Abstract
Background:
Use of recombinant human bone morphogenetic protein-2 (rhBMP-2) is expensive and may cause local side effects. A small synthetic molecule, SVAK-12, has recently been shown in vitro to potentiate rhBMP-2-induced transdifferentiation of myoblasts into the osteoblastic phenotype. The aims of this study were to test the ability of SVAK-12 to enhance bone formation in a rodent ectopic model and to test whether a single percutaneous injection of SVAK-12 can accelerate callus formation in a rodent femoral fracture model.
Methods:
Collagen disks with rhBMP-2 alone or with rhBMP-2 and SVAK-12 were implanted in a standard athymic rat chest ectopic model, and radiographic analysis was performed at four weeks. In a second set of rats (Sprague-Dawley), SVAK-12 was percutaneously injected into the site of a closed femoral fracture. The fractures were analyzed radiographically and biomechanically (with torsional testing) five weeks after surgery.
Results:
In the ectopic model, there was dose-dependent enhancement of rhBMP-2 activity with use of SVAK-12 at doses of 100 to 500 μg. In the fracture model, the SVAK-12-treated group had significantly higher radiographic healing scores than the untreated group (p = 0.028). Biomechanical testing revealed that the fractured femora in the 200 to 250-μg SVAK-12 group were 43% stronger (p = 0.008) and 93% stiffer (p = 0.014) than those in the control group. In summary, at five weeks the femoral fracture group injected with SVAK-12 showed significantly improved radiographic and biomechanical evidence of healing compared with the controls.
Conclusions:
A single local dose of a low-molecular-weight compound, SVAK-12, enhanced bone-healing in the presence of low-dose exogenous rhBMP-2 (in the ectopic model) and endogenous rhBMPs (in the femoral fracture model).
Clinical Relevance:
This study demonstrates that rhBMP-2 responsiveness can be enhanced by a novel small molecule, SVAK-12. Local application of anabolic small molecules has the potential for potentiating and accelerating fracture-healing. Use of this small molecule to lower required doses of rhBMPs might both decrease their cost and improve their safety profile.
The clinical use of recombinant human bone morphogenetic proteins (rhBMPs) to enhance bone-healing has been limited by the relatively high cost of recombinant protein and the local side effects. A synthetic small molecule that could potentiate the activity of rhBMPs would be highly desirable because it could lower the cost and potentially reduce the side effects. We initiated a program to design and test small molecules that would enhance rhBMP responsiveness by interrupting the function of Smad ubiquitin regulatory factor 1 (Smurf1), a key inhibitory molecule of the canonical Smad intracellular rhBMP signaling pathway. We have recently shown that one such molecule, SVAK-12, potentiates rhBMP-2-induced transdifferentiation of pleuripotent myoblasts into the osteoblastic phenotype1. This low-molecular-weight synthetic compound was selected from a group of compounds as having the best activity as assessed with a series of validated cell-based assay systems2. However, SVAK-12 has not been tested in vivo. Therefore, the purpose of this study was (1) to determine whether SVAK-12 could enhance a suboptimal dose of exogenous rhBMP-2 to form ectopic bone in a rodent chest implant model, and (2) to test whether a single percutaneous injection of SVAK-12 could enhance healing induced by endogenous rhBMPs in a rodent femoral fracture model. We have applied for a patent on SVAK-12. There has been no revenue or licensing to date.
Materials and Methods
SVAK-12 Synthetic Compound
SVAK-12 is a small 137.14-Da synthetic compound designed to specifically interact with the Smurf1-WW2 domain and block the binding of natural targets of Smurf1. Its chemical name is 2-Vinyl-4,6-diamino-1,3,5-triazine. It has been shown in vitro to potentiate the activity of rhBMP-2 and to promote the rhBMP-induced expression of phenotypic markers characteristic of a differentiated osteoblast1. Computational pharmacoinformatic analysis (Accelrys, San Diego, California) of SVAK-12 indicates that it has desirable pharmacokinetic and pharmacodynamic properties for use as a therapeutic agent at the doses tested.
Experiment 1: Ectopic Bone Formation Model
All animal procedures were approved by the local Institutional Animal Care and Use Committee. The SVAK-12 compound was first tested in a standard athymic rat chest ectopic-bone-formation model with use of a dose of rhBMP-2 that had been established to be inadequate to induce bone formation consistently3. rhBMP-2 with or without SVAK-12 was loaded with use of a pipette onto sterile bovine Type-I collagen disks (8 mm in diameter and 3 mm thick; Kensey Nash, Exton, Pennsylvania) in a biosafety cabinet. The disks were then transported in a sterile container to the surgical operating room. Each implant was loaded with a total volume of 100-μL solution containing 1.5 μg of rhBMP-2 (Medtronic, Minneapolis, Minnesota) alone or combined with 0, 25, 50, 100, 250, 500, 750, or 1000 μg of SVAK-12 solubilized in the organic solvent dimethyl sulfoxide (DMSO, 10%; Sigma-Aldrich, St. Louis, Missouri). A total of sixty-four disks were implanted. Eight disks were implanted at all doses except for the no-vehicle control and the 1000-μg dose (n = 4 for each). In a pilot experiment, 10% to 100% DMSO was determined to have no effect on rhBMP-2-induced ectopic bone formation (data not shown).
Male athymic nude five to six-week-old rats (Harlan Laboratories, Indianapolis, Indiana) were anesthetized with 1% to 2% isoflurane mixed with oxygen at a flow rate of 0.5 to 1 L/min and maintained during surgery with the same dose. Surgery was performed with the animal positioned supine on a circulating-water heating pad. Four 1-cm transverse incisions were made about 3 cm apart on the chest of each rat, and subcutaneous pockets were created by blunt dissection with scissors. The implants were inserted into the pockets, and closure was accomplished with closely spaced interrupted absorbable polyglactin-910 sutures (Vicryl; Ethicon, Johnson & Johnson, Somerville, New Jersey).
The rats were housed in autoclaved cages (two per cage) that had a microisolator top and contained autoclaved bedding, and they were given autoclaved food and water ad libitum. Cages were changed in a biosafety cabinet. All of the rats fed well after the surgery. There were no postoperative complications associated with the surgical procedure.
The rats were killed four weeks postoperatively. The implants were harvested and were evaluated with manual palpation, high-resolution digital radiography, and nondecalcified histological analysis.
Radiographic Analysis
The amount of bone in each implant was assigned a semiquantitative score by a blinded observer on the basis of the volume of the implant that was mineralized on radiographs (0 = no bone, 1 = less than 25% of the implant containing bone, 2 = 25% to 49% of the implant containing bone, 3 = 50% to 74% of the implant containing bone, 4 = 75% to 99% of the implant containing bone, and 5 = 100% or more of the original implant size containing bone).
Histological Analysis
The collagen disks were fixed in 10% formalin and embedded in methylmethacrylate (Sigma-Aldrich) with use of standard procedures recommended by the manufacturer. Nondecalcified sections (5 μm) were cut on a microtome. Staining was performed with Gomori one-step trichrome (Richard-Allan Scientific, Kalamazoo, Michigan).
Statistical Analysis
The significance of differences among the mean radiographic bone scores associated with the various treatment doses was calculated with a one-way analysis of variance (ANOVA) with the Holm-Sidak post hoc multiple comparison test. A p value of <0.05 was considered significant.
Experiment 2: Femoral Fracture Model
A standard drop-weight closed femoral fracture model with intramedullary fixation was used4. This model, which results in a predictable and reproducible closed transverse fracture of the femur, has been used extensively to study the stages of fracture-healing. Forty-eight male Sprague-Dawley rats (Charles River, Wilmington, Massachusetts) were randomized into three groups (at a ratio of 1:2:2): an untreated fracture group (n = 10), a vehicle-injected group (n = 19), and a SVAK-12-injected group (n = 19). The contralateral femora were not fractured. The rats were anesthetized with 3% isoflurane induction followed by a 2% maintenance dose. The hindlimbs of the rats were then shaved, prepared, and draped in sterile fashion. Bupivacaine (0.25%; Hospira, Lake Forest, Illinois) was infiltrated locally at the incision site. A medial parapatellar incision was made, and the patella was reflected laterally. A 20-gauge stainless-steel hypodermic needle was inserted through the femoral notch into the intramedullary canal via a retrograde approach. An intramedullary Kirschner wire (0.45 mm; Synthes, West Chester, Pennsylvania) was cut to the appropriate length and introduced into the femoral canal. The skin incision was closed with staples. A closed femoral fracture was then created with a standard fracture apparatus by dropping a weight of 300 g from a height of 30 cm onto the stabilized femur4. Radiographs were made immediately after the fractures were created, and only rats with a transverse midshaft fracture with an appropriately positioned intramedullary wire were included in the study. Postoperatively, the rats were allowed to bear weight as tolerated. Subcutaneous buprenorphine (0.05 mg/kg) was administered every eight hours for three days postoperatively for pain control.
Randomization and Percutaneous Injection of SVAK-12
All fractures were injected with a 50-μL Hamilton syringe (Hamilton Company, Reno, Nevada) with a 50-mm 22-gauge needle. The percutaneous injection was performed twenty-four hours after creation of the closed fracture to avoid disruption of the fracture hematoma. The location of the fracture was determined on immediate postoperative radiographs and by palpation of the fracture site with the needle tip at the time of the percutaneous injection. A total of 25 μL was injected into five different regions of the fracture (5 μL inside the fracture site as well as the anterior, posterior, lateral, and medial aspects of the site). Before the injections were administered to the actual study group, trial injections were performed with use of methylene blue to ensure that the fracture site was correctly targeted.
Rats with an acceptable fracture and wire position were randomly assigned to have the left femur (fractured side) remain untreated (n = 10) or be injected with the vehicle (10% to 100% DMSO) used to dissolve SVAK-12 (n = 19), with SVAK-12 at a dose of 200 μg (n = 9), or with SVAK-12 at a dose of 250 μg (n = 10). (There was no difference between the 200 and 250-μg-dose SVAK-12 groups, so we combined them.) The SVAK-12 dose was chosen on the basis of the peak activity in the ectopic experiments. DMSO at concentrations of 10% to 100% was determined to have no effect on fracture-healing in a pilot experiment (data not shown). All of the rats were killed with use of CO2 five weeks after the surgery.
Radiographic Analysis
The femora were harvested and, after removal of soft tissues, radiographs were obtained in the anteroposterior orientation with use of a high-resolution digital radiography system (Faxitron MX-20 with DC-2 option; Faxitron Bioptics, Tucson, Arizona) at an exposure of 24 kV for three seconds. A radiographic healing score was utilized by four observers to independently grade the fracture repair. The three parameters of the scoring system were gap size, bone in the gap, and mineralized callus, each of which was assigned a score of 0, 1, or 2. The composite score ranged from 0 to 6.
Biomechanical Analysis
Both the right and the left femur of nine to twelve rats per group (ten with an untreated fracture, nine with a vehicle-injected fracture, and twelve with a SVAK-12-treated fracture) were harvested for destructive torsional testing. The intramedullary Kirschner wire was removed, and the femora were frozen at −20°C until the time of testing. The harvested femora were slowly thawed to room temperature, and the ends of the femora were then embedded in Cerrobend alloy (Bolton Metal Products, Bellafonte, Pennsylvania). The ends were mounted in the grips of a material testing machine (MTS 858 Mini-Bionix, Eden Prairie, Minnesota) and loaded to failure at a rotation of 1°/sec. Torsional stiffness (Nmm/deg) was calculated from the slope of the torque against the angular-displacement curve obtained from the X-Y plotter. The ultimate torsional strength (Nmm) was obtained from the digital read-out. A two-factor analysis of variance was used to evaluate the effect of SVAK-12 on fracture repair. The biomechanical properties of the healing fractures were either compared directly or expressed as a percentage of the unfractured control femur (right side).
Histological Analysis of Femoral Callus
The femoral specimens were labeled, fixed in 10% buffered formalin for five days, processed through graded alcohols and xylene, and embedded longitudinally in methylmethacrylate. Nondecalcified sections were cut on an EXAKT-300 Microgrinding System (Appartebau, Gauting, Germany), ground to a 50-μm thickness, and stained with methylene blue/acid fuchsin (Sigma-Aldrich).
Statistical Analyses
Statistical analysis was performed with use of Sigma Plot 11 (Systat Software, San Jose, California). One-way ANOVA with Holm-Sidak post hoc multiple comparison testing was used to determine the significance of differences among the fracture treatment groups (SVAK-12, vehicle, and untreated). When the means of only two groups were compared, significance was calculated with use of the Student t test. A p value of <0.05 was considered significant.
Source of Funding
This study was funded by the Atlanta Research and Education Foundation.
Results
Experiment 1: Ectopic Bone Formation Model
Radiographic Analysis
Our published in vitro data based on the use of C2C12 cells suggested that SVAK-12 enhanced rhBMP-2-induced osteoblast differentiation2. To extend those observations in vivo, we first determined whether SVAK-12 increased ectopic bone formation induced by a suboptimal dose of rhBMP-2 in a rat model of ectopic bone formation. Increasing doses of SVAK-12 ranging from 100 to 500 μg in the presence of 1.5 μg of rhBMP-2 resulted in an increase in ectopic bone formation. The enhancement of ectopic bone formation in the 100, 250, and 500-μg groups was significantly greater than that in the vehicle group, as evidenced by the significantly improved mean bone scores (p < 0.02) (Fig. 1). This experiment demonstrated for the first time that addition of SVAK-12 enhances bone formation induced by rhBMP-2, as determined by radiographic analysis.
Fig. 1.
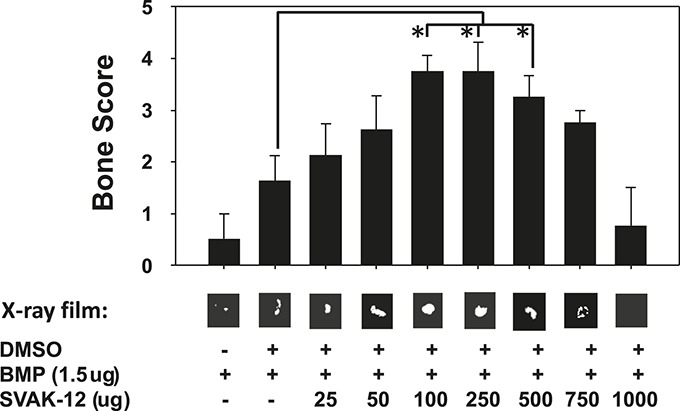
Ectopic bone induction at four weeks by SVAK-12. There was a dose-dependent increase in the rhBMP-2-induced bone formation as evidenced by the increasing mean bone scores with increasing doses of SVAK-12. Significant differences compared with implants treated with the vehicle (DMSO)—i.e., the control group—were found at the 100, 250, and 500-μg doses of SVAK-12 (*p < 0.02). Typical radiographs of the ectopic ossicles in the various treatment groups are shown below their respective bone scores. The radiopaque areas of the SVAK-12-treated groups (25, 50, 100, 250, 500, and 750 μg/100 μL) were larger and more dense than those in the control group.
Histological Analysis
We wanted to confirm that the radiopaque images observed in the radiographic analysis represented normal bone. Histological sections of the implants showed the characteristics of normal bone with trabeculae and hematopoietic marrow in the intertrabecular space. There was also unmineralized osteoid adjacent to osteoblasts confirming that the bone was deposited by live cells and not merely by chemical calcification. A representative section of bone formation in a collagen disk is shown in Figure 2. This analysis confirmed that the enhanced ossification visualized with radiographic analysis had normal histological characteristics of immature bone.
Fig. 2.
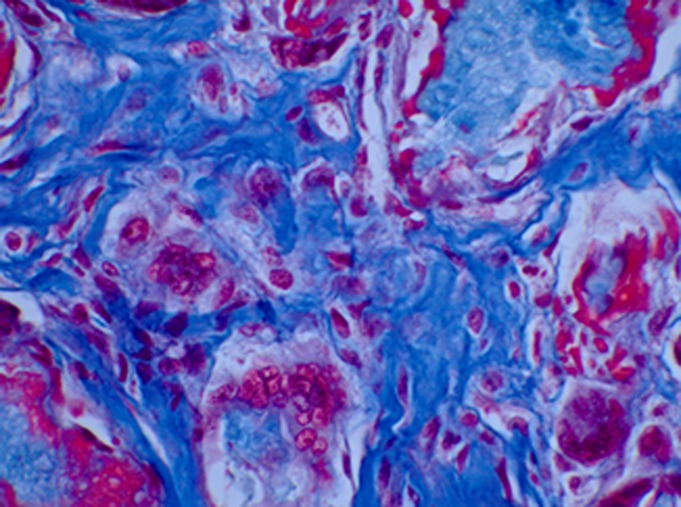
Representative histological image of a collagen disk containing rhBMP-2 and SVAK-12 four weeks after subcutaneous transplantation, showing ectopic trabecular bone formation and mineralized osteoid. This photomicrograph demonstrates that the bone formed has been deposited by osteoblasts and is not a chemical deposition of mineral (Gomori one-step trichrome stain, ×33).
Experiment 2: Femoral Fracture Model
Radiographic Analysis
After successfully enhancing ectopic bone formation, we extended the study to examine the effects of SVAK-12 on fracture-healing. There were no significant differences between any of the groups of rats with regard to age, weight at surgery, or weight change during the experiments. As described above, a semiquantitative radiographic healing score was used to grade the fracture repair (Fig. 3), and this score differed significantly between the control vehicle (DMSO)-injected group and the SVAK-12-injected group (p = 0.028) (Fig. 4). The mean radiographic score in the SVAK-12-treated group was 1 point higher (on a 6-point scale) than that in the vehicle-treated group. In addition, the fracture callus was better mineralized and there was more bone in the fracture gap in the SVAK-12-treated group.
Fig. 3.
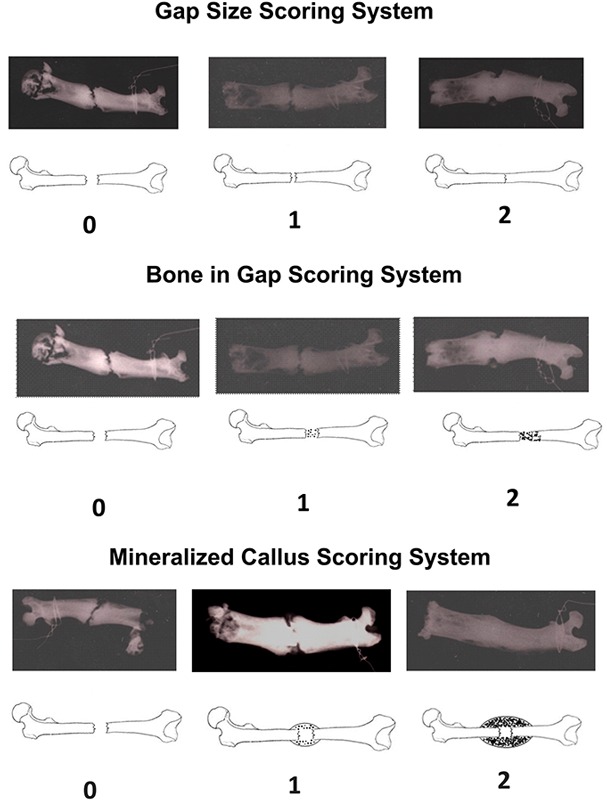
Radiographic healing scoring system. The radiographs of the femora were graded by four blinded observers independently using this scoring system. The three components of the scoring system included gap size, bone in the gap, and mineralized callus size. The composite score ranged from 0 to 6.
Fig. 4.
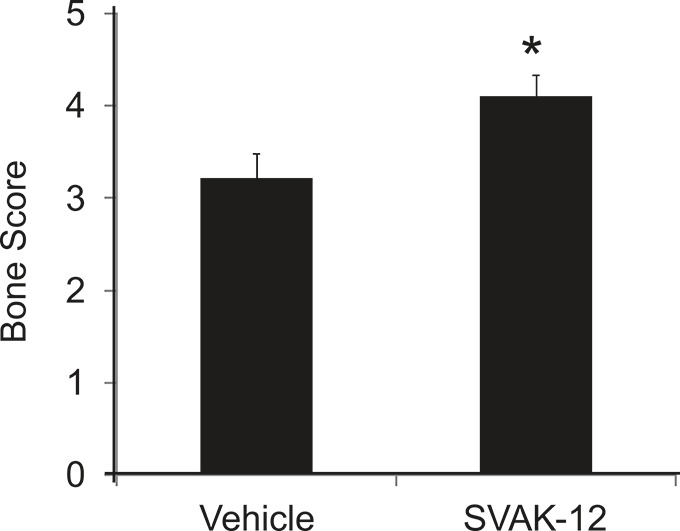
The composite radiographic healing scores, which differed significantly between the vehicle-treated group and the SVAK-12-treated group (*p = 0.028).
Biomechanical Testing
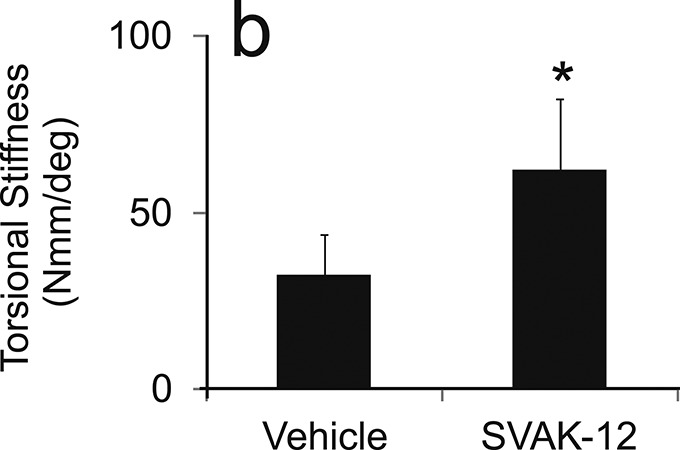
We analyzed the biomechanical properties of the healing fracture site to determine whether SVAK-12 enhanced torsional strength and stiffness compared with that in the intact and vehicle-treated controls. Significant differences in the callus biomechanical properties were found among the treatment groups five weeks after fracture. The mean torsional strength (606.6 versus 424.8 Nmm) and stiffness (62.3 versus 32.4 Nmm/deg) were significantly greater in the SVAK-12 treated group than the vehicle control group (p = 0.008 and p = 0.014, respectively). The single injection of SVAK-12 enhanced the healing fracture callus properties compared with the injection of the vehicle alone (Fig. 5).
Effects of a single injection of SVAK-12 compared with injection of the vehicle on the mean torsional strength (a) and mean torsional stiffness (b) of the fractured femora five weeks after fracture. The mean torsional strength and stiffness in the SVAK-12-treated group were significantly different from those in the vehicle control group at five weeks (*p = 0.008 and p = 0.014, respectively).
Fig. 5-A.
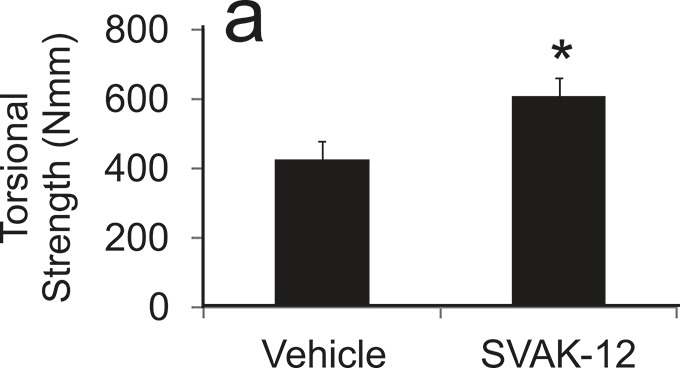
Fig. 5-B.
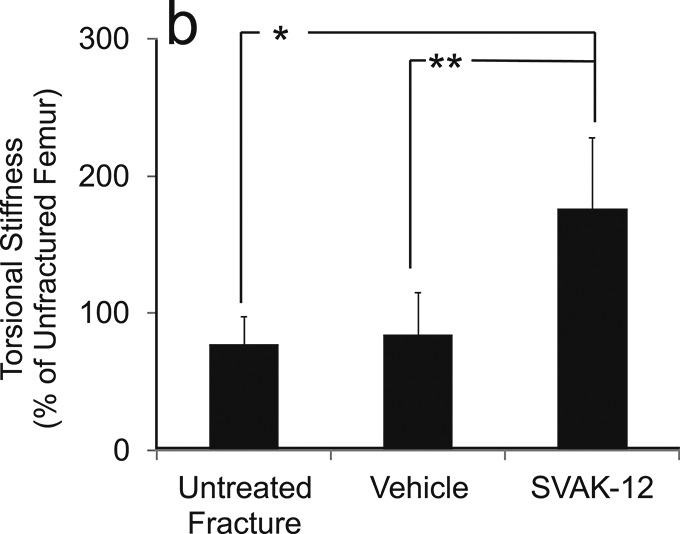
When the biomechanical results were analyzed by normalizing the absolute values to those of the intact femora, the relative torsional strength was 35.4% greater in the SVAK-12-treated group than in the vehicle-injected group (118.2% versus 82.8%, p = 0.004). The vehicle-injected group had not yet regained 100% of the strength of the intact unfractured femur, whereas the untreated (no-injection) fracture group did regain 100% of the intact strength. The SVAK-12-injected group, in contrast, was approximately 20% stronger than the intact (unfractured) femur group, despite the apparent adverse effects of the injection. The relative torsional stiffness in the SVAK-12 group was more than twice that of the untreated fracture group (176.0% versus 77.4%, p = 0.002) and the vehicle-treated group (176.0% versus 84.8%, p = 0.04) (Fig. 6).
Effects of SVAK-12 injection on the relative torsional strength (a) and stiffness (b) five weeks after fracture. The value for the treated fracture was calculated as a percentage of the value for the unfractured femur. There was a significant difference in the relative torsional strength (p = 0.004) between the vehicle-treated group and the SVAK-12-treated group. The relative torsional stiffness in the SVAK-12-treated group was more than twice that in the untreated fracture group (*p = 0.002) and the vehicle-treated group (**p = 0.04).
Fig. 6-A.
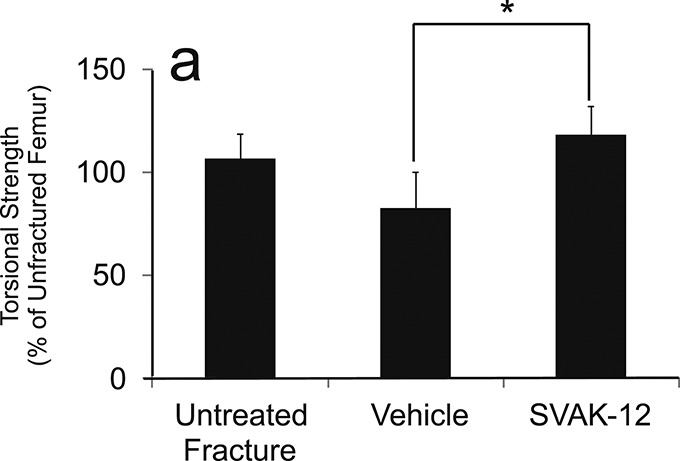
Fig. 6-B.
Histological Analysis
Several femora were processed for histological analysis of nondecalcified sections following biomechanical testing. On the basis of qualitative analysis, the fracture callus appeared to be thicker in the SVAK-12-treated group than in the DMSO control group (Fig. 7). Histological preparation of many of the post-testing specimens was not ideal due to destruction through the site, so we would consider these observations qualitative at best and would defer to the biomechanical testing data as more definitive.
Fig. 7.
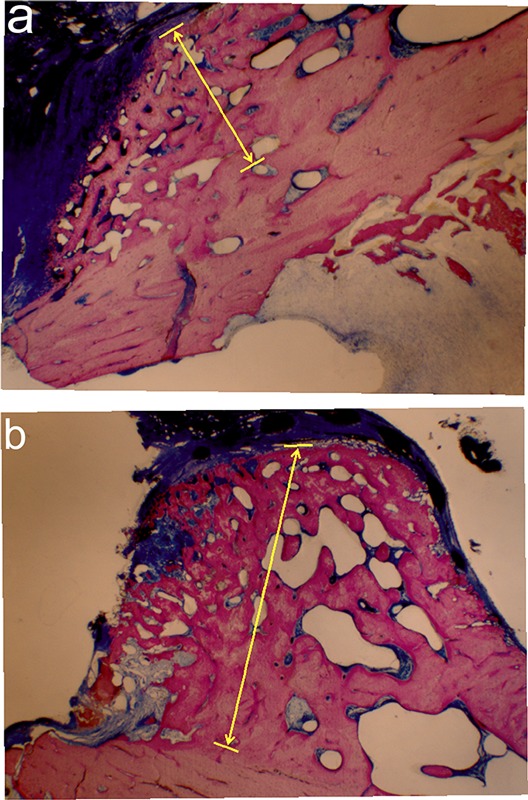
Effect of SVAK-12 injection on the healing fracture callus. A single percutaneous injection of SVAK-12 twenty-four hours after the fracture resulted in a thicker fracture callus (area defined by arrows) after five weeks (b) compared with that in the vehicle-injection group (a) (methylene blue/acid fuchsin stain, ×4).
Discussion
Although rhBMP-2 has been approved by the U.S. Food and Drug Administration (FDA) for clinical application as a bone-graft substitute in interbody lumbar spine fusions and for open tibial fractures, the need for relatively large doses of a costly recombinant protein makes its use prohibitive for many patients5,6. One approach to improving the efficiency of rhBMP-2 with regard to its ability to induce bone formation is to potentiate its efficacy by using a synthetic small molecule. In this study, we tested for the first time in vivo a compound, SVAK-12, that we had screened in vitro and selected on the basis of its in vitro ability to potentiate rhBMP-2.
In the first experiment, SVAK-12 increased the amount of ectopic bone formation induced by a suboptimal amount of rhBMP-2 in vivo. A dose-response relationship was evident on radiographs. Histological sections revealed that the pattern of bone formation included normal osteoblast-lined trabeculae, unmineralized osteoid, and some marrow elements, confirming that the ossification was due to live bone formation, not merely chemical deposition of calcium.
rhBMP-2 induces differentiation of osteoprogenitor cells into osteoblastic cells7. rhBMP-2 also has chemotactic effects on mesenchymal cells, osteoblastic cells, and endothelial cells, suggesting that the enhancement of bone formation by rhBMP-2 may be related to an increase in recruitment of bone-forming cells and enhancement of neovascularization8. On the basis of its designed target (Smurf1), we believe that SVAK-12 blocks the Smurf1-induced degradation of critical members of the rhBMP-2 signaling cascade, resulting in increased intracellular responsiveness to rhBMP-2. The design of the ectopic bone formation experiment did not permit us to determine if there was also an increase in cellular recruitment or other functions of rhBMPs. Since we did not test SVAK-12 without rhBMP, we cannot draw conclusions about its ability to serve as a stand-alone osteoinductive molecule, but these results have confirmed its ability to potentiate exogenously administered rhBMP-2.
The fracture repair portion of this study (Experiment 2) was conducted without the use of any exogenous rhBMP. Endogenous rhBMPs, including rhBMP-2, are expressed around the fracture site, which provided an opportunity to test the ability of SVAK-12 to enhance endogenous rhBMPs9. We used a well-characterized closed femoral fracture model that has been validated in multiple laboratories. A study by Einhorn et al. showed that the healing in this model could be accelerated by use of a single percutaneous injection of rhBMP-210. On the basis of that study, we chose an end point of five weeks to evaluate potential acceleration of fracture repair. At this time point, the fractures treated with SVAK-12 had superior healing characteristics when compared with both the untreated and vehicle-treated groups. The increased stiffness that we observed is indicative of a more mature fracture callus. Like Einhorn et al., we observed that injecting the early fracture hematoma with vehicle alone apparently resulted in delayed healing as measured by torsional strength (but not stiffness) compared with the untreated fractures. Thus, SVAK-12 had to overcome the nonspecific negative effect of injection to show acceleration of fracture repair.
In addition to biomechanical testing, we wanted to determine if we could radiographically demonstrate evidence of accelerated fracture repair. Repair of long-bone fractures entails both endochondral and intramembranous bone formation so we tried to incorporate both healing mechanisms in our radiographic scoring system11. The radiographic scores led to a similar conclusion: that a single percutaneous injection of SVAK-12 could accelerate long-bone-fracture repair. This scoring system has not been validated; therefore, we consider these data to be complementary to the biomechanical results but not conclusive in their own right.
One limitation of this model is that eventually all of these closed fractures are expected to heal, so it is not a particularly challenging model. The end point of five weeks was chosen in an effort to measure acceleration of healing in the treatment groups. The numbers in any such animal study are never large enough to demonstrate a lower nonunion rate, so that issue cannot be addressed on the basis of this model. Another potential limitation of our study is that we cannot be certain that the side-effect profile of an osteoinductive small molecule will be less than that seen with rhBMP-2. Most of the side effects of rhBMP-2 are related to the burst phase of release kinetics (with the clinically required high dose of rhBMP-2) off the collagen sponge: 50% of the rhBMP-2 is released in four days. The advantage of a small-molecule enhancer is that it can be integrated with alternative drug delivery systems with more tightly controllable release kinetics linked to degradation of the carrier/scaffold. The gradual delivery of an rhBMP-enhancer would allow a much lower loading dose of rhBMP and avoid the side effects seen from the burst release phase of the larger doses. Moreover, when SVAK-12 is used as a stand-alone therapeutic to enhance endogenous rhBMPs, which are physiologically released over time at low levels (as in fracture repair), we would again expect to avoid the sudden release of large doses of rhBMPs.
Many bioactive factors play an important role in bone tissue engineering12. Several bioactive materials have been used in other studies to enhance fracture-healing; these include activin, fibroblast growth factor-2 (FGF-2), Nell-1 protein, platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), and stromal cell-derived factor-1 alpha (SDF-1 alpha)13-17. Other small chemicals used in several studies include N-methylpyrrolidone (NMP) and BMP-binding peptide (BBP)18,19. Our group has shown that local implantation of bone marrow cells transfected with LIM mineralization protein-1 (LMP-1) cDNA induced good spine fusion20. Statins also may enhance rhBMP activity and have a positive effect in fracture repair models21.
SVAK-12 is unique in that it was specifically selected to inhibit an intracellular negative regulator of the rhBMP-2 signaling pathway. As a result, its mechanism of action and biologic activity should be more predictable than those of some of the other factors that also have multiple physiologic effects. rhBMP-2 intracellular signaling requires critically important transduction proteins known as Smads. The half-life of these proteins is regulated by Smurf1, an E3 ligase that targets Smads for degradation. Increased levels or activity of Smurf1 results in decreased rhBMP signal transduction due to degradation of Smads22. Since SVAK-12 was selected as an effective blocker of Smurf1, SVAK-12 would be expected to enhance the responsiveness of cells to rhBMP-2 in vitro1. This novel interaction of a synthetic small molecule with the WW2 domain of Smurf1 to block Smad binding results in increased cellular responsiveness to rhBMP-2 and provides proof of concept that Smurf1 can indeed serve as a novel regulatory target for the rhBMP-2 signaling pathway23,24. The current experiment extended our in vitro observations of the Smurf1 blockade strategy to two in vivo models, both of which showed the ability of SVAK-12 to enhance bone formation.
In summary, the results for the chest ectopic bone formation model clearly show that SVAK-12 can enhance, in a dose-dependent manner, bone induction by a suboptimal dose of exogenously applied rhBMP-2. The findings from the femoral fracture experiments also indicate that SVAK-12 can accelerate bone formation and improve the quality of bone-healing in a femoral fracture model in which endogenous rhBMP-2 has been shown to be present9. SVAK-12, designed to specifically inhibit Smurf1, is a novel low-molecular-weight synthetic compound that is cost-effective and has the potential to be used in the clinical setting to enhance rhBMP-2 efficacy. SVAK-12 warrants additional study of its ability to enhance and possibly promote bone-healing and regeneration.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Acknowledgments
Note: The authors thank Sandy Yurevich for surgical assistance, Manjula Viggeswarapu and Mesfin Teklemariam for preparing the compound for in vivo use, and Ludmilla Freidman for performing radiography and preparing the histological specimens.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Kato S, Sangadala S, Tomita K, Titus L, Boden SD. A synthetic compound that potentiates bone morphogenetic protein-2-induced transdifferentiation of myoblasts into the osteoblastic phenotype. Mol Cell Biochem. 2011 Mar;349(1-2):97-106 Epub 2010 Nov 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okada M, Sangadala S, Liu Y, Yoshida M, Reddy BV, Titus L, Boden SD. Development and optimization of a cell-based assay for the selection of synthetic compounds that potentiate bone morphogenetic protein-2 activity. Cell Biochem Funct. 2009 Dec;27(8):526-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall J, Sorensen RG, Wozney JM, Wikesjö UM. Bone formation at rhBMP-2-coated titanium implants in the rat ectopic model. J Clin Periodontol. 2007 May;34(5):444-51 [DOI] [PubMed] [Google Scholar]
- 4.Bonnarens F, Einhorn TA. Production of a standard closed fracture in laboratory animal bone. J Orthop Res. 1984;2(1):97-101 [DOI] [PubMed] [Google Scholar]
- 5.Boden SD. Bone growth enhancing substances for spinal fusion. : Garfin SR, Vaccaro AR, Orthopaedic knowledge update. Spine. Rosemont: American Academy of Orthopaedic Surgeons; 1997. p 63-70 [Google Scholar]
- 6.Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, Aro H, Atar D, Bishay M, Börner MG, Chiron P, Choong P, Cinats J, Courtenay B, Feibel R, Geulette B, Gravel C, Haas N, Raschke M, Hammacher E, van der Velde D, Hardy P, Holt M, Josten C, Ketterl RL, Lindeque B, Lob G, Mathevon H, McCoy G, Marsh D, Miller R, Munting E, Oevre S, Nordsletten L, Patel A, Pohl A, Rennie W, Reynders P, Rommens PM, Rondia J, Rossouw WC, Daneel PJ, Ruff S, Rüter A, Santavirta S, Schildhauer TA, Gekle C, Schnettler R, Segal D, Seiler H, Snowdowne RB, Stapert J, Taglang G, Verdonk R, Vogels L, Weckbach A, Wentzensen A, Wisniewski T; BMP-2 Evaluation in Surgery for Tibial Trauma (BESTT) Study Group Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002 Dec;84-A(12):2123-34 [DOI] [PubMed] [Google Scholar]
- 7.Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000 Aug;21(4):393-411 [DOI] [PubMed] [Google Scholar]
- 8.Li G, Corsi-Payne K, Zheng B, Usas A, Peng H, Huard J. The dose of growth factors influences the synergistic effect of vascular endothelial growth factor on bone morphogenetic protein 4-induced ectopic bone formation. Tissue Eng Part A. 2009 Aug;15(8):2123-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bostrom MP, Lane JM, Berberian WS, Missri AA, Tomin E, Weiland A, Doty SB, Glaser D, Rosen VM. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res. 1995 May;13(3):357-67 [DOI] [PubMed] [Google Scholar]
- 10.Einhorn TA, Majeska RJ, Mohaideen A, Kagel EM, Bouxsein ML, Turek TJ, Wozney JM. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003 Aug;85-A(8):1425-35 [DOI] [PubMed] [Google Scholar]
- 11.Ketenjian AY, Jafri AM, Arsenis C. Studies on the mechanism of callus cartilage differentiation and calcification during fracture healing. Orthop Clin North Am. 1978 Jan;9(1):43-65 [PubMed] [Google Scholar]
- 12.Boden SD. Bioactive factors for bone tissue engineering. Clin Orthop Relat Res. 1999 Oct;(367 Suppl):S84-94 [DOI] [PubMed] [Google Scholar]
- 13.Sakai R, Miwa K, Eto Y. Local administration of activin promotes fracture healing in the rat fibula fracture model. Bone. 1999 Aug;25(2):191-6 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y, Tensho K, Nakaya H, Nawata M, Okabe T, Wakitani S. Low dose fibroblast growth factor-2 (FGF-2) enhances bone morphogenetic protein-2 (BMP-2)-induced ectopic bone formation in mice. Bone. 2005 Mar;36(3):399-407 [DOI] [PubMed] [Google Scholar]
- 15.Siu RK, Lu SS, Li W, Whang J, McNeill G, Zhang X, Wu BM, Turner AS, Seim HB, 3rd, Hoang P, Wang JC, Gertzman AA, Ting K, Soo C. Nell-1 protein promotes bone formation in a sheep spinal fusion model. Tissue Eng Part A. 2011 Apr;17(7-8):1123-35 Epub 2011 Feb 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nash TJ, Howlett CR, Martin C, Steele J, Johnson KA, Hicklin DJ. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994 Mar-Apr;15(2):203-8 [DOI] [PubMed] [Google Scholar]
- 17.Higashino K, Viggeswarapu M, Bargouti M, Liu H, Titus L, Boden SD. Stromal cell-derived factor-1 potentiates bone morphogenetic protein-2 induced bone formation. Tissue Eng Part A. 2011 Feb;17(3-4):523-30 Epub 2010 Nov 2 [DOI] [PubMed] [Google Scholar]
- 18.Miguel BS, Ghayor C, Ehrbar M, Jung RE, Zwahlen RA, Hortschansky P, Schmoekel HG, Weber FE. N-methyl pyrrolidone as a potent bone morphogenetic protein enhancer for bone tissue regeneration. Tissue Eng Part A. 2009 Oct;15(10):2955-63 [DOI] [PubMed] [Google Scholar]
- 19.Behnam K, Phillips ML, Silva JD, Brochmann EJ, Duarte ME, Murray SS. BMP binding peptide: a BMP-2 enhancing factor deduced from the sequence of native bovine bone morphogenetic protein/non-collagenous protein. J Orthop Res. 2005 Jan;23(1):175-80 [DOI] [PubMed] [Google Scholar]
- 20.Boden SD. Biology of lumbar spine fusion and use of bone graft substitutes: present, future, and next generation. Tissue Eng. 2000 Aug;6(4):383-99 [DOI] [PubMed] [Google Scholar]
- 21.Pauly S, Luttosch F, Morawski M, Haas NP, Schmidmaier G, Wildemann B. Simvastatin locally applied from a biodegradable coating of osteosynthetic implants improves fracture healing comparable to BMP-2 application. Bone. 2009 Sep;45(3):505-11 Epub 2009 May 21 [DOI] [PubMed] [Google Scholar]
- 22.Zhao M, Qiao M, Harris SE, Oyajobi BO, Mundy GR, Chen D. Smurf1 inhibits osteoblast differentiation and bone formation in vitro and in vivo. J Biol Chem. 2004 Mar 26;279(13):12854-9 Epub 2003 Dec 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sangadala S, Boden SD, Viggeswarapu M, Liu Y, Titus L. LIM mineralization protein-1 potentiates bone morphogenetic protein responsiveness via a novel interaction with Smurf1 resulting in decreased ubiquitination of Smads. J Biol Chem. 2006 Jun 23;281(25):17212-9 Epub 2006 Apr 11 [DOI] [PubMed] [Google Scholar]
- 24.Sangadala S, Boden SD, Metpally RP, Reddy BV. Modeling and analysis of molecularinteraction between Smurf1-WW2 domain and various isoforms of LIM mineralization protein. Proteins. 2007 Aug 15;68(3):690-701 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest


