Abstract
Background:
Wear, oxidation, and particularly rim impingement damage of ultra-high molecular weight polyethylene total disc replacement components have been observed following surgical revision. However, neither in vitro testing nor retrieval-based evidence has shown the effect(s) of impingement on the characteristics of polyethylene wear debris. Thus, we sought to determine (1) differences in polyethylene particle size, shape, number, or biological activity that correspond to mild or severe rim impingement and (2) in an analysis of all total disc replacements, regardless of impingement classification, whether there are correlations between the extent of regional damage and the characteristics of polyethylene wear debris.
Methods:
The extent of dome and rim damage was characterized for eleven retrieved polyethylene cores obtained at revision surgery after an average duration of implantation of 9.7 years (range, 4.6 to 16.1 years). Polyethylene wear debris was isolated from periprosthetic tissues with use of nitric acid and was imaged with use of environmental scanning electron microscopy. Subsequently, particle size, shape, number, biological activity, and chronic inflammation scores were determined.
Results:
Grouping of particles by size ranges that represented high biological relevance (<0.1 to 1-μm particles), intermediate biological relevance (1 to 10-μm particles), and low biological relevance (>10-μm particles) revealed an increased volume fraction of particles in the <0.1 to 1-μm and 1 to 10-μm size ranges in the mild-impingement cohort as compared with the severe-impingement cohort. The increased volume fractions resulted in a higher specific biological activity per unit particle volume in the mild-impingement cohort than in the severe-impingement cohort. However, functional biological activity, which is normalized by particle volume (mm3/g of tissue), was significantly higher in the severe-impingement cohort. This increase was due to a larger volume of particles in all three size ranges. In both cohorts, the functional biological activity correlated with the chronic inflammatory response, and the extent of rim penetration positively correlated with increasing particle size, number, and functional biological activity.
Conclusions:
The results of this study suggest that severe rim impingement increases the production of biologically relevant particles from motion-preserving lumbar total disc replacement components.
Level of Evidence:
Prognostic Level IV. See Instructions for Authors for a complete description of levels of evidence.
Total disc replacement surgery is intended to both preserve motion and reduce pain in patients with severe lumbar disc degeneration1,2. Two lumbar total disc replacement devices have been approved for use in the United States: the Charité III (DePuy Spine, Raynham, Massachusetts) and the ProDisc (Synthes, West Chester, Pennsylvania). The Charité artificial disc is no longer used, but it has the longest clinical history, having been implanted in Europe since the 1980s; it consists of two CoCr end plates fixed to the adjacent vertebral bodies1. Between the end plates, a mobile, biconvex conventional ultra-high molecular weight polyethylene (hereafter referred to as polyethylene) core articulates against the concave bearing surfaces. Similar to hip and knee joint replacements, motion-preserving total disc replacements are prone to generate wear debris during a patient’s daily activities3-9. The Charité, like the ProDisc, consists of two metallic end plates and a polyethylene core. However, the polyethylene core is firmly attached to the inferior end plate with the use of a locking mechanism. The superior surface of the core is dome-shaped and articulates against a concave superior metallic plate10. Until recently, published data on the role of polyethylene wear debris as a factor limiting the longevity of total disc replacements consisted of only a few case studies11-13. Additionally, the clinical implications of polyethylene debris in spine tissue are still poorly understood6,14,15.
Polyethylene implant wear is affected by many variables, including surface roughness, cross-linking, wear path (distance, direction), and applied load16-20; however, the generation of wear debris can be attributed to four predominant wear modes18. Mode-1 wear occurs with articulation of intended bearing surfaces. Mode 2 occurs during articulation of a bearing and nonbearing surface, and Mode 3 occurs when abrasive third-body particles become entrapped between articulating surfaces. Finally, Mode 4 is the result of unintended articulation between nonbearing surfaces18. Impingement, typically ascribed to Mode-4 wear, is a source of concern in total hip and knee replacement.
The effects of impingement have been addressed by numerous studies of revised artificial hip, knee, and shoulder components21-26. Specifically, malfunctioning metal-on-metal27-31 and ceramic-on-ceramic32-37 hip bearings as well as rim fracture of highly cross-linked polyethylene liners38,39 have been considered in the context of impingement. Recently, total disc replacement retrieval studies have also identified impingement patterns on the polyethylene core of Charité and ProDisc implants13,40-43. In the Charité implant, the kinematics of the superior bearing surface cause locking of the core, resulting in an accumulation of rim damage, radial cracking, and rim fracture12,40,44.
While impingement remains a clinical concern in total disc replacement, the extent to which impingement affects polyethylene particle formation remains uncertain. Thus, we sought to determine (1) differences in polyethylene particle size, shape, number, or biological activity that correspond to mild or severe rim impingement and (2) in an analysis of all total disc replacements, regardless of impingement classification, whether there are correlations between the extent of regional damage and the characteristics of polyethylene wear debris.
Materials and Methods
Patient Selection
Forty-eight SB Charité III total disc replacements (Link, Hamburg, Germany) were retrieved during revision surgery, and analyzed between 2002 and 2008. Components were made of polyethylene GUR 412 resin and gamma-air sterilized or GUR 1020 resin and gamma-inert sterilized and polymer barrier packaged, which allowed exposure to air. We recently reported the clinical information, oxidative properties, and surface damage of this cohort44,45. In eleven of these forty-eight cases, periprosthetic tissue samples were retrieved as well. The revisions in these eleven patients was indicated at an average of 9.7 years (range, 4.6 to 16.1 years) because of persistent back and leg pain and, in one case, osteolysis (Table I). To confirm that the subset of total disc replacements was representative of the published collection, oxidation, the hydroperoxide index (see Appendix), and surface damage (see Appendix) were compared with those of all forty-eight total disc replacements; no significant differences were observed. The extent of rim impingement was determined on each device. Components with evidence of minimal rim contact (e.g., minor burnishing; Fig. 1, right) were considered to have mild impingement, whereas evidence of severe rim contact (e.g., large areas of burnishing, fatigue, and subsurface cracking; Fig. 1, left) was considered to indicate severe impingement. The frequency of severe impingement (seven of the eleven total disc replacement retrievals that included removal of tissue and thirty-seven of the forty-eight replacements overall) was higher than that of mild impingement (four of eleven and ten of forty-eight). One total disc replacement exhibiting signs of severe impingement was considered separately because of the presence of osteolysis.
TABLE I.
Summary of Clinical Information (Sorted by Increasing Implantation Time within Each Impingement Classification)
| Case | Sample ID | Impingement Classification | Implantation Time (yr) | Revision Reason | Age at Implantation (yr) | Level |
| 1 | Maa003 | Severe | 6.24 | Subsidence | 46 | L4-L5 |
| 2 | Maa006 | Severe | 6.50 | Grossly loose with osteolysis in sacrum | 46 | L5-S1 |
| 3 | Maa002 | Severe | 9.18 | Persistent pain in low back and both legs; flattening of polyethylene core; broken metal wire; subsidence | 39 | L5-S1 |
| 4* | Maa018 | Severe | 10.58 | Persistent lumbar and right leg pain; disc degeneration at L3-L4, above a successful posterior fusion at L4-S1 | 33 | L4-L5 |
| 5 | Maa004 | Severe | 12.70 | Anterior position of L4-L5 disc | 32 | L4-L5 |
| 6 | Maa013 | Severe | 12.75 | Instability—retrolisthesis at L1-L2 and L2-L3; pseudarthrosis at L4-L5; anterior position and possible wear | 34 | L3-L4 |
| 7 | Maa019 | Severe | 16.10 | Progressive anterior migration; pressure against aorta; back and leg pain | 72 | L4-L5 |
| 8 | Maa023 | Mild | 4.61 | Severe back and leg pain; multiple disc degeneration above prosthesis; lateral displacement of upper end plate | 45 | L5-S1 |
| 9 | Maa010 | Mild | 8.47 | Persisting pain after failed posterior fusion | 34 | L5-S1 |
| 10 | Sal007 | Mild | 9.72 | Faulty polyethylene | 39 | L5-S1 |
| 11 | Maa009 | Mild | 10.21 | Pain due to severe facet joint degeneration at L4-L5 and disc degeneration at L1-L2 and L3-L4 | 39 | L4-L5 |
Bisegmental Charité implants at L2-L3 and L4-L5.
Fig. 1.
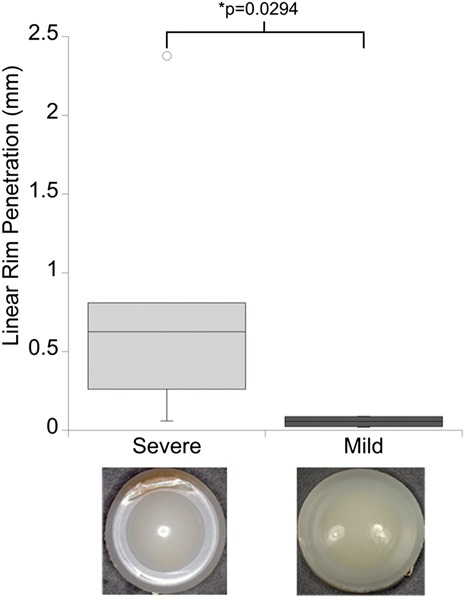
Linear rim penetration was increased for retrieved Charité total disc replacement components exhibiting severe impingement. Provided are boxed ranges of the 25th to 75th percentile and whiskers showing the 1st and 4th quartiles. The length of the whiskers is equal to 1.5 times the box height; anything outside of this range is considered an outlier and is shown as an open circle. Representative images are also provided to illustrate both impingement classifications. A significant difference between impingement groups was determined with use of independent t tests.
Implant and Wear Particle Analyses
Wear particles from the eleven tissue specimens were isolated, imaged, and morphologically characterized. Their osteolytic potential was determined with use of modifications of the functional biological activity and specific biological activity indices described by Fisher et al. (see Appendix)46,47. Specific biological activity is the estimated biological activity (per unit volume) of wear debris based on particle sizes within three ranges—those that stimulate the highest inflammatory responses (<0.1 to 1-μm particles), those that stimulate an intermediate response (1 to 10-μm particles), and those that stimulate the lowest response (>10-μm particles). Functional biological activity is the product of the specific biological activity and total component-wear volume. This metric provides the weighted biological importance of wear debris by taking into account the total volume/number of particles, which directly reflects the amount of component wear.
Particle purity was validated by means of Fourier transform infrared spectroscopy (see Appendix). Tissue specimen inflammatory response was assessed histologically. Gross mechanical damage of the polyethylene cores (penetration depth) was measured with use of a calibrated micrometer. The cores’ oxidation status was assessed (after lipid extraction) with Fourier transform infrared spectroscopy, as was hydroperoxide content. The technical details of these various protocols and indices are presented in the Appendix.
Statistical Analysis
It was determined that, with a statistical power of p = 0.80 and a significance level of α = 0.05, ten total disc replacement samples were sufficient to detect a 1.5-fold change in functional biological activity between impingement groups. Distributions of particle morphological characteristics were assessed for normality with use of the Shapiro-Wilk test. For normally distributed data, differences between impingement groups were evaluated with use of independent t tests. For nonparametric data, Mann-Whitney U tests were used to evaluate differences in particle characteristics. Correlations between particle characteristics and inflammation, implantation time, penetration, and oxidation were determined with use of the Spearman rank correlation test.
Source of Funding
Funding was provided by grant R01AR056264 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and grant HHSF223200930112G from the U.S. Food and Drug Administration (FDA).
Results
Differences in Particle Characteristics and Inflammatory Responses Between Mild and Severe-Impingement Groups
No significant differences in mean particle size, shape, or number were observed on the basis of the total disc replacement-impingement classification. The mean particle size (equivalent circular diameter) was 0.56 ± 0.11 μm in the mild-impingement group compared with 0.81 ± 0.30 μm in the severe-impingement group (p = 0.15) (see Appendix). In both impingement groups, characterization of particle shapes revealed a combination of granular and, to a lesser extent, fibrillar morphologies (Fig. 2). The mean aspect ratio did not differ significantly between the mild and severe-impingement groups (p = 0.77) (see Appendix). Roundness values were also comparable between the groups (p = 0.78). Form factor was similar between the impingement groups as well (p = 0.58); however, in both groups, the form factor of the submicrometer particles was increased (more rounded) compared with that of the larger particles (p < 0.01). Finally, the mean particle number per gram did not differ significantly between the impingement groups (p = 0.10).
Fig. 2.
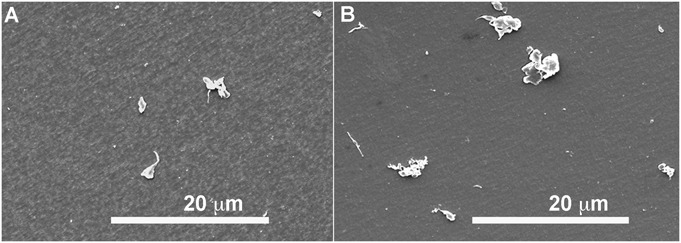
Representative environmental scanning electron microscope images of polyethylene particles from Charité components with mild (Fig. 2-A) and severe (Fig. 2-B) impingement.
A chronic inflammatory response to the presence of wear particles was observed in all patients (see Appendix). The majority of the inflammatory cells observed throughout the fibrous tissue were enlarged macrophages/histiocytes filled with small wear debris. Similarly, when large wear debris was present, giant cells surrounded the particles. The macrophage/histiocyte responses were similar in the severe and mild-impingement groups (p = 0.75), given the comparable volumes of particles in the <0.1 to 1-μm size range. However, there was a significant increase in giant cells (p = 0.04) associated with the increased volume of >10-μm particles in the severe-impingement group. The overall chronic inflammatory responses (combined macrophage and giant-cell scores) in the severe-impingement group tended to be higher than that in the mild group (p = 0.08), presumably as a result of the increased volume of particles of all three sizes.
We also evaluated particles according to size ranges with high biological relevance (<0.1 to 1-μm particles), intermediate biological relevance (1 to 10-μm particles), and low biological relevance (>10-μm particles). This analysis showed the mean equivalent circular diameter of the particles in the >10-μm range to be larger in the severe-impingement group (p = 0.03), and tissues from this group contained more particles in the 1 to 10-μm size range (p = 0.03) (Table II).
TABLE II.
Particle Sizes and Numbers for Individual Particle Size Ranges
| Equivalent Circular Diameter (μm) |
Particle Number |
|||||
| Impingement Classification | <0.1-1 μm | 1-10 μm | >10 μm | <0.1-1 μm | 1-10 μm | >10 μm |
| Severe* | 0.42 ± 0.05 | 1.97 ± 0.21 | 18.70 ± 4.83 | 1.95 ± 0.52 × 109 | 4.52 ± 3.04 ×107 | 3.17 ± 2.98 × 105 |
| Mild* | 0.38 ± 0.08 | 1.91 ± 0.11 | 12.86 ± 3.35 | 1.40 ± 0.44 × 109 | 1.13 ± 0.73 ×107 | 0.71 ± 0.28 × 105 |
| P value | 0.26 | 0.54 | 0.03† | 0.11 | 0.03† | 0.07 |
The values are given as the mean and standard deviation.
Significantly higher in the severe-impingement group. Significance was evaluated with use of Mann-Whitney U tests and set at p < 0.05.
Differences in Biological Activity of Particles Between Mild and Severe-Impingement Groups
Significant increases in the volume fraction, V(r), of particles in the <0.1 to 1-μm and 1 to 10-μm size ranges were observed in the group of total disc replacements with mild impingement (p = 0.04 and p = 0.03, respectively, compared with the severe-impingement group), whereas the volume fraction of >10-μm particles was increased in the severe-impingement group (p = 0.01) (Fig. 3-A). After application of ranked biological activity scalars, B(r), particles from total disc replacements with mild impingement had increased specific biological activity compared with those in the severe-impingement group (p = 0.02) (Fig. 3-B).
Fig. 3.
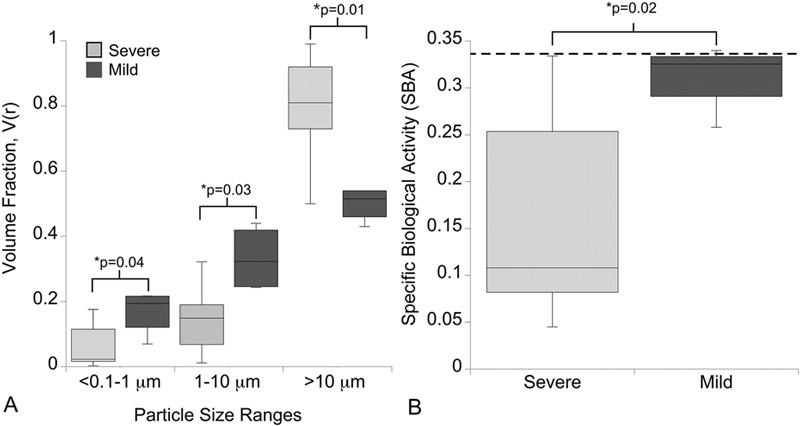
Differences in volume fraction (V[r]) and specific biological activity were observed on the basis of impingement classification. Fig. 3-A Total disc replacements with mild impingement had an increase in the volume fraction of particles in the <0.1 to 1-μm and 1 to 10-μm-size ranges, whereas the severe-impingement group had an increase in the volume fraction of particles of >10 μm. Fig. 3-B As a result, specific biological activity was increased for the mild-impingement group. Provided are boxed ranges of the 25th to 75th percentile and whiskers showing the 10th and 90th percentiles. The dotted line represents the specific biological activity of polyethylene wear debris from the single total disc replacement revised because of osteolysis. Significant differences between impingement groups were determined with use of Mann-Whitney U tests.
The total particle volume (mm3/g of tissue), with respect to both the 1 to 10-μm and the >10-μm size range (p = 0.01) and the cumulative value for all three size ranges (p = 0.01), was significantly higher in the severe-impingement group than it was in the mild-impingement group (Fig. 4-A). As a result, functional biological activity (specific biological activity normalized by particle volume [mm3/g of tissue]) was significantly higher in the severe-impingement group (p = 0.01) (Fig. 4-B). The single total disc replacement revised because of osteolysis had among the highest specific biological activity and functional biological activity values of any specimen in either impingement group.
Fig. 4.
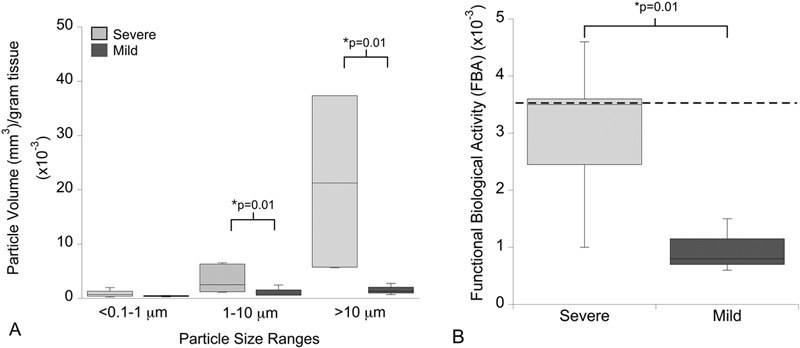
Differences in the total particle volume and functional biological activity were observed on the basis of impingement classification. Fig. 4-A Total disc replacements with severe impingement had an increase in the volume of particles in the 1 to 10-μm and >10-μm size ranges as well as the cumulative value for particles in all three size ranges. Fig. 4-B Functional biological activity (specific biological activity normalized by particle volume [mm3/g of tissue]) was increased in the severe-impingement group as compared with that in the mild-impingement group. Provided are boxed ranges of 25th to 75th percentile and whiskers showing the 10th and 90th percentiles. The dotted line represents the functional biological activity of polyethylene wear debris from the single total disc replacement revised because of osteolysis. Significant differences between impingement groups were determined with use of Mann-Whitney U tests.
When both groups were considered together, the functional biological activity showed a positive correlation with giant-cell number and the overall chronic inflammatory response (combined macrophage and giant-cell scores) (ρ = 0.62; p = 0.04) (see Appendix). However, there was no correlation, in either impingement group, between the functional biological activity and the level of implantation (Kruskal-Wallis test, p = 0.76).
Correlations Between Regional Total Disc Replacement Damage or Oxidation and the Characteristics of Polyethylene Wear Debris
Analysis of the retrieved total disc replacements revealed varying amounts of regional penetration, oxidation, and hydroperoxide (see Appendix). Neither oxidation nor hydroperoxide indices were correlated with particle morphology or calculated biological activities. However, linear rim penetration was significantly increased in the severe-impingement group (p = 0.03) (Fig. 1). In addition, the extent of linear rim penetration was positively correlated with increasing particle size (ρ = 0.68, p = 0.02) and particle number (ρ = 0.72, p = 0.01) (Figs. 5-A and 5-B). Furthermore, linear rim penetration was positively correlated with increasing functional biological activity values (ρ = 0.75, p = 0.01) (Fig. 5-C).
Fig. 5.
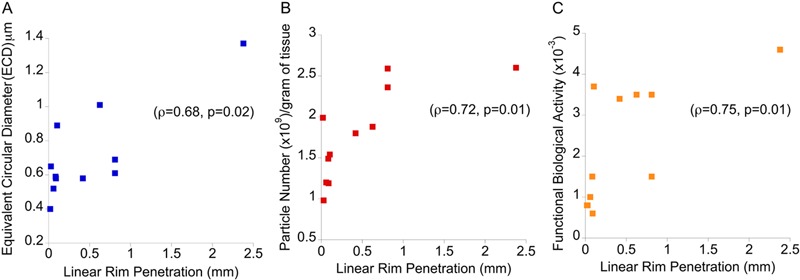
Positive correlations were observed between increasing linear rim penetration (mm) and mean equivalent circular diameter (μm) (Fig. 5-A), polyethylene wear particle number (×109/g tissue) (Fig. 5-B), and functional biological activity (Fig. 5-C) with use of Spearman rank correlation. Shown are the ranked magnitudes of each variable.
Discussion
Collectively, to our knowledge, these data represent the largest characterization of submicrometer and micrometer-sized polyethylene debris from revised lumbar total disc replacements. In addition, this first implementation of biological activity to particles isolated from periprosthetic tissues highlights the differences in particle characteristics due to impingement. Specifically, this study shows that severe impingement exacerbates the production of biologically relevant polyethylene wear debris, which may represent a serious negative consequence of this unintended wear mode. Thus, the results of this study serve as an important benchmark both in terms of understanding the clinical relevance of polyethylene wear particles in the spine and for the future development of test methods to simulate total disc replacement rim impingement in vitro.
We acknowledge that this study had limitations. First, the number of tissue samples available for particle analysis was small. However, when we compared all forty-eight total disc replacements with the subset of eleven with available periprosthetic tissues, we observed no differences between measurements of oxidation or penetration45. Second, the lower cutoff of wear particle size was based on the 0.05-μm pore size of the polycarbonate membranes used to filter the samples. According to Scott et al.48, this pore size is expected to exclude 2.8% of the total particle number, thereby having a negligible effect on the total particle volume. In addition, Richards et al.49 showed that nanoparticles are rarely observed when a pore size of 0.017 μm is used, a finding that agrees with that of Lapcikova et al.50, who observed nanoparticles in only two of 100 hip-tissue samples from patients. The biological activity of nanoparticles is currently under investigation51-54. A third limitation of our investigation was its focus on only one of the implant designs (the Charité device) currently approved by the FDA. This design was chosen because it has the longest clinical record and represented the largest cohort with retrieved tissue in our collection. While there are substantial differences between the ProDisc and Charité devices, both exhibit mainly adhesive/abrasive wear on the conforming dome region. Thus, impingement and its effect on wear debris remain a clinical concern for current and future Charité and ProDisc motion-preserving devices. Finally, the use of particle volume per gram of tissue to determine the functional biological activity differs from the original method of Fisher et al., who utilized component wear volume (mm3/106 cycles)46,47. The volume of particles per gram of tissue depends on both the amount of tissue used and the tissue region55. While the implant volumetric wear rate is a direct measurement, this measurement was not possible in the present study because of the iatrogenic damage of the polyethylene cores. This damage manifested as deep, parallel scratches or complete tearing of the polyethylene core that appeared to have occurred during extraction. There is, however, a moderate positive correlation (ρ = 0.55) between functional biological activity based on particles isolated from hip tissues and functional biological activity based on hip implant volumetric wear (unpublished data). Therefore, the functional biological activity values in our study56 are analogous to, but not directly comparable with, the results reported by Fisher’s group46.
Studies other than our own6,57 focusing on the characteristics of polyethylene particles in the lumbar spine are, for the most part, limited to an in vitro study by Serhan et al.58. In their study, particle sizes visualized with an environmental scanning electron microscope ranged from submicrometer to >10 μm; however, the mean particle size (5 μm) was an order of magnitude larger than the mean particle size (0.72 μm) in the current study. Additionally, the total disc replacement components tested in that in vitro study were not loaded to induce rim impingement. Overall, the sizes of the particles from the total disc replacements with mild and severe impingement in our study were similar to those in previous studies of particles isolated from hip and knee tissues59,60, and the particle number in our severe-impingement group was in the same range as particle loads found in hip implant tissues (2.3 × 109 particles in the size range of 0.05 to 2.0 μm)57. However, while the range of particle sizes observed in our patients with mild (0.40 to 0.65 μm) and severe (0.52 to 1.37 μm) impingement of the total disc replacement appeared to be influenced by differences in conformity and wear patterns, the mean particle size, shape, and number did not differ significantly between groups. Importantly, grouping of particles according to biologically relevant size ranges revealed differences. Specifically, particles from total disc replacements with evidence of severe impingement were larger and more numerous in the >10-μm and 1 to 10-μm size ranges, respectively. Thus, the use of mean particle characteristics alone as opposed to size range stratification is less sensitive in distinguishing differences associated with multiple modes of component wear.
In general, prevalent wear mechanisms affecting motion-preserving total disc replacements include adhesive/abrasive wear of the highly conforming dome region and predominantly fatigue wear at isolated points of rim contact12,40,44. In a radiographic study of sixty-six ProDisc total disc replacements, Käfer et al. found the prevalence of posterior component impingement to be 11% to 15% (depending on the lumbar level and/or extension angle) and that impingement was more frequently observed at the L4-L5 vertebral level and for bisegmental implants at the L4-L5 and L5-S1 levels13. Importantly, it was not possible to directly evaluate whether total disc replacement impingement affected wear particle characteristics in that in vivo study.
Our findings of correlations of increasing linear rim penetration with increasing particle size and number are also supported by hip and knee retrieval studies. After both total hip arthroplasty and total knee arthroplasty, contact resulting from impingement contributes to localized cracking and fragmentation, to an increase in wear, and to an overall altered distribution of forces24,39,61,62. Rundell et al. observed similar findings using a validated computational model of mobile-bearing total disc replacements in the lumbar spine: they showed that peak contact stress significantly correlated with rim penetration rate (mm/yr) at the interface of the superior footplate and the central polyethylene core41.
Since the original biological activity model was initially published, it has been implemented in simulator studies to investigate the effect of roughness63,64, wear path complexity65-69, polymer cross-linking64,68-71, and/or type of material72,73 on particle generation. On the basis of differences in the biomaterials and/or testing parameter(s) used in vitro, published values of specific biological activity have ranged from 0.08 to 0.9446,70,74. In the current study, specific biological activity values exhibited substantial variability, ranging from 0.05 to 0.34 as a result of changes in the particle volume fraction of each size range. In the mild-impingement group, the decreased mean particle size in the >10-μm range contributed to the lower volume fraction in that range and concurrently to the increased volume fraction in both the <0.1 to 1-μm and the 1 to 10-μm size ranges. In contrast, the decreased volume fraction of the <0.1 to 1-μm and 1 to 10-μm-sized particles from the total disc replacements with severe impingement resulted in reduced values of specific biological activity. To eliminate the volume-fraction bias, functional biological activity was implemented, to account for the overall volume of particles in each size range. Functional biological activity was increased in the severe-impingement group as a result of the increased volume of particles per gram of tissue across all size ranges. In the single case of osteolysis, the increases in both specific biological activity and functional biological activity were due to the large volume fraction of biologically relevant particles and a large overall volume of wear debris. However, given the similar levels of penetration at the dome and rim, it is unclear whether the particles originated during normal articulation at the dome or during severe impingement at the rim.
At present, the biological activity model does not include the contributions of pro-inflammatory factors in vivo (e.g., adsorption of endotoxin/protein75-80 and/or the extent of polyethylene particle oxidation81-83), so further investigation of the inflammatory cytokine responses to wear debris in the spine is warranted. Nevertheless, these results lend support to the use of biological activity calculations in combination with particle size and number in individual size ranges to illustrate differences in total disc replacement wear modes. The effect of impingement on the overall biological activity potential highlights the importance of developing test methods that reproduce a comprehensive range of total disc replacement wear modes in vitro.
Appendix
Details of the methods for wear particle isolation, imaging, wear particle analysis, particle validation, inflammatory response evaluation, penetration analysis, and oxidation analysis; tables showing implant oxidation values, linear penetration values, equations and definitions of wear particle characteristics and biological activity indices, and values for particle size, shape, and number; and a figure demonstrating a comparison of the Fourier transform infrared spectroscopy findings with previous spectra are available with the online version of this article as a data supplement at jbjs.org.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Details of the methods for wear particle isolation, imaging, wear particle analysis, particle validation, inflammatory response evaluation, penetration analysis, and oxidation analysis; tables showing implant oxidation values, linear penetration values, equations and definitions of wear particle characteristics and biological activity indices, and values for particle size, shape, and number; and a figure demonstrating a comparison of the Fourier transform infrared spectroscopy findings with previous spectra
Acknowledgments
Note: The authors acknowledge Dr. Andre van Ooij and Ilona Punt from Maastricht, Dr. ERS Ross from Salford, and Dr. Jorge Isaza from Baton Rouge for collection of periprosthetic tissue samples and implant retrievals. They thank Dr. Nancy Pleshko and Arash Hanifi for their assistance with Fourier transform infrared spectroscopy filter measurements. Additionally, they thank Ryan Siskey, Exponent, Inc., and Dr. Jonathan Peck and Genevieve Hill from the FDA for their assistance and many helpful discussions.
Footnotes
Disclosure: One or more of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of an aspect of this work. In addition, one or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Link HD. History, design and biomechanics of the LINK SB Charité artificial disc. Eur Spine J. 2002 Oct;11(Suppl 2):S98-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochschuler SH, Ohnmeiss DD, Guyer RD, Blumenthal SL. Artificial disc: preliminary results of a prospective study in the United States. Eur Spine J. 2002 Oct;11(Suppl 2):S106-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agarwal S. Osteolysis – basic science, incidence and diagnosis. Curr Orthop. 2004;18(3):220-31 [Google Scholar]
- 4.Amstutz HC, Campbell P, Kossovsky N, Clarke IC. Mechanism and clinical significance of wear debris-induced osteolysis. Clin Orthop Relat Res. 1992 Mar;(276):7-18 [PubMed] [Google Scholar]
- 5.Kurtz SM, van Ooij A, Ross R, de Waal Malefijt J, Peloza J, Ciccarelli L, Villarraga ML. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J. 2007 Jan-Feb;7(1):12-21 [DOI] [PubMed] [Google Scholar]
- 6.Punt IM, Cleutjens JP, de Bruin T, Willems PC, Kurtz SM, van Rhijn LW, Schurink GW, van Ooij A. Periprosthetic tissue reactions observed at revision of total intervertebral disc arthroplasty. Biomaterials. 2009 Apr;30(11):2079-84 [DOI] [PubMed] [Google Scholar]
- 7.Purdue PE, Koulouvaris P, Nestor BJ, Sculco TP. The central role of wear debris in periprosthetic osteolysis. HSS J. 2006 Sep;2(2):102-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Revell PA, al-Saffar N, Kobayashi A. Biological reaction to debris in relation to joint prostheses. Proc Inst Mech Eng H. 1997;211(2):187-97 [DOI] [PubMed] [Google Scholar]
- 9.Willert HG, Bertram H, Buchhorn GH. Osteolysis in alloarthroplasty of the hip. The role of ultra-high molecular weight polyethylene wear particles. Clin Orthop Relat Res. 1990 Sep;(258):95-107 [PubMed] [Google Scholar]
- 10.Kurtz SM. The UHMWPE biomaterials handbook: ultra-high molecular weight polyethylene in total joint replacement and medical devices. 2nd ed Burlington: Academic Press; 2009 [Google Scholar]
- 11.Devin CJ, Myers TG, Kang JD. Chronic failure of a lumbar total disc replacement with osteolysis. Report of a case with nineteen-year follow-up. J Bone Joint Surg Am. 2008 Oct;90(10):2230-4 [DOI] [PubMed] [Google Scholar]
- 12.van Ooij A, Kurtz SM, Stessels F, Noten H, van Rhijn L. Polyethylene wear debris and long-term clinical failure of the Charité disc prosthesis: a study of 4 patients. Spine (Phila Pa 1976). 2007 Jan 15;32(2):223-9 [DOI] [PubMed] [Google Scholar]
- 13.Käfer W, Clessienne CB, Däxle M, Kocak T, Reichel H, Cakir B. Posterior component impingement after lumbar total disc replacement: a radiographic analysis of 66 ProDisc-L prostheses in 56 patients. Spine (Phila Pa 1976). 2008 Oct 15;33(22):2444-9 [DOI] [PubMed] [Google Scholar]
- 14.van den Eerenbeemt KD, Ostelo RW, van Royen BJ, Peul WC, van Tulder MW. Total disc replacement surgery for symptomatic degenerative lumbar disc disease: a systematic review of the literature. Eur Spine J. 2010 Aug;19(8):1262-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murtagh RD, Quencer RM, Cohen DS, Yue JJ, Sklar EL. Normal and abnormal imaging findings in lumbar total disk replacement: devices and complications. Radiographics. 2009 Jan-Feb;29(1):105-18 [DOI] [PubMed] [Google Scholar]
- 16.Buford A, Goswami T. Review of wear mechanisms in hip implants: Paper I - general. Mater Des. 2004 Aug;25(5):385-93 [Google Scholar]
- 17.Fisher J. Wear of ultra high molecular weight polyethylene in total artificial joints. Curr Orthop. 1994;8:164-9 [Google Scholar]
- 18.McKellop HA. The lexicon of polyethylene wear in artificial joints. Biomaterials. 2007 Dec;28(34):5049-57 [DOI] [PubMed] [Google Scholar]
- 19.Tsao AK, Jones LC, Lewallen DG; Implant Wear Symposium 2007 Clinical Work Group What patient and surgical factors contribute to implant wear and osteolysis in total joint arthroplasty? J Am Acad Orthop Surg. 2008;16(Suppl 1):S7-13 [DOI] [PubMed] [Google Scholar]
- 20.Williams PA, Clarke IC. Understanding polyethylene wear mechanisms by modeling of debris size distributions. Wear. 2009 Jun;267(1-4):646-52 [Google Scholar]
- 21.ASTM International F 2582-08 standard test method for impingement of acetabular prostheses. http://www.astm.org/Standards/F2582.htm. Accessed 2010 Sep [Google Scholar]
- 22.Brown TD, Callaghan JJ. Impingement in Total Hip Replacement: Mechanisms and Consequences. Curr Orthop. 2008 Dec 1;22(6):376-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favre P, Moor B, Snedeker JG, Gerber C. Influence of component positioning on impingement in conventional total shoulder arthroplasty. Clin Biomech (Bristol, Avon). 2008 Feb;23(2):175-83 [DOI] [PubMed] [Google Scholar]
- 24.Haas BD. Tibial post impingement in posterior-stabilized total knee arthroplasty. Orthopedics. 2006 Sep;29(9)(Suppl):S83-5 [PubMed] [Google Scholar]
- 25.Hamai S, Miura H, Higaki H, Shimoto T, Matsuda S, Iwamoto Y. Evaluation of impingement of the anterior tibial post during gait in a posteriorly-stabilised total knee replacement. J Bone Joint Surg Br. 2008 Sep;90(9):1180-5 [DOI] [PubMed] [Google Scholar]
- 26.Holley KG, Furman BD, Babalola OM, Lipman JD, Padgett DE, Wright TM. Impingement of acetabular cups in a hip simulator: comparison of highly cross-linked and conventional polyethylene. J Arthroplasty. 2005 Oct;20(7)(Suppl 3):77-86 [DOI] [PubMed] [Google Scholar]
- 27.Labek G, Brabec E, Frischhut S, Krismer M. High failure rate of the Duraloc Constrained Inlay. Acta Orthop. 2009 Oct;80(5):545-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malik A, Maheshwari A, Dorr LD. Impingement with total hip replacement. J Bone Joint Surg Am. 2007 Aug;89(8):1832-42 [DOI] [PubMed] [Google Scholar]
- 29.Powers CC, Fricka KB, Austin MS, Sr Engh CA. Five Duraloc locking ring failures. J Arthroplasty. 2010 Oct;25(7):e15-8 [DOI] [PubMed] [Google Scholar]
- 30.Malik A, Dorr LD, Long WT. Impingement as a mechanism of dissociation of a metasul metal-on-metal liner. J Arthroplasty. 2009 Feb;24(2):e13-6 [DOI] [PubMed] [Google Scholar]
- 31.Onda K, Nagoya S, Kaya M, Yamashita T. Cup-neck impingement due to the malposition of the implant as a possible mechanism for metallosis in metal-on-metal total hip arthroplasty. Orthopedics. 2008 Apr;31(4):396. [DOI] [PubMed] [Google Scholar]
- 32.Lee YK, Ha YC, Yoo JJ, Koo KH, Yoon KS, Kim HJ. Alumina-on-alumina total hip arthroplasty: a concise follow-up, at a minimum of ten years, of a previous report. J Bone Joint Surg Am. 2010 Jul 21;92(8):1715-9 [DOI] [PubMed] [Google Scholar]
- 33.Matar WY, Restrepo C, Parvizi J, Kurtz SM, Hozack WJ. Revision hip arthroplasty for ceramic-on-ceramic squeaking hips does not compromise the results. J Arthroplasty. 2010 Sep;25(6)(Suppl):81-6 [DOI] [PubMed] [Google Scholar]
- 34.Murali R, Bonar SF, Kirsh G, Walter WK, Walter WL. Osteolysis in third-generation alumina ceramic-on-ceramic hip bearings with severe impingement and titanium metallosis. J Arthroplasty. 2008 Dec;23(8):e13-9 [DOI] [PubMed] [Google Scholar]
- 35.Hwang DS, Kim YM, Lee CH. Alumina femoral head fracture in uncemented total hip arthroplasty with a ceramic sandwich cup. J Arthroplasty. 2007 Apr;22(3):468-71 [DOI] [PubMed] [Google Scholar]
- 36.Restrepo C, Matar WY, Parvizi J, Rothman RH, Hozack WJ. Natural history of squeaking after total hip arthroplasty. Clin Orthop Relat Res. 2010 Sep;468(9):2340-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popescu D, Gallart X, Garcia S, Bori G, Tomas X, Riba J. Fracture of a ceramic liner in a total hip arthroplasty with a sandwich cup. Arch Orthop Trauma Surg. 2008 Aug;128(8):783-5 [DOI] [PubMed] [Google Scholar]
- 38.Furmanski J, Anderson M, Bal S, Greenwald AS, Halley D, Penenberg B, Ries M, Pruitt L. Clinical fracture of cross-linked UHMWPE acetabular liners. Biomaterials. 2009 Oct;30(29):5572-82 [DOI] [PubMed] [Google Scholar]
- 39.Duffy GP, Wannomae KK, Rowell SL, Muratoglu OK. Fracture of a cross-linked polyethylene liner due to impingement. J Arthroplasty. 2009 Jan;24(1):e15-9 [DOI] [PubMed] [Google Scholar]
- 40.Goreham-Voss CM, Vicars R, Hall RM, Brown TD. Preferential superior surface motion in wear simulations of the Charité total disc replacement. Eur Spine J. 2012 Jun;21(Suppl 5):S700-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rundell SA, Day JS, Isaza J, Siskey R, MacDonald D, Kurtz SM. Derivation of clinically relevant boundary conditions suitable for evaluation of chronic impingement of lumbar total disc replacement: application to standard development. J Am Soc Test Mater Int. 2011 May;8(5):e1-14 [Google Scholar]
- 42.Shkolnikov YP, Bowden A, MacDonald D, Kurtz SM. Wear pattern observations from TDR retrievals using autoregistration of voxel data. J Biomed Mater Res B Appl Biomater. 2010 Aug;94(2):312-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurtz SM, Patwardhan A, MacDonald D, Ciccarelli L, van Ooij A, Lorenz M, Zindrick M, O’Leary P, Isaza J, Ross R. What is the correlation of in vivo wear and damage patterns with in vitro TDR motion response? Spine (Phila Pa 1976). 2008 Mar 1;33(5):481-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurtz SM, van Ooij A, Ross R, de Waal Malefijt J, Peloza J, Ciccarelli L, Villarraga ML. Polyethylene wear and rim fracture in total disc arthroplasty. Spine J. 2007 Jan-Feb;7(1):12-21 Epub 2006 Dec 6 [DOI] [PubMed] [Google Scholar]
- 45.Kurtz SM, MacDonald D, Ianuzzi A, van Ooij A, Isaza J, Ross ER, Regan J. The natural history of polyethylene oxidation in total disc replacement. Spine (Phila Pa 1976). 2009 Oct 15;34(22):2369-77 [DOI] [PubMed] [Google Scholar]
- 46.Fisher J, Bell J, Barbour PS, Tipper JL, Matthews JB, Besong AA, Stone MH, Ingham E. A novel method for the prediction of functional biological activity of polyethylene wear debris. Proc Inst Mech Eng H. 2001;215(2):127-32 [DOI] [PubMed] [Google Scholar]
- 47.Matthews JB, Besong AA, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. Evaluation of the response of primary human peripheral blood mononuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J Biomed Mater Res. 2000 Nov;52(2):296-307 [DOI] [PubMed] [Google Scholar]
- 48.Scott M, Widding K, Jani S. Do current wear particle isolation procedures underestimate the number of particles generated by prosthetic bearing components? Wear. 2001 Oct;251(1-12):1213-7 [Google Scholar]
- 49.Richards L, Brown C, Stone MH, Fisher J, Ingham E, Tipper JL. Identification of nanometre-sized ultra-high molecular weight polyethylene wear particles in samples retrieved in vivo. J Bone Joint Surg Br. 2008 Aug;90(8):1106-13 [DOI] [PubMed] [Google Scholar]
- 50.Lapcikova M, Slouf M, Dybal J, Zolotarevova E, Entlicher G, Pokorny D, Gallo J, Sosna A. Nanometer size wear debris generated from ultrahigh molecular weight polyethylene in vivo. Wear. 2009 Jan;266(1-2):349-55 [Google Scholar]
- 51.Tipper JL. The biological response to nanometre-sized particles. Read at the 4th UHMWPE International Meeting; 2009 Sep 16-17; Torino, Italy [Google Scholar]
- 52.Howling GI, Barnett PI, Tipper JL, Stone MH, Fisher J, Ingham E. Quantitative characterization of polyethylene debris isolated from periprosthetic tissue in early failure knee implants and early and late failure Charnley hip implants. J Biomed Mater Res. 2001;58(4):415-20 [DOI] [PubMed] [Google Scholar]
- 53.Gallo J, Slouf M, Goodman SB. The relationship of polyethylene wear to particle size, distribution, and number: A possible factor explaining the risk of osteolysis after hip arthroplasty. J Biomed Mater Res B Appl Biomater. 2010 Jul;94(1):171-7 [DOI] [PubMed] [Google Scholar]
- 54.Revell PA. The biological effects of nanoparticles. Nanotechnology Perceptions. 2006;2:283-98 [Google Scholar]
- 55.Tipper JL, Ingham E, Hailey JL, Besong AA, Fisher J, Wroblewski BM, Stone MH. Quantitative analysis of polyethylene wear debris, wear rate and head damage in retrieved Charnley hip prostheses. J Mater Sci Mater Med. 2000 Feb;11(2):117-24 [DOI] [PubMed] [Google Scholar]
- 56.Baxter RM, Macdonald DW, Kurtz SM, Steinbeck MJ. Characteristics of highly crosslinked polyethylene wear debris in vivo. J Biomed Mater Res B Appl Biomater. 2013 Feb 22. doi: 10.1002/jbm.b.32902. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Punt I, Baxter R, van Ooij A, Willems P, van Rhijn L, Kurtz S, Steinbeck M. Submicron sized ultra-high molecular weight polyethylene wear particle analysis from revised SB Charité III total disc replacements. Acta Biomater. 2011 Sep;7(9):3404-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Serhan HA, Dooris AP, Parsons ML, Ares PJ, Gabriel SM. In vitro wear assessment of the Charité Artificial Disc according to ASTM recommendations. Spine (Phila Pa 1976). 2006 Aug 1;31(17):1900-10 [DOI] [PubMed] [Google Scholar]
- 59.Campbell P, Doorn P, Dorey F, Amstutz HC. Wear and morphology of ultra-high molecular weight polyethylene wear particles from total hip replacements. Proc Inst Mech Eng H. 1996;210(3):167-74 [DOI] [PubMed] [Google Scholar]
- 60.Shanbhag AS, Bailey HO, Hwang DS, Cha CW, Eror NG, Rubash HE. Quantitative analysis of ultrahigh molecular weight polyethylene (UHMWPE) wear debris associated with total knee replacements. J Biomed Mater Res. 2000;53(1):100-10 [DOI] [PubMed] [Google Scholar]
- 61.Birman MV, Noble PC, Conditt MA, Li S, Mathis KB. Cracking and impingement in ultra-high-molecular-weight polyethylene acetabular liners. J Arthroplasty. 2005 Oct;20(7)(Suppl 3):87-92 [DOI] [PubMed] [Google Scholar]
- 62.Verborgt O, Victor J. Post impingement in posterior stabilised total knee arthroplasty. Acta Orthop Belg. 2004 Feb;70(1):46-50 [PubMed] [Google Scholar]
- 63.Bowsher JG, Williams PA, Clarke IC, Green DD, Donaldson TK. “Severe” wear challenge to 36 mm mechanically enhanced highly crosslinked polyethylene hip liners. J Biomed Mater Res B Appl Biomater. 2008 Jul;86(1):253-63 [DOI] [PubMed] [Google Scholar]
- 64.Galvin AL, Tipper JL, Ingham E, Fisher J. Nanometre size wear debris generated from crosslinked and non-crosslinked ultra high molecular weight polyethylene in artificial joints. Wear. 2005;259(7-12):977-83 [Google Scholar]
- 65.Ge S, Wang S, Gitis N, Vinogradov M, Xiao J. Wear behavior and wear debris distribution of UHMWPE against Si3N4 ball in bi-directional sliding. Wear. 2008;264(7-8):571-8 [Google Scholar]
- 66.Fisher J, McEwen HM, Tipper JL, Jennings LM, Farrar R, Stone M, Ingham E. Wear-simulation analysis of rotating-platform mobile-bearing knees. Orthopedics. 2006 Sep;29(9)(Suppl):S36-41 [PubMed] [Google Scholar]
- 67.Tipper JL, Galvin AL, Williams S, McEwen HM, Stone MH, Ingham E, Fisher J. Isolation and characterization of UHMWPE wear particles down to ten nanometers in size from in vitro hip and knee joint simulators. J Biomed Mater Res A. 2006 Sep 1;78(3):473-80 [DOI] [PubMed] [Google Scholar]
- 68.McEwen HM, Barnett PI, Bell CJ, Farrar R, Auger DD, Stone MH, Fisher J. The influence of design, materials and kinematics on the in vitro wear of total knee replacements. J Biomech. 2005 Feb;38(2):357-65 [DOI] [PubMed] [Google Scholar]
- 69.Utzschneider S, Paulus A, Datz JC, Schroeder C, Sievers B, Wegener B, Jansson V. Influence of design and bearing material on polyethylene wear particle generation in total knee replacement. Acta Biomater. 2009 Sep;5(7):2495-502 [DOI] [PubMed] [Google Scholar]
- 70.Endo M, Tipper JL, Barton DC, Stone MH, Ingham E, Fisher J. Comparison of wear, wear debris and functional biological activity of moderately crosslinked and non-crosslinked polyethylenes in hip prostheses. Proc Inst Mech Eng H. 2002;216(2):111-22 [DOI] [PubMed] [Google Scholar]
- 71.Williams PA, Yamamoto K, Masaoka T, Oonishi H, Clarke IC. Highly crosslinked polyethylenes in hip replacements: improved wear performance or paradox? Trib Trans. 2007;50(2):277-90 [Google Scholar]
- 72.Ingram J, Matthews JB, Tipper J, Stone M, Fisher J, Ingham E. Comparison of the biological activity of grade GUR 1120 and GUR 415HP UHMWPE wear debris. Biomed Mater Eng. 2002;12(2):177-88 [PubMed] [Google Scholar]
- 73.Tipper JL, Galvin AL, Ingham E, Fisher J. Estimation of the osteolytic potential of noncrosslinked and crosslinked polyethylenes and ceramic total hip prostheses. J Am Soc Test Mater Int. 2006 May;3(6):e1-17 [Google Scholar]
- 74.Fisher J, Matthews B, Besong AA, Tipper JL, Stone MH, Ingham E. Predictions of the biological activity of polyethylene wear debris generated under different conditions in vitro. Read at the Annual Meeting of the Orthopaedic Research Society; 2000 Mar 12-15; Orlando, Florida [Google Scholar]
- 75.Xing Z, Pabst MJ, Hasty KA, Smith RA. Accumulation of LPS by polyethylene particles decreases bone attachment to implants. J Orthop Res. 2006 May;24(5):959-66 [DOI] [PubMed] [Google Scholar]
- 76.Bi Y, Seabold JM, Kaar SG, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Adherent endotoxin on orthopedic wear particles stimulates cytokine production and osteoclast differentiation. J Bone Miner Res. 2001 Nov;16(11):2082-91 [DOI] [PubMed] [Google Scholar]
- 77.Wilkins R, Tucci M, Benghuzzi H. Evaluation of endotoxin binding to uhmwpe and inflammatory mediator production by macrophages. Biomed Sci Instrum. 2008;44:459-64 [PubMed] [Google Scholar]
- 78.Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Nalepka JL, Seabold JM. Does endotoxin contribute to aseptic loosening of orthopedic implants? J Biomed Mater Res B Appl Biomater. 2005 Jan 15;72(1):179-85 [DOI] [PubMed] [Google Scholar]
- 79.Elfick AP, Green SM, McCaskie AW, Birch MA. Opsonization of polyethylene wear particles regulates macrophage and osteoblast responses in vitro. J Biomed Mater Res B Appl Biomater. 2004 Nov 15;71(2):244-51 [DOI] [PubMed] [Google Scholar]
- 80.Cho DR, Shanbhag AS, Hong CY, Baran GR, Goldring SR. The role of adsorbed endotoxin in particle-induced stimulation of cytokine release. J Orthop Res. 2002 Jul;20(4):704-13 [DOI] [PubMed] [Google Scholar]
- 81.Rocha MF, Mansur AA, Martins CP, Barbosa-Stancioli EF, Mansur HS. Macrophage Response to UHMWPE Submitted to Accelerated Ageing in Hydrogen Peroxide. Open Biomed Eng J. 2010;4:107-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maitra R, Clement CC, Crisi GM, Cobelli N, Santambrogio L. Immunogenecity of modified alkane polymers is mediated through TLR1/2 activation. PLoS ONE. 2008;3(6):e2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brach del Prever EM, Bistolfi A, Costa L, Bracco P, Linari A, Botto Micca F, Crova M, Gallinaro P. The biological reaction to polyethylene wear debris can be related with oxidation of the UHMWPE cups. Chir Organi Mov. 2003 Jul-Sep;88(3):291-303 [PubMed] [Google Scholar]
- 84.Margevicius KJ, Bauer TW, McMahon JT, Brown SA, Merritt K. Isolation and characterization of debris in membranes around total joint prostheses. J Bone Joint Surg Am. 1994 Nov;76(11):1664-75 [DOI] [PubMed] [Google Scholar]
- 85.Baxter RM, Steinbeck MJ, Tipper JL, Parvizi J, Marcolongo M, Kurtz SM. Comparison of periprosthetic tissue digestion methods for ultra-high molecular weight polyethylene wear debris extraction. J Biomed Mater Res B Appl Biomater. 2009 Oct;91(1):409-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.ASTM International F1877–05e1 standard practice for characterization of particles. 2005. http://www.astm.org/DATABASE.CART/HISTORICAL/F1877-05E1.htm. Accessed 2007 Sep [Google Scholar]
- 87.Mirra JM, Amstutz HC, Matos M, Gold R. The pathology of the joint tissues and its clinical relevance in prosthesis failure. Clin Orthop Relat Res. 1976 Jun;(117):221-40 [PubMed] [Google Scholar]
- 88.ASTM International F2102–06e1 standard guide for evaluating the extent of oxidation in ultra-high-molecular-eight polyethylene fabricated forms intended for surgical implants. 2006. http://www.astm.org/Standards/F2102.htm. Accessed 2009 Dec [Google Scholar]
- 89.Costa L, Bracco P, Brach del Prever EM, Kurtz SM, Gallinaro P. Oxidation and oxidation potential in contemporary packaging for polyethylene total joint replacement components. J Biomed Mater Res B Appl Biomater. 2006 Jul;78(1):20-6 [DOI] [PubMed] [Google Scholar]
- 90.Carlsson DJ, Lacoste J. A critical comparison of methods for hydroperoxide measurement in oxidized polyolefins. Polym Degrad Stabil. 1991;32(3):377-86 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest
Details of the methods for wear particle isolation, imaging, wear particle analysis, particle validation, inflammatory response evaluation, penetration analysis, and oxidation analysis; tables showing implant oxidation values, linear penetration values, equations and definitions of wear particle characteristics and biological activity indices, and values for particle size, shape, and number; and a figure demonstrating a comparison of the Fourier transform infrared spectroscopy findings with previous spectra


