Abstract
Abstract:
We previously reported on the metal ion concentrations of cobalt, chromium, and titanium that were found in the serum of patients three years after they had undergone primary total hip arthroplasty as compared with the concentrations found in the serum of control patients who did not have an implant. This study is a concise update on the serum metal levels found in a cohort of these patients ten years after the time of hip implantation. Of the original seventy-five subjects, metal ion levels were available for forty patients (53%). Ten patients (hybrid group) had received a hybrid total hip replacement that consisted of a modular cobalt-alloy femoral stem with a cobalt-alloy femoral head that had been inserted with cement and a titanium acetabular socket that had been inserted without cement. Nine patients (cobalt-chromium [CoCr] group) had received an implant with an extensively porous-coated modular cobalt-alloy femoral stem and femoral head along with a titanium acetabular socket; the femoral and acetabular components had each been inserted without cement. Eight patients (titanium group) had undergone insertion of a proximally porous-coated modular titanium-alloy femoral stem with a cobalt-alloy femoral head and a titanium acetabular socket; the femoral and acetabular components had each been inserted without cement. Thirteen patients (control group) from the original control group of patients who had not received an implant served as control subjects. Serum metal levels were measured with use of high-resolution sector field inductively coupled plasma mass spectrometry. The hybrid total hip arthroplasty group had mean cobalt levels that were 3.2 times higher at 120 months than they were at baseline, and the cobalt levels in that group were significantly higher than those in the titanium total hip arthroplasty group at thirty-six, sixty, eighty-four, ninety-six, and 120 months (p < 0.01). The hybrid group had mean chromium levels that were 3.9 times higher at 120 months than they were at baseline, and the CoCr total hip arthroplasty group had chromium levels that were 3.6 times higher at 120 months than they were at baseline. The serum titanium levels were higher in the titanium group at all follow-up time intervals as compared with the levels in all other groups, and the level in the titanium group at 120 months was eighteen times higher than it was at baseline (p < 0.01). Patients with well-functioning primary metal-on-polyethylene total hip replacements had elevated serum metal levels for as many as ten years postoperatively. Furthermore, metal release at the modular femoral head-neck junctions, rather than passive dissolution from porous ingrowth surfaces, was likely the dominant source of serum cobalt and chromium.
Level of Evidence:
Therapeutic Level II. See Instructions for Authors for a complete description of levels of evidence.
Background
It has been established that serum metal levels are increased following primary total hip arthroplasty with all types of femoral head-acetabular liner articulation couples1-4. In recent years there has been mounting concern regarding adverse local and systemic effects of elevated metal levels from total joint replacement components. Studies have documented local soft-tissue reactions5-8, pseudotumor formation6, systemic effects7, and end-organ deposition9 with elevated serum metal concentrations from metal-on-metal total hip replacements8. However, increased serum metal levels are not unique to metal-on-metal bearings, and other implant devices may present with elevated metal levels and adverse local and remote tissue responses1,2,9,10. All metallic components of joint replacements are subject to electrochemical corrosion, potentially resulting in the formation of chemically active metal degradation products4.
Over the years, as implants became more durable and were associated with increased longevity, a demographic change took place in the total hip arthroplasty population as younger, more active patients became eligible for the procedure. The long-term physiologic response to elevated serum metal concentrations from total hip arthroplasty implants is unknown. In addition, there is no accepted threshold above which serum concentrations of metals such as cobalt, chromium, and titanium are known to be toxic in patients with a joint replacement. Cobalt and chromium are toxic in high concentrations in vivo, and titanium is potentially carcinogenic at high levels in animal models11. Our original report examined serum metal ion concentrations after primary total hip arthroplasty in a prospective, controlled, longitudinal study with three-year follow-up12. The purpose of the present study was to prospectively determine the serum metal concentrations in patients with primary metal-on-polyethylene total hip replacements at the time of the ten-year follow-up.
Methods
Study Groups
All patients provided informed consent prior to study initiation and the protocol was approved by the institutional review board at our university. Patients eligible for study inclusion were those with a diagnosis of unilateral hip osteoarthritis with no preexisting metallic implants. All prostheses were inserted within the time period of 1989 to 1993 and no metallic implants other than the total hip arthroplasty components were used in any patient. Seventy-five patients were originally enrolled in this study, with fifty-five receiving a unilateral primary total hip arthroplasty for the treatment of osteoarthritis and twenty serving as control subjects, as previously described12.
For the current study, twenty-seven (49%) of the original fifty-five patients who received a primary total hip arthroplasty had ten-year follow-up (Table I). All twenty-seven patients had a well-functioning prosthesis according to the Harris hip score, and none had radiographic evidence of implant loosening or osteolysis. Twenty-eight total hip arthroplasty and seven control patients were disqualified from the study for reasons including contralateral or adjacent joint replacement or implantation of additional metal implants (twelve), revision surgery (eight), failure to appear for follow-up at 120 months (four), refusal to participate in the study protocols (three), and advanced systemic disease or death (eight). Of the remaining twenty-seven study patients, ten patients (the hybrid group) had received a hybrid total hip replacement (cobalt-alloy cemented femoral stem with a titanium acetabular socket without cement), nine patients (the cobalt-chromium [CoCr] group) had received an extensively porous-coated cobalt-alloy femoral stem and titanium acetabular socket without cement, and eight patients (the titanium group) had received a proximally porous-coated titanium-alloy femoral stem and titanium acetabular socket without cement. Cobalt-alloy femoral heads were used in all patients, with a femoral head size of 28 mm used in twenty-one hips and a head size of 32 mm used in six hips. Thirteen (65%) of the original twenty control subjects had ten-year follow-up and continued to serve as control patients (the control group).
TABLE I.
Study Groups at Ten-Year Follow-up
| Original Study Group (N = 75) | Study Group at Ten Years (N = 40) | Number of Men/Women | Years of Age (Range)* | |
| Hybrid† | 20 | 10 | 5/5 | 60 (37 to 74) |
| CoCr† | 15 | 9 | 4/5 | 58 (40 to 80) |
| Ti† | 20 | 8 | 5/3 | 59 (41 to 79) |
| Control† | 20 | 13 | 5/8 | 62 (45 to 70) |
Age values and range are expressed in mean number of years.
Hybrid total hip arthroplasty consisted of a modular cobalt-alloy femoral stem and head inserted with cement and a titanium acetabular socket inserted without cement; cobalt-chromium (CoCr) total hip arthroplasty consisted of an extensively porous-coated modular cobalt-alloy femoral stem with a cobalt-alloy femoral head and a titanium-alloy socket, inserted without cement; titanium (Ti) total hip arthroplasty had a proximally porous-coated modular titanium-alloy femoral stem with a cobalt-alloy femoral head and a titanium socket, inserted without cement; and control subjects had no implants.
The hybrid total hip arthroplasty group consisted of ten (50%) of the original twenty patients who received a hybrid total hip arthroplasty. The femoral component consisted of a modular uncoated cobalt-alloy stem, inserted with cement, along with a 28-mm-diameter cobalt-alloy head with a so-called six-degree taper geometry. Three Iowa and seven Precoat femoral components (both types manufactured by Zimmer, Warsaw, Indiana) had been used. The acetabular component had been inserted without cement and consisted of a commercially pure titanium shell with a diffusion-bonded, commercially pure, titanium fiber-metal porous-coated surface (Harris-Galante II; Zimmer). The acetabular socket was secured to the pelvis with two titanium-alloy self-tapping screws. A snap-fit ultra-high molecular weight polyethylene liner served as the bearing surface.
The CoCr total hip arthroplasty group consisted of nine (60%) of the original fifteen patients who received extensively porous-coated cobalt-alloy femoral and titanium-alloy acetabular components that had been inserted without cement. Six AML (14/16 taper geometry), one Solution (14/16 taper geometry), and two Prodigy (12/14 taper geometry) femoral components (all three types manufactured by DePuy, Warsaw, Indiana) had been inserted, with the decision for the use of each based on preoperative templating and intraoperative bone quality. The acetabular socket consisted of a titanium-alloy shell with a beaded commercially pure titanium porous coating. Seven Duraloc and two Solution acetabular components (both types manufactured by DePuy) had been used. The Duraloc shells had been secured to the pelvis with three porous-coated titanium-alloy spikes, and the Solution shells had been secured with two titanium-alloy screws. The bearing couple consisted of a modular cobalt-alloy femoral head (a 28-mm-diameter head had been used in three hips and a 32-mm-diameter head had been used in six) and an ultra-high molecular weight polyethylene liner.
The titanium total hip arthroplasty group consisted of eight (40%) of the original twenty patients who had received a proximally porous-coated titanium-alloy femoral stem with a cobalt-alloy femoral head and an acetabular component that was identical to that used in the patients in the hybrid group; both the femoral and the acetabular components had been inserted without cement. The femoral stem was made of Ti-6A1-4V with a commercially pure titanium fiber-metal porous-coated surface that was diffusion-bonded onto the proximal aspect of the stem. Six Anatomic femoral components, one Harris-Galante Multilock component, and one Harris-Galante porous-coated (HGP) component (all three types manufactured by Zimmer) had been used. The bearing surface consisted of a modular 28-mm-diameter cobalt-alloy femoral head (with a six-degree taper) and an ultra-high molecular weight polyethylene liner.
The control group comprised thirteen (65%) of the twenty patients who had served as controls in the original study. The thirteen remaining control patients had had no subsequent total joint replacement, implantation of metal components, or progressive systemic diseases as of the time of the ten-year follow-up.
Serum Metal Analysis
Blood samples for serum metal analysis were obtained in accordance with a previously described collection process4. Blood samples were obtained prior to arthroplasty and at the time of the follow-up intervals of twelve, thirty-six, sixty, eighty-four, ninety-six, 108, and 120 months. All vessels and utensils used for the collection of specimens were verified to be free of metal contamination, and blood samples were obtained with use of siliconized butterfly needles in triplicate polypropylene syringes4. Three ten-milliliter syringes were used to draw blood during each testing period, and each syringe was labeled to indicate the sequence of collection. The first ten milliliters was drawn to rinse the needle and adaptor. Blood was then allowed to clot naturally and was centrifuged for thirty minutes, separating the samples into cell and serum fractions that were stored in labeled vials at −80°C. All specimen manipulations following collection were carried out in a class-100 environment with use of a SterilGARD Biological Safety Cabinet (Baker, Sanford, Maine) along with class-100 gloves (Oak Technical, Ravenna, Ohio) to minimize atmospheric and manual contamination.
The serum concentrations of cobalt, chromium, and titanium were measured with use of high-resolution sector field inductively coupled plasma mass spectrometry (HR-SF-ICP-MS) (Element2; Thermo Fisher Scientific GmbH, Bremen, Germany) and with use of the method of additions as described previously13. All calibration solutions and internal standard solutions were prepared by gradual dilutions of single-element standard solutions (1000 μg/mL standard solution; High Purity Standards, Charleston, South Carolina). The certified reference material Seronorm Trace Elements in serum (SERO AS, Billingstad, Norway) was routinely analyzed with samples. To maintain consistent data and improve detection limits, all samples from our original study were retested with HR-SF-ICP-MS. Detection limits of 0.04 nanograms per milliliter (ng/mL) for cobalt, 0.015 ng/mL for chromium, and 0.2 ng/mL for titanium were obtained. Concentrations below these detection limits were assigned a value of one-half of the detection limit by convention in order to calculate means. Since nonparametric statistics were utilized, this had no effect on the statistical analysis for intergroup and intragroup difference. There were no values below the detection limit for titanium, cobalt, or chromium after the initial zero-month assessments.
Statistical Analysis
The data reported in accompanying figures are the means for each group at each time interval with standard errors. Intergroup comparisons were made with use of the Wilcoxon-Mann-Whitney test. Intragroup longitudinal comparisons were made with use of the Friedman test, with significance set at p < 0.05.
Source of Funding
A grant from the National Institutes of Health (NIH)/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) was received in support of preparation of this work (AR 39310).
Results
Cobalt concentrations were elevated in all three implant groups at all follow-up periods as compared with the concentrations in the control group (Fig. 1). The hybrid group had significantly higher cobalt levels at all postoperative time periods as compared with the levels in the control group (p < 0.03). The hybrid group had cobalt levels that were 3.2 times higher at 120 months than they were at baseline, and the cobalt levels in that group were significantly higher than those in the titanium group at thirty-six, sixty, eighty-four, ninety-six, and 120 months (p < 0.01). Cobalt concentrations were significantly higher in the hybrid group than they were in the CoCr group at the ninety-six-month follow-up point (p < 0.05). Intragroup analysis revealed that serum cobalt concentrations in the hybrid group were significantly higher than they were at baseline at all follow-up intervals (p < 0.05).
Fig. 1.
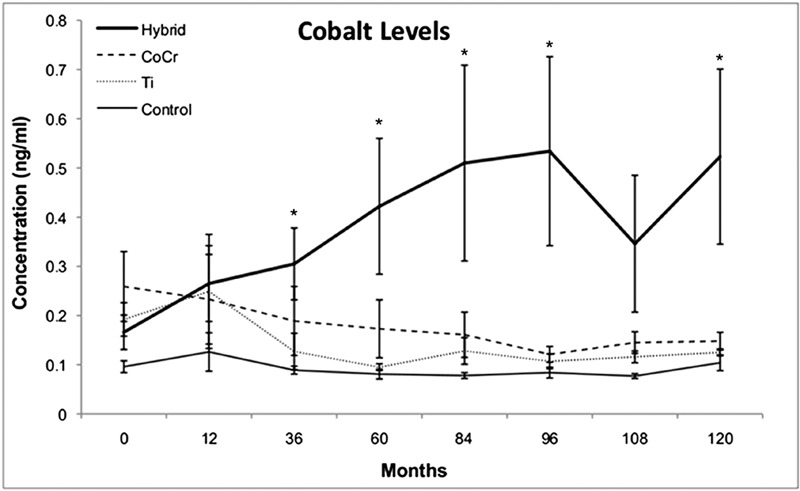
Serum cobalt concentrations. Line graph showing the concentrations of cobalt in the serum, in nanograms per milliliter (ng/mL, parts per billion), as a function of time in the four groups investigated. The hybrid group had cobalt levels that were 3.2 times higher at 120 months than they were at baseline and had cobalt concentrations that were significantly higher than the levels in the titanium (Ti) group at thirty-six, sixty, eighty-four, ninety-six, and 120 months (p < 0.01). *Cobalt levels in the hybrid group were significantly different than those in the titanium group (p < 0.05).
Serum chromium levels tended to be higher for all implant groups at all follow-up periods than they were in the control group, with levels reaching significance at twelve and eighty-four months. At 120 months, chromium levels were 3.9 times higher in the hybrid group than they were at baseline (Fig. 2) and 3.6 times higher in the CoCr group than they were at baseline. At sixty months, the chromium levels in the hybrid group were significantly elevated as compared with those in the CoCr group (p < 0.01). Intragroup analysis revealed that serum chromium concentrations in the hybrid group were significantly higher at all follow-up intervals than they were at baseline (p < 0.04). In addition, the chromium levels in the hybrid group were significantly higher from sixty through 120 months than they were at twelve months (p < 0.04), and the serum chromium levels in the CoCr group were significantly higher at all follow-up periods as compared with baseline levels (p < 0.02) and reached a peak at 120 months. The titanium group experienced early elevations of serum chromium at twelve months (p < 0.03 in comparison with the control group), with a return to near baseline levels at 108 months.
Fig. 2.
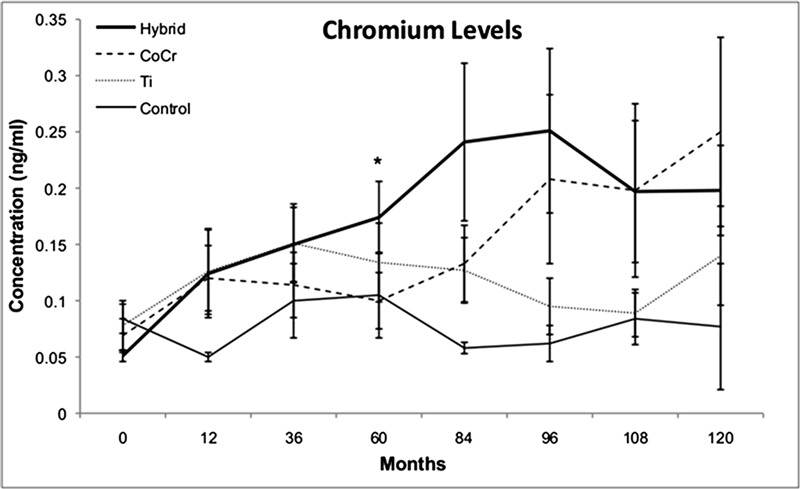
Serum chromium concentrations. Line graph showing the concentrations of chromium in the serum, in nanograms per milliliter (ng/mL, parts per billion), as a function of time in the four groups investigated. At 120 months, chromium levels were 3.9 times higher in the hybrid group than they were at baseline and 3.6 times higher in the CoCr group than they were at baseline. At sixty months, the hybrid group had elevated chromium levels as compared with the levels in the CoCr group (p < 0.01). *Chromium levels in the hybrid group were significantly different than the levels in the CoCr group (p < 0.01). Ti = titanium group.
The titanium concentrations in all three implant groups were significantly elevated at all follow-up periods as compared with the levels seen in the control group (p < 0.01) (Fig. 3). Serum titanium levels in the titanium group were higher than those in all other groups at all follow-up intervals, and they were eighteen times higher than baseline levels at 120 months (p < 0.01). Titanium levels in the titanium group reached a peak early, at thirty-six months, and then proceeded to decline until after the 108-month follow-up interval. The titanium levels at 120 months were 8.9 times higher than they were at baseline in the hybrid group and 9.2 times higher than at baseline in the CoCr group (p < 0.01).
Fig. 3.
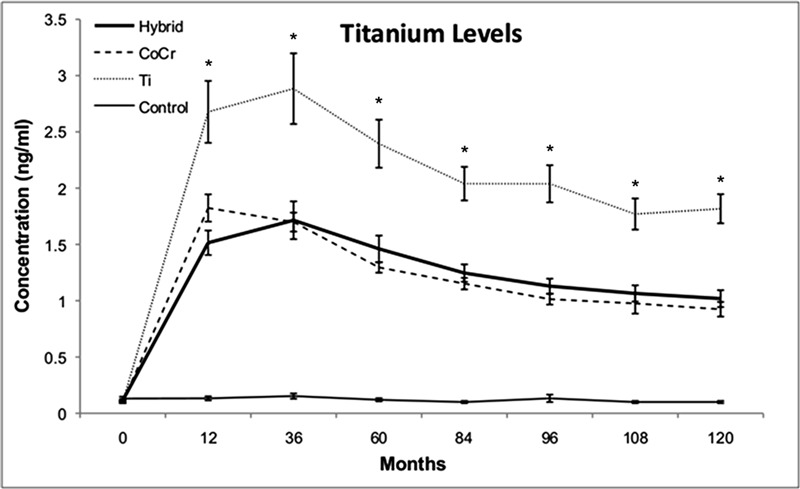
Serum titanium concentrations. Line graph showing the concentrations of titanium in the serum, in nanograms per milliliter (ng/mL, parts per billion), as a function of time in the four groups investigated. The titanium levels at 120 months were 8.9 times higher than they were at baseline in the hybrid group and 9.2 times higher than at baseline in the CoCr group. Serum titanium levels in the titanium (Ti) group were higher than those in all other groups at all follow-up intervals, and they were eighteen times higher than baseline levels at 120 months (p < 0.01). *Titanium levels in the titanium group were significantly different than those in the hybrid and CoCr groups (p < 0.01).
Conclusions
This is a follow-up report of a prospective longitudinal controlled study investigating serum metal ion concentrations in three patient cohorts with well-functioning unilateral metal-on-polyethylene total hip replacements ten years after surgery. Elevation of serum metal levels after primary total hip arthroplasty is well-documented4,12,14-17, and several studies have investigated the serum levels of cobalt and chromium after joint arthroplasty14-16,18,19. However, there is limited information available regarding the long-term impact of primary metal-on-polyethylene total hip replacements on the serum concentrations of metals, particularly for serum titanium in titanium-containing implants4,12,20,21.
Results from our previous study led us to conclude that fretting corrosion at the modular femoral head-neck junction was the likely source of increased chromium release rather than passive dissolution. This conclusion was reached on the basis of elevations in serum chromium in the hybrid group (the group with cobalt alloy femoral stems, inserted with cement) as compared with the CoCr group (the group with fully porous-coated femoral stems, inserted without cement). The hybrid group had a head-neck taper geometry with less flexural rigidity of the neck, a factor which has been shown to correlate with mechanically assisted crevice corrosion (MACC) at these interfaces22,23.
In contrast with our previous findings of low cobalt concentrations12, the results at the time of the ten-year follow-up showed elevated cobalt levels in all three implant groups as compared with the levels in the control group. This difference is due to the nearly tenfold improvement in the detection limit of cobalt that can be achieved with HR-SF-ICP-MS (detection limit, 0.04 ng/mL) as compared with atomic absorption spectrometry (detection limit, 0.3 ng/mL), which was the analysis system used in the previous study. Increases in serum levels of cobalt were previously observed in the setting of metal-on-metal articulations and failed implants with modular head-neck junction corrosion and poorly functioning primary total hip prostheses24,25. Factors that could affect the fretting corrosion process, causing increased cobalt concentrations, include taper size of the femoral stem, geometry, tolerances, assembly forces, surface finish, and metallurgy22,26,27. In the current study, there were no significant differences in cobalt levels between the CoCr group, in which extensively porous-coated cobalt-alloy femoral stems had been used, and the titanium group, in which proximally porous-coated titanium-alloy femoral stems had been used, despite the fact that there were significant elevations at all follow-up intervals in comparison with the levels seen in the control group. Since the only source of cobalt in the titanium group is the femoral head, the origin of the disseminated cobalt must be either the articular surface or the femoral head bore. On the basis of the report by Goldberg et al., the bore of the femoral head is the most likely source23. In addition, the lack of difference in cobalt levels between these two groups supports the conclusions of our earlier study that passive dissolution from porous surface areas is not a dominant mode of metal release.
The finding of elevated cobalt levels in the hybrid group as compared with the levels seen in the titanium group at thirty-six to 120 months is noteworthy. One potential source of the elevated cobalt levels in the hybrid group is fretting corrosion of the femoral stem in the cement mantle. This source seems unlikely in these patients who were judged as having a well-functioning total hip prosthesis on the basis of follow-up clinical evaluation and stable radiographs at the time of the latest follow-up. A more likely cause is the difference in the composition of the head-neck junctions. While these components have identical taper geometries, the titanium group had a femoral head-neck junction that consisted of titanium-alloy against cobalt-alloy, whereas the hybrid group had a femoral head-neck junction that consisted of cobalt-alloy against cobalt-alloy. Thus, the cobalt-alloy surface area of the femoral head-neck junction in the hybrid group was roughly twice that of the femoral head-neck junction in the titanium group. Furthermore, lower cobalt levels in patients with the mixed-metal taper connection suggest that galvanic corrosion is not a dominant mode of metal release.
Our previous results indicated that there were higher levels of chromium in the titanium group than there were in the hybrid and CoCr groups throughout the thirty-six-month follow-up. However, in the current study these increased values did not persist after thirty-six months. The results seen for chromium levels in the present study were similar to those seen for cobalt levels in that the chromium concentration levels were similar between the CoCr and the titanium groups (in which porous-coated components were used) but were increased in the hybrid group (in which no porous-coated component was used). Chromium results gave further support to the theory that MACC at the modular head-neck junction (rather than passive dissolution at porous surfaces) was a primary source of metal release. The fact that cobalt levels in the hybrid group were more consistently elevated than chromium levels in this study is consistent with our recent report documenting disproportionate elevations in serum cobalt with respect to serum chromium in patients with adverse local tissue reactions due to MACC associated with modular metal-on-polyethylene implants28. In this report, revision surgery consisted of retention of the femoral component with insertion of a modular ceramic head with a titanium alloy sleeve. This resulted in resolution of symptoms and a decrease in serum cobalt levels consistent with the findings reported here of lower cobalt levels in mixed-metal head-neck junctions28.
Patients in the titanium group of our study previously had titanium levels that were 3.4 times greater than those of patients in the control group at thirty-six months. Previous studies have reported that serum titanium levels are elevated only in patients with a loose prosthesis requiring revision surgery4,20. In the current study, the titanium group had a peak titanium level at thirty-six months that progressively decreased until after the 108-month follow-up interval. The patients who had titanium-alloy femoral stems as well as titanium acetabular sockets had the highest titanium levels at all time intervals, indicating that femoral and acetabular components were both sources of disseminated titanium degradation products.
The fact that only roughly half of the original patients from the three-year follow-up study were qualified and available for long-term metal ion analysis is a major limitation of this study and represents attrition bias29. Thirty-five patients were disqualified or removed from the original study group as previously described. Our decreased sample size may have prevented further trends and differences between and within groups from being detected. It is likely that small group size contributed to large error bars and variable significant differences found in metal ion levels. However, no one specific study group lost significantly more patients than the other, making statistical analysis of metal level differences for the different implant types and controls valid.
Another limitation of this study is that it was not randomized and there was selection bias with regard to the type of implant used as well as the method of fixation, both of which were chosen based on surgeon preference. One of the general limitations in the literature regarding metal ion concentrations is the wide variability in how measurements are gathered, analyzed, and reported, thus making comparisons between studies difficult18. Even within studies there is substantial technical difficulty in ensuring that samples are drawn, processed, and analyzed in the exact same manner for consistency of results. Detecting differences between groups is challenging due to small detection limits, variations among individuals, and potential outside interferences18. An advantage of the present study was that all study samples were drawn and analyzed with identical technique within a single laboratory at our institution, which has expertise in analyzing serum metal ion concentrations, thus allowing more subtle differences between groups to be detected.
The biologic and clinical effects of increased serum metal concentrations derived from total joint prostheses remain unclear. The direct causal relationship between elevated metal levels and clinical symptoms has not been well defined, despite individual reports of severe local and systemic reactions in the presence of increased serum metal concentrations after total joint replacement6,7. Systemic responses to metal debris from joint replacement remain rare events3, but the timing and threshold of these events are unknown. Accurate and longitudinal monitoring of serum metal concentrations may be an important part of postoperative care, especially in patients who have unexplained symptoms that raise concerns regarding the possibility of an adverse metal reaction. It is important to note that, even in patients with well-functioning prostheses who have not experienced increases in serum metal concentrations, deposition and accumulation of metal ions can occur locally and in remote organs3.
The present study contributes to the understanding of long-term changes in serum metal levels in patients after primary total joint replacement and how these levels are influenced by component composition and taper geometry. In addition, this study provides normative data on patients with a well-functioning metal-on-polyethylene total hip arthroplasty, which can be helpful in evaluating patients who are suspected of having an adverse local tissue reaction to MACC. The toxicological risks associated with metal ions from joint prostheses represent an area of much-needed investigation, as the number of patients who will receive implants is estimated to increase rapidly over the next decade.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Agins HJ, Alcock NW, Bansal M, Salvati EA, Wilson PD, Jr, Pellicci PM, Bullough PG. Metallic wear in failed titanium-alloy total hip replacements. A histological and quantitative analysis. J Bone Joint Surg Am. 1988;70(3):347-56 [PubMed] [Google Scholar]
- 2.Black J, Sherk H, Bonini J, Rostoker WR, Schajowicz F, Galante JO. Metallosis associated with a stable titanium-alloy femoral component in total hip replacement. A case report. J Bone Joint Surg Am. 1990;72(1):126-30 [PubMed] [Google Scholar]
- 3.Jacobs JJ, Shanbhag A, Glant TT, Black J, Galante JO. Wear Debris in Total Joint Replacements. J Am Acad Orthop Surg. 1994;2(4):212-20 [DOI] [PubMed] [Google Scholar]
- 4.Jacobs JJ, Skipor AK, Black J, Urban R, Galante JO. Release and excretion of metal in patients who have a total hip-replacement component made of titanium-base alloy. J Bone Joint Surg Am. 1991;73(10):1475-86 [PubMed] [Google Scholar]
- 5.Hsu AR, Kim JD, Fabi D, Levine BR. Adverse reactions in metal-on-metal total hip arthroplasty: two cases presenting as pseudoseptic acetabular component loosening. Am J Orthop (Belle Mead NJ). 2011;40(10):509-13 [PubMed] [Google Scholar]
- 6.Watters TS, Eward WC, Hallows RK, Dodd LG, Wellman SS, Bolognesi MP. Pseudotumor with superimposed periprosthetic infection following metal-on-metal total hip arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(7):1666-9 [DOI] [PubMed] [Google Scholar]
- 7.Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty: a case report. J Bone Joint Surg Am. 2010;92(17):2847-51 [DOI] [PubMed] [Google Scholar]
- 8.Willert HG, Buchhorn GH, Fayyazi A, Flury R, Windler M, Köster G, Lohmann CH. Metal-on-metal bearings and hypersensitivity in patients with artificial hip joints. A clinical and histomorphological study. J Bone Joint Surg Am. 2005;87(1):28-36 [DOI] [PubMed] [Google Scholar]
- 9.Urban RM, Tomlinson MJ, Hall DJ, Jacobs JJ. Accumulation in liver and spleen of metal particles generated at nonbearing surfaces in hip arthroplasty. J Arthroplasty. 2004;19(8)(Suppl 3):94-101 [DOI] [PubMed] [Google Scholar]
- 10.Jacobs JJ, Urban RM, Wall J, Black J, Reid JD, Veneman L. Unusual foreign-body reaction to a failed total knee replacement: simulation of a sarcoma clinically and a sarcoid histologically. A case report. J Bone Joint Surg Am. 1995;77(3):444-51 [DOI] [PubMed] [Google Scholar]
- 11.Angle CR. Organ-specific therapeutic intervention. : Goyer RA, Klaassen CD, Waalkes MP, Metal toxicology. San Diego: Academic Press; 1995. p 71-110 [Google Scholar]
- 12.Jacobs JJ, Skipor AK, Patterson LM, Hallab NJ, Paprosky WG, Black J, Galante JO. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998;80(10):1447-58 [DOI] [PubMed] [Google Scholar]
- 13.Iavicoli I, Falcone G, Alessandrelli M, Cresti R, De Santis V, Salvatori S, Alimonti A, Carelli G. The release of metals from metal-on-metal surface arthroplasty of the hip. J Trace Elem Med Biol. 2006;20(1):25-31 [DOI] [PubMed] [Google Scholar]
- 14.Bartolozzi A, Black J. Chromium concentrations in serum, blood clot and urine from patients following total hip arthroplasty. Biomaterials. 1985;6(1):2-8 [DOI] [PubMed] [Google Scholar]
- 15.Black J, Maitin EC, Gelman H, Morris DM. Serum concentrations of chromium, cobalt and nickel after total hip replacement: a six month study. Biomaterials. 1983;4(3):160-4 [DOI] [PubMed] [Google Scholar]
- 16.Brien WW, Salvati EA, Betts F, Bullough P, Wright T, Rimnac C, Buly R, Garvin K. Metal levels in cemented total hip arthroplasty. A comparison of well-fixed and loose implants. Clin Orthop Relat Res. 1992;(276):66-74 [PubMed] [Google Scholar]
- 17.Jacobs JJ, Silverton C, Hallab NJ, Skipor AK, Patterson L, Black J, Galante JO. Metal release and excretion from cementless titanium alloy total knee replacements. Clin Orthop Relat Res. 1999;(358):173-80 [PubMed] [Google Scholar]
- 18.Michel R, Nolte M, Reich M, Löer F. Systemic effects of implanted prostheses made of cobalt-chromium alloys. Arch Orthop Trauma Surg. 1991;110(2):61-74 [DOI] [PubMed] [Google Scholar]
- 19.Sunderman FW, Jr, Hopfer SM, Swift T, Rezuke WN, Ziebka L, Highman P, Edwards B, Folcik M, Gossling HR. Cobalt, chromium, and nickel concentrations in body fluids of patients with porous-coated knee or hip prostheses. J Orthop Res. 1989;7(3):307-15 [DOI] [PubMed] [Google Scholar]
- 20.Dorr LD, Bloebaum R, Emmanual J, Meldrum R. Histologic, biochemical, and ion analysis of tissue and fluids retrieved during total hip arthroplasty. Clin Orthop Relat Res. 1990;(261):82-95 [PubMed] [Google Scholar]
- 21.Kärrholm J, Frech W, Nilsson KG, Snorrason F. Increased metal release from cemented femoral components made of titanium alloy. 19 hip prostheses followed with radiostereometry (RSA). Acta Orthop Scand. 1994;65(6):599-604 [DOI] [PubMed] [Google Scholar]
- 22.Gilbert JL, Buckley CA, Jacobs JJ. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res. 1993;27(12):1533-44 [DOI] [PubMed] [Google Scholar]
- 23.Goldberg JR, Gilbert JL, Jacobs JJ, Bauer TW, Paprosky W, Leurgans S. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin Orthop Relat Res. 2002;(401):149-61 [DOI] [PubMed] [Google Scholar]
- 24.Jacobs JJ, Urban RM, Gilbert JL, Skipor AK, Black J, Jasty M, Galante JO. Local and distant products from modularity. Clin Orthop Relat Res. 1995;(319):94-105 [PubMed] [Google Scholar]
- 25.Jacobs JJ, Skipor AK, Doorn PF, Campbell P, Schmalzried TP, Black J, Amstutz HC. Cobalt and chromium concentrations in patients with metal on metal total hip replacements. Clin Orthop Relat Res. 1996;(329)(Suppl):S256-63 [DOI] [PubMed] [Google Scholar]
- 26.Brown SA, Flemming CA, Kawalec JS, Placko HE, Vassaux C, Merritt K, Payer JH, Kraay MJ. Fretting corrosion accelerates crevice corrosion of modular hip tapers. J Appl Biomater. 1995;6(1):19-26 [DOI] [PubMed] [Google Scholar]
- 27.Young DL, Bobyn JD, Krygier JJ, Dujovne AR. Factors affecting fretting damage at the Morse taper junction of modular hip implants. Trans Soc Biomater. 1995;18:49 [Google Scholar]
- 28.Cooper HJ, Della Valle CJ, Berger RA, Tetreault M, Paprosky WG, Sporer SM, Jacobs JJ. Corrosion at the head-neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am. 2012 Sep 19;94:1655-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jüni P, Egger M. Commentary: Empirical evidence of attrition bias in clinical trials. Int J Epidemiol. 2005;34(1):87-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest


