of neural, endothelial, or interstitial cell contaminants and the presence of PCR product in cultured muscle cells demonstrate the expression of MT1 mRNA in smooth muscle cells. Expression of MT1 and MT2 receptor protein was examined by western blot analysis using selective antibody to MT1 or MT2. The results demonstrate the expression of MT1 of predicted size (∼ 40 kDa) in the homogenates of isolated smooth muscle cells. Expression of MT2 was not detected in the homogenates of dispersed gastric muscle cells (Fig. 1B). Expression of both MT1 and MT2 receptors was detected in the homogenates of rabbit brain (Fig. 1B)
Figure 1. Expression of MT1 receptors and activation of Gαq by melatonin in gastric smooth muscle cells.
(A) RT-PCR. Total RNA isolated from cultured (first passage) rabbit gastric muscle cells and the brain was reverse transcribed, and cDNA was amplified with specific primers for MT1 or MT2. Experiments were done in the presence (+ RT) or absence (−RT) of reverse transcriptase (RT). PCR product with predicted size was obtained in the presence of reverse transcriptase with primers for MT1 (194 bp), but not with primers for MT2, in smooth muscle cells (SMC), whereas PCR products were obtained with primers for MT1 (194 bp) and MT2 (392 bp) in the brain. (B) Western blot. Lysates prepared from dispersed smooth muscle cells (lane 1), cultured gastric smooth muscle cells (lane 2), and the brain (lane 3) of rabbit were run on SDS-PAGE and analyzed by western blot. Proteins were probed with polyclonal antibodies to MT1 (1:1000) or MT2 (1:1000). A protein band corresponding to 40 kDa was obtained with only MT1 antibody in smooth muscle cells, whereas a protein bands corresponding to 40 kDa were obtained with MT1 and MT2 antibody in the brain.
3.2. Identification of G proteins coupled to MT1 receptors
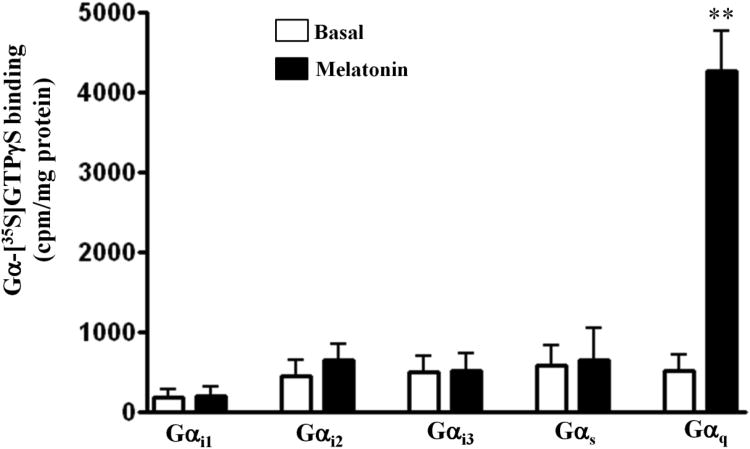
Studies in various tissues and cell lines suggest that MT1 receptors are coupled to activation of G proteins, but the specific G proteins coupled to MT1 receptors in smooth muscle has not been identified. Muscle cells membranes were incubated with [35S]GTPγS (60 nM) in the presence or absence of melatonin (1 μM) and the aliquots were added to wells precoated with different Gα antibodies; an increase in the binding of [35S]GTPγS complexes to a specific Gα antibody reflected the activation of the corresponding G protein. In some experiments low concentrations of GTPγS stimulated binding of [35S]GTPγS to Gαi2, Gαq and Gαs. However, incubation of muscle membranes with melatonin in the presence of low concentrations of GTPγS caused a significant increase in the binding of [35S]GTPγS selectively to Gαq (392+45% increase above basal levels, p< 0.001, n=8), but not to Gαi1, Gαi2, Gα3, or Gαs. These results suggest that MT1 receptors are preferentially coupled to activation of Gq in gastric smooth muscle (Fig. 2B).
Figure 2. Selective activation of Gq proteins by melatonin.
Membranes were isolated from dispersed gastric muscle cells and incubated with [35S]GTPγS for 20 min in the presence or absence of melatonin (1 μM). Aliquots were added to wells coated with antibody to Gαi2, Gαi3, Gαs, or Gαq for 2 h and bound radioactivity from each well was counted by liquid scintillation. The amount of [35S]GTPγS bound to the activated Gα subunit was expressed as counts per minute (cpm) per milligram of protein. Melatonin induced significant increase in the binding of [35S]GTPγS.Gα complexes to wells coated with Gαq antibody only. Values are mean±SEM of 4 experiments. **p<0.001 significant increase in Gαq-[35S]GTPγS binding in respone to melatonin.
3.3. Signaling pathways activated by MT1 in gastric smooth muscle
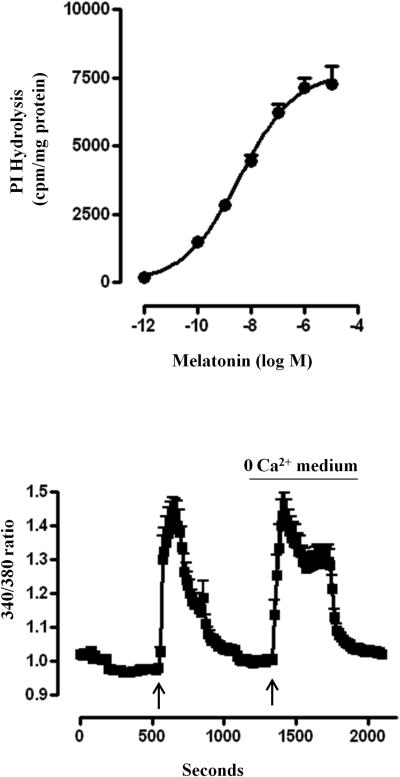
Previous studies in smooth muscle have shown that activation of Gq by excitatory neurotransmitters such as acetylcholine and substance P results in the stimulation of phosphoinositide (PI)-specific phospholipase C (PLC-β) activity, generation of inositiol 1,4,5-trisphosphate (IP3) and IP3-dependent Ca2+ release leading to smooth muscle contraction. The effector enzyme stimulated by Gq proteins coupled to MT1 receptors was examined by measurements of inositol formation in response to melatonin in cells labeled with [3H]myoinositol. As expected from the activation of Gq, incubation of muscle cells with melatonin for 60 s caused an increased in inositol formation in a concentration-dependent fashion with an IC50 of 4±1 nM (Fig. 3A). The increase was significant at 0.1 nM (1473±104 cpm/mg protein above basal levels of 896±101cpm/mg protein, p<0.01, n=4) and a maximal increase was obtained at 1 μM (7142±351 cpm/mg protein/mg protein above basal levels, p<0.001, n=4). The extent of stimulation of PLC-β activity with melatonin was similar to that obtained with other contractile agonists such as acetylcholine or substance P in gastric smooth muscle cells.
Figure 3. Stimulation of PLC-β activity and release of Ca2+ by melatonin.
(A) Phosphoinositide-specific (PI) hydrolysis (PLC-β activity) in response to melatonin was measured in dispersed muscle cells labeled with myo-[3H]inositol. Freshly dispersed muscle cells were treated for 60 s with different concentrations of melatonin and PLC-β activity was measured as increase in water-soluble [3H]inositol formation. The results are expressed as [3H]inositol phosphate formation in counts per minute (cpm) per mg protein above basal levels (basal: 642±99 cpm/mg protein). Values are means±SEM of 4 experiments. (B) Isolated smooth cells were loaded with 5 μM fura-2 and treated with 1 μM melatonin in the absence of extracellular Ca2+. The cells were alternately excited at 380 nm and 340 nm. The background and autofluorescence were corrected from images of a cell without the fura 2. Results are expressed as 340/380 ratio and an increase in ratio reflects an increase in cytosolic Ca2+. The figure shows results obtained from 38 cells.
Activation of PLC-β results in the generation of inositol 1, 4, 5-trisphosphate (IP3) and IP3-dependent Ca2+ release from intracellular sarcoplasmic reticulum stores [34]. Consistent with the activation of Gq and stimulation of PLC-β activity, addition of melatonin to cells loaded with fura-2 resulted in an increase in cytosolic Ca2+. The increase in Ca2+ was not affected by removal of extracellular Ca2+ suggesting that the increase is due to release of Ca2+ from intracellular stores (Fig. 3B).
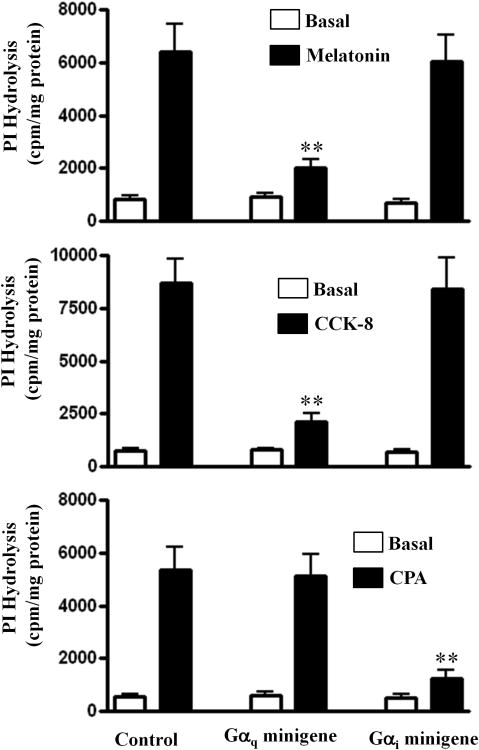
The G protein involved in the activation of PLC-β activity in response to melatonin was obtained by expression of Gα minigenes in cultured smooth muscle cells. The synthetic peptide corresponding to the COOH terminus of Gα subunits selectively antagonized G protein activation by blocking receptor-G protein interaction [25-29]. Minigene plasmid constructs that encode COOH-terminal peptide sequence of Gαi and Gαq were expressed to selectively block Gi and Gq activation, respectively. Treatment of cultured muscle cells with melatonin (1 μM) caused a significant increase in PLC-β activity and the extent of stimulation was closely similar to that obtained in freshly dispersed smooth muscle cells (5867±980 cpm/mg protein above basal levels in cultured smooth muscle cells and 6245±1005 cpm/mg protein above basal levels in freshly dispersed smooth muscle cells). Expression of Gαq minigene blocked stimulation of PI hydrolysis in response to melatonin. In contrast, expression of Gαi minigene had no effect on stimulation of PI hydrolysis PLC-β activity (Fig. 4A) in response to melatonin. The results suggest that MT1 receptor coupled to activation of PLC-β activity via Gαq and is consistent with the selective activation of Gαq by melatonin. The validity of Gi minigene approach to selectively block G protein-dependent PLC-β activity has been demonstrated in previous studies [28-30] and confirmed in the present study using cholecystokinin (CCK) and the cyclopentyladenosine (CPA). Previous studies in gastrointestinal smooth muscle have shown that CCK acts via Gq-coupled receptors to activate Gαq-dependent PLC-β1, whereas CPA acts via Gi3-coupled A1 receptors to stimulate Gβγ-dependent PLC-β3 [23, 35]. Expression of Gαq minigene also blocked stimulation of PI hydrolysis in response to CCK (1 nM), whereas expression of Gαi blocked stimulation of PI hydrolysis in response to (CPA, 1 μM) (Figs. 4B and 4C).
Figure 4. Gαq-dependent activation of PI hydrolysis by melatonin.
Cultured gastric muscle cells labeled with myo-[3H]inositol and expressing Gαq minigene, Gαi minigene, or control vector were treated with melatonin (1 μM), cholecycstokinin (CCk, 1 nm) or cyclopentyladenosine (CPA, 1 μM) for 60 s. Total [3H]inositol phosphates were separated by ion-exchange chromatography. PI hydrolysis activity stimulated by melatonin or CCK was abolished in cells expressing Gαq minigene, but was not affected in cells expressing Gαi minigene. In contrast, PI hydrolysis activity stimulated by CPA was abolished in cells expressing Gαi minigene, but was not affected in cells expressing Gαq minigene. Results are expressed as total [3H]inositol phosphate formation in cpm/mg protein. Values are means ± SEM of four experiments. ** Significant inhibition from control response (P<0.01).
Stimulation of PLC-β activity and increase in intracellular Ca2+ by contractile agonists in smooth muscle leads to muscle contraction. The functional significance of MT1 receptor-mediated stimulation of PLC-β activity and increase in cytosolic Ca2+ was examined by measurements of muscle contraction by scanning micrometry. Contraction was measured as decrease in muscle cell length in response to melatonin compared to control cell length. Treatment of muscle cells with melatonin caused contraction in a concentration-dependent manner with a maximal contraction of 28±4% (p<0.001, n=6) decrease in cell length (basal cell length in the absence of melatonin treatment 125±4 μm) (Fig. 5). The extent of muscle contraction induced by melatonin is similar to that obtained with other contractile agonists such as acetylcholine, substance P, ATP, sphingosine 1-phosphate (27±3% to 32±4% decrease in cell length) in smooth muscle cells.
Figure 5. Stimulation of muscle contraction by melatonin.
Contraction of muscle cells was measured as decrease in basal cell length in response to various concentrations of melatonin. Muscle cells (0.5 ml cell suspension) were treated with melatonin for 30 s and the reaction was terminated with 0.1% acrolein. The mean length of 50 muscle cells was measured by scanning micrometry and was compared with the length of untreated muscle cells (125±4 μm). The contractile response was expressed as the percent decrease in the mean cell length from control cell length. Values are means±SEM of 6 experiments.
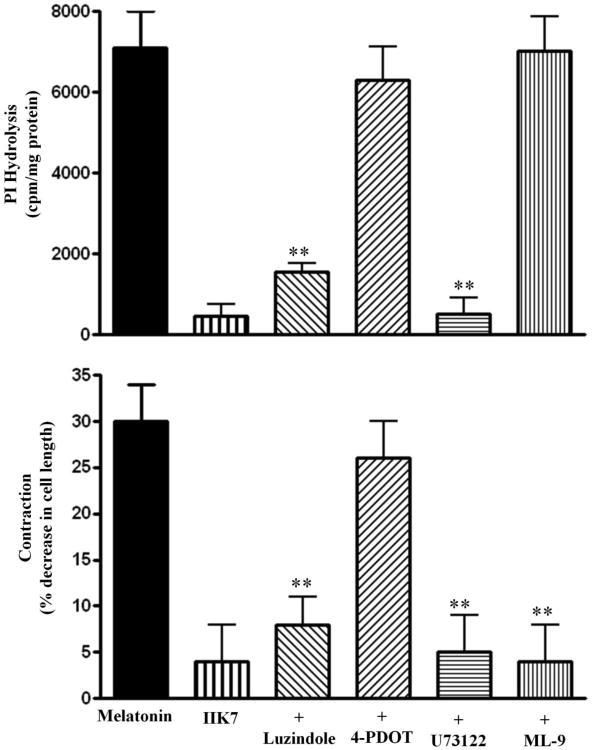
Melatonin-induced PI hydrolysis and muscle contraction were blocked by the selective PLC-β inhibitor, U-73122 (10 μM) and by a non-selective MT1/MT2 receptor antagonist luzindole (100 nM), but not by a selective MT2 receptor antagonist, 4P-PDOT (100 nM). Consistent with this a selective MT2 receptor against IIK7 had no effect on PI hydrolysis or muscle contraction. Muscle contraction in response to melatonin was also inhibited a selective MLCK inhibitor ML-9 (1 μM) (6±5% decrease in cell length) (Fig. 6). These results suggest that contraction in response to melatonin was mediated via activation MT1 receptors coupled to activation of PLC-β via Gαq, generation of IP3 and IP3-dependent Ca2+ release, and stimulation of Ca2+/calmodulin-dependent MLC kinase activity.
Figure 6. Stimulation PI hydrolysis and muscle contraction by melatonin via MT1 receptors.
(A) Phosphoinositide-specific (PI) hydrolysis (PLC-β activity) was measured in dispersed muscle cells labeled with myo-[3H]inositol. Cells were treated for 60 s with melatonin in the presence or absence of a non-selective MT1/MT2 receptor antagonist luzindole (100 nM), a selective MT2 receptor antagonist 4P- PDOT (100 nM), PI hydrolysis inhibitor (10 μM) or MLCK inhibitor ML-9 (1 μM), or with a selective MT2 receptor agonist IIK7 alone (100 nM). PLC-β activity was measured as increase in water-soluble [3H]inositol formation. The results are expressed as [3H]inositol phosphate formation in counts per minute (cpm) per mg protein above basal levels (basal: 562±102 cpm/mg protein). Values are means+SEM of 4 experiments. ** Significant inhibition from control melatonin response (P<0.01). (B) Dispersed muscle cells were treated for 30 s with melatonin in the presence or absence of luzindole (100 nM), 4P-PDOT (100 nM), (10 μM), or MLCK inhibitor ML-9 (1 μM), or with IIK7 alone (100 nM). Contraction of muscle cells was measured as decrease in basal cell length in response to various concentrations of melatonin. Muscle cells (0.5 ml cell suspension) were treated with melatonin (1 μM) in the presence or absence of U73122 (10 μM) or ML-9 (1 μM). The mean length of 50 muscle cells was measured by scanning micrometry and was compared with the length of untreated muscle cells (119±6 μm). The contractile response was expressed as the percent decrease in the mean cell length from control cell length. Values are means±SEM of 6 experiments. ** Significant inhibition from control melatonin response (P<0.01).
4. Discussion
Melatonin release from pineal gland displays a circadian rhythmic pattern with the increased release during nighttime and decreased release during the daytime [1]. Melatonin regulates rhythmic changes in gastrointestinal motility [6, 7]. Several studies in isolated muscle strips demonstrated that the actions of melatonin are complex and involve both direct and indirect effects on smooth muscle including antagonistic relationship between serotonin and melatonin [8-10, 16-21]. Melatonin acting via MT1 and MT2 receptors is shown to activate various intracellular signaling pathways including inhibition of adenylyl cyclase and soluble guanylyl cylcase activity, and stimulation of PLC-β activity [36]. The present study characterized the signaling pathways mediated by melatonin receptors in gastric smooth muscle cells using biochemical, molecular and functional methods. The results demonstrate the expression of MT1, but not MT2 receptors in gastric smooth muscle cells and their ability to stimulate PLC-β activity via Gαq, increase intracellular Ca2+ and induce smooth muscle contraction.
The evidence for the coupling of MT1 receptors to Gq-dependent stimulation of PLC-β activity and to elicit muscle contraction was based on a combination of experimental strategies. (i) mRNA and protein expression of MT1 was demonstrated in cultured muscle cells by RT-PCR and in isolated muscle cells by western blot. MT2 receptors are not detected by RT-PCR or western blot analysis, raising the possibility that the expression of these proteins is either absent or not abundant in these cells. mRNA transcripts for all both MT1 and MT2 receptors have been detected in rat intestine [7, 18, 19]. Radioligand binding studies in the gastrointestinal (GI) tract of duck have identified regional differences in the densities with the following descending order of density: ileum > colon > esophagus [37]. Western blot analysis MT2 receptors in rat GI tract have demonstrated highest expression expression in colon [38]. These and other studies suggest that expression levels also vary with different regions and with species [39, 40]. However, cell-specific expression of MT1 and MT2 receptor in the GI is not clear. (ii) The MT1 receptors are coupled to activation of Gq. Selective activation of Gq was demonstrated using [35S]GTPγS and subtype-selective G protein antibodies. [35S]GTPγS.Gα complexes activated by melatonin bound selectively to Gαq antibodies reflecting activation of Gq proteins. No melatonin induced increase in the binding to Gαi1, Gαi2, Gαi3, or Gαs antibodies to [35S]GTPγS could be detected. Studies in various cell lines suggest that MT1 are coupled to both PTx-sensitive and PTx-insensitive G proteins [36]. Our studies demonstrate that MT1 receptors are coupled to PTx-insensitive Gq protein. (iii) Melatonin caused an increase in PLC-β activity (PI hydrolysis) in a concentration-dependent fashion. The extent of increase was similar to that obtained with other Gq-coupled receptors in gastric smooth muscle cells [28-30]. Previous studies in these muscle cells have shown that receptors coupled to both Gq and Gi proteins stimulate PI hydrolysis via distinct PLC-β isoforms [23, 28-30, 34]. Gq coupled receptors such as muscarinic m3, sphingosine-1-phosphate 2 (S1P2), endothelin ETA, purinergic P2Y2, and NPY2 are coupled to stimulation of PI hydrolysis via Gαq-dependent PLC-β1 isozyme, whereas Gi3- adenosine A1 receptors are coupled to stimulation of PI hydrolysis via Gβγi -dependent PLC-β3 isozyme [28-30, 34, 35]. The specific G proteins involved in the stimulation of PLC-β activity by melatonin was examined using the minigene approach. Previous studies have shown that the COOH-terminus of G protein α subunits is critical in mediating receptor-G protein interaction and peptides corresponding to COOH-terminus serve as competitive inhibitors of receptor-G protein interaction [25-29]. The minigene plasmid vectors were designed to express the COOH-terminal peptide sequences of various Gα subunits after transfection into cells. In gastric muscle cells transfection of minigene plasmid constructs that encode oligonucleotide sequences corresponding to Gαq completely blocked the activation of PLC-β activity by melatonin. The results provide evidence that MT1 receptors are coupled to stimulation of PLC-β activity via Gq and this is consistent with the selective activation of Gq. (iv) Melatonin, as other Gq-coupled receptors, induced an increase in intracellular Ca2+ in single muscle cells. The increase in Ca2+ was not affected by removal of extracellular Ca2+ suggesting that the source of Ca2+ was intracellular. This is consistent with the activation of PLC-β activity which results in the generation of Ca2+ mobilizing messenger IP3. The increase Ca2+ and the binding of Ca2+ to calmodulin results in the stimulation of Ca2+/calmodulin-dependent myosin light chain (MLC) kinase activity and phosphorylation of MLC20 at Ser19, a prerequisite for initiation of actomyosin interaction and muscle contraction [34]. (v) Consistent with the stimulation of PLC-β activity and increase in intracellular Ca2+ in response to melatonin, addition of melatonin to dispersed gastric muscle cells elicited rapid (30 s) muscle contraction. Contraction was blocked by the inhibitors of PLC-β or MLC kinase. The extent of muscle contraction was similar to the other contractile agonists such as acetylcholine and substance P [28-30]. (vi) PI hydrolysis and muscle contraction were blocked by a non-selective MT1/MT2 receptor antagonist, whereas a selective MT2 agonist did not stimulate PI hydrolysis or muscle contraction and a selective MT2 receptor antagonist had no effect on PI hydrolysis and muscle contraction in response to melatonin.
From a physiological point of view, melatonin produced in the enterochromaffin cells or by pineal gland act on myenteric neurons and smooth muscle cells to regulate gut motility [2]. Both contraction and relaxation of smooth muscle have been reported in previous studies [16-22]. In vivo effects of melatonin also depend on the dose of melatonin with small doses accelerating intestinal transit and high doses inhibiting the transit [16]. In vitro spontaneous and serotonin-induced contractions of rat duodenum are inhibited by high doses of melatonin [21]. The mechanism by which melatonin regulates motility is not clear and some studies suggested blockade of nicotinic channels and interaction with Ca2+ activated K+ channels [18]. Studies by Lucchelli et al [21] demonstrated direct contractile effect of melatonin in gastrointestinal smooth muscle. These studies demonstrated that melatonin and its analogues induced contractile responses in guinea pig proximal colon in a concentration-dependent manner. The rank of agonist potency was: 2-indomelatonin>6-chloromelatonin>N-acetyl-5-HT>5-MCA-NAT>melatonin, an order typical for MT2 receptors. However, prazosin, an α-adrenoreceptor antagonist possessing moderate/high affinity for melatonin MT2 sites had no effect on melatonin-induced contractions. While the rank order of agonist potencies would suggest the participation of MT2 receptors, the ineffectiveness of prazosin on melatonin-induced contractions suggests the contrary. In contrast to contractile effect, studies by Storr et al [19] have demonstrated that addition of melatonin to isolated gastric and intestine muscle strips caused inhibition of NANC-mediated muscle relaxation via MT1 receptors. These studies suggest that the effect of melatonin may vary in different species and different regions of the gut depending on whether the activated receptor is present predominantly on smooth muscle cells or enteric neurons. Our studies provide evidence for the involvement of MT1 receptors in melatonin-induced contraction in isolated muscle cell devoid of neural elements.
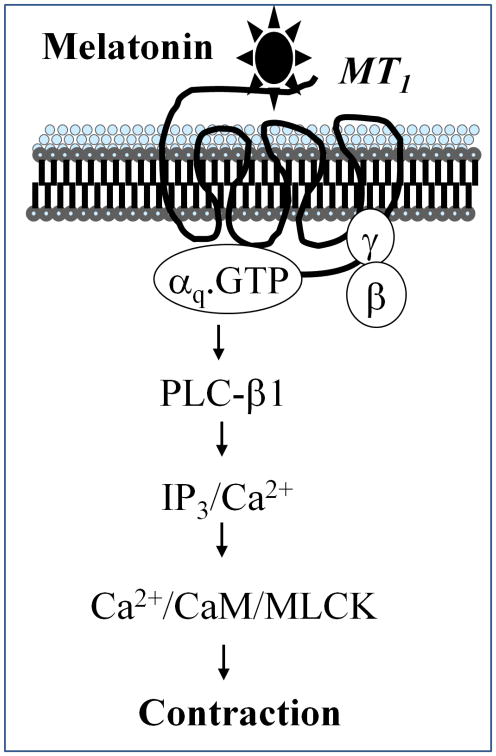
In summary, the present study demonstrated that gastric smooth muscle cells express receptors (MT1) for melatonin preferentially coupled to Gq. Activation of these receptors by melatonin causes stimulation of PLC-β activity and Ca2+ release from intracellular stores resulting in muscle contraction (Fig. 7).
Figure 7. Pathway mediating muscle contraction by melatonin.
In gastric smooth muscle, melatonin interacts with MT1 receptors, which is coupled to stimulation of phosphoinositide-specific phospholipase C (PLC-β) via Gq. Stimulation of PLC-β activity results in the generation of inositol 1,4,5-trisphosphate (IP3) and IP3-dependent Ca2+ release leading to muscle contraction.
Highlights.
Gastric smooth muscle, cells express MT1 receptors for melatonin
MT1 receptors are coupled to Gq/PLC-β1/IP3/Ca2+ pathway and muscle contraction
Signaling by MT1 receptors revealed a mechanism for regulation of gut motility
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants (DK28300 and DK15564 to KS Murthy and DK 34153 to JR Grider). We thank Dr. Lyall and S. Mummalaneni for their expert technical help with the Ca2+ measurements in single muscle cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep. 2009;61:383–410. doi: 10.1016/s1734-1140(09)70081-7. [DOI] [PubMed] [Google Scholar]
- 2.Raikhlin NT, Kvetnoy IM. Melatonin and enterochromaffine cells. Acta Histochem. 1976;55:19–24. doi: 10.1016/S0065-1281(76)80092-X. [DOI] [PubMed] [Google Scholar]
- 3.Raikhlin NT, Kvetnoy IM, Tolkachev VN. Melatonin may be synthesised in enterochromaffin cells. Nature. 1975;255:344–345. doi: 10.1038/255344a0. [DOI] [PubMed] [Google Scholar]
- 4.Kvetnoy IM, Ingel IE, Kvetnaia TV, Malinovskaya NK, Rapoport SI, Raikhlin NT, Trofimov AV, Yuzhakov VV. Gastrointestinal melatonin: cellular identification and biological role. Neuro Endocrinol Lett. 2002;23:121–132. [PubMed] [Google Scholar]
- 5.Bubenik GA, Hacker RR, Brown GM, Bartos L. Melatonin concentrations in the luminal fluid, mucosa, and muscularis of the bovine and porcine gastrointestinal tract. J Pineal Res. 1999;26:56–63. doi: 10.1111/j.1600-079x.1999.tb00567.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee PP, Pang SF. Melatonin and its receptors in the gastrointestinal tract. Biol Signals. 1993;2:181–193. doi: 10.1159/000109491. [DOI] [PubMed] [Google Scholar]
- 7.Chen CQ, Fichna J, Bashashati M, Li YY, Storr M. Distribution, function and physiological role of melatonin in the lower gut. World J Gastroenterol. 2007;17:3888–3898. doi: 10.3748/wjg.v17.i34.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thor PJ, Krolczyk G, Gil K, Zurowski D, Nowak L. Melatonin and serotonin effects on gastrointestinal motility. J Physiol Pharmacol. 2007;58(6):97–103. [PubMed] [Google Scholar]
- 9.Teresa Martin M, Azpiroz F, Malagelada JR. Melatonin as a modulator of the ileal brake mechanism. Scand J Gastroenterol. 2005;40:559–563. doi: 10.1080/00365520510012316. [DOI] [PubMed] [Google Scholar]
- 10.Martin MT, Azpiroz F, Malagelada JR. Melatonin and the gastrointestinal tract. Therapie. 1998;53:453–458. [PubMed] [Google Scholar]
- 11.Reppert SM. Melatonin receptors: molecular biology of a new family of G protein-coupled receptors. J Biol Rhythms. 1997;12:528–531. doi: 10.1177/074873049701200606. [DOI] [PubMed] [Google Scholar]
- 12.Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci U S A. 1995;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 14.Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27:101–110. doi: 10.1385/ENDO:27:2:101. [DOI] [PubMed] [Google Scholar]
- 15.von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002;309:151–162. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- 16.Drago F, Macauda S, Salehi S. Small doses of melatonin increase intestinal motility in rats. Dig Dis Sci. 2002;47:1969–1974. doi: 10.1023/a:1019696006677. [DOI] [PubMed] [Google Scholar]
- 17.Kasimay O, Cakir B, Devseren E, Yegen BC. Exogenous melatonin delays gastric emptying rate in rats: role of CCK2 and 5-HT3 receptors. J Physiol Pharmacol. 2005;56:543–553. [PubMed] [Google Scholar]
- 18.Storr M, Schusdziarra V, Allescher HD. Inhibition of small conductance K+ -channels attenuated melatonin-induced relaxation of serotonin-contracted rat gastric fundus. Can J Physiol Pharmacol. 2000;78:799–806. [PubMed] [Google Scholar]
- 19.Storr M, Koppitz P, Sibaev A, Saur D, Kurjak M, Franck H, Schusdziarra V, Allescher HD. Melatonin reduces non-adrenergic, non-cholinergic relaxant neurotransmission by inhibition of nitric oxide synthase activity in the gastrointestinal tract of rodents in vitro. J Pineal Res. 2002;33:101–108. doi: 10.1034/j.1600-079x.2002.02909.x. [DOI] [PubMed] [Google Scholar]
- 20.Merle A, Delagrange P, Renard P, Lesieur D, Cuber JC, Roche M, Pellissier S. Effect of melatonin on motility pattern of small intestine in rats and its inhibition by melatonin receptor antagonist S 22153. J Pineal Res. 2000;29:116–124. doi: 10.1034/j.1600-079x.2000.290208.x. [DOI] [PubMed] [Google Scholar]
- 21.Lucchelli A, Santagostino-Barbone MG, Tonini M. Investigation into the contractile response of melatonin in the guinea-pig isolated proximal colon: the role of 5-HT4 and melatonin receptors. Br J Pharmacol. 1997;121:1775–1781. doi: 10.1038/sj.bjp.0701287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murthy KS, Makhlouf GM. Phosphoinositide metabolism in intestinal smooth muscle: preferential production of IP3 in circular muscle cells. Am J Physiol. 1991;261:G945–951. doi: 10.1152/ajpgi.1991.261.6.G945. [DOI] [PubMed] [Google Scholar]
- 23.Murthy KS, Makhlouf GM. Functional characterization of phosphoinositide-specific phospholipase C-beta1 and beta 3 in intestinal smooth muscle. Am J Physiol. 1995;269:C969–78. doi: 10.1152/ajpcell.1995.269.4.C969. [DOI] [PubMed] [Google Scholar]
- 24.Teng B, Murthy KS, Kuemmerle JF, Grider JR, Sase K, Michel T, Makhlouf GM. Expression of endothelial nitric oxide synthase in human and rabbit gastrointestinal smooth muscle cells. Am J Physiol. 1998;275:G342–351. doi: 10.1152/ajpgi.1998.275.2.G342. [DOI] [PubMed] [Google Scholar]
- 25.Gilchrist A, Li A, Hamm HE. Design and use of C-terminal minigene vectors for studying role of heterotrimeric G proteins. Methods Enzymol. 2002;344:58–69. doi: 10.1016/s0076-6879(02)44705-2. [DOI] [PubMed] [Google Scholar]
- 26.Gilchrist A, Vanhauwe JF, Li A, Thomas TO, Voyno-Yasenetskaya T, Hamm HE. G alpha minigenes expressing C-terminal peptides serve as specific inhibitors of thrombin-mediated endothelial activation. J Biol Chem. 2001;276:25672–25679. doi: 10.1074/jbc.M100914200. [DOI] [PubMed] [Google Scholar]
- 27.Gilchrist A, Bunemann M, Li A, Hosey MM, Hamm HE. A dominant-negative strategy for studying roles of G proteins in vivo. J Biol Chem. 1999;274:6610–6616. doi: 10.1074/jbc.274.10.6610. [DOI] [PubMed] [Google Scholar]
- 28.Zhou H, Murthy KS. Distinctive G protein-dependent signaling in smooth muscle by sphingosine 1-phosphate receptors S1P1 and S1P2. Am J Physiol Cell Physiol. 2004;286:C1130–1138. doi: 10.1152/ajpcell.00429.2003. [DOI] [PubMed] [Google Scholar]
- 29.Sriwai W, Zhou H, Murthy KS. G(q)-dependent signalling by the lysophosphatidic acid receptor LPA(3) in gastric smooth muscle: reciprocal regulation of MYPT1 phosphorylation by Rho kinase and cAMP-independent PKA. Biochem J. 2008;411:543–551. doi: 10.1042/bj20071299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang J, Zhou H, Mahavadi S, Sriwai W, Lyall V, Murthy KS. Signaling pathways mediated gastrointestinal smooth muscle contraction and MLC20 phosphorylation by motilin receptors. Am J Physiol. 2005;288:G23–31. doi: 10.1152/ajpgi.00305.2004. [DOI] [PubMed] [Google Scholar]
- 31.Ting KN, Blaylock NA, Sugden D, Delagrange P, Scalbert E, Wilson VG. Molecular and pharmacological evidence for MT1 melatonin receptor subtype in the tail artery of juvenile Wistar rats. Br J Pharmacol. 1999;127:987–995. doi: 10.1038/sj.bjp.0702612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fisher SP, Sugden D. Sleep-promoting action of IIK7, a selective MT2 melatonin receptor agonist in the rat. Neurosci Lett. 2009;457:93–96. doi: 10.1016/j.neulet.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dortch-Carnes J, Tosini G. Melatonin receptor agonist-induced reduction of SNP-released nitric oxide and cGMP production in isolated human non-pigmented ciliary epithelial cells. Exp Eye Res. 2012;107:1–10. doi: 10.1016/j.exer.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 35.Murthy KS, Makhlouf GM. Adenosine A1 receptor-mediated activation of phospholipase C-beta 3 in intestinal muscle:dual requirement for alpha and beta gamma subunits of Gi3. Mol Pharmacol. 1995;47:1172–1179. [PubMed] [Google Scholar]
- 36.Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62:343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee PP, Shiu SY, Chow PH, Pang SF. Regional and diurnal studies of melatonin and melatonin binding sites in the duck gastro-intestinal tract. Biol Signals. 1995;4:212–224. doi: 10.1159/000109445. [DOI] [PubMed] [Google Scholar]
- 38.Stebelova K, Anttila K, Manttari S, Saarela S, Zeman M. Immunohistochemical definition of MT(2) receptors and melatonin in the gastrointestinal tissues of rat. Acta Histochem. 2010;112:26–33. doi: 10.1016/j.acthis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Poon AM, Mak AS, Luk HT. Melatonin and 2[125I]iodomelatonin binding sites in the human colon. Endocr Res. 1996;22:77–94. doi: 10.3109/07435809609030499. [DOI] [PubMed] [Google Scholar]
- 40.Pontoire C, Bernard M, Silvain C, Collin JP, Voisin P. Characterization of melatonin binding sites in chicken and human intestines. Eur J Pharmacol. 1993;247:111–118. doi: 10.1016/0922-4106(93)90067-j. [DOI] [PubMed] [Google Scholar]