Abstract
Background:
In the United States, hip fracture rates have declined by 30% coincident with bisphosphonate use. However, bisphosphonates are associated with sporadic cases of atypical femoral fracture. Atypical femoral fractures are usually atraumatic, may be bilateral, are occasionally preceded by prodromal thigh pain, and may have delayed fracture-healing. This study assessed the occurrence of bisphosphonate-associated nonhealing femoral fractures through a review of data from the U.S. FDA (Food and Drug Administration) Adverse Event Reporting System (FAERS) (1996 to 2011), published case reports, and international safety efforts.
Methods:
We analyzed the FAERS database with use of the proportional reporting ratio (PRR) and empiric Bayesian geometric mean (EBGM) techniques to assess whether a safety signal existed. Additionally, we conducted a systematic literature review (1990 to February 2012).
Results:
The analysis of the FAERS database indicated a PRR of 4.51 (95% confidence interval [CI], 3.44 to 5.92) for bisphosphonate use and nonhealing femoral fractures. Most cases (n = 317) were attributed to use of alendronate (PRR = 3.32; 95% CI, 2.71 to 4.17). In 2008, international safety agencies issued warnings and required label changes. In 2010, the FDA issued a safety notification, and the American Society for Bone and Mineral Research (ASBMR) issued recommendations about bisphosphonate-associated atypical femoral fractures.
Conclusions:
Nonhealing femoral fractures are unusual adverse drug reactions associated with bisphosphonate use, as up to 26% of published cases of atypical femoral fractures exhibited delayed healing or nonhealing.
Although hip fracture rates among older adults have declined coincident with use of bisphosphonates1-5, concern exists regarding the sporadic occurrence of atypical femoral fractures and prolonged suppression of bone remodeling by bisphosphonates6. Atypical femoral fractures are usually atraumatic, are occasionally preceded by prodromal thigh pain, and have been reported in individuals on bisphosphonate therapy7. These fractures are transverse and may be bilateral; up to 26% of published cases exhibited delayed healing or nonhealing (Table I). Radiographs show thickened cortices and a “beaked” appearance to the transverse fracture (Fig. 1). Considerable concern has been raised by such fractures and has resulted in a decline in bisphosphonate use8.
TABLE I.
Major and Minor Criteria for Atypical Femoral Fractures39
| Major features* |
| Located anywhere along the femur from just distal to the lesser trochanter to just proximal to the supracondylar flare |
| Associated with no or minimal trauma, as in a fall from a standing height or less |
| Transverse or short oblique configuration |
| Noncomminuted |
| Complete fractures extend through both cortices and may be associated with a medial spike; incomplete fractures involve only the lateral cortex |
| Minor features |
| Localized periosteal reaction of the lateral cortex† |
| Generalized increase in cortical thickness of the diaphysis |
| Prodromal symptoms such as dull or aching pain in the groin or thigh |
| Bilateral fractures and symptoms |
| Delayed healing |
| Comorbid conditions (e.g., vitamin-D deficiency, rheumatoid arthritis, hypophosphatasia) |
| Use of pharmaceutical agents (e.g., bisphosphonates, glucocorticoids, proton pump inhibitors) |
Fractures of the femoral neck, intertrochanteric fractures with spiral subtrochanteric extension, pathological fractures associated with primary or metastatic bone tumors, and periprosthetic fractures are specifically excluded. All major features are required in order to satisfy the case definition of atypical femoral fracture. None of the minor features are required, but these have been associated with some such fractures.
Often referred to in the literature as “beaking” or “flaring.”
Fig. 1.
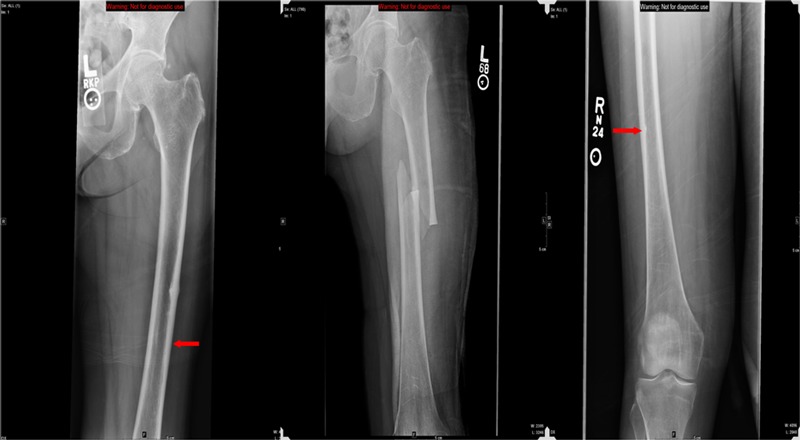
Radiographic appearance of an atypical femoral fracture in a sixty-three-year-old woman who had been on bisphosphonate therapy for seven years prior to developing left thigh pain. The left panel is an initial anteroposterior radiograph of the left femur showing focal cortical thickening along the lateral proximal femoral diaphysis that represents an incomplete fracture (arrow). The central panel is an anteroposterior radiograph of the femur taken two days later showing a displaced fracture centered at the prior site of cortical thickening even though the patient had been placed on protected weight-bearing and did not sustain a trauma during the intervening time. The right panel is an anteroposterior radiograph of the right femur of the same patient, made after she had begun experiencing milder right thigh pain, demonstrating subtle cortical thickening along the lateral proximal aspect of the right femur that also represents a developing insufficiency fracture (arrow).
Bisphosphonates are synthetic analogues of pyrophosphate (O3P-O-PO3)9, inhibit the formation and aggregation of calcium phosphate crystals, and are potent inhibitors of bone resorption. Their use is typically accompanied by an increase in bone mineral content10. After a certain duration of exposure, bone formation also decreases, which has been attributed to the “uncoupling” that occurs between bone formation and resorption11. Atypical femoral fractures were not observed in clinical trials of bisphosphonate use in osteoporosis12,13. However, a growing number of cases have been reported internationally since 2004. Our objectives were to analyze the presence of nonhealing femoral fractures and atypical femoral fractures by a multifaceted approach: through a systematic review of atypical femoral fractures in the literature, an analysis of atypical femoral fractures and nonhealing femoral fractures in the U.S. Food and Drug Administration (FDA) Adverse Event Reporting System (FAERS) (1996 to 2011) that addressed the identification of a safety signal, and a review of notifications and warnings established by international safety agencies.
Materials and Methods
A literature search was conducted with use of PubMed and Embase to identify English-language articles dated January 1990 to February 2012. Literature search terms included atypical femoral fracture, subtrochanteric fracture, nonhealing femoral fracture, nonunion diaphyseal fracture, malunion shaft fracture, bilateral femoral fractures, transverse fracture, and the names of individual bisphosphonates, among others (Fig. 2). Meeting abstracts were reviewed by one author (B.J.E.); if there were questions, three additional members of the RADAR (Research on Adverse Drug events And Reports) group (D.P.W., J.M.M., and P.S.) reviewed the data. The extracted data items were the subjects in each study, type of bisphosphonate(s), dose and duration of bisphosphonate exposure, clinical presentation, prodromal symptoms, characteristics of the fracture(s), level of trauma, and radiographic changes. Cases were reviewed for the presence of diseases and drugs affecting bone metabolism, the presence of vitamin-D deficiency, bone histology, management, and outcome. Case reports, case series, analyses of randomized clinical trials, and epidemiologic studies were eligible. Data were abstracted into a standardized case report form, and any discrepancies were discussed with the original authors.
Fig. 2.
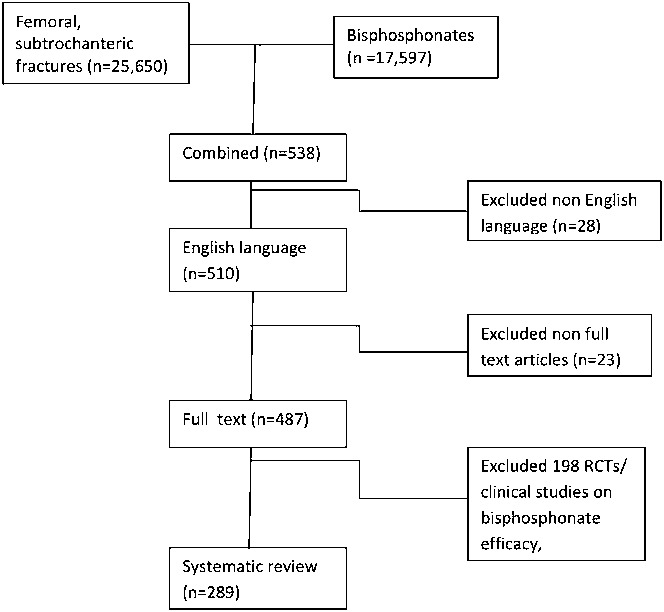
Results of the literature search strategy. RCT = randomized controlled trial.
The FAERS database from January 1996 through September 2011 was searched. Search terms included atypical femoral fractures, nonunion, or nonhealing femoral or subtrochanteric fractures, among others, in the absence of the terms malignancy or metabolic bone, combined with bisphosphonate drug names14-18. Reports that did not include the terms “atypical,” “non healing,” “nonhealing,” or “fracture nonunion” were excluded as they could be related to the underlying osteoporosis.
We then conducted a disproportionality analysis within the FAERS data with use of proportional reporting ratio (PRR) and empiric Bayesian geometric mean (EBGM) values with accompanying 95% confidence intervals (CIs) to determine whether the number of atypical femoral fractures associated with bisphosphonates was greater than that for other drugs19-21. The PRR is a statistical aid to identify safety signals on the basis of the proportions of specified adverse reactions for drugs of interest, where the comparator is all other drugs in the database. The PRR and EBGM methods utilize a proportionate approach that utilizes the stability of a large database19. The EBGM method is a quantitative method for signal detection that stratifies by age, sex, and time, and it is less prone to false positive signals than the PRR method19. Judgments about the existence of a safety signal and signal strength are made on the basis of three pieces of information: the PRR (or EBGM), the chi-square value or 95% CI of the PRR, and the number of cases. A signal is defined as a PRR of ≥2, a chi-square of ≥4, and three or more cases (see Appendix).
Causality assessment for the published cases (n = 422) was conducted with use of the Naranjo and Bradford-Hill criteria22,23. The 10-point Naranjo probability scale assigns a weighted value to each possible answer to ten questions. The probability that the data indicates an adverse drug reaction is classified as definite if the total score is 9 or 10, probable if it is 5 to 8, possible if it is 1 to 4, and doubtful if it is 022. To avoid inclusion of duplicate cases, the original authors were contacted when multiple publications involved the same case series. The Bradford-Hill criteria, which evaluate the likelihood of causality in a chronic disease, rely on multiple factors including temporal association, dose response, related experimental data, consistency of the findings, alternative explanations, coherence of the findings, and plausibility22.
In addition, we searched for safety notifications disseminated by the FDA, the European Medicines Agency (EMA), Health Canada, and Australia’s Adverse Drug Reactions Advisory Committee (ADRAC).
Source of Funding
This study was partially funded by grants 3 R01CA 102713-01, 1 K01 CA134554-01, and 1 R01 CA125077-01 A1 from the National Institutes of Health.
Results
The literature search involving atypical femoral fractures and bisphosphonates identified 538 manuscripts. Twenty-eight of these were excluded because they were not in the English language, twenty-three because the full text was not available, and 198 because they were randomized clinical trials focusing on bisphosphonate efficacy. The remaining 289 manuscripts were used for the systematic review (Fig. 2).
We identified an association between bisphosphonate therapy and nonhealing femoral fractures. The FAERS database contained 362 cases of nonhealing femoral fractures associated with bisphosphonates. The PRR for these fractures and bisphosphonate use was 4.51 (95% CI, 3.44 to 5.92), with most cases (n = 317) being attributed to alendronate (PRR, 3.32; 95% CI, 2.71 to 4.17) (Table II). Comorbidities were rare and included rheumatoid arthritis (n = 26, 7%) and breast cancer (n = 6, 2%). Concomitant medications included glucocorticoids (n = 35, 10%), etanercept (n = 36, 10%), estrogen (n = 12, 3%), and aromatase inhibitors (n = 3, <1%). The time line of safety reporting is depicted in Figure 3. No cases specifically diagnosed as atypical femoral fractures were identified in the FAERS database.
TABLE II.
Proportional Reporting Ratio (PRR), Empiric Bayesian Geometric Mean (EBGM), and Odds Ratio for Nonhealing Femoral Fractures in the FAERS database
| Drug | No. of Reports | Parameter | Value (95% CI) |
| Any bisphosphonate | 362 | PRR | 4.51 (3.44, 5.92) |
| Odds ratio | 4.99 (3.76, 6.62) | ||
| EBGM | 1.46 (1.32, 1.62) | ||
| Alendronate | 317 | PRR | 3.32 (2.71, 4.17) |
| Odds ratio | 3.71 (2.95, 4.67) | ||
| EBGM | 1.55 (1.39, 1.73) | ||
| Ibandronic acid | 36 | PRR | 1.38 (1.01, 1.90) |
| Odds ratio | 1.43 (0.95, 2.05) | ||
| EBGM | 1.19 (0.90, 1.54) | ||
| Pamidronate | 14 | PRR | 2.78 (1.74, 4.46) |
| Odds ratio | 3.29 (1.81, 6.01) | ||
| EBGM | 2.37 (1.37, 3.86) | ||
| Risedronate | 38 | PRR | 1.84 (1.35, 2.51) |
| Odds ratio | 1.98 (1.38, 2.83) | ||
| EBGM | 1.71 (1.23, 2.32) | ||
| Zoledronic acid | 26 | PRR | 1.43 (0.98, 2.08) |
| Odds ratio | 1.49 (0.98, 2.26) | ||
| EBGM | 1.18 (0.87, 1.58) |
Fig. 3.
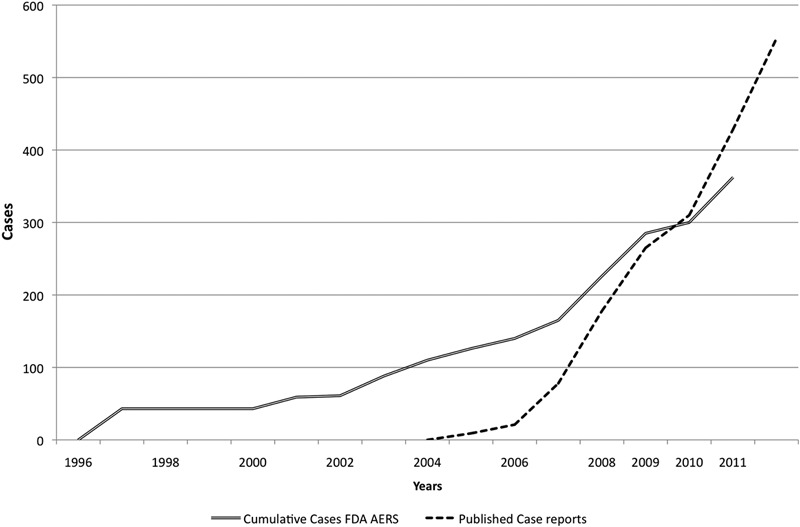
Case reports in the literature and reports in the FAERS database of bisphosphonate-associated atypical femoral fractures from January 1996 to September 2011.
Case Series
From 2005 to February 2012, investigators published reports of 422 cases of atypical femoral fracture in patients on bisphosphonates. In 2005, Odvina et al. reported five cases of alendronate-associated femoral fracture with delayed fracture-healing, evidence of severe suppression of bone turnover, and reduced or absent osteoblastic surfaces7. Another report presented nine cases of atypical femoral fracture in 2005 and 2006 in Singapore24. Severe suppression of bone remodeling, resembling adynamic bone disease, was reported in 2006 in one patient in Hong Kong after ten years of alendronate use25. Twenty-five of seventy patients with atypical femoral fractures in New York had used alendronate for a median duration of 6.2 years26. The odds ratio (OR) for atypical femoral fracture in the alendronate users was 139.33 (95% CI, 19.0 to 939.4; p < 0.001)26. The fracture pattern was described as transverse or oblique, showing thickened cortices, with a unicortical-beak “chalk stick fracture” (Fig. 1). Femoral cortical stress reactions may herald the onset of an atypical femoral neck fracture27 (see Appendix).
Epidemiologic Studies
Population-Based Studies
Abrahamsen et al. conducted an age-matched case control study of alendronate users who had sustained a prior non-hip fracture and found that the hazard ratio (HR) for femoral diaphyseal fracture with alendronate use was 1.46 (95% CI, 0.91 to 2.35; p = 0.12) compared with 1.45 (95% CI, 1.21 to 1.74; p ≤ 0.001) for proximal femoral fracture. High compliance with alendronate use reduced the risk of fracture; however, the subgroup of prolonged compliant users (six years or longer) was limited to 178 patients who sustained thirty-nine hip fractures and five atypical femoral fractures28. Moreover, fractures were assessed with use of ICD (International Classification of Diseases)-9 codes, which are subject to ascertainment errors. Schilcher and Aspenberg identified five cases of femoral “insufficiency” fractures in bisphosphonate users (duration of use, 3.5 to 8.5 years). The incidence of atypical femoral fractures per 1000 person-years was 1 (95% CI, 0.3 to 2) among women on continuous bisphosphonate treatment compared with 0.02 (95% CI, 0.004 to 0.1) among nontreated women. Thus, the risk of atypical femoral fracture was increased by a factor of 46 (95% CI, 11 to 200) with bisphosphonate use29. A population-based study in Sweden (n = 12,777 femoral fractures) identified fifty-nine atypical femoral fractures (95% CI, 25.6 to 87.3). The increase in absolute risk of atypical femoral fractures with bisphosphonate use was five cases (95% CI, four to seven cases) per 10,000 person-years. The OR for atypical femoral fracture with bisphosphonate use was 33.3 (95% CI, 14.3 to 77.8), and the duration of bisphosphonate use influenced the risk, with an OR of 1.3 (95% CI, 1.1 to 1.6) per 100 daily doses of alendronate. The risk diminished by 70% per year after bisphosphonate use was discontinued; the OR was 0.28 (95% CI, 0.21 to 0.38) per year30. Kim et al. analyzed health-care utilization data and reported that a total of 104 subtrochanteric or diaphyseal femoral fractures were observed among 33,815 patients31. The incidence of subtrochanteric or diaphyseal femoral fractures per 1000 person-years was 1.46 (95% CI, 1.11 to 1.88) among the bisphosphonate users and 1.43 (95% CI, 1.06 to 1.89) among raloxifene and calcitonin users. The resulting HR for bisphosphonate use compared with raloxifene and calcitonin use was 1.03 (95% CI, 0.70 to 0.52). A retrospective study of the Kaiser Permanente database, which included 1,271,575 person-years of observations, indicated that the cumulative incidence of non-atypical femoral fractures was 18.2 (95% CI, 16.0 to 20.7) per 100,000 person-years compared with 5.9 (95% CI, 4.6 to 7.4) per 100,000 person-years for atypical femoral fractures. These rates were stable over time32. Age-adjusted rates of typical hip fractures in two national databases decreased by 31.6% among women and 20.5% among men from 1996 to 2007. In contrast, age-adjusted rates of subtrochanteric fragility fractures per 100,000 individuals remained unchanged among men (p = 0.34) but increased 20.4% among women, from 28.4 (95% CI, 27.7 to 29.1) in 1999 to 34.2 (95% CI, 33.4 to 34.9) in 2007. On the basis of the age-adjusted rates, there was an increase of one subtrochanteric fragility fracture for every decrease of approximately 100 typical femoral neck or intertrochanteric fractures33.
Cohort Studies
Black et al. analyzed clinical trials and identified 284 recorded hip or femoral fractures among 14,195 participants. Ten of the participants had twelve femoral fractures, a rate of 2.3 per 10,000 patient-years. The relative hazard of atypical femoral fracture was 1.03 (95% CI, 0.06 to 16.46) for alendronate use in the FIT trial, 1.50 (95% CI, 0.25 to 9.00) for zoledronic acid use in the HORIZON trial, and 1.33 (95% CI, 0.12 to 14.67) for alendronate use in the FLEX trial. However, the study by Black et al. was underpowered34. Another study indicated that bisphosphonates were associated with an increased risk of femoral fracture (OR, >1000; p = 0.0001)35. Other authors estimated risks of 1 per 250,000 person-years and 1 per 1,000,000 person-years for alendronate and risedronate, respectively, although there may have been substantial underreporting and miscoding36-38. More recently, Dell et al. analyzed the records of a large health maintenance organization and identified 102 patients with atypical femoral fractures who had been on oral bisphosphonates for a mean of 5.5 years. The risk of atypical femoral fracture increased with increasing duration of treatment in patients in the Kaiser Permanente Healthy Bones Program, which included 188,814 patients who had used bisphosphonates. The age-adjusted incidence rate in those patients was 1.78 (95% CI, 1.5 to 2.0) per 100,000 person-years for an exposure of 0.1 to 0.9 year but increased to 113.1 (95% CI, 69.3 to 156.8) per 100,000 person-years for an exposure of 8.0 to 9.9 years39. We conclude that the incidence of atypical fracture of the femur increases with increasing duration of bisphosphonate use, but the rate remains much lower than the expected rate of devastating hip fractures in elderly osteoporotic patients. Lenart et al. reported that bisphosphonate use was more common in individuals with subtrochanteric and/or femoral shaft fractures compared with controls with intertrochanteric and/or femoral neck fractures (OR, 4.44; 95% CI, 1.77 to 11.35; p = 0.002). A typical radiographic pattern was identified in ten of the fifteen bisphosphonate users with subtrochanteric and/or shaft fractures. This radiographic pattern was strongly associated with bisphosphonate use (OR, 15.33; 95% CI, 3.06 to 76.90; p < 0.001). The duration of bisphosphonate use was longer in patients with subtrochanteric and/or shaft fractures compared with both hip fracture control groups (p = 0.001)40. A recent population-based study indicated a higher risk of atypical femoral fracture in alendronate users (OR, 1.74; 95% CI, 1.8 to 7.3); however, this risk was also elevated prior to initiation of therapy, pointing to an effect of the underlying disease being treated41.
Causality Assessment
Causality was assessed with use of the Naranjo and Bradford-Hill criteria22,42. For the Naranjo criteria, the median value for the published case reports was five (indicating a probable adverse drug reaction) and ranged from two (possible) to seven (probable) in the individual reports; the quality of the reports was variable. For the Bradford-Hill criteria, a temporal relationship was evident as bisphosphonate use preceded the occurrence of atypical femoral fractures, although some cases occurred in bisphosphonate-naive patients43. There was consistency in the findings as results were replicated in studies in different settings such as North America, Europe, Australia, and Asia. The condition of plausibility was satisfied by known preclinical findings44-47 and findings of bisphosphonate-induced osteopetrosis in pediatric cases and of atypical femoral fractures associated with severe suppression of bone turnover7,48,49. Alternative explanations are conceivable as a prior unidentified metabolic abnormality could potentially predispose individuals to femoral fracture. Supporting experimental evidence is exemplified by the prevention of femoral fractures by limited weight-bearing after the identification of cortical stress reaction27. A dose-response relationship was noted in a study by Dell et al. in which a higher incidence of atypical femoral fractures was associated with longer bisphosphonate use39. Coherence of the findings is detailed below.
Basic Science Indicating Coherence of Data on Atypical Femoral Fractures According to the Bradford-Hill Criteria
In 2007, Yang et al. found that pamidronate administration for six months in a murine model resulted in elevated osseous levels of pamidronate (61.8 ± 15.7 ng/mg of bone) and that these levels were associated with a decrease in the time to failure during biomechanical testing50. Thus, there is concern that prolonged bisphosphonate residence in bone may result in skeletal damage51. This damage may result from the nonmetabolized nature of bisphosphonates and the ability of bisphosphonates to induce osteoclast failure and associated apoptosis51. Prevailing theories about possible mechanisms by which bisphosphonates induce atypical femoral fractures include cellular abnormalities with severe suppression of bone turnover, collagen abnormalities with increased cross-linking, mineral abnormalities with changes in crystal structure, and preexisting osteomalacia demonstrating a “hypophosphatasia-like appearance.”
Bisphosphonates may produce severe suppression of bone turnover, histomorphometric findings of excess microcracks, decreased anisotropy, and increased secondary mineralization51,52. Bisphosphonates may “uncouple” bone formation from resorption in the context of suppressed remodeling, as reported by Somford et al.53. Iatrogenic osteopetrosis has been ascribed to aggressive bisphosphonate therapy leading to suppression of bone resorption48,49. Furthermore, bisphosphonates at micromolar concentrations can induce apoptosis of both osteoclasts54 and osteoblasts55.
Bisphosphonates exert effects on collagen cross-linking and collagen isomerization in cancellous and cortical bone, and such changes are determined by the degree of turnover suppression in bone56,57. Bone matrix is a two-phase system in which the mineral phase provides the stiffness and the collagen fibers provide the ductility and the ability to absorb energy (i.e., the toughness)58. The modulus of toughness is defined as the area under the stress-strain curve (the energy required to cause failure of the material, expressed in units that are independent of its size or geometry)59, and nonenzymatic cross-linking contributes to bone toughness60,61. However, high levels of cross-linking of collagen decrease energy absorption via microdamage formation, which in turn accelerates brittle fracture62-64. Additionally, aging is associated with an increase in advanced glycosylated end products, which contribute to bone brittleness65. The mechanical integrity of collagen fibers from human cortical bones, assessed after demineralization, deteriorates with increasing subject age, and this deterioration is associated with a 30% to 50% decrease in the work to fracture, particularly its post-yield portion.
Bisphosphonates increase mean tissue age and mineralization, resulting in an increased propensity for microcracks and reduced bone resilience that collectively increase the fracture risk52,66. Furthermore, the mineral content (mineral/matrix ratio) of cortical bone but not cancellous bone has been shown to be increased by bisphosphonate treatment52,57,67. These consistent observations suggest that although alendronate treatment increases bone mass, it also decreases tissue heterogeneity and thus affects the mechanical properties of the tissue67. Healthy trabecular bone has broadly heterogeneous crystals, and crystal homogeneity in trabecular regions adversely affects the mechanical properties of bone57. Bisphosphonate therapy reduces the heterogeneity of crystals and contributes to increased brittleness of treated bone. Fourier-transform infrared spectrometry (FTIR) imaging of bone tissue revealed a more uniform composition in bisphosphonate-treated patients than in bisphosphonate-naive patients. The observed reductions in mineral and matrix heterogeneity may diminish tissue-level toughening mechanisms68-71. In a sheep model of rapid bone turnover, the use of zolendronate reduced the mineralization gradient from surface to core regions. Zolendronate restored mineralization levels, stiffness, and hardness but did not restore the gradients present in healthy tissue, and mineral crystal properties were altered72.
Hypophosphatasia is a rare genetic disorder characterized by low serum alkaline phosphatase activity secondary to mutation(s) within the gene that encodes the isoenzyme of alkaline phosphatase73,74. As a consequence, inorganic pyrophosphate accumulates extracellularly and blocks skeletal mineralization, resulting in osteomalacia73,74. It has been proposed that bisphosphonates may cause a hypophosphatasia-like syndrome, at least focally where the fractures are occurring. Patients with hypophosphatasia develop Looser lines (milkman’s fractures, pseudofractures) that closely resemble what is occurring in subjects receiving bisphosphonates. The symptoms, radiographic appearance, failure to heal, and requirement for intramedullary rodding associated with atypical femoral fractures are reminiscent of hypophosphatasia and X-linked hypophosphatemia75.
Bisphosphonates have been shown to affect fracture-healing (in murine models). Alendronate’s effects are dose-dependent, and supranormal doses adversely affect osteoclastic and osteoblastic function76. Fracture repair resulted in large calluses but was more delayed with alendronate treatment (because of delayed remodeling of woven bone into lamellar bone) compared with estrogen and raloxifene treatment77,78. Similar findings were noted with high-dose risedronate and zoledronic acid79,80. Through its direct effects on preosteoclasts, alendronate appears to regulate expression of ephrin-B1, which acts through the EphB1 and EphB3 receptors on osteoblasts to suppress their differentiation81. In a canine model, alendronate did not cause adverse effects on union, strength, or mineralization of bone82. In humans, bisphosphonate use was associated with longer times to radiographic union of distal radial fractures83,84. However, use of alendronate resulted in improved spinal fusion after laminectomy, despite alendronate’s usually detrimental biological effect on the healing process85. In one study, femoral insufficiency fractures after prolonged bisphosphonate therapy seldom healed spontaneously and most patients required surgery86. Treatment of atypical femoral fractures has an elevated failure rate, and revision surgery involving intramedullary nailing may be required87. Thus, the orthopaedic procedures required to treat atypical femoral fractures may be more complex, with the need for bone-stimulating agents such as a bone morphogenetic protein (BMP) or nailing. When nailing of fractures is performed in patients who have been on bisphosphonate therapy, healing is fair and occurs through endochondral union with development of a large callus88. However, the use of plates tends to be unsuccessful as healing occurs through intramembranous remodeling, which is typically delayed in patients who have been on bisphosphonate therapy. Consequently, the cost of such complex surgical care is estimated to be higher88.
Safety Agency Reporting on Atypical Femoral Fracture and Bisphosphonates
Australia’s ADRAC identified forty-four cases of femoral fractures associated with bisphosphonate use89. The Pharmacovigilance Working Party of the EMA’s Committee for Medicinal Products for Human Use (CHMP) initiated a class review on bisphosphonates and atypical femoral fractures in July 2008 following published reports and a label change requested by the Australian authorities. The CHMP recommended that the risk of atypical femoral fractures be added to the product information for alendronate as an association was evident. In 2008, Australia’s Therapeutic Goods Administration (TGA) mandated a label change for alendronate to include a warning about atypical femoral fractures47. The FDA issued a safety notification on March 10, 2010, and it deferred recommendations to the American Society for Bone and Mineral Research (ASBMR)90, whose recommendations were published in September 201038. The FDA subsequently announced label changes for bisphosphonates on October 13, 201091. Health Canada issued a warning on October 14, 201092. The time line for reporting of bisphosphonate-associated atypical femoral fractures is shown in Figure 3. The European Society of Clinical and Economic Aspects of Osteoporosis and the International Osteoporosis Foundation estimate the incidence at 1 per 1000 person-years93.
Discussion
To our knowledge, our study is the first to identify a safety signal between bisphosphonates and nonhealing femoral fractures within the FAERS database. In March 2010, the FDA had stated that it was not able to identify a safety signal involving bisphosphonates and atypical femoral fractures within this database90. In the published case series, 26% of atypical femoral fractures were reported to have delayed fracture-healing27,53,94-96. Some of the nonhealing femoral fractures in the FAERS database could well be atypical femoral fractures. Analyses of adverse events with use of the Naranjo criteria identified atypical femoral fractures as a possible or probable adverse reaction to bisphosphonate therapy. Although atypical femoral fractures were not evident in premarketing clinical trials12,97, such trials have limited subject numbers because they focus on efficacy, and they can thus identify only the most common adverse drug reactions. A median of seven years elapses between the time of drug approval and the time that notifications of serious adverse drug reactions are disseminated by pharmaceutical companies or the FDA98. Furthermore, Ioannidis and Lau showed that safety reporting in pharmaceutical clinical trials is largely inadequate99.
There are several policy implications related to our findings, including an expected negative impact on the bisphosphonate prescribing patterns of health-care providers, concerns about long-term safety of patients who use bisphosphonates, the need for evidence-based clinical protocols for long-term use of bisphosphonates, and increased medical liability. Negative reporting in the media about bisphosphonates resulted in a decrease in bisphosphonate use by 29,633 individuals in Australia, and this was projected to result in an estimated seventy hip fractures, sixty other fractures, and fourteen deaths8. It is feared that concerns about bisphosphonate safety and the attendant decrease in prescriptions and reimbursement for bisphosphonate use might erase the 30% reduction in hip fracture incidence in the U.S. that has been attributed to bisphosphonates5; these concerns are especially important given the aging of the population4.
Bisphosphonates are highly effective medications that increase bone mass and prevent fractures in individuals with osteoporosis. They prevent functional decline associated with fractures, prevent hospitalizations, and reduce disability, need for long-term care, and mortality5,100-104. Bisphosphonates are important in the treatment of cancer patients as they delay the onset of metastasis and reduce the risk of skeletal-related events, and they also palliate or control bone pain in multiple cancer types, thus preserving quality of life105. Preclinical studies suggest that bisphosphonates may have antitumor activity106,107. Zoledronic acid improves disease-free survival and overall survival, reduces the persistence of circulating and disseminated tumor cells, and decreases residual invasive tumor size in patients with early breast cancer and myeloma108. These data suggest that, in addition to providing benefits related to prevention of skeletal fractures, zolendronate may also potentially provide clinically meaningful benefits through anticancer activity109.
We highlight the timeline of safety notifications by the EMA and ADRAC in 2008 and Health Canada and the FDA in 201090. The ASBMR task force produced a case definition that will assist in future identification of cases (Table I)38. Following the occurrence of bisphosphonate-associated osteonecrosis of the jaw and atypical femoral fractures, clinicians appear to have begun using intermittent bisphosphonate therapy, in which treatment with a bisphosphonate for five years is followed by a “drug holiday” to minimize long-term drug exposure110. It is noteworthy that no specific clinical guidelines for drug holidays have been published.
Class-related toxicities of bisphosphonates are anticipated, as is the case with osteonecrosis of the jawx111. Therefore, greater pharmacovigilance involving bisphosphonates is required. Collaborative research partnerships among orthopaedic surgeons, bone and mineral metabolism clinicians and scientists, and pharmacovigilance teams are essential. Although the FAERS received reports of bisphosphonate toxicity as early as 1996, there is generally a substantial delay before information of this nature becomes publicly available. In contrast, investigators rapidly reported bisphosphonate-related case series in peer-reviewed specialty publications. This highlights the critical importance of the observant clinician in pharmacovigilance. Comprehensive reporting on a small number of cases can identify a safety signal14,113. Initiatives by the RADAR group have identified safety signals in a number of severe adverse drug reactions (pure red cell aplasia15,114, clopidogrel-associated thrombotic thrombocytopenic purpura115, and osteonecrosis of the jaw112) on the basis of less than 100 cases; conversely, pharmacovigilance by the FDA and pharmaceutical manufacturers focuses on mining large data sets.
The association of atypical femoral fractures with bisphosphonate use is evident on the basis of a number of criteria and is strengthening by the likelihood of causality116,117. The existence of this rare adverse drug reaction is also supported by the entire preclinical science regarding bisphosphonate in bone. Most of the reported cases have been attributed to alendronate, the most commonly prescribed and longest-marketed bisphosphonate. It is unlikely that this is due to greater exposure as the proportional reporting ratio takes into account the number of nonhealing femoral fractures reported and total number of reports associated with the drug compared with the number of such fractures reported and total number of reports associated with all other drugs. Therefore, the longer a drug is marketed and the greater its use, the larger the number of reports becomes in all boxes of the cross-tabulation analysis simultaneously.
Limitations of this study include the risk of reporting bias in the original case reports118 and case series, incomplete retrieval of identified research, the limited quality of the FAERS reports, and publication bias. Selective reporting within studies is also a limitation; for instance, we were not able to review radiographs, identify the duration of or compliance with bisphosphonates therapy, or have access to clinical data. Additionally, patient identifiers in these reports have been removed, which further limits the ability to eliminate redundancy. There may be reporting bias in reports from academic centers; however, there is little evidence of duplication in published cases from these sources. Limitations of the PRR and EBGM techniques stem from the voluntary nature of adverse drug reaction reporting to the FDA. It is estimated that <10% of adverse drug reactions are reported to MedWatch. The PRR or EBGM methodology represents an estimate of the comparative reporting rates for specific reactions to individual drugs, and these rates may be affected by external factors. Therefore, the PRR or EBGM results are not used as definitive evidence of the reaction, but rather as findings that must be combined with supporting information to serve as a signal that a direct relationship between the drug and reaction exists115.
In conclusion, nonhealing femoral fractures are associated with bisphosphonate use. These fractures may be related to atypical femoral fractures, a rare adverse reaction to prolonged bisphosphonate therapy. Hip fracture rates and consequent morbidity and mortality among older adults in the U.S. have declined by 30% coincident with the use of bisphosphonates1-5. The benefits of bisphosphonates are 100-fold greater than the risk of atypical femoral fractures. Further research in this area is needed, such as further evaluation of the incidence of this adverse drug reaction, the creation of an international registry for bisphosphonate-related atypical femoral fractures, the development of novel imaging technologies to identify impending fractures, the development of predictive clinical models for the development of atypical femoral fractures, and genetic studies to identify genetic variants associated with atypical femoral fractures. In the final analysis, a better understanding of the mechanisms leading to these atypical femoral fractures may enable us to develop prediction rules for this uncommon adverse drug reaction and to stratify and target our care accordingly.
Appendix
A table summarizing case reports of atypical femoral fractures and a more detailed description of pharmacovigilance analysis tools are available with the online version of this article as a data supplement at jbjs.org.
Supplementary Material
Disclosure of Potential Conflicts of Interest
A table summarizing case reports of atypical femoral fractures and a more detailed description of pharmacovigilance analysis tools
Acknowledgments
Note: The authors express their appreciation to Dr. Michael Whyte for his assistance in the development of this manuscript.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. Also, one or more of the authors has had another relationship, or has engaged in another activity, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Browner WS, Pressman AR, Nevitt MC, Cummings SR. Mortality following fractures in older women. The study of osteoporotic fractures. Arch Intern Med. 1996 Jul 22;156(14):1521-5 [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA, Odén A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jönsson B. Mortality after osteoporotic fractures. Osteoporos Int. 2004 Jan;15(1):38-42 Epub 2003 Oct 30 [DOI] [PubMed] [Google Scholar]
- 3.Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11(7):556-61 [DOI] [PubMed] [Google Scholar]
- 4.US Department of Health and Human Services Bone health and osteoporosis: a report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, Office of the Surgeon General; 2004 [Google Scholar]
- 5.Brauer CA, Coca-Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA. 2009 Oct 14;302(14):1573-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB. Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res. 2000 Apr;15(4):613-20 [DOI] [PubMed] [Google Scholar]
- 7.Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY. Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab. 2005 Mar;90(3):1294-301 Epub 2004 Dec 14 [DOI] [PubMed] [Google Scholar]
- 8.Sambrook PN, Chen JS, Simpson JM, March LM. Impact of adverse news media on prescriptions for osteoporosis: effect on fractures and mortality. Med J Aust. 2010 Aug 2;193(3):154-6 [DOI] [PubMed] [Google Scholar]
- 9.Fleisch H, Reszka A, Rodan G, Rogers M. Bisphosphonates: mechanisms of action. : Bilezikian JP, Raisz LG, Rodan GA, Principles of bone biology. 2nd ed; vol 2 San Diego: Academic Press; 2002. p 1361-85 [Google Scholar]
- 10.Gasser AB, Morgan DB, Fleisch HA, Richelle LJ. The influence of two diphosphonates on calcium metabolism in the rat. Clin Sci. 1972 Jul;43(1):31-45 [DOI] [PubMed] [Google Scholar]
- 11.Jamal SA, Dion N, Ste-Marie LG. Atypical femoral fractures and bone turnover. N Engl J Med. 2011 Sep 29;365(13):1261-2 [DOI] [PubMed] [Google Scholar]
- 12.Ste-Marie LG, Sod E, Johnson T, Chines A. Five years of treatment with risedronate and its effects on bone safety in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004 Dec;75(6):469-76 Epub 2004 Oct 14 [DOI] [PubMed] [Google Scholar]
- 13.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR; FLEX Research Group Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006 Dec 27;296(24):2927-38 [DOI] [PubMed] [Google Scholar]
- 14.Bennett CL, Nebeker JR, Lyons EA, Samore MH, Feldman MD, McKoy JM, Carson KR, Belknap SM, Trifilio SM, Schumock GT, Yarnold PR, Davidson CJ, Evens AM, Kuzel TM, Parada JP, Cournoyer D, West DP, Sartor O, Tallman MS, Raisch DW. The Research on Adverse Drug Events and Reports (RADAR) project. JAMA. 2005 May 4;293(17):2131-40 [DOI] [PubMed] [Google Scholar]
- 15.Bennett CL, Luminari S, Nissenson AR, Tallman MS, Klinge SA, McWilliams N, McKoy JM, Kim B, Lyons EA, Trifilio SM, Raisch DW, Evens AM, Kuzel TM, Schumock GT, Belknap SM, Locatelli F, Rossert J, Casadevall N. Pure red-cell aplasia and epoetin therapy. N Engl J Med. 2004 Sep 30;351(14):1403-8 [DOI] [PubMed] [Google Scholar]
- 16.Bennett CL, Nebeker JR, Yarnold PR, Tigue CC, Dorr DA, McKoy JM, Edwards BJ, Hurdle JF, West DP, Lau DT, Angelotta C, Weitzman SA, Belknap SM, Djulbegovic B, Tallman MS, Kuzel TM, Benson AB, Evens A, Trifilio SM, Courtney DM, Raisch DW. Evaluation of serious adverse drug reactions: a proactive pharmacovigilance program (RADAR) vs safety activities conducted by the Food and Drug Administration and pharmaceutical manufacturers. Arch Intern Med. 2007 May 28;167(10):1041-9 [DOI] [PubMed] [Google Scholar]
- 17.Bennett CL, Schumock GT, Desai AA, Kwaan HC, Raisch DW, Newlin R, Stadler W. Thalidomide-associated deep vein thrombosis and pulmonary embolism. Am J Med. 2002 Nov;113(7):603-6 [DOI] [PubMed] [Google Scholar]
- 18.Bennett CL, Tigue CC, Angelotta C, McKoy JM, Edwards BJ. Adverse effects of drugs used to treat hematologic malignancies: surveillance efforts from the Research on Adverse Drug events And Reports project. Semin Thromb Hemost. 2007 Jun;33(4):365-72 [DOI] [PubMed] [Google Scholar]
- 19.Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001 Oct-Nov;10(6):483-6 [DOI] [PubMed] [Google Scholar]
- 20.Hauben M, Zhou X. Quantitative methods in pharmacovigilance: focus on signal detection. Drug Saf. 2003;26(3):159-86 [DOI] [PubMed] [Google Scholar]
- 21.DuMouchel W, Smith ET, Beasley R, Nelson H, Yang X, Fram D, Almenoff JS. Association of asthma therapy and Churg-Strauss syndrome: an analysis of postmarketing surveillance data. Clin Ther. 2004 Jul;26(7):1092-1104 [DOI] [PubMed] [Google Scholar]
- 22.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981 Aug;30(2):239-45 [DOI] [PubMed] [Google Scholar]
- 23.Kelly WN. The quality of published adverse drug event reports. Ann Pharmacother. 2003 Dec;37(12):1774-8 [DOI] [PubMed] [Google Scholar]
- 24.Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS. Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br. 2007 Mar;89(3):349-53 [DOI] [PubMed] [Google Scholar]
- 25.Cheung RK, Leung KK, Lee KC, Chow TC. Sequential non-traumatic femoral shaft fractures in a patient on long-term alendronate. Hong Kong Med J. 2007 Dec;13(6):485-9 [PubMed] [Google Scholar]
- 26.Neviaser AS, Lane JM, Lenart BA, Edobor-Osula F, Lorich DG. Low-energy femoral shaft fractures associated with alendronate use. J Orthop Trauma. 2008 May-Jun;22(5):346-50 [DOI] [PubMed] [Google Scholar]
- 27.Koh JS, Goh SK, Png MA, Kwek EB, Howe TS. Femoral cortical stress lesions in long-term bisphosphonate therapy: a herald of impending fracture? J Orthop Trauma. 2010 Feb;24(2):75-81 [DOI] [PubMed] [Google Scholar]
- 28.Abrahamsen B, Eiken P, Eastell R. Subtrochanteric and diaphyseal femur fractures in patients treated with alendronate: a register-based national cohort study. J Bone Miner Res. 2009 Jun;24(6):1095-102 [DOI] [PubMed] [Google Scholar]
- 29.Schilcher J, Aspenberg P. Incidence of stress fractures of the femoral shaft in women treated with bisphosphonate. Acta Orthop. 2009 Aug;80(4):413-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schilcher J, Michaëlsson K, Aspenberg P. Bisphosphonate use and atypical fractures of the femoral shaft. N Engl J Med. 2011 May 5;364(18):1728-37 [DOI] [PubMed] [Google Scholar]
- 31.Kim SY, Schneeweiss S, Katz JN, Levin R, Solomon DH. Oral bisphosphonates and risk of subtrochanteric or diaphyseal femur fractures in a population-based cohort. J Bone Miner Res. 2011 May;26(5):993-1001 doi: 10.1002/jbmr.288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldstein AC, Black D, Perrin N, Rosales AG, Friess D, Boardman D, Dell R, Santora A, Chandler JM, Rix MM, Orwoll E. Incidence and demography of femur fractures with and without atypical features. J Bone Miner Res. 2012 May;27(5):977-86 doi: 10.1002/jbmr.1550 [DOI] [PubMed] [Google Scholar]
- 33.Wang Z, Bhattacharyya T. Trends in incidence of subtrochanteric fragility fractures and bisphosphonate use among the US elderly, 1996-2007. J Bone Miner Res. 2011 Mar;26(3):553-60 doi: 10.1002/jbmr.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Black DM, Kelly MP, Genant HK, Palermo L, Eastell R, Bucci-Rechtweg C, Cauley J, Leung PC, Boonen S, Santora A, de Papp A, Bauer DC; Fracture Intervention Trial Steering Committee; HORIZON Pivotal Fracture Trial Steering Committee Bisphosphonates and fractures of the subtrochanteric or diaphyseal femur. N Engl J Med. 2010 May 13;362(19):1761-71 Epub 2010 Mar 24 [DOI] [PubMed] [Google Scholar]
- 35.Isaacs JD, Shidiak L, Harris IA, Szomor ZL. Femoral insufficiency fractures associated with prolonged bisphosphonate therapy. Clin Orthop Relat Res. 2010 Dec;468(12):3384-92 Epub 2010 Aug 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.American Dental Association Council on Scientific Affairs Dental management of patients receiving oral bisphosphonate therapy: expert panel recommendations. J Am Dent Assoc. 2006 Aug;137(8):1144-50 [DOI] [PubMed] [Google Scholar]
- 37.Edwards BJ. Risedronate exposure 2001-2010. Chicago: Warner Chilcott Labs; 2010 [Google Scholar]
- 38.Shane E, Burr D, Ebeling PR, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster D, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Koval K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Sen HT, van der Meulen MC, Weinstein RS, Whyte M; American Society for Bone and Mineral Research Atypical subtrochanteric and diaphyseal femoral fractures: report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res. 2010 Nov;25(11):2267-94 [DOI] [PubMed] [Google Scholar]
- 39.Dell RM, Adams AL, Greene DF, Funahashi TT, Silverman SL, Eisemon EO, Zhou H, Burchette RJ, Ott SM. Incidence of all atypical nontraumatic diaphyseal fractures of the femur. J Bone Miner Res. 2012 Jul 26. doi: 10.1002/jbmr. 1719. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, van der Meulen MC, Lorich DG, Lane JM. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009 Aug;20(8):1353-62 Epub 2008 Dec 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vestergaard P, Schwartz F, Rejnmark L, Mosekilde L. Risk of femoral shaft and subtrochanteric fractures among users of bisphosphonates and raloxifene. Osteoporos Int. 2011 Mar;22(3):993-1001 Epub 2010 Dec 17 [DOI] [PubMed] [Google Scholar]
- 42.Shakir SA, Layton D. Causal association in pharmacovigilance and pharmacoepidemiology: thoughts on the application of the Austin Bradford-Hill criteria. Drug Saf. 2002;25(6):467-71 [DOI] [PubMed] [Google Scholar]
- 43.Tan SC, Koh SB, Goh SK, Howe TS. Atypical femoral stress fractures in bisphosphonate-free patients. Osteoporos Int. 2011 Jul;22(7):2211-2 Epub 2010 Sep 14 [DOI] [PubMed] [Google Scholar]
- 44.Boskey AL, DiCarlo E, Paschalis E, West P, Mendelsohn R. Comparison of mineral quality and quantity in iliac crest biopsies from high- and low-turnover osteoporosis: an FT-IR microspectroscopic investigation. Osteoporos Int. 2005 Dec;16(12):2031-8 Epub 2005 Aug 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boskey AL, Marks SC., Jr Mineral and matrix alterations in the bones of incisors-absent (ia/ia) osteopetrotic rats. Calcif Tissue Int. 1985 May;37(3):287-92 [DOI] [PubMed] [Google Scholar]
- 46.Boskey AL, Wright TM, Blank RD. Collagen and bone strength. J Bone Miner Res. 1999 Mar;14(3):330-5 [DOI] [PubMed] [Google Scholar]
- 47.European Medicines Agency. Assessment report for Fosavance. 2009 Feb 19. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000619/WC500024252.pdf. Accessed 2010 Nov 20.
- 48.Whyte MP, McAlister WH, Novack DV, Clements KL, Schoenecker PL, Wenkert D. Bisphosphonate-induced osteopetrosis: novel bone modeling defects, metaphyseal osteopenia, and osteosclerosis fractures after drug exposure ceases. J Bone Miner Res. 2008 Oct;23(10):1698-707 [DOI] [PubMed] [Google Scholar]
- 49.Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003 Jul 31;349(5):457-63 [DOI] [PubMed] [Google Scholar]
- 50.Yang KH, Won JH, Yoon HK, Ryu JH, Choo KS, Kim JS. High concentrations of pamidronate in bone weaken the mechanical properties of intact femora in a rat model. Yonsei Med J. 2007 Aug 31;48(4):653-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mashiba T, Mori S, Burr DB, Komatsubara S, Cao Y, Manabe T, Norimatsu H. The effects of suppressed bone remodeling by bisphosphonates on microdamage accumulation and degree of mineralization in the cortical bone of dog rib. J Bone Miner Metab. 2005;23 Suppl:36-42 [DOI] [PubMed] [Google Scholar]
- 52.Boivin G, Meunie PJ. Changes in bone remodeling rate influence the degree of mineralization of bone which is a determinant of bone strength: therapeutic implications. Adv Exp Med Biol. 2001;496:123-7 [DOI] [PubMed] [Google Scholar]
- 53.Somford MP, Draijer FW, Thomassen BJ, Chavassieux PM, Boivin G, Papapoulos SE. Bilateral fractures of the femur diaphysis in a patient with rheumatoid arthritis on long-term treatment with alendronate: clues to the mechanism of increased bone fragility. J Bone Miner Res. 2009 Oct;24(10):1736-40 [DOI] [PubMed] [Google Scholar]
- 54.Benford HL, McGowan NW, Helfrich MH, Nuttall ME, Rogers MJ. Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone. 2001 May;28(5):465-73 [DOI] [PubMed] [Google Scholar]
- 55.Idris AI, Rojas J, Greig IR, Van’t Hof RJ, Ralston SH. Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int. 2008 Mar;82(3):191-201 Epub 2008 Feb 8 [DOI] [PubMed] [Google Scholar]
- 56.Allen MR, Gineyts E, Leeming DJ, Burr DB, Delmas PD. Bisphosphonates alter trabecular bone collagen cross-linking and isomerization in beagle dog vertebra. Osteoporos Int. 2008 Mar;19(3):329-37 Epub 2007 Dec 18 [DOI] [PubMed] [Google Scholar]
- 57.Gourion-Arsiquaud S, Allen MR, Burr DB, Vashishth D, Tang SY, Boskey AL. Bisphosphonate treatment modifies canine bone mineral and matrix properties and their heterogeneity. Bone. 2010 Mar;46(3):666-72 Epub 2009 Nov 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Viguet-Carrin S, Garnero P, Delmas PD. The role of collagen in bone strength. Osteoporos Int. 2006;17(3):319-36 Epub 2005 Dec 9 [DOI] [PubMed] [Google Scholar]
- 59.Burr DB. The contribution of the organic matrix to bone’s material properties. Bone. 2002 Jul;31(1):8-11 [DOI] [PubMed] [Google Scholar]
- 60.Yamauchi M, Young DR, Chandler GS, Mechanic GL. Cross-linking and new bone collagen synthesis in immobilized and recovering primate osteoporosis. Bone. 1988;9(6):415-8 [DOI] [PubMed] [Google Scholar]
- 61.Yamauchi M, Woodley DT, Mechanic GL. Aging and cross-linking of skin collagen. Biochem Biophys Res Commun. 1988 Apr 29;152(2):898-903 [DOI] [PubMed] [Google Scholar]
- 62.Wu P, Koharski C, Nonnenmann H, Vashishth D. Loading on non-enzymatically glycated and damaged bone results in an instantaneous fracture. Trans Orthop Res Soc. 2003;28:404 [Google Scholar]
- 63.Gourion-Arsiquaud S, Faibish D, Myers E, Spevak L, Compston J, Hodsman A, Shane E, Recker RR, Boskey ER, Boskey AL. Use of FTIR spectroscopic imaging to identify parameters associated with fragility fracture. J Bone Miner Res. 2009 Sep;24(9):1565-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Li X, Shen X, Agrawal CM. Age-related changes of noncalcified collagen in human cortical bone. Ann Biomed Eng. 2003 Dec;31(11):1365-71 [DOI] [PubMed] [Google Scholar]
- 65.Compston J. Pathophysiology of atypical femoral fractures and osteonecrosis of the jaw. Osteoporos Int. 2011 Dec;22(12):2951-61 Epub 2011 Oct 14. Erratum in: Osteoporos Int. 2012 Feb;23(2):793 [DOI] [PubMed] [Google Scholar]
- 66.Burr DB. Bone material properties and mineral matrix contributions to fracture risk or age in women and men. J Musculoskelet Neuronal Interact. 2002 Mar;2(3):201-4 [PubMed] [Google Scholar]
- 67.Boskey AL, Spevak L, Weinstein RS. Spectroscopic markers of bone quality in alendronate-treated postmenopausal women. Osteoporos Int. 2009 May;20(5):793-800 Epub 2008 Sep 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gladnick BP, Donnelly E, Lorich DG, Unnanuntana A, DiCarlo EF, Doty F, Boskey AL, Lane JM, Kane SM, Wolinsky PR. The effects of long-term bisphosphonate use on bone quality. Read at the Annual Meeting of the American Academy of Orthopaedic Surgeons; 2010 Mar 9-13; New Orleans, LA. [Google Scholar]
- 69.Geusens P. Bisphosphonates for postmenopausal osteoporosis: determining duration of treatment. Curr Osteoporos Rep. 2009 Mar;7(1):12-7 [DOI] [PubMed] [Google Scholar]
- 70.Renders GA, Mulder L, Langenbach GE, van Ruijven LJ, van Eijden TM. Biomechanical effect of mineral heterogeneity in trabecular bone. J Biomech. 2008 Sep 18;41(13):2793-8 Epub 2008 Aug 22 [DOI] [PubMed] [Google Scholar]
- 71.Keaveny TM, Hayes WC. A 20-year perspective on the mechanical properties of trabecular bone. J Biomech Eng. 1993 Nov;115(4B):534-42 [DOI] [PubMed] [Google Scholar]
- 72.Calton EF, Macleay J, Boskey AL. Fourier transform infrared imaging analysis of cancellous bone in alendronate- and raloxifene-treated osteopenic sheep. Cells Tissues Organs. 2011;194(2-4):302-6 Epub 2011 May 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Whyte MP. Hypophosphatasia. : Scriver CR, Beaudet AL, Valle D, Sly WS, The metabolic & molecular bases of inherited disease. 8th ed New York: McGraw-Hill; 2001. p 5313-29 [Google Scholar]
- 74.Whyte MP. Hypophosphatasia: nature’s window on alkaline phosphatase function in humans. : Bilezikian JP, Raisz LG, Martin TJ, Principles of bone biology. 3rd ed San Diego, CA: Academic Press; 2008. p 1573-98 [Google Scholar]
- 75.Whyte MP. Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res. 2009 Jun;24(6):1132-4 [DOI] [PubMed] [Google Scholar]
- 76.Sama AA, Khan SN, Myers ER, Huang RC, Cammisa FP, Jr, Sandhu HS, Lane JM. High-dose alendronate uncouples osteoclast and osteoblast function: a study in a rat spine pseudarthrosis model. Clin Orthop Relat Res. 2004 Aug;(425):135-42 [DOI] [PubMed] [Google Scholar]
- 77.Cao Y, Mori S, Mashiba T, Westmore MS, Ma L, Sato M, Akiyama T, Shi L, Komatsubara S, Miyamoto K, Norimatsu H. Raloxifene, estrogen, and alendronate affect the processes of fracture repair differently in ovariectomized rats. J Bone Miner Res. 2002 Dec;17(12):2237-46 [DOI] [PubMed] [Google Scholar]
- 78.Saito M, Shiraishi A, Ito M, Sakai S, Hayakawa N, Mihara M, Marumo K. Comparison of effects of alfacalcidol and alendronate on mechanical properties and bone collagen cross-links of callus in the fracture repair rat model. Bone. 2010 Apr;46(4):1170-9 Epub 2009 Dec 22 [DOI] [PubMed] [Google Scholar]
- 79.Kidd LJ, Cowling NR, Wu AC, Kelly WL, Forwood MR. Bisphosphonate treatment delays stress fracture remodeling in the rat ulna. J Orthop Res. 2011 Dec;29(12):1827-33 doi: 10.1002/jor.21464. Epub 2011 May 19 [DOI] [PubMed] [Google Scholar]
- 80.McDonald MM, Dulai S, Godfrey C, Amanat N, Sztynda T, Little DG. Bolus or weekly zoledronic acid administration does not delay endochondral fracture repair but weekly dosing enhances delays in hard callus remodeling. Bone. 2008 Oct;43(4):653-62 Epub 2008 Jun 3 [DOI] [PubMed] [Google Scholar]
- 81.Shimizu E, Tamasi J, Partridge NC. Alendronate affects osteoblast functions by crosstalk through EphrinB1-EphB. J Dent Res. 2012 Mar;91(3):268-74 Epub 2011 Dec 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peter CP, Cook WO, Nunamaker DM, Provost MT, Seedor JG, Rodan GA. Effect of alendronate on fracture healing and bone remodeling in dogs. J Orthop Res. 1996 Jan;14(1):74-9 [DOI] [PubMed] [Google Scholar]
- 83.Rozental TD, Vazquez MA, Chacko AT, Ayogu N, Bouxsein ML. Comparison of radiographic fracture healing in the distal radius for patients on and off bisphosphonate therapy. J Hand Surg Am. 2009 Apr;34(4):595-602 [DOI] [PubMed] [Google Scholar]
- 84.Gerstenfeld LC, Sacks DJ, Pelis M, Mason ZD, Graves DT, Barrero M, Ominsky MS, Kostenuik PJ, Morgan EF, Einhorn TA. Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res. 2009 Feb;24(2):196-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nagahama K, Kanayama M, Togawa D, Hashimoto T, Minami A. Does alendronate disturb the healing process of posterior lumbar interbody fusion? A prospective randomized trial. J Neurosurg Spine. 2011 Apr;14(4):500-7 Epub 2011 Jan 28 [DOI] [PubMed] [Google Scholar]
- 86.Ha YC, Cho MR, Park KH, Kim SY, Koo KH. Is surgery necessary for femoral insufficiency fractures after long-term bisphosphonate therapy? Clin Orthop Relat Res. 2010 Dec;468(12):3393-8 Epub 2010 Sep 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weil YA, Rivkin G, Safran O, Liebergall M, Foldes AJ. The outcome of surgically treated femur fractures associated with long-term bisphosphonate use. J Trauma. 2011 Jul;71(1):186-90 [DOI] [PubMed] [Google Scholar]
- 88.Lane J. Subtrochanteric fractures: healing characteristics. In: Edwards BJ, editor. Chicago: ;2010. p [Google Scholar]
- 89.Adverse Drug Advisory Committee (ADRAC) Bisphosphonate and fractures medicines summary. 2010 Oct 26 [Google Scholar]
- 90.US Food and Drug Administration FDA Drug Safety Communication: Ongoing safety review of oral bisphosphonates and atypical subtrochanteric femur fractures. 2010 Mar 10. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203891.htm. Accessed 2010 Mar 11 [Google Scholar]
- 91.US Food and Drug Administration Bisphosphonates (osteoporosis drugs): label change—atypical fractures update. 2010 Oct 13. http://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm229244.htm. Accessed 2010 Oct 19 [Google Scholar]
- 92.Health Canada Safety review of bisphosphonate drugs and the possible risk of rare but serious thigh bone fractures. 2010 Oct 14. http://www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/_2010/2010_175-eng.php. Accessed 2010 Oct 19 [Google Scholar]
- 93.Rizzoli R, Akesson K, Bouxsein M, Kanis JA, Napoli N, Papapoulos S, Reginster JY, Cooper C. Subtrochanteric fractures after long-term treatment with bisphosphonates: a European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int. 2011 Feb;22(2):373-90 Epub 2010 Nov 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ing-Lorenzini K, Desmeules J, Plachta O, Suva D, Dayer P, Peter R. Low-energy femoral fractures associated with the long-term use of bisphosphonates: a case series from a Swiss university hospital. Drug Saf. 2009;32(9):775-85 doi: 10.2165/00002018-200932090-00002 [DOI] [PubMed] [Google Scholar]
- 95.Armamento-Villareal R, Napoli N, Panwar V, Novack D. Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med. 2006 Nov 9;355(19):2048-50 [DOI] [PubMed] [Google Scholar]
- 96.Visekruna M, Wilson D, McKiernan FE. Severely suppressed bone turnover and atypical skeletal fragility. J Clin Endocrinol Metab. 2008 Aug;93(8):2948-52 Epub 2008 Jun 3 [DOI] [PubMed] [Google Scholar]
- 97.Black DM, Thompson DE, Bauer DC, Ensrud K, Musliner T, Hochberg MC, Nevitt MC, Suryawanshi S, Cummings SR; Fracture Intervention Trial Fracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab. 2000 Nov;85(11):4118-24 [DOI] [PubMed] [Google Scholar]
- 98.Lasser KE, Allen PD, Woolhandler SJ, Himmelstein DU, Wolfe SM, Bor DH. Timing of new black box warnings and withdrawals for prescription medications. JAMA. 2002 May 1;287(17):2215-20 [DOI] [PubMed] [Google Scholar]
- 99.Ioannidis JP, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA. 2001 Jan 24-31;285(4):437-43 [DOI] [PubMed] [Google Scholar]
- 100.Juby AG, De Geus-Wenceslau CM. Evaluation of osteoporosis treatment in seniors after hip fracture. Osteoporos Int. 2002 Mar;13(3):205-10 [DOI] [PubMed] [Google Scholar]
- 101.Kennedy CC, Papaioannou A, Adachi JD. Treating osteoporosis: economic aspects of bisphosphonate therapy. Expert Opin Pharmacother. 2006 Aug;7(11):1457-67 [DOI] [PubMed] [Google Scholar]
- 102.Center JR, Bliuc D, Nguyen ND, Nguyen TV, Eisman JA. Osteoporosis medication and reduced mortality risk in elderly women and men. J Clin Endocrinol Metab. 2011 Apr;96(4):1006-14 Epub 2011 Feb 2 [DOI] [PubMed] [Google Scholar]
- 103.Beaupre LA, Morrish DW, Hanley DA, Maksymowych WP, Bell NR, Juby AG, Majumdar SR. Oral bisphosphonates are associated with reduced mortality after hip fracture. Osteoporos Int. 2011 Mar;22(3):983-91 Epub 2010 Nov 4 [DOI] [PubMed] [Google Scholar]
- 104.Cree MW, Juby AG, Carriere KC. Mortality and morbidity associated with osteoporosis drug treatment following hip fracture. Osteoporos Int. 2003 Sep;14(9):722-7 Epub 2003 Aug 7 [DOI] [PubMed] [Google Scholar]
- 105.Saad F, Lipton A. Clinical benefits and considerations of bisphosphonate treatment in metastatic bone disease. Semin Oncol. 2007 Dec;34(6 Suppl 4):S17-23 [DOI] [PubMed] [Google Scholar]
- 106.Coleman R. On the horizon: can bisphosphonates prevent bone metastases? Breast. 2007 Dec;16 Suppl 3:S21-7 Epub 2007 Nov 7 [DOI] [PubMed] [Google Scholar]
- 107.Lipton A. Improving progression-free and overall survival in patients with cancer: a potential role for bisphosphonates. Expert Opin Pharmacother. 2011 Apr;12(5):749-62 Epub 2011 Jan 20 [DOI] [PubMed] [Google Scholar]
- 108.Lipton A. Zoledronic acid: multiplicity of use across the cancer continuum. Expert Rev Anticancer Ther. 2011 Jul;11(7):999-1012 [DOI] [PubMed] [Google Scholar]
- 109.Costa L, Harper P, Coleman RE, Lipton A. Anticancer evidence for zoledronic acid across the cancer continuum. Crit Rev Oncol Hematol. 2011 Feb;77 Suppl 1:S31-7 [DOI] [PubMed] [Google Scholar]
- 110.Wilkinson JM, Little DG. Bisphosphonates in orthopedic applications. Bone. 2011 Jul;49(1):95-102 Epub 2011 Jan 20 [DOI] [PubMed] [Google Scholar]
- 111.Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. Clin Endocrinol Metab. 2010 Apr;95(4):1555-65 Epub 2010 Feb 19 [DOI] [PubMed] [Google Scholar]
- 112.Edwards BJ, Gounder M, McKoy JM, Boyd I, Farrugia M, Migliorati C, Marx R, Ruggiero S, Dimopoulos M, Raisch DW, Singhal S, Carson K, Obadina E, Trifilio S, West D, Mehta J, Bennett CL. Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol. 2008 Dec;9(12):1166-72 [DOI] [PubMed] [Google Scholar]
- 113.Fisher MJ, Bennett CL, Brown J. Lack of coordinated pharmacovigilance adversely affects veteran care. 2009 National meeting of the Health Services Research and Development Service, Veterans Administration; 2009 May 1-3; Chicago, IL. Paper no. 3005. [Google Scholar]
- 114.McKoy JM, Stonecash RE, Cournoyer D, Rossert J, Nissenson AR, Raisch DW, Casadevall N, Bennett CL. Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion. 2008 Aug;48(8):1754-62 Epub 2008 May 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bennett CL, Connors JM, Carwile JM, Moake JL, Bell WR, Tarantolo SR, McCarthy LJ, Sarode R, Hatfield AJ, Feldman MD, Davidson CJ, Tsai HM. Thrombotic thrombocytopenic purpura associated with clopidogrel. N Engl J Med. 2000 Jun 15;342(24):1773-7 [DOI] [PubMed] [Google Scholar]
- 116.Russell RG, Croucher PI, Rogers MJ. Bisphosphonates: pharmacology, mechanisms of action and clinical uses. Osteoporos Int. 1999;9 Suppl 2:S66-80 [DOI] [PubMed] [Google Scholar]
- 117.Rodan GA, Reszka AA. Bisphosphonate mechanism of action. Curr Mol Med. 2002 Sep;2(6):571-7 [DOI] [PubMed] [Google Scholar]
- 118.Hazell L, Shakir SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf. 2006;29(5):385-96 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest
A table summarizing case reports of atypical femoral fractures and a more detailed description of pharmacovigilance analysis tools


