Abstract
Background:
Femoral stems with dual-taper modularity were introduced to allow additional options for hip-center restoration independent of femoral fixation in total hip arthroplasty. Despite the increasing availability and use of these femoral stems, concerns exist about potential complications arising from the modular neck-body junction.
Methods:
This was a multicenter retrospective case series of twelve hips (eleven patients) with adverse local tissue reactions secondary to corrosion at the modular neck-body junction. The cohort included eight women and three men who together had an average age of 60.1 years (range, forty-three to seventy-seven years); all hips were implanted with a titanium-alloy stem and cobalt-chromium-alloy neck. Patients presented with new-onset and increasing pain at a mean of 7.9 months (range, five to thirteen months) following total hip arthroplasty. After serum metal-ion studies and metal artifact reduction sequence (MARS) magnetic resonance imaging (MRI) revealed abnormal results, the patients underwent hip revision at a mean of 15.2 months (range, ten to twenty-three months). Tissue specimens were examined by a single histopathologist, and the retrieved implants were studied with use of light and scanning electron microscopy.
Results:
Serum metal levels demonstrated greater elevation of cobalt (mean, 6.0 ng/mL) than chromium (mean, 0.6 ng/mL) or titanium (mean, 3.4 ng/mL). MRI with use of MARS demonstrated adverse tissue reactions in eight of nine patients in which it was performed. All hips showed large soft-tissue masses and surrounding tissue damage with visible corrosion at the modular femoral neck-body junction. Available histology demonstrated large areas of tissue necrosis in seven of ten cases, while remaining viable capsular tissue showed a dense lymphocytic infiltrate. Microscopic analysis was consistent with fretting and crevice corrosion at the modular neck-body interface.
Conclusions:
Corrosion at the modular neck-body junction in dual-tapered stems with a modular cobalt-chromium-alloy femoral neck can lead to release of metal ions and debris resulting in local soft-tissue destruction. Adverse local tissue reaction should be considered as a potential cause for new-onset pain in patients with these components, and early revision should be considered given the potentially destructive nature of these reactions. A workup including serologic studies (erythrocyte sedimentation rate and C-reactive protein), serum metal levels, and MARS MRI can be helpful in establishing this diagnosis.
Level of Evidence:
Therapeutic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Modularity in total hip arthroplasty has become increasingly common in the last two decades. Following the successful introduction of modular heads, femoral stems with an additional modular junction between the neck and body of the stem soon became available. These so-called dual-taper stems allow for increased options for hip-center restoration by allowing for adjustments in offset, length, and version, which can be made independent of femoral stem fixation1-3. Most major device manufacturers offer stem designs with a modular neck option.
This additional modular junction creates new concerns, including the potential for corrosion between the neck and body of the stem. Although corrosion at this junction has been documented in retrieval analyses4-6, in vitro studies7,8, and case reports of modular neck fracture9-11, the biological implications of taper corrosion at this junction are poorly understood. Recent studies have documented the potential for taper corrosion at the head-neck junction to lead to the release of metal debris and severe adverse local tissue reactions12-16, such as that seen in patients with failed metal-on-metal bearings17-20. Similar reactions have been reported in a small series of patients with dual-taper stems21, but these reactions have not yet been reported in a larger cohort of patients.
The purpose of the present study was to describe a series of patients who presented with painful adverse local tissue reactions secondary to corrosion at the modular neck-body junction and document the clinical presentation, diagnostic workup, and surgical findings.
Materials and Methods
Study Population
This study was designed as a retrospective descriptive case series of twelve hips in eleven patients who underwent revision of a total hip arthroplasty as a result of adverse local tissue reaction secondary to corrosion at the modular femoral neck-body taper junction. This was a multicenter, multisurgeon series involving six surgeons at five different institutions. Revision arthroplasties were performed over a nineteen-month period (December 2010 through June 2012). Images from one of the eleven patients have been recently published as a part of a review article22. The study group consisted of eight women and three men with an average age of 60.1 years (see Appendix). Approval was obtained from two institutional review boards where the authors worked; for the other three centers, specimens were sent to the “main” institution along with clinical data (prior to study initiation) to help those treating surgeons make the appropriate diagnosis. The study was initiated later, and the institutional review board at the “main institution” specified that specimens already in the implant retrieval laboratory could be used.
Patients initially presented with new-onset pain at a mean of 7.9 months (range, 5.1 to 13.3 months) after the index surgery, following an uneventful and asymptomatic initial recovery period (see Appendix). All patients presented with pain; the pain was localized to the groin in eleven of twelve hips, but commonly involved the buttock (five hips), trochanteric region (four hips), or thigh (three hips). A serious limp or weakness was evidenced in three patients (four hips), and one of these patients complained of marked leg swelling. A frequent finding on physical examination was pain localizing to the groin with straight leg-raise testing against gravity or resistance. Harris hip scores23 averaged 43.2 points prior to revision surgery (range, 22 to 71 points) in the five patients for whom they were available.
Eleven primary procedures were performed via a posterior approach, while one was performed via an anterolateral approach; all were performed for a diagnosis of degenerative joint disease (see Appendix). The implanted acetabular components included ten Trident PSL shells (Stryker, Mahwah, New Jersey), one Tritanium shell (Stryker), and one Anatomic Dual Mobility cup (Stryker) that was used as part of a Mobile Bearing Hip System (Stryker). The bearing surface was ceramic (BIOLOX Delta; CeramTec, Plochingen, Germany) on highly crossed-linked polyethylene (X3; Stryker) in eight cases, and cobalt-chromium alloy on highly cross-linked polyethylene in the other four. All of the femoral components were a single design (Rejuvenate; Stryker) made from a titanium-molybdenum-zirconium-iron alloy (TMZF; Stryker) with a cobalt-chromium-alloy modular neck. This implant was voluntarily recalled by the manufacturer in June 2012. The implanted femoral components, femoral neck lengths, and femoral head sizes are provided in the Appendix.
Preoperative Workup
Inflammatory markers were assessed in every patient, including erythrocyte sedimentation rate and C-reactive protein. Aspiration of the hip was performed preoperatively in ten of twelve hips. Using analytical methodology previously described24, serum metal ion levels were obtained at a specialized trace-metal-analysis laboratory for eight of the twelve hips; the metal levels for two additional hips were analyzed at a commercial laboratory. All patients underwent radiography of the hip at the time of initial symptoms. Magnetic resonance imaging (MRI) scans were performed with use of metal artifact reduction sequence (MARS) protocol in ten of the twelve hips (nine of the eleven patients) and were read by a musculoskeletal radiologist at the institution in which they were performed.
Revision Surgery
Revision surgery was performed at a mean of 15.2 months after the index procedure (range, 9.7 to 23.1 months). A posterior approach was utilized in all cases. Frozen sections and intraoperative cultures were taken upon entry into the joint in five hips. Capsular tissue was preserved in formalin, and was sent to a single specialized laboratory for histological analysis in ten of the twelve hips. Ten of the twelve explanted femoral components were sent for analysis to a specialized implant retrieval laboratory.
Laboratory Analysis
The excised capsule and surrounding tissue were graded (on a scale of 1 to 4 [see Appendix]) by the pathologist for presence of necrosis, inflammatory exudate, diffuse lymphocytes, perivascular lymphocytes, plasma cells, eosinophils, and polymorphonuclear leukocytes using light microscopy of routine paraffin sections stained with hematoxylin and eosin. The retrieved modular neck-body and head-neck junctions were examined for evidence of fretting and corrosion with use of a stereo light microscope at eight to fifty times magnification and with a scanning electron microscope (model 6490LV, JEOL U.S.A., Peabody, Massachusetts) at magnifications from 500 to 5000 (see Appendix). The degree of corrosion was graded by one of the authors (R.M.U.) as none (grade 1), mild (grade 2), moderate (grade 3), or severe (grade 4), according to the criteria of Goldberg25.
Source of Funding
This study was supported in part by funding from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS) Grant AR39310, and in part by a research grant from Zimmer, Inc. (Warsaw, Indiana); both of these grants aided in the development and operations of the specialized laboratories used for implant retrieval analysis, histopathological analysis, and trace metal ion analysis.
Results
The erythrocyte sedimentation rate was elevated in seven of eleven patients (mean, 31.2 mm/hr; range, 3 to 62 mm/hr; normal, <27 mm/hr), while CRP was elevated in five of eleven patients (mean, 14 mg/L; range, <5 to 36 mg/L; normal, <8 mg/L) (see Appendix). Both markers were elevated in four of eleven patients. Due to large amounts of amorphous material or cellular destruction in the aspirated samples of five of the ten hips that had undergone aspiration, the laboratory was unable to perform a cell count of the aspirated synovial fluid for those five hips; in the remaining five samples, the mean synovial fluid white blood-cell (WBC) count was 3427 WBC/μL (range, 2 to 11,500 WBC/μL) with a mean differential of 54% neutrophils (range, 32% to 90%), 22% lymphocytes (range, 1% to 39%), and 9% monocytes (range, 1% to 17%). On the basis of previously established thresholds26, the synovial fluid WBC count was suggestive of infection in one case (11,500 WBC/μL), but this was not supported by the differential (34% neutrophils). This same patient had an elevated erythrocyte sedimentation rate (59 mm/hr) but a normal C-reactive protein level (7 mg/L). Aerobic and anaerobic cultures failed to demonstrate growth in any of the ten hips; and the patients were not taking antibiotics prior to aspiration.
Serum metal ion levels demonstrated a substantial elevation in serum cobalt, which was elevated to a greater extent than serum chromium; serum titanium levels were generally within the normal reference range of patients with a well-functioning total hip arthroplasty (see Appendix). Radiographs were interpreted as normal in most cases, but one demonstrated subtle scalloping of the bone along the medial femoral neck. MRIs were interpreted as normal in one patient, but the remaining studies demonstrated large fluid collections and/or hypertrophic soft-tissue reactions or pseudotumor formation (Fig. 1).
Fig. 1.
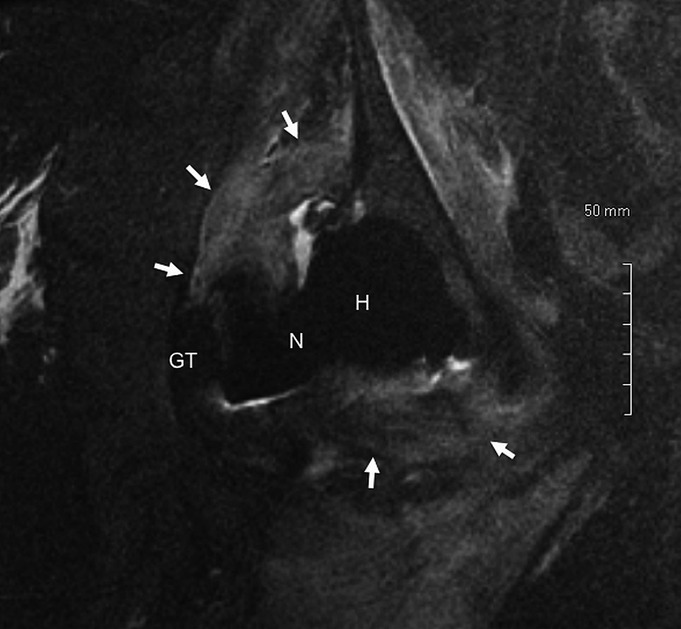
Fig. 1 Coronal short tau inversion recovery (STIR) magnetic resonance image acquired with use of metal artifact reduction sequence (MARS), demonstrating a large pseudotumor (arrows) surrounding the prosthetic hip joint, approximately twenty-one months following total hip arthroplasty (Case 3). As a reference, the greater trochanter (GT), modular prosthetic femoral neck (N), and modular femoral head (H) are labeled.
On the average, it took 8.6 months (range, 2.2 to 14.7 months) to establish a diagnosis and perform revision surgery after the patient’s initial presentation of symptoms. Patients often sought second opinions when the etiology of the pain was unclear. One patient (Case 3) underwent a lumbar spinal fusion, which was not successful in alleviating any of her symptoms, before the underlying diagnosis of taper corrosion was made. Another patient (Case 1) underwent an exploratory irrigation and debridement, tissue biopsy, and modular head, neck, and liner exchange without relief of symptoms.
Surgical Findings
At the time of revision surgery, a large amount of fluid was typically encountered upon entry into the hip joint. In all tissue specimens obtained, frozen sections demonstrated no evidence of acute inflammation and intraoperative cultures eventually yielded no growth. Capsular hypertrophy and necrosis of the soft-tissue structures surrounding the hip joint were typical, resulting in large soft-tissue masses in several patients. A thorough debridement was performed, removing all abnormal hypertrophic and necrotic soft tissue. In each hip, the modular junction between the neck and body of the femoral component demonstrated obvious corrosion, with fretting of the taper and deposition of black, flaky material at its base. The modular necks remained engaged on the tapers in all hips without any signs of loosening. The femoral components were revised in all hips. All components were well fixed with osseous ingrowth, with seven of twelve hips requiring an extended trochanteric osteotomy for component removal. Three hips that did not have an extended trochanteric osteotomy sustained a proximal femoral fracture during or after revision; one fracture was bypassed, the second was treated with a proximal cerclage cable intraoperatively with uneventful healing, and the third developed a postoperative trochanteric fracture requiring internal fixation with additional surgery. The acetabular component was well fixed in all hips but was concomitantly revised in the hip of one patient because the treating surgeon chose not to use the existing dual-mobility bearing; the modular liner was exchanged in the remaining eleven hips. In each hip, the existing bearing surface appeared normal without any evidence of wear.
Laboratory Analysis
There was severe corrosion at the modular femoral neck-body junction in all ten of the retrieved devices, with evidence of fretting, corrosion, and fretting-corrosion debris seen with use of light microscopy (Fig. 2) and scanning electron microscopy (Fig. 3). At the head-neck junction, pitting corrosion (Fig. 4) was severe in six cases (Cases 2, 3, 5, 6, 8, and 9) (see Appendix).
Fig. 2.

Fig. 2 Posterior (left) and medial (right) light microscopic images of modular femoral neck that was retrieved after approximately sixteen months in vivo (Case 2). Surface damage, consisting of fretting, corrosion, and black debris, was apparent on all retrieved cobalt-chromium modular femoral neck-body junctions.
Fig. 3.
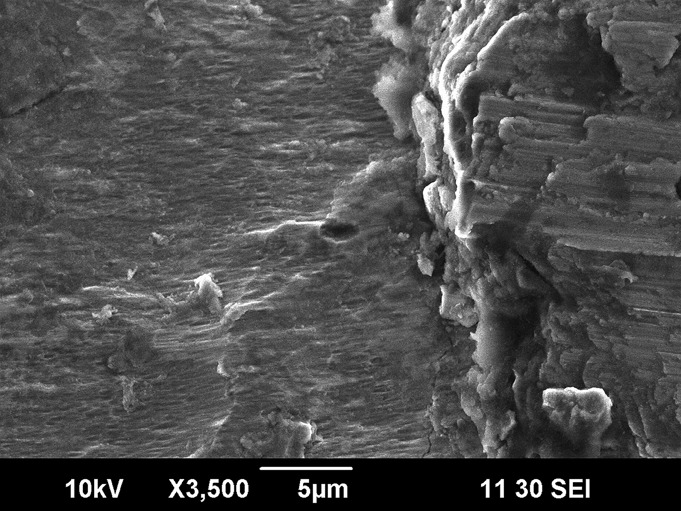
Fig. 3 Scanning electron microscope image (×3500) demonstrating fretting and corrosion (left and center) as well as a thick deposit of fretting-corrosion debris (right) on the lateral aspect of a damaged neck-body junction that had been in vivo for approximately twenty months (Case 1). Similar findings were seen in all ten retrieved implants.
Fig. 4.
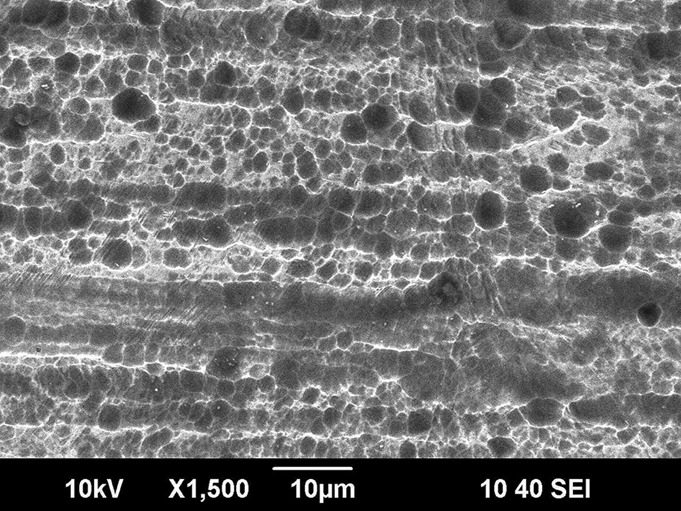
Fig. 4 Scanning electron microscope image (×1500) of a femoral head-neck junction that had been in vivo for sixteen months, demonstrating marked pitting corrosion of a cobalt-chromium femoral neck that was mated with a ceramic head (Case 6). Femoral head-neck corrosion was found in six of the ten implants analyzed (five with ceramic heads, and one with a metal head).
Stained histological sections were available from ten of the twelve revised hips. Seven of these had marked necrosis of the joint pseudocapsule, and seven showed mild to moderate surface fibrin (see Appendix). Viable areas of the pseudocapsule showed abundant perivascular and diffuse lymphocytes (Fig. 5). In six cases, diffuse lymphocytes dominated, and a more laminated appearance was evident (Fig. 6). The lymphocytes were often accompanied by plasmacytes and/or eosinophils (Fig. 7). Polymorphonuclear leukocytes were rare. The histology was normal in Case 12, for which only a small tissue sample was available. Numerous particles of translucent, pale-green chromium phosphate (CrPO4) corrosion products were present in the tissues or within fibrin exudate covering the surface of the joint pseudocapsule (Appendix; Fig. 8). Opaque metallic or birefringent polyethylene wear particles in macrophages were only rarely observed in the histological sections. Notably, there were no qualitative or quantitative differences in the appearance of the periprosthetic tissue between patients with elevated or normal inflammatory markers.
Fig. 5.
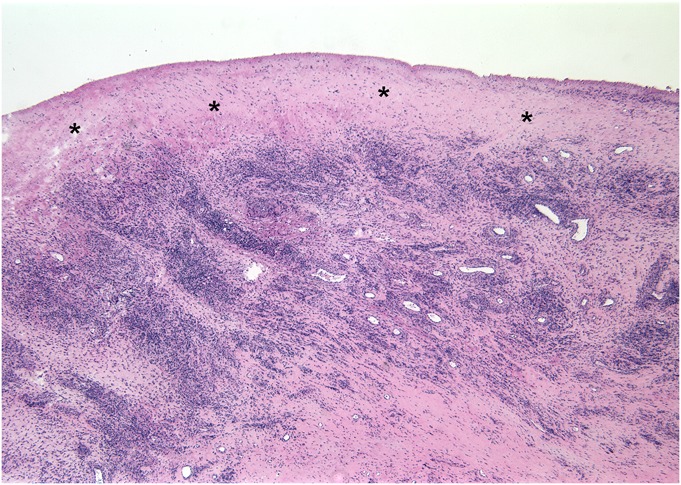
Fig. 5 Histological surgical specimen obtained 12.6 months following hip arthroplasty (Case 4), demonstrating abundant diffuse and perivascular lymphocytes in the joint pseudocapsule. The synovial lining is absent, and a layer of organizing fibrin exudate (*) covers the inner surface of the capsule (hematoxylin and eosin stain, ×25 magnification).
Fig. 6.
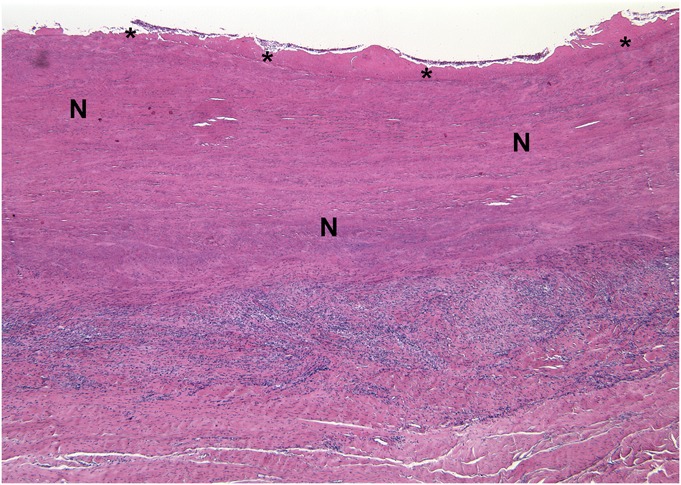
Fig. 6 Histological specimen obtained during revision surgery after 12.5 months in vivo (Case 5). A broad layer of the joint pseudocapsule is necrotic (N) and covered by a surface layer of fibrin (*). Diffuse lymphocytes and a lesser number of eosinophils form a distinct layer (hematoxylin and eosin stain, ×25 magnification).
Fig. 7.
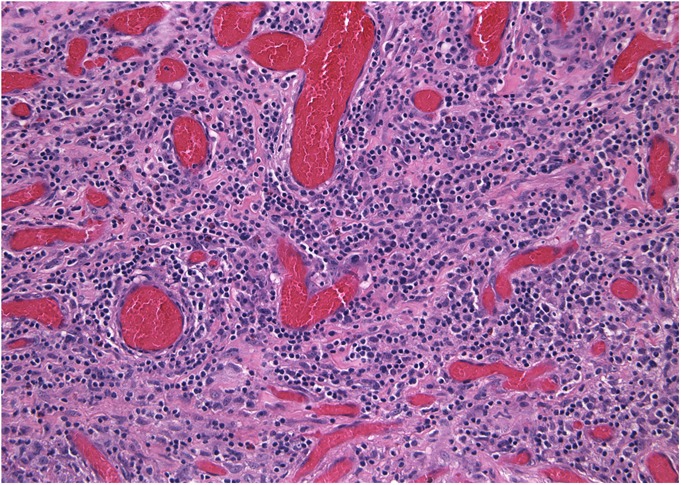
Histological specimen removed during revision surgery (Case 1). The majority of the specimen was necrotic, but viable areas of pseudocapsule showed chronic inflammation dominated by diffuse lymphocytes and scattered eosinophils (hematoxylin and eosin stain, ×200 magnification).
Fig. 8.
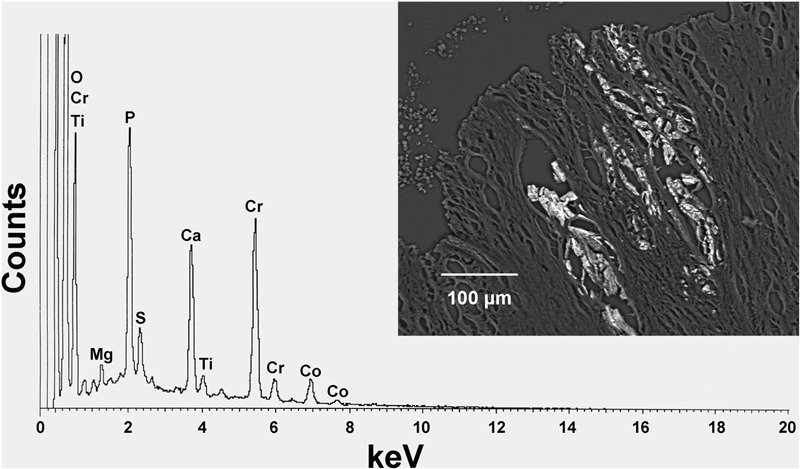
An energy-dispersive x-ray spectrum (Case 5) reveals the elemental composition of particles rich in chromium (Cr) and consistent with chromium phosphate corrosion product. Traces of cobalt (Co) and titanium (Ti) are also present, the latter suggesting that these particles were generated by corrosion at the junction between the cobalt-chromium modular femoral neck and the titanium alloy stem. The particles (inset) lie in necrotic fibrous tissue of the joint pseudocapsule (backscattered electron micrograph, ×200). O = oxygen, Mg = magnesium, P = phosphorus, S = sulfur, and Ca = calcium.
Discussion
The potential for corrosion at modular taper junctions in total hip implants was first described in the early 1980s27 and, since that time, has been documented in numerous retrieval studies25,28-36. While there have long been concerns that corrosion at these modular junctions might produce soluble and particulate debris with the potential to migrate locally and systemically37-39, there have been relatively few reports of adverse local tissue reactions and pseudotumor formation secondary to taper corrosion and the resultant metal debris; most have been described only recently12-16,40. These soft-tissue reactions appear clinically and histologically similar to the adverse local tissue reactions seen in failed metal-on-metal bearings17-20. To date, these reports have generally been limited to corrosion occurring at the modular head-neck junction rather than the modular neck-body junction.
Numerous concerns have arisen in recent years over potential complications specific to dual-tapered stem designs, including the potential for fracture of the modular neck component9-11,41-45, dissociation of the modular neck from the body46,47, and corrosion at the modular neck-body interface4-8. However, to date, there have been only two small case series describing a total of five cases of adverse local tissue reaction in response to corrosion at the modular neck-body junction; these occurred in different dual-taper stem designs that also utilized modular cobalt-chromium-alloy necks5,21. The current study documents the potential for this process to cause pain and severe soft-tissue destruction around the hip in a larger cohort of patients, which can lead to early failure within the first several years of implantation.
Fretting and corrosion at the head-neck junction can also contribute to the generation of particulate and soluble products of corrosion and to an adverse local-tissue response when a cobalt-chromium-alloy head is mated with a cobalt-chromium-alloy or titanium-alloy stem12. In vivo fretting corrosion tests of one design indicated that zirconia heads mated with cobalt-chromium-alloy stems produced less fretting than that seen with cobalt-chromium-alloy heads mated with cobalt-chromium-alloy stems48. In the present study, fretting and extensive pitting corrosion were observed in six of ten head-neck junctions studied, five of which employed a ceramic head. Similar findings have been documented in a previous retrieval analysis of dual-tapered stems4. Surgeons should be aware that in vivo fretting and corrosion can occur with a ceramic head and a cobalt-chromium-alloy neck.
The etiology of the adverse local tissue reactions appears to be linked to release of metal ions and debris from the modular taper junction. This likely occurs through a process of mechanically assisted crevice corrosion, which was described as a combination of fretting and crevice corrosion25,49; scanning electron microscopy analysis of the retrieved components was consistent with this process. Factors that may contribute to corrosion at the modular interface include extended neck lengths50 or contamination of the taper interface with debris51. There may also be factors related to the design of the taper junction itself. All of the stems in this series were from a single manufacturer. While this may relate to a susceptibility of this specific stem design to corrosion, adverse local tissue reactions have also been reported in association with other dual-taper stem designs that feature a modular cobalt-chromium-alloy neck5,21.
The material composition of the modular components may also influence their susceptibility to corrosion; it is less likely that a modular neck made from a titanium alloy (thereby allowing for a titanium-titanium modular junction) would lead to adverse local tissue reaction5 as there is no potential source for cobalt or chromium release from such a junction52. However, these titanium modular necks have been associated with fracture9-11,41-45. Furthermore, it is unknown if the unique composition of the titanium-alloy stem (TMZF; Stryker), which is a proprietary blend of titanium, iron, molybdenum, and zirconium, may influence the potential for corrosion or nature of the adverse local tissue reaction.
In the present series, several patients underwent extended evaluations before the diagnosis of taper corrosion was made; this may be because suspicion of an adverse metal reaction was quite low in patients with ceramic-on-polyethylene or metal-on-polyethylene bearing surfaces. However, once a diagnosis of taper corrosion is considered as a potential etiology, the diagnostic workup is fairly straightforward. Physical examination is able to localize pain to the hip joint. MRI with use of MARS protocols typically demonstrates large fluid collections or adverse soft-tissue reactions. Serum metal-ion testing routinely demonstrates a greater elevation in serum cobalt than serum chromium, which has also been documented in reports of head-neck taper corrosion12,53-55. We have found this constellation of findings to be reliable in making a diagnosis of adverse local tissue reaction secondary to taper corrosion12. There are several reasons why serum levels of cobalt may be higher than those for chromium. The content of cobalt in cobalt-chromium alloy is higher than that in chromium. Furthermore, in some forms of corrosion, cobalt is selectively leached from the alloy49. Finally, some of the chromium released due to corrosion is precipitated as chromium phosphate corrosion product and sequestered locally rather than forming organometallic complexes in the serum.
It is important to note that erythrocyte sedimentation rate and C-reactive protein were frequently elevated in this series, as has been seen in previous studies of adverse local tissue reaction secondary to taper corrosion12. Although this may raise the suspicion for periprosthetic joint infection, an aspiration and synovial fluid analysis with cultures can be quite helpful to rule out this possibility. In our experience, the presence of metal debris and degenerated cells often hinders the ability of the laboratory to perform a cell count on this fluid.
We note several limitations to the present study design. First, the multicenter and retrospective nature of this study led to some degree of heterogeneity in the patients’ workup and does not allow us to estimate the incidence of this problem. In addition, we only report on the preoperative and operative findings in the absence of follow-up. We believe that these limitations are outweighed by the need to report these findings, given the potential for adverse patient outcomes associated with these devices, particularly as this clinical entity remains poorly understood and has been rarely described to date.
In conclusion, corrosion at the modular neck-body taper of a dual-taper femoral component can produce severe soft-tissue damage about the hip and should be included in the differential diagnosis for occult hip pain following total hip arthroplasty in patients with these types of prostheses. After infection was excluded, the diagnosis was aided by obtaining serum metal ion levels and a specialized MRI. We recommend these tests for patients who have dual-taper femoral designs that utilize a modular neck component and who present with pain in the absence of a more obvious cause. Importantly, given the potentially destructive nature of these adverse soft-tissue reactions, consideration should be given to early revision surgery, with femoral component exchange, to remove the ongoing source of metal-ion and debris generation.
Appendix
Tables showing the demographic, clinical, laboratory, surgical, and implant data, the results of microscopic and histologic examination of implants and tissue removed at the time of surgery, and the preoperative metal levels for the twelve hips (eleven patients) in the study group are available with the online version of this article as a data supplement at jbjs.org.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Tables showing the demographic, clinical, laboratory, surgical, and implant data, the results of microscopic and histologic examination of implants and tissue removed at the time of surgery, and the preoperative metal levels for the twelve hips (eleven patients) in the study group
Acknowledgments
Note: The authors thank Dr. Wayne Paprosky, Dr. Fred Flandry, and Dr. Brett Levine for their contributions to this case series, and Deborah J. Hall for her assistance in analysis with use of the scanning electron microscope.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Archibeck MJ, Cummins T, Carothers J, Junick DW, White RE., Jr A comparison of two implant systems in restoration of hip geometry in arthroplasty. Clin Orthop Relat Res. 2011 Feb;469(2):443-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y. Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Joint Surg Br. 2009 Mar;91(3):333-40 [DOI] [PubMed] [Google Scholar]
- 3.Duwelius PJ, Hartzband MA, Burkhart R, Carnahan C, Blair S, Wu Y, Grunkemeier GL. Clinical results of a modular neck hip system: hitting the “bull’s-eye” more accurately. Am J Orthop (Belle Mead NJ). 2010 Oct;39(10)(Suppl):2-6 [PubMed] [Google Scholar]
- 4.Kop AM, Swarts E. Corrosion of a hip stem with a modular neck taper junction: a retrieval study of 16 cases. J Arthroplasty. 2009 Oct;24(7):1019-23 [DOI] [PubMed] [Google Scholar]
- 5.Kop AM, Keogh C, Swarts E. Proximal component modularity in THA—at what cost? An implant retrieval study. Clin Orthop Relat Res. 2012 Jul;470(7):1885-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine BR, Hall DJ, Urban RM, Sporer SM, Galante JO, Jacobs JJ, Lachiewicz PF, Lombardi AV. Fretting corrosion and fracture of the modular neck-body junctions in hip replacement femoral components. Read at the Annual Meeting of the American Academy of Orthopaedic Surgeons. 2010 Mar 9-14; New Orleans, LA.
- 7.Viceconti M, Ruggeri O, Toni A, Giunti A. Design-related fretting wear in modular neck hip prosthesis. J Biomed Mater Res. 1996 Feb;30(2):181-6 [DOI] [PubMed] [Google Scholar]
- 8.Viceconti M, Baleani M, Squarzoni S, Toni A. Fretting wear in a modular neck hip prosthesis. J Biomed Mater Res. 1997 May;35(2):207-16 [DOI] [PubMed] [Google Scholar]
- 9.Atwood SA, Patten EW, Bozic KJ, Pruitt LA, Ries MD. Corrosion-induced fracture of a double-modular hip prosthesis: a case report. J Bone Joint Surg Am. 2010 Jun;92(6):1522-5 [DOI] [PubMed] [Google Scholar]
- 10.Dangles CJ, Altstetter CJ. Failure of the modular neck in a total hip arthroplasty. J Arthroplasty. 2010 Oct;25(7):e5-7 [DOI] [PubMed] [Google Scholar]
- 11.Wright G, Sporer S, Urban R, Jacobs J. Fracture of a modular femoral neck after total hip arthroplasty: a case report. J Bone Joint Surg Am. 2010 Jun;92(6):1518-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper HJ, Della Valle CJ, Berger R, Tetreault M, Paprosky W, Sporer S, Jacobs J. Corrosion at the head-neck taper as a cause for adverse local tissue reactions in total hip arthroplasty. J Bone Joint Surg Am. 2012;94(18):1655-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mao X, Tay GH, Godbolt DB, Crawford RW. Pseudotumor in a well-fixed metal-on-polyethylene uncemented hip arthroplasty. J Arthroplasty. 2012 Mar;27(3):e13-7 [DOI] [PubMed] [Google Scholar]
- 14.Walsh AJ, Nikolaou VS, Antoniou J. Inflammatory pseudotumor complicating metal-on-highly cross-linked polyethylene total hip arthroplasty. J Arthroplasty. 2012 Feb;27(2):e5-8 [DOI] [PubMed] [Google Scholar]
- 15.Lindgren JU, Brismar BH, Wikstrom AC. Adverse reaction to metal release from a modular metal-on-polyethylene hip prosthesis. J Bone Joint Surg Br. 2011 Oct;93(10):1427-30 [DOI] [PubMed] [Google Scholar]
- 16.Meftah M, Nicolaou N, Rodriguez JA. Metal allergy response to femoral head-neck corrosion after total hip replacement. Curr Orthop Pract. 2010;21(5):530-3 [Google Scholar]
- 17.Campbell P, Ebramzadeh E, Nelson S, Takamura K, De Smet K, Amstutz HC. Histological features of pseudotumor-like tissues from metal-on-metal hips. Clin Orthop Relat Res. 2010 Sep;468(9):2321-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doorn PF, Mirra JM, Campbell PA, Amstutz HC. Tissue reaction to metal on metal total hip prostheses. Clin Orthop Relat Res. 1996 Aug;329(329)(Suppl):S187-205 [DOI] [PubMed] [Google Scholar]
- 19.Mahendra G, Pandit H, Kliskey K, Murray D, Gill HS, Athanasou N. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop. 2009 Dec;80(6):653-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008 Jul;90(7):847-51 [DOI] [PubMed] [Google Scholar]
- 21.Gill IPS, Webb J, Sloan K, Beaver RJ. Corrosion at the neck-stem junction as a cause of metal ion release and pseudotumour formation. J Bone Joint Surg Br. 2012 Jul;94(7):895-900 [DOI] [PubMed] [Google Scholar]
- 22.Meneghini RM, Hallab NJ, Jacobs JJ. Evaluation and treatment of painful total hip arthroplasties with modular metal taper junctions. Orthopedics. 2012 May;35(5):386-91 [DOI] [PubMed] [Google Scholar]
- 23.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969 Jun;51(4):737-55 [PubMed] [Google Scholar]
- 24.Jacobs JJ, Skipor AK, Patterson LM, Hallab NJ, Paprosky WG, Black J, Galante JO. Metal release in patients who have had a primary total hip arthroplasty. A prospective, controlled, longitudinal study. J Bone Joint Surg Am. 1998 Oct;80(10):1447-58 [DOI] [PubMed] [Google Scholar]
- 25.Goldberg JR, Gilbert JL, Jacobs JJ, Bauer TW, Paprosky W, Leurgans S. A multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin Orthop Relat Res. 2002 Aug;(401):149-61 [DOI] [PubMed] [Google Scholar]
- 26.Schinsky MF, Della Valle CJ, Sporer SM, Paprosky WG. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008 Sep;90(9):1869-75 [DOI] [PubMed] [Google Scholar]
- 27.Lucas LC, Buchanan RA, Lemons JE. Investigations on the galvanic corrosion of multialloy total hip prostheses. J Biomed Mater Res. 1981 Sep;15(5):731-47 [DOI] [PubMed] [Google Scholar]
- 28.Collier JP, Mayor MB, Williams IR, Surprenant VA, Surprenant HP, Currier BH. The tradeoffs associated with modular hip prostheses. Clin Orthop Relat Res. 1995 Feb;(311):91-101 [PubMed] [Google Scholar]
- 29.Cook SD, Barrack RL, Baffes GC, Clemow AJT, Serekian P, Dong N, Kester MA. Wear and corrosion of modular interfaces in total hip replacements. Clin Orthop Relat Res. 1994 Jan;(298):80-8 [PubMed] [Google Scholar]
- 30.Collier JP, Surprenant VA, Jensen RE, Mayor MB. Corrosion at the interface of cobalt-alloy heads on titanium-alloy stems. Clin Orthop Relat Res. 1991 Oct;(271):305-12 [PubMed] [Google Scholar]
- 31.Collier JP, Surprenant VA, Jensen RE, Mayor MB, Surprenant HP. Corrosion between the components of modular femoral hip prostheses. J Bone Joint Surg Br. 1992 Jul;74(4):511-7 [DOI] [PubMed] [Google Scholar]
- 32.Bobyn JD, Tanzer M, Krygier JJ, Dujovne AR, Brooks CE. Concerns with modularity in total hip arthroplasty. Clin Orthop Relat Res. 1994 Jan;(298):27-36 [PubMed] [Google Scholar]
- 33.Brown SA, Flemming CAC, Kawalec JS, Placko HE, Vassaux C, Merritt K, Payer JH, Kraay MJ. Fretting corrosion accelerates crevice corrosion of modular hip tapers. J Appl Biomater. 1995 Spring;6(1):19-26 [DOI] [PubMed] [Google Scholar]
- 34.Cook SD, Barrack RL, Clemow AJT. Corrosion and wear at the modular interface of uncemented femoral stems. J Bone Joint Surg Br. 1994 Jan;76(1):68-72 [PubMed] [Google Scholar]
- 35.Lieberman JR, Rimnac CM, Garvin KL, Klein RW, Salvati EA. An analysis of the head-neck taper interface in retrieved hip prostheses. Clin Orthop Relat Res. 1994 Mar;300(300):162-7 [PubMed] [Google Scholar]
- 36.Mathiesen EB, Lindgren JU, Blomgren GG, Reinholt FP. Corrosion of modular hip prostheses. J Bone Joint Surg Br. 1991 Jul;73(4):569-75 [DOI] [PubMed] [Google Scholar]
- 37.Urban RM, Jacobs JJ, Gilbert JL, Galante JO. Migration of corrosion products from modular hip prostheses. Particle microanalysis and histopathological findings. J Bone Joint Surg Am. 1994 Sep;76(9):1345-59 [DOI] [PubMed] [Google Scholar]
- 38.Jacobs JJ, Urban RM, Gilbert JL, Skipor AK, Black J, Jasty M, Galante JO. Local and distant products from modularity. Clin Orthop Relat Res. 1995 Oct;(319):94-105 [PubMed] [Google Scholar]
- 39.Goldberg JR, Gilbert JL. In vitro corrosion testing of modular hip tapers. J Biomed Mater Res B Appl Biomater. 2003 Feb 15;64(2):78-93 [DOI] [PubMed] [Google Scholar]
- 40.Svensson O, Mathiesen EB, Reinholt FP, Blomgren G. Formation of a fulminant soft-tissue pseudotumor after uncemented hip arthroplasty. A case report. J Bone Joint Surg Am. 1988 Sep;70(8):1238-42 [PubMed] [Google Scholar]
- 41.Ellman MB, Levine BR. Fracture of the modular femoral neck component in total hip arthroplasty. J Arthroplasty. 2013 Jan;28(1):e1-5 [DOI] [PubMed] [Google Scholar]
- 42.Grupp TM, Weik T, Bloemer W, Knaebel HP. Modular titanium alloy neck adapter failures in hip replacement—failure mode analysis and influence of implant material. BMC Musculoskelet Disord. 2010;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skendzel JG, Blaha JD, Urquhart AG. Total hip arthroplasty modular neck failure. J Arthroplasty. 2011 Feb;26(2):e1-4 [DOI] [PubMed] [Google Scholar]
- 44.Sotereanos NG, Sauber TJ, Tupis TT. Modular femoral neck fracture after primary total hip arthroplasty. J Arthroplasty. 2013 Jan;28(1):e7-9 [DOI] [PubMed] [Google Scholar]
- 45.Wilson DAJ, Dunbar MJ, Amirault JD, Farhat Z. Early failure of a modular femoral neck total hip arthroplasty component: a case report. J Bone Joint Surg Am. 2010 Jun;92(6):1514-7 [DOI] [PubMed] [Google Scholar]
- 46.Kouzelis A, Georgiou CS, Megas P. Dissociation of modular total hip arthroplasty at the neck-stem interface without dislocation. J Orthop Traumatol. 2012 Dec;13(4):221-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sporer SM, DellaValle C, Jacobs J, Wimmer M. A case of disassociation of a modular femoral neck trunion after total hip arthroplasty. J Arthroplasty. 2006 Sep;21(6):918-21 [DOI] [PubMed] [Google Scholar]
- 48.Hallab NJ, Messina C, Skipor A, Jacobs JJ. Differences in the fretting corrosion of metal-metal and ceramic-metal modular junctions of total hip replacements. J Orthop Res. 2004 Mar;22(2):250-9 [DOI] [PubMed] [Google Scholar]
- 49.Gilbert JL, Buckley CA, Jacobs JJ. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res. 1993 Dec;27(12):1533-44 [DOI] [PubMed] [Google Scholar]
- 50.Doehring TC, Rubash HE, Dore DE. Micromotion measurements with hip center and modular neck length alterations. Clin Orthop Relat Res. 1999 May;(362):230-9 [PubMed] [Google Scholar]
- 51.Jauch SY, Huber G, Hoenig E, Baxmann M, Grupp TM, Morlock MM. Influence of material coupling and assembly condition on the magnitude of micromotion at the stem-neck interface of a modular hip endoprosthesis. J Biomech. 2011 Jun 3;44(9):1747-51 [DOI] [PubMed] [Google Scholar]
- 52.Kretzer JP, Jakubowitz E, Krachler M, Thomsen M, Heisel C. Metal release and corrosion effects of modular neck total hip arthroplasty. Int Orthop. 2009 Dec;33(6):1531-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langton DJ, Jameson SS, Joyce TJ, Gandhi JN, Sidaginamale R, Mereddy P, Lord J, Nargol AV. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg Br. 2011 Aug;93(8):1011-6 [DOI] [PubMed] [Google Scholar]
- 54.Garbuz DS, Tanzer M, Greidanus NV, Masri BA, Duncan CP. The John Charnley Award: Metal-on-metal hip resurfacing versus large-diameter head metal-on-metal total hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2010 Feb;468(2):318-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bolland BJ, Culliford DJ, Langton DJ, Millington JP, Arden NK, Latham JM. High failure rates with a large-diameter hybrid metal-on-metal total hip replacement: clinical, radiological and retrieval analysis. J Bone Joint Surg Br. 2011 May;93(5):608-15 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest
Tables showing the demographic, clinical, laboratory, surgical, and implant data, the results of microscopic and histologic examination of implants and tissue removed at the time of surgery, and the preoperative metal levels for the twelve hips (eleven patients) in the study group


