Abstract
Liver fibrosis is a wound healing process, the end result of chronic liver injury elicited by different noxious stimuli. Activated hepatic stellate cells or myofibroblasts and portal myofibroblasts are considered as the main producers of the extracellular matrix in the liver. Upon liver injury the quiescent stellate cells transdifferentiate into myofibroblasts a process highlighted by the loss of vitamin A stores, upregulation of interstitial type collagens, smooth muscle α actin, matrix metalloproteinases, proteoglycans, and the induction of cell survival pathways. Activation of hepatic stellate cells is a result of a complex interplay between the parenchymal cells, immune cells, extracellular matrix mechanics and extrahepatic milieu such as the gut microbiome. In this review we will focus on the pathomechanism of stellate cell activation following chronic liver injury; with the aim of identifying possible treatment targets for anti-fibrogenic agents.
Keywords: Liver fibrosis, Stellate cell activation, Hepatocyte apoptosis, Sterile inflammation, Inflammasome, Reactive oxidative species, Gut microbiota, Epigenetic signaling, Myofibroblast, Pathobiology
Introduction
Cirrhosis and its complications are the major causes of morbidity and mortality worldwide (1, 2). The only treatment currently available is liver transplantation however; the need for organs is continuously increasing and many patients die while on the waiting list. Despite significant improvement in our understanding of the fibrogenic process there is still no FDA-approved treatment available.
Hepatic stellate cells (HSC) or fat storing cells are normally localized in the parasinusoidal space and produce only small amounts of ECM components for the formation of the basement membrane. When exposed to soluble factors or changes in matrix stiffness (3), they undergo morphological transition to myofibroblast-like cells (4–6). The transdifferentiation is characterized by the loss of retinoid stores and acquiring a myofibroblast (MF) phenotype, becoming highly proliferative, migratory and depositing collagen I in the parenchyma (7). MF express fibroblastic markers including α-smooth muscle actin, (α-SMA), and produce transforming growth factor β (TGF-β), platelet derived growth factor (PDGF) and connective tissue growth factor (CTGF) and other cytokines. A change in mechanical tension is also an important initiating factor in HSC activation. Upon changes in matrix stiffness TGF-β1 becomes activated and released from its latent form (LAP), and this results in an increase in α-SMA expression in HSC (8). Activated HSC also express a combination of MMPs and their specific tissue inhibitors (TIMPs) (9). In the fibrotic liver HSC produce pro-MMP-2 and membrane type 1 (MT1)-MMP, which drive pericellular generation of active MMP2 and local degradation of the normal liver matrix. In addition, there is a marked increase in the expression of TIMP-1 leading to the inhibition of degradation of fibrillar liver collagens (10). Portal fibroblasts are located in the portal area and under physiological environment regulate the normal ECM turnover (11). They are a distinct cell population from HSC as they express elastin, fibulin-2 and glycophosphatidylinositol-linked glycoprotein (Thy1.1) but do not store vitamin A and are negative for glial fibrillary acidic protein (GFAP), desmin or cytoglobin (12). Portal fibroblasts are an important source of TGFβ and ECM during cholestatic liver injury (13, 14).
The common, key initiating factor of liver fibrogenesis is a persistent injury to the parenchymal cells. The cause could be toxic (alcohol, CCl4), metabolic (lipoapoptosis in non-alcoholic steatohepatitis [NASH]) viral (hepatitis C and B [HCV, HBV]), cholestatic injury (primary biliary cirrhosis or sclerosing cholangitis), genetic defect (e.g. accumulation of mis-folded α1 anti-trypsin) and result initially in the dysfunction of the epithelial cells with adaptive stress responses, and eventually culminate in cell death by apoptosis or necrosis (15). Induction of such pathways during cell stress can generate reactive oxidative species (ROS), inflammatory cytokines and chemokines which are profibrogenic and/or induce sterile inflammation which leads to the propagation of injury.
The role of hepatocyte stress, adaptive responses and cell death in fibrosis
Hepatocytes are constantly exposed to cellular stress and are equipped with efficient adaptive pathways to maintain homeostasis. Recent studies suggest that during NASH dysregulation of insulin signaling, β-oxidation, and an increased influx and uptake of free fatty acids from the adipose tissue result in the accumulation of saturated fatty acids in hepatocytes which is the major cause of lipotoxicity (16, 17). Lipotoxicity activates of endoplasmic reticulum (ER) stress pathways and the unfolded protein response (UPR) in an attempt to reestablish homeostasis. This may result in a translational arrest, the induction of protein folding and degradation, and the activation of autophagy. Autophagy in hepatocytes occurs as macroautophagy or chaperone-mediated autophagy whereby the degradation of damaged organelles limits the extent of injury (18). Autophagy was recently shown to confer a resistance to TNFα-mediated cell death of hepatocytes by inhibiting caspase 8 activation and the mitochondrial pathway of apoptosis (19).
Prolonged and persistent ER stress however; can cause failure of the adaptive mechanisms and the induction of the c-Jun N-terminal kinase (JNK), apoptosis signal-regulating kinase 1 (ASK1) and the transcription factor CCAAT/enhancer binding homologous protein (CHOP), launching apoptotic cascades (20, 21). The association between ER stress and apoptotic responses have been recognized not only in NASH but in alcoholic liver disease (22, 23), and viral hepatitis (24, 25).
Apoptosis of hepatocytes can also be elicited by extrinsic signals such as TNFα, the FasL (CD95L) and TNF-related apoptosis-inducing ligand (TRAIL) which are well-characterized inducers of hepatocyte death. The cell death pathways during liver injury are described in detail in the excellent reviews by Reinehr et al. (26) and Guicciardi et al. (27).
The paracrine effects of the dying cells and the fate of apoptotic cells deserves attention as cell death was shown to induce the activation of HSC by multiple mechanisms. Apoptotic cells can release ATP or UTP into the extracellular milieu serving as bait for phagocytic cells and causing phagocytes bind to their prey via the purinergic P2Y receptor (28). HSC were also shown to express P2Y receptors; and during their activation the expression of P2Y receptor subtypes is altered. As activation of P2Y receptors especially the P2Y6 in the activated HSC regulates procollagen α1(I) transcription it could be an attractive target for anti fibrogenic strategies (29).
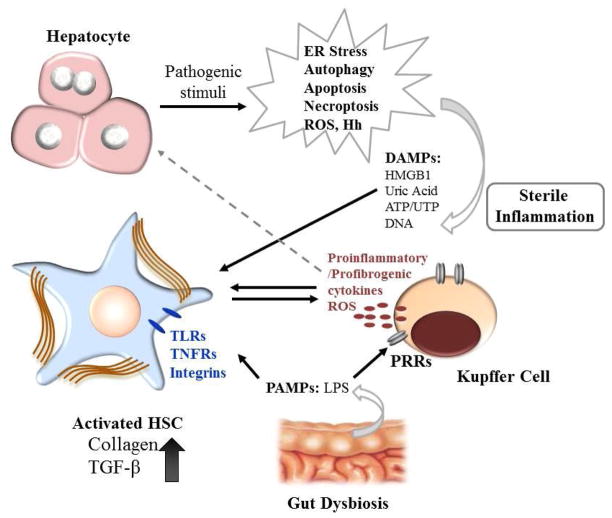
Apoptotic cells could be engulfed by the professional phagocytes the Kupffer cells by efferocytosis, or by the HSC. Phagocytosis of the apoptotic bodies activates the NADPH oxidase 2, superoxide production and in the HSC the collagen α1 (I) promoter activity, and the production TGF-β (30) whereas in Kupffer cells it stimulates death ligand expression (31). Apoptotic bodies contain DNA and are the source of unmethylated cytosine-phosphate guanosine (CpG)-DNA motifs that are recognized by Toll-like receptor 9 (TLR 9). Engagement of the TLR 9 in turn, activates signaling cascades resulting in the production of collagen 1 and the pro-fibrogenic cytokine TGF-β (32). In a mouse model of NASH, TLR9 activation induced production of IL-1β by the Kupffer cells, leading to inflammation, and fibrosis (33). Elucidating the time course and the hierarchy of these pathways would be essential in order to develop targets for fibrosis treatment (Figure 1).
Figure 1. Mechanism of liver injury-mediated stellate cell activation.
Under pathologic conditions, hepatocytes first initiate adaptive responses such as induction of ER stress and the unfolded protein response (UPR), and the induction of autophagy. After prolonged insult however, hepatocytes undergo apoptosis or necrosis and during the latter, release DAMPs reacting with PRRs on the target cells, to launch the sterile inflammation responses. This leads to inflammasome formation and the production of proinflammatory and profibrogenic cytokines, and the recruitment of inflammatory cells. DAMPs from injured hepatocytes may directly activate HSC by the engagement of TLRs on HSC. Compounding the above events, dysbiosis and increased permeability of the gut also contributes to fibrosis by releasing high levels of LPS, a PAMP molecule inducing macrophage and HSC activation via TLR4 signaling.
The Role of Sterile Inflammation
Non-infectious stimuli such as chronic cholestasis, alcoholic or non-alcoholic hepatitis, ischemia/reperfusion and drug toxicity can cause damage to hepatocytes in the absence of exogenous pathogens. The consequent sterile inflammation is characterized by the release of endogenous damage-associated molecular patterns (DAMPs) and the activation of cellular pattern recognition receptors (PRRs) which result in the production of proinflammatory cytokines and the recruitment of immune cells to the site of injury (34, 35). Sterile inflammation results in the assembly of a cytosolic protein complex, the inflammasome with the activation of the caspase-1, and the secretion of interleukin-1β, IL-18 and other cytokines (36). The redox state of cells is an important modulator of inflammasome activation as it determines the efficiency of its activation, and downstream signals (37).
DAMPs that are playing a role in liver injury include the high-mobility group box1 (HMGB1), galectins, ATP, uric acid and extracellular DNA. The role of these DAMPs in sterile inflammation was recently reviewed in detail by Kubes and Mehal (38). DAMPs play a major role in the recruitment of inflammatory cells. Recruitment of neutrophils is a hallmark of progressive NASH, alcoholic and autoimmune hepatitis and portends a worse prognosis. Neutrophils responding to sterile inflammation were found to be highly migratory through the sinusoids toward the focus of tissue necrosis, ultimately infiltrating the area of the damage (39). Recently, Wang et al. showed that HMGB1 induced IL-23 production via the activation of TLR4 in macrophages in the acetaminophen overdosed mouse model; this in turn activated hepatic γδ T cells to release IL-17A, a potent inducer of neutrophil infiltration (40). In a different study using the alcohol diet model, HMGB1 was shown to attenuate macrophage efferocytosis thereby facilitating secondary necrosis and perpetuating injury (41).
Galectins, a group of β-galactoside binding-lectins, are classified as DAMPs (42). Galectin-3 has been shown to play an important role in liver diseases as galectin 3 deficient animals were protected from fibrotic stimuli and acute liver injuries (43–48). It is expressed mainly in macrophages and cholangiocytes in normal livers, but upregulated and secreted from the MF in the cirrhotic liver (43, 44). Galectin-3 is a chemoattractant for macrophages (42), and playing a role in their alternative activation (49, 50).
Targeting the inflammasomes could be a promising therapeutic approach in NASH and ASH. For instance, the caspase 1−/− mice were protected against high fat diet-induced steatosis, inflammation and early fibrosis (51). In a different study caspase 1 was responsible for the induction of IL-1β signaling which was mediated by the IL-1 receptor; and IL-1R antagonism improved the inflammasome-dependent alcoholic steatohepatitis in mice (52).
The Role of Hepatic Immune Regulation
There is extensive experimental evidence associates fibrosis with T helper type 2 (Th2) differentiation, characterized by the production of cytokines IL-4 and IL-13 and the induction of the MF collagen production (53) (54). IL-13 was shown to be involved in HCV-induced fibrosis (53), and in shistosomiasis (5), and the Th2 cytokines also regulate the alternative activation of macrophages (55). Recently, IL-17-producing effector CD4+T (Th17) cells have been discovered regulating hepatic fibrosis (56). IL-17 stimulated Kupffer cells to express the inflammatory cytokines IL-6, IL-1β, TNF-α and the fibrogenic mediator TGF-β1. IL-17 also directly stimulated HSC to express collagen type I via STAT3. Using IL-22 in these studies was shown to attenuate fibrosis.
NK cells in the liver on the other hand have been described as anti-fibrotic by clearing active HSC and producing the anti-fibrotic cytokine inteferon-γ (IFNγ) (57). Using an NK cell-HSC co-culture system, NK cells were shown to selectively kill the early activated HSC but not the quiescent or fully activated myofibroblasts. The possible explanation for this is that transitional activation of HSC causes the release of retinoic acid from HSC, which induces the expression of retinoic acid inducible gene1 (REA1), an NK cell activator (58). IFNγ mainly produced by T helper 1 and NK cells is known to be proapoptotic to active HSC, partially via the activation of the NK cell receptor group 2 member D (NKG2D) and the downstream activation of STAT1 (59). Animal studies have shown that alcohol consumption blunts the IFNγ/NKG2D transduction activity, thereby enhancing HSC survival and causing propagation of fibrosis (60). However, IL-10 and TGF-β were shown to inhibit the anti-fibrotic effects of NK cells especially during chronic alcohol consumption (61, 62).
NKT cells may have diverging roles in acute injury and fibrosis. NKT cells were protective in acute CCl4-induced injury, but potent NKT activation (induced by α-galactosylceramide) exacerbated the injury (63). In chronic CCl4 injury, invariant NKT (iNKT) cells were protective at early but not at late stage; and depletion of NKT cells showed no effects. A possible explanation is that the active iNKT cells produce both Th1 cytokines and Th2 cytokines (63). In mice on the methionine choline deficient diet (MCD), the activation of hedgehog (Hh) signaling induced the CXCL16, an NKT cell chemoattractant. The accumulation of NKT cells accompanied by Hh induction was also found in cirrhotic NASH patients (64). Recently, it was shown that the ligand/receptor interaction between CXCL16 and its receptor CXCR6 was crucial for the accumulation of NKT cells in the CCl4- and MCD-induced fibrosis. The CXCR6−/− mice were resistant to these stimuli and the fibrotic phenotype was reestablished by the adoptive transfer of wild type NKT cell (65). It will be of interest to see further studies on how the role(s) of NK and NKT cells are integrated in fibrogenesis and whether their activation is fibrosis stage-dependent.
The Role of Oxidative Stress
Virtually all known causes of liver disease can lead to oxidative stress. In physiological settings, the cytotoxic potential of ROS is buffered by the different scavenging and antioxidant systems, including glutathione and thiol containing proteins, superoxide dismutases, catalases, peroxiredoxins, thioredoxin. Oxidative radicals can promote hepatocyte oncotic necrosis, apoptosis or necroptosis by inducing mitochondrial dysfunction (66). Beside direct cytotoxicity, ROS can induce a sensitizing effect to prolonged insults, or induce the generation of pro-inflammatory mediators. Oxidative radicals can thus be indirectly (via hepatocyte damage), or directly (Kupffer cell and HSC activation) profibrogenic (67, 68). Furthermore, HSC activation can result from osteopontin, a ROS sensitive, extracellular matrix cytokine which was recently was shown to induce collagen production involving integrin αvβ3 engagement and PI3K/pAkt/NFκB signaling (69).
ROS production in myofibroblasts occurs in response to profibrogenic mediators including PDGF, TGF-β, leptin and angiotensin II (70), induction of ER stress and autophagy in HSC (71), and by the phagocytosis of apoptotic debris in Kupffer cells and myofibroblasts (30). In general, ROS can be generated via the mitochondrial electron chain, activation of the cytochrome P450, arachidonic acid oxidation, xanthine oxidase activation and the induction of the nicotinamide adenine dinucleotide phosphate (NADPH) oxidases. Activation of the phagocytic, NOX2 (68), (30) or non-phagocytic forms NADPH oxidases NOX1 (72, 73), and NOX4 (74), are the major sources of ROS in the myofibroblasts, and the induction of profibrogenic pathways. ROS are also important second messengers, regulating signaling pathways leading to cell survival of myofibroblasts (75, 76). As NADPH oxidase activation may represent a core pathway in fibrosis NOX inhibitors are emerging as targets for anti-fibrotic therapy. Recently using an inhibitor to NOX1/4 was shown promise to prevent fibrosis progression in the CCl4 and bile duct ligation (BDL) models (77, 78).
Intrinsic Metabolic Pathways and HSC Activation
Chronic liver injury is intimately linked to the modulation of intrinsic metabolic pathways in HSC and thereby to their activation and cell fate. Dying hepatocytes release hedgehog (Hh) ligands that activate Hh signaling in the neighboring cells (79). Hedgehog ligands are developmental morphogens that control the progenitor cell fate, liver repair, regeneration and wound healing (80, 81). An interesting feature of hedgehog signaling in HSC that it is involved in the induction of glycolytic pathways during the transdifferentiation of quiescent HSCs into myofibroblasts causing lactate accumulation since gluconeogenesis and lipogenesis are suppressed (82). Inhibitors of Hh signaling or glycolysis or lactate accumulation converted myofibroblasts to quiescent HSCs, and conditional disruption of Hh signaling in myofibroblasts reduced the numbers of glycolytic myofibroblasts and liver fibrosis in mice. Targeting Hh signaling thus could be a promising therapeutic strategy.
Loss of retinoids from HSC is a hallmark of myofibroblastic transdifferentiation. Recently autophagy emerged as a mechanism involved in lipid loss in HSC, providing energy for their activation (83). Autophagy-deficient HSC were unable to process the lipid droplets by acid lipases, resulting in the accumulation and decreased free fatty acid availability, leading to decreased mitochondrial β-oxidation and ATP production. Genetic inhibition of autophagy in HSC attenuated liver fibrosis. Similar effect was seen in the fibrogenic cells from other tissues (lung, kidney, embryonic fibroblasts) deriving from mice or humans. It is also interesting to note that in the in lecithin-retinol acyltransferase (LRAT)-deficient mouse model the absence of HSC retinoid droplets did not affect hepatic fibrosis in the BDL and CCl4 models however, decreased carcinogenesis (84).
The Role of the Gut Microbiome
Chronic liver injury could be significantly perpetuated by extrahepatic causes. In NASH and alcoholic steatohepatitis, changes in the gut microbiota, increased intestinal permeability, and bacterial translocation significantly influence the inflammatory pathways in the liver (85, 86). The mucosal surface between the bacteria and the host immune system is responsible for the maintenance of innate and adaptive immune responses in order to maintain intestinal homeostasis. This function relies upon the specific pattern recognition receptors, including Toll-like receptors (TLRs) and NOD-like receptors that recognize the conserved microbial patterns termed pathogen-associated molecular patterns (PAMPs). One of the best-known PAMPs is lipopolysaccharide (LPS) deriving from Gram negative bacteria. LPS was shown to activate TLR4 in Kupffer cells via the CD14 complex resulting in the activation of Jun N-terminal kinase, p38, interferon regulatory factor 3 and nuclear factor-κB pathway NF-κB (87, 88) which in turn results in the activation of proinflammatory pathways including TNFα and IL-1β. HSC are crucial part of the TLR4-mediated signaling cascades as they respond to LPS by induction of NF-κB and JNK and stimulation of surface expression of the adhesion molecules ICAM-1 and VCAM-1 (89). Furthermore, the gut microbiota and TLR4 were shown to be important not only in fibrosis (88) but also in liver carcinogenesis (90). An interesting aspect on the role of gut microbiota was recently revealed by Henao-Mejia et al (91). In their study defective NLRP6 and NLRP3 inflammasomes resulted in intestinal dysbiosis which was associated with progressive hepatic steatosis and inflammation through the influx of TLR4 and TLR9 agonists, leading to the upregulation of TNF-α expression in the liver. TLR2 has also been found to play a pivotal role in regulating intestinal microbiota and liver fibrosis (92). Upon BDL both TLR2−/− and TNFRI−/− mice showed reduced fibrosis, lower serum LPS levels and decreased bacterial translocation. TNF-α activated the TNFRI on the intestinal epithelial cells and promoted gut permeability, facilitating bacterial translocation. The subsequent increase in LPS in the circulation activated the TLR4 receptors on Kupffer cells, HSC and hepatocytes (92). Therapeutic modulation of the intestinal microflora thus could be an alternative strategy to develop a novel anti-fibrotic therapy.
MiRNAs and HSC activation
MicroRNAs are noncoding RNAs that regulate gene expression by specifically binding with 3′-untranslated region (3′-UTR) of target gene mRNAs (93). In terms of HSC activation, targeting the inhibition of TGFβ signaling pathway by miR-19b has shown a great promise (94). MiR-19b binding to the 3′ untranslated region of TGF-βRII decreased the expression of type I collagen. In addition, the miR-29 family was emerging as a very important regulator of liver fibrosis. MiR-29b could be a novel regulator of type I collagen expression in addition to its involvement in the well-known Smad cascade by its interaction with SP1 expression. Overexpression of miR-29b suppressed primary, cultured mouse HSC viability and the expression of α-SMA (95). Exosomes have emerged as carriers of extracellular miRNAs (96), and modulators of cell-cell interactions. In alcoholic liver disease increase in circulating miR-122 and miR-155 was seen whereas the induction of miR-122 in the plasma after CpG treatment suggests that circulating miR-122 could be a marker of liver injury caused by DAMPs (97). The clinical relevance of circulating miRNAs in fibrosis however, still remains to be investigated.
Epigenetic Modulation of HSC Activation
Epigenetic changes including DNA methylation, histone modifications, and silencing by miRNAs, as above; significantly impact on wound healing and progression of fibrosis. According to current concepts HSC transdifferentiation is facilitated by the repression of the adipogenic genes such as PPAR-γ, inhibitor of nuclear factor-κB protein-α (Iκ-Bα), or C/EBP, SREBP-1c (98). During HSC activation, the epigenetic repression of PPAR-γ is mediated by the depletion of miR132 and this results in the induction of the methyl-CpG binding protein 2 (MeCP2) and its increased recruitment to PPARγ promoter; whereas the enhancer of zeste homolog 2 (EZH2) causes histone methylation preventing the recruitment of the RNA polymerase II. Rosmarinic acid and baicalin have been shown to induce the de-repression of these pathways also suppressing the canonical Wnt signaling (99). An interesting concept is the epigenetic suppressive adaptation. It was shown that the history of liver damage corresponds with the transmission of an epigenetic suppression of the fibrogenesis in the liver in the offspring. This was hallmarked by the higher expression of PPARγ in the subsequent generation and it was hypothesized that a myofibroblast-secreted soluble factor stimulated heritable epigenetic signatures in sperm; affecting the offspring’s adaptation to future fibrogenic hepatic insults (100). In this study it was also described that patients with mild liver fibrosis have PPARγ hypomethylation compared to patients with severe liver fibrosis. Better understanding and of the epigenetic factors involved in liver fibrogenesis may shed light on the etiology of the clinically observed susceptibility of patients to fibrogenic stimuli.
Conclusion
The complexity of fibrogenic pathways, their regulation, and the cross talk between the different cell types requires further studies in order to identify the core pathways involved in stellate cell activation. Identification and validation of these pathways will generate new therapeutic targets preventing or halting fibrosis progression and will reduce the complications of portal hypertension, thereby improving the quality of life and survival of patients with liver disease.
Footnotes
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Compliance with Ethics Guidelines
Conflict of Interest
Joy X. Jiang declares that she has no conflict of interest.
Natalie J. Török has received research funding from Genkyotex and National Institutes of Health.
References
- 1.D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44:217–231. doi: 10.1016/j.jhep.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Lee UE, Friedman SL. Mechanisms of hepatic fibrogenesis. Best Pract Res Clin Gastroenterol. 2011;25:195–206. doi: 10.1016/j.bpg.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wells RG. Tissue mechanics and fibrosis. Biochim Biophys Acta. 2013 doi: 10.1016/j.bbadis.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mormone E, George J, Nieto N. Molecular pathogenesis of hepatic fibrosis and current therapeutic approaches. Chem Biol Interact. 2011;193:225–231. doi: 10.1016/j.cbi.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cong M, Iwaisako K, Jiang C, Kisseleva T. Cell signals influencing hepatic fibrosis. Int J Hepatol. 2012;2012:158547. doi: 10.1155/2012/158547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 8.Buscemi L, Ramonet D, Klingberg F, Formey A, Smith-Clerc J, Meister JJ, Hinz B. The single-molecule mechanics of the latent TGF-beta1 complex. Curr Biol. 2011;21:2046–2054. doi: 10.1016/j.cub.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 9.Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21:373–384. doi: 10.1055/s-2001-17552. [DOI] [PubMed] [Google Scholar]
- 10.Han YP. Matrix metalloproteinases, the pros and cons, in liver fibrosis. J Gastroenterol Hepatol. 2006;21 (Suppl 3):S88–91. doi: 10.1111/j.1440-1746.2006.04586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinnman N, Francoz C, Barbu V, Wendum D, Rey C, Hultcrantz R, Poupon R, et al. The myofibroblastic conversion of peribiliary fibrogenic cells distinct from hepatic stellate cells is stimulated by platelet-derived growth factor during liver fibrogenesis. Lab Invest. 2003;83:163–173. doi: 10.1097/01.lab.0000054178.01162.e4. [DOI] [PubMed] [Google Scholar]
- 12.Bosselut N, Housset C, Marcelo P, Rey C, Burmester T, Vinh J, Vaubourdolle M, et al. Distinct proteomic features of two fibrogenic liver cell populations: hepatic stellate cells and portal myofibroblasts. Proteomics. 2010;10:1017–1028. doi: 10.1002/pmic.200900257. [DOI] [PubMed] [Google Scholar]
- 13.Wells RG, Kruglov E, Dranoff JA. Autocrine release of TGF-beta by portal fibroblasts regulates cell growth. FEBS Lett. 2004;559:107–110. doi: 10.1016/S0014-5793(04)00037-7. [DOI] [PubMed] [Google Scholar]
- 14.Dranoff JA, Wells RG. Portal fibroblasts: Underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malhi H, Gores GJ. Cellular and molecular mechanisms of liver injury. Gastroenterology. 2008;134:1641–1654. doi: 10.1053/j.gastro.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim SH, Kohli R, Gores GJ. Mechanisms of lipotoxicity in NAFLD and clinical implications. J Pediatr Gastroenterol Nutr. 2011;53:131–140. doi: 10.1097/MPG.0b013e31822578db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Czaja MJ. Two types of autophagy are better than one during hepatocyte oxidative stress. Autophagy. 2011;7:96–97. doi: 10.4161/auto.7.1.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amir M, Zhao E, Fontana L, Rosenberg H, Tanaka K, Gao G, Czaja MJ. Inhibition of hepatocyte autophagy increases tumor necrosis factor-dependent liver injury by promoting caspase-8 activation. Cell Death Differ. 2013 doi: 10.1038/cdd.2013.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ibrahim SH, Gores GJ. Who pulls the trigger: JNK activation in liver lipotoxicity? J Hepatol. 2012;56:17–19. doi: 10.1016/j.jhep.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishitoh H. CHOP is a multifunctional transcription factor in the ER stress response. J Biochem. 2012;151:217–219. doi: 10.1093/jb/mvr143. [DOI] [PubMed] [Google Scholar]
- 22.Ji C, Kaplowitz N, Lau MY, Kao E, Petrovic LM, Lee AS. Liver-specific loss of glucose-regulated protein 78 perturbs the unfolded protein response and exacerbates a spectrum of liver diseases in mice. Hepatology. 2011;54:229–239. doi: 10.1002/hep.24368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J, Lai KK, Verlinsky A, Lugea A, French SW, Cooper MP, Ji C, et al. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J Hepatol. 2011;55:673–682. doi: 10.1016/j.jhep.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saeed M, Suzuki R, Watanabe N, Masaki T, Tomonaga M, Muhammad A, Kato T, et al. Role of the endoplasmic reticulum-associated degradation (ERAD) pathway in degradation of hepatitis C virus envelope proteins and production of virus particles. J Biol Chem. 2011;286:37264–37273. doi: 10.1074/jbc.M111.259085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merquiol E, Uzi D, Mueller T, Goldenberg D, Nahmias Y, Xavier RJ, Tirosh B, et al. HCV Causes Chronic Endoplasmic Reticulum Stress Leading to Adaptation and Interference with the Unfolded Protein Response. Plos One. 2011:6. doi: 10.1371/journal.pone.0024660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinehr R, Haussinger D. CD95 death receptor and epidermal growth factor receptor (EGFR) in liver cell apoptosis and regeneration. Arch Biochem Biophys. 2012;518:2–7. doi: 10.1016/j.abb.2011.12.004. [DOI] [PubMed] [Google Scholar]
- *27.Guicciardi ME, Gores GJ. Apoptosis as a mechanism for liver disease progression. Semin Liver Dis. 2010;30:402–410. doi: 10.1055/s-0030-1267540. The authors review the main pathways of apoptosis and its contribution to fibrosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461:282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dranoff JA, Ogawa M, Kruglov EA, Gaca MD, Sevigny J, Robson SC, Wells RG. Expression of P2Y nucleotide receptors and ectonucleotidases in quiescent and activated rat hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol. 2004;287:G417–424. doi: 10.1152/ajpgi.00294.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang JX, Venugopal S, Serizawa N, Chen X, Scott F, Li Y, Adamson R, et al. Reduced nicotinamide adenine dinucleotide phosphate oxidase 2 plays a key role in stellate cell activation and liver fibrogenesis in vivo. Gastroenterology. 2010;139:1375–1384. doi: 10.1053/j.gastro.2010.05.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canbay A, Feldstein AE, Higuchi H, Werneburg N, Grambihler A, Bronk SF, Gores GJ. Kupffer cell engulfment of apoptotic bodies stimulates death ligand and cytokine expression. Hepatology. 2003;38:1188–1198. doi: 10.1053/jhep.2003.50472. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, Mehal WZ. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 33.Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, et al. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323–334. e327. doi: 10.1053/j.gastro.2010.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *34.Henao-Mejia J, Elinav E, Strowig T, Flavell RA. Inflammasomes: far beyond inflammation. Nat Immunol. 2012;13:321–324. doi: 10.1038/ni.2257. Recent update on the role of inflammasomes and their role in disease progression. [DOI] [PubMed] [Google Scholar]
- 35.Kono H, Rock KL. How dying cells alert the immune system to danger. Nature Reviews Immunology. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodrigue-Gervais IG, Saleh M. Caspases and immunity in a deadly grip. Trends Immunol. 2013;34:41–49. doi: 10.1016/j.it.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 37.Rubartelli A, Gattorno M, Netea MG, Dinarello CA. Interplay between redox status and inflammasome activation. Trends Immunol. 2011;32:559–566. doi: 10.1016/j.it.2011.08.005. [DOI] [PubMed] [Google Scholar]
- **38.Kubes P, Mehal WZ. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. The authors provide a detailed and excellent summary on DAMP-induced sterile inflammation and inflammasome signaling in liver diseases. [DOI] [PubMed] [Google Scholar]
- 39.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330:362–366. doi: 10.1126/science.1195491. [DOI] [PubMed] [Google Scholar]
- 40.Wang X, Sun R, Wei H, Tian Z. High-mobility group box 1 (HMGB1)-Toll-like receptor (TLR)4-interleukin (IL)-23-IL-17A axis in drug-induced damage-associated lethal hepatitis: Interaction of gammadelta T cells with macrophages. Hepatology. 2013;57:373–384. doi: 10.1002/hep.25982. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Bu HF, Zhong W, Asai A, Zhou Z, Tan XD. MFG-E8 and HMGB1 are involved in the mechanism underlying alcohol-induced impairment of macrophage engulfing apoptotic cells. Mol Med. 2013 doi: 10.2119/molmed.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs) Immunol Rev. 2009;230:172–187. doi: 10.1111/j.1600-065X.2009.00790.x. [DOI] [PubMed] [Google Scholar]
- 43.Henderson NC, Mackinnon AC, Farnworth SL, Poirier F, Russo FP, Iredale JP, Haslett C, et al. Galectin-3 regulates myofibroblast activation and hepatic fibrosis. Proc Natl Acad Sci U S A. 2006;103:5060–5065. doi: 10.1073/pnas.0511167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jiang JX, Chen X, Hsu DK, Baghy K, Serizawa N, Scott F, Takada Y, et al. Galectin-3 modulates phagocytosis-induced stellate cell activation and liver fibrosis in vivo. Am J Physiol Gastrointest Liver Physiol. 2012;302:G439–446. doi: 10.1152/ajpgi.00257.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nomoto K, Nishida T, Nakanishi Y, Fujimoto M, Takasaki I, Tabuchi Y, Tsuneyama K. Deficiency in galectin-3 promotes hepatic injury in CDAA diet-induced nonalcoholic fatty liver disease. Scientific World Journal. 2012;2012:959824. doi: 10.1100/2012/959824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iacobini C, Menini S, Ricci C, Blasetti Fantauzzi C, Scipioni A, Salvi L, Cordone S, et al. Galectin-3 ablation protects mice from diet-induced NASH: a major scavenging role for galectin-3 in liver. J Hepatol. 2011;54:975–983. doi: 10.1016/j.jhep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Dragomir AC, Sun R, Choi H, Laskin JD, Laskin DL. Role of galectin-3 in classical and alternative macrophage activation in the liver following acetaminophen intoxication. J Immunol. 2012;189:5934–5941. doi: 10.4049/jimmunol.1201851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volarevic V, Milovanovic M, Ljujic B, Pejnovic N, Arsenijevic N, Nilsson U, Leffler H, et al. Galectin-3 deficiency prevents concanavalin A-induced hepatitis in mice. Hepatology. 2012;55:1954–1964. doi: 10.1002/hep.25542. [DOI] [PubMed] [Google Scholar]
- 49.MacKinnon AC, Farnworth SL, Hodkinson PS, Henderson NC, Atkinson KM, Leffler H, Nilsson UJ, et al. Regulation of alternative macrophage activation by galectin-3. J Immunol. 2008;180:2650–2658. doi: 10.4049/jimmunol.180.4.2650. [DOI] [PubMed] [Google Scholar]
- 50.Henderson NC, Sethi T. The regulation of inflammation by galectin-3. Immunol Rev. 2009;230:160–171. doi: 10.1111/j.1600-065X.2009.00794.x. [DOI] [PubMed] [Google Scholar]
- 51.Dixon LJ, Flask CA, Papouchado BG, Feldstein AE, Nagy LE. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PLoS One. 2013;8:e56100. doi: 10.1371/journal.pone.0056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, Barrieau M, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest. 2012;122:3476–3489. doi: 10.1172/JCI60777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weng HL, Liu Y, Chen JL, Huang T, Xu LJ, Godoy P, Hu JH, et al. The etiology of liver damage imparts cytokines transforming growth factor beta1 or interleukin-13 as driving forces in fibrogenesis. Hepatology. 2009;50:230–243. doi: 10.1002/hep.22934. [DOI] [PubMed] [Google Scholar]
- 54.Holt AP, Salmon M, Buckley CD, Adams DH. Immune interactions in hepatic fibrosis. Clin Liver Dis. 2008;12:861–882. x. doi: 10.1016/j.cld.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765–776. e761–763. doi: 10.1053/j.gastro.2012.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao B, Radaeva S. Natural killer and natural killer T cells in liver fibrosis. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbadis.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Radaeva S, Wang L, Radaev S, Jeong WI, Park O, Gao B. Retinoic acid signaling sensitizes hepatic stellate cells to NK cell killing via upregulation of NK cell activating ligand RAE1. Am J Physiol Gastrointest Liver Physiol. 2007;293:G809–816. doi: 10.1152/ajpgi.00212.2007. [DOI] [PubMed] [Google Scholar]
- 59.Saile B, Eisenbach C, Dudas J, El-Armouche H, Ramadori G. Interferon-gamma acts proapoptotic on hepatic stellate cells (HSC) and abrogates the antiapoptotic effect of interferon-alpha by an HSP70-dependant pathway. Eur J Cell Biol. 2004;83:469–476. doi: 10.1078/0171-9335-00409. [DOI] [PubMed] [Google Scholar]
- 60.Jeong WI, Park O, Gao B. Abrogation of the antifibrotic effects of natural killer cells/interferon-gamma contributes to alcohol acceleration of liver fibrosis. Gastroenterology. 2008;134:248–258. doi: 10.1053/j.gastro.2007.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jeong WI, Park O, Suh YG, Byun JS, Park SY, Choi E, Kim JK, et al. Suppression of innate immunity (natural killer cell/interferon-gamma) in the advanced stages of liver fibrosis in mice. Hepatology. 2011;53:1342–1351. doi: 10.1002/hep.24190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *62.Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology. 2013;57:1654–1662. doi: 10.1002/hep.26115. The authors summarized the most advanced findings on the role of NK cells in liver diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park O, Jeong WI, Wang L, Wang H, Lian ZX, Gershwin ME, Gao B. Diverse roles of invariant natural killer T cells in liver injury and fibrosis induced by carbon tetrachloride. Hepatology. 2009;49:1683–1694. doi: 10.1002/hep.22813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Syn WK, Oo YH, Pereira TA, Karaca GF, Jung Y, Omenetti A, Witek RP, et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. 2010;51:1998–2007. doi: 10.1002/hep.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wehr A, Baeck C, Heymann F, Niemietz PM, Hammerich L, Martin C, Zimmermann HW, et al. Chemokine Receptor CXCR6-Dependent Hepatic NK T Cell Accumulation Promotes Inflammation and Liver Fibrosis. J Immunol. 2013 doi: 10.4049/jimmunol.1202909. [DOI] [PubMed] [Google Scholar]
- 66.Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26 (Suppl 1):173–179. doi: 10.1111/j.1440-1746.2010.06592.x. [DOI] [PubMed] [Google Scholar]
- 67.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 68.De Minicis S, Brenner DA. NOX in liver fibrosis. Arch Biochem Biophys. 2007;462:266–272. doi: 10.1016/j.abb.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Urtasun R, Lopategi A, George J, Leung TM, Lu Y, Wang X, Ge X, et al. Osteopontin, an oxidant stress sensitive cytokine, up-regulates collagen-I via integrin alpha(V)beta(3) engagement and PI3K/pAkt/NFkappaB signaling. Hepatology. 2012;55:594–608. doi: 10.1002/hep.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Novo E, Parola M. The role of redox mechanisms in hepatic chronic wound healing and fibrogenesis. Fibrogenesis Tissue Repair. 2012;5 (Suppl 1):S4. doi: 10.1186/1755-1536-5-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hernandez-Gea V, Hilscher M, Rozenfeld R, Lim MP, Nieto N, Werner S, Devi LA, et al. Endoplasmic Reticulum Stress Induces Fibrogenic Activity in Hepatic Stellate Cells through Autophagy. J Hepatol. 2013 doi: 10.1016/j.jhep.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paik YH, Iwaisako K, Seki E, Inokuchi S, Schnabl B, Osterreicher CH, Kisseleva T, et al. The nicotinamide adenine dinucleotide phosphate oxidase (NOX) homologues NOX1 and NOX2/gp91(phox) mediate hepatic fibrosis in mice. Hepatology. 2011;53:1730–1741. doi: 10.1002/hep.24281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cui W, Matsuno K, Iwata K, Ibi M, Matsumoto M, Zhang J, Zhu K, et al. NOX1/nicotinamide adenine dinucleotide phosphate, reduced form (NADPH) oxidase promotes proliferation of stellate cells and aggravates liver fibrosis induced by bile duct ligation. Hepatology. 2011;54:949–958. doi: 10.1002/hep.24465. [DOI] [PubMed] [Google Scholar]
- 74.Sancho P, Mainez J, Crosas-Molist E, Roncero C, Fernandez-Rodriguez CM, Pinedo F, Huber H, et al. NADPH oxidase NOX4 mediates stellate cell activation and hepatocyte cell death during liver fibrosis development. PLoS One. 2012;7:e45285. doi: 10.1371/journal.pone.0045285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kis K, Liu X, Hagood JS. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med. 2011;13:e27. doi: 10.1017/S1462399411001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang JX, Mikami K, Venugopal S, Li Y, Torok NJ. Apoptotic body engulfment by hepatic stellate cells promotes their survival by the JAK/STAT and Akt/NF-kappaB-dependent pathways. J Hepatol. 2009;51:139–148. doi: 10.1016/j.jhep.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology. 2012;56:2316–2327. doi: 10.1002/hep.25938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schroder K, Brandes RP, et al. Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo. Free Radic Biol Med. 2012;53:289–296. doi: 10.1016/j.freeradbiomed.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Omenetti A, Choi S, Michelotti G, Diehl AM. Hedgehog signaling in the liver. J Hepatol. 2011;54:366–373. doi: 10.1016/j.jhep.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robbins DJ, Fei DL, Riobo NA. The Hedgehog signal transduction network. Sci Signal. 2012;5:re6. doi: 10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *81.Choi SS, Omenetti A, Syn WK, Diehl AM. The role of Hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol. 2011;43:238–244. doi: 10.1016/j.biocel.2010.10.015. A state-of -the art summary on the current knowledge of Hedgehog signaling in liver diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y, Choi SS, Michelotti GA, Chan IS, Swiderska-Syn M, Karaca GF, Xie G, et al. Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 2012;143:1319–1329. e1311–1311. doi: 10.1053/j.gastro.2012.07.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hernandez-Gea V, Ghiassi-Nejad Z, Rozenfeld R, Gordon R, Fiel MI, Yue Z, Czaja MJ, et al. Autophagy releases lipid that promotes fibrogenesis by activated hepatic stellate cells in mice and in human tissues. Gastroenterology. 2012;142:938–946. doi: 10.1053/j.gastro.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kluwe J, Wongsiriroj N, Troeger JS, Gwak GY, Dapito DH, Pradere JP, Jiang H, et al. Absence of hepatic stellate cell retinoid lipid droplets does not enhance hepatic fibrosis but decreases hepatic carcinogenesis. Gut. 2011;60:1260–1268. doi: 10.1136/gut.2010.209551. [DOI] [PubMed] [Google Scholar]
- 85.Yan AW, Schnabl B. Bacterial translocation and changes in the intestinal microbiome associated with alcoholic liver disease. World J Hepatol. 2012;4:110–118. doi: 10.4254/wjh.v4.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fouts DE, Torralba M, Nelson KE, Brenner DA, Schnabl B. Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J Hepatol. 2012;56:1283–1292. doi: 10.1016/j.jhep.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Machado MV, Cortez-Pinto H. Gut microbiota and nonalcoholic fatty liver disease. Ann Hepatol. 2012;11:440–449. [PubMed] [Google Scholar]
- *88.Seki E, Schnabl B. Role of innate immunity and the microbiota in liver fibrosis: crosstalk between the liver and gut. J Physiol. 2012;590:447–458. doi: 10.1113/jphysiol.2011.219691. The authors summarized the most important mechanisms by which the gut-liver axis is regulated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37:1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 90.Dapito DH, Mencin A, Gwak GY, Pradere JP, Jang MK, Mederacke I, Caviglia JM, et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell. 2012;21:504–516. doi: 10.1016/j.ccr.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hartmann P, Haimerl M, Mazagova M, Brenner DA, Schnabl B. Toll-like receptor 2-mediated intestinal injury and enteric tumor necrosis factor receptor I contribute to liver fibrosis in mice. Gastroenterology. 2012;143:1330–1340. e1331. doi: 10.1053/j.gastro.2012.07.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.He Y, Huang C, Zhang SP, Sun X, Long XR, Li J. The potential of microRNAs in liver fibrosis. Cell Signal. 2012;24:2268–2272. doi: 10.1016/j.cellsig.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 94.Lakner AM, Steuerwald NM, Walling TL, Ghosh S, Li T, McKillop IH, Russo MW, et al. Inhibitory effects of microRNA 19b in hepatic stellate cell-mediated fibrogenesis. Hepatology. 2012;56:300–310. doi: 10.1002/hep.25613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ogawa T, Iizuka M, Sekiya Y, Yoshizato K, Ikeda K, Kawada N. Suppression of type I collagen production by microRNA-29b in cultured human stellate cells. Biochem Biophys Res Commun. 2010;391:316–321. doi: 10.1016/j.bbrc.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 96.Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr Opin Lipidol. 2012;23:91–97. doi: 10.1097/MOL.0b013e328350a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, Alao H, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology. 2012;56:1946–1957. doi: 10.1002/hep.25873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.She H, Xiong S, Hazra S, Tsukamoto H. Adipogenic transcriptional regulation of hepatic stellate cells. J Biol Chem. 2005;280:4959–4967. doi: 10.1074/jbc.M410078200. [DOI] [PubMed] [Google Scholar]
- 99.Yang MD, Chiang YM, Higashiyama R, Asahina K, Mann DA, Mann J, Wang CC, et al. Rosmarinic acid and baicalin epigenetically derepress peroxisomal proliferator-activated receptor gamma in hepatic stellate cells for their antifibrotic effect. Hepatology. 2012;55:1271–1281. doi: 10.1002/hep.24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zeybel M, Hardy T, Wong YK, Mathers JC, Fox CR, Gackowska A, Oakley F, et al. Multigenerational epigenetic adaptation of the hepatic wound-healing response. Nat Med. 2012;18:1369–1377. doi: 10.1038/nm.2893. [DOI] [PMC free article] [PubMed] [Google Scholar]