Abstract
➤ Tendon injuries often result from excessive or insufficient mechanical loading, impairing the ability of the local tendon cell population to maintain normal tendon function.
➤ The resident cell population composing tendon tissue is mechanosensitive, given that the cells are able to alter the extracellular matrix in response to modifications of the local loading environment.
➤ Natural tendon healing is insufficient, characterized by improper collagen fibril diameter formation, collagen fibril distribution, and overall fibril misalignment.
➤ Current tendon repair rehabilitation protocols focus on implementing early, well-controlled eccentric loading exercises to improve repair outcome.
➤ Tissue engineers look toward incorporating mechanical loading regimens to precondition cell populations for the creation of improved biological augmentations for tendon repair.
Tendon Structure and Function
Tendons connect muscle to bone for the transmission of forces producing joint movement. Composed of primarily type-I collagen fibers in a parallel alignment1, tendons are viscoelastic, possessing both solid and fluid-like characteristics and exhibiting changes to the stress-strain relationship with respect to the rate at which they are loaded2. In addition to type-I collagen, tendons are composed of minor collagens3, including type III, an immature fibrillar collagen that matures into type-I collagen, and type-X collagen, a short-chained collagen found localized in the tendon-to-bone insertion site3. Given the highly organized, hierarchical collagen structure (Fig. 1), tendons exhibit high tensile strength4-6, allowing for the efficient transmission of large loads, a result of the local cell population to adapt to changes in loading conditions7. Further contributing to the structure and biomechanical properties are proteoglycans and glycoproteins, which function to regulate the process of collagen fibrillogenesis and control fibril diameter throughout tendon development and homeostasis8-13. Studies using genetically manipulated mouse models, in which decorin has been knocked out, have investigated the role of decorin, a small leucine-rich proteoglycan important to tendon structure, and have shown that the absence of decorin results in improper collagen fibril formation and decreases mechanical properties13. Undoubtedly, proper tendon structure relies on the interaction of a number of factors to establish normal tendon function.
Fig. 1.
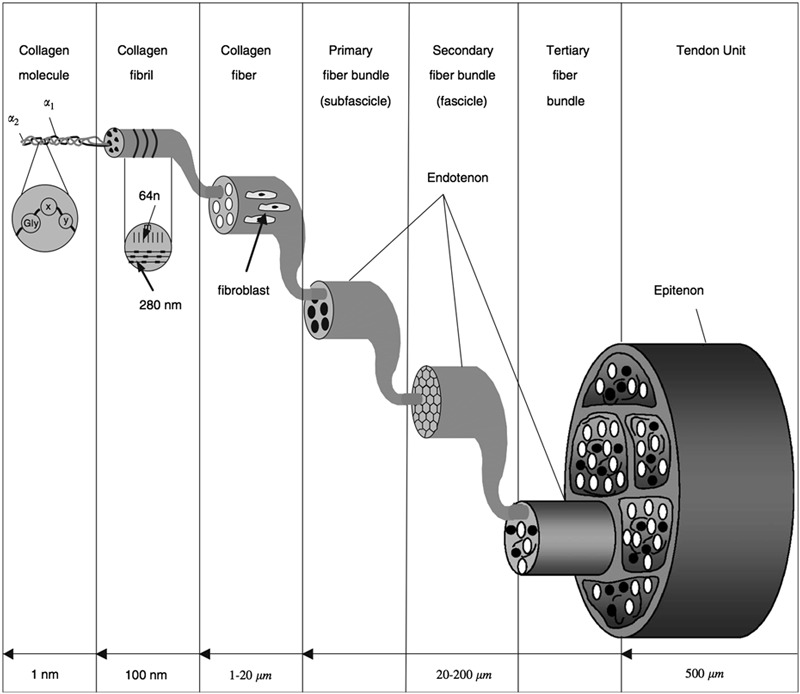
The tendon’s hierarchical structure begins at the molecular level with tropocollagen1. Approximately five tropocollagen molecules form a microfibril, which then aggregate to create a subfibril1. Several subfibrils form a single fibril. Multiple fibrils form a tendon fascicle, and fascicles, separated by the endotenon, join to form the macroscopic tendon1. Tendon fibroblasts, or tenocytes, are found on collagen fibers allowing for the regulation of the extracellular environment in response to chemical and mechanical cues. (Reproduced, with permission of Elsevier, from: Silver FH, Freeman JW, Seehra GP. Collagen self-assembly and the development of tendon mechanical properties. J. Biomech. 2003 Oct;36(10):1529-33, Copyright 2003; and Wang JH. Mechanobiology of tendon. J Biomech. 2006;39(9):1563-82, Copyright 2006.)
Tendon fibroblasts, also referred to as tenoyctes, are the primary cell type regulating tendon homeostasis. These spindle-shaped cells, located along collagen fibers, interact with one another and adjacent collagen fibers, allowing for the formation of collagen cross-links and recognition of chemical and mechanical changes in the extracellular environment14. Tenocytes are mechanosensitive since they can respond to mechanical loading events by modulating the extracellular environment through the formation and degradation of matrix proteins via a process termed mechanotransduction14,15. This process involves interactions among extracellular matrix proteins, cell surface receptors, the internal actin cytoskeleton, and signaling molecules, which ultimately regulate protein expression in response to loading alterations15. While normal physiologic loads are necessary for appropriate tendon development and maintenance, abnormal loading inhibits the capacity of the cell population to maintain homeostasis, contributing to injury16. Reestablishing these mechanotransductive processes may be key to improving repair outcome following tendon injury16.
The Role of Loading in Tendon Development and Homeostasis
Tendon Development
Mechanical forces during development are vital to successful limb and musculoskeletal tissue formation during embryogenesis17-24. Given limitations in technologies and model systems to isolate single mechanical events, investigating the role of tendon loading during embryogenesis is difficult17. Nevertheless, investigators have shown through in vivo embryonic immobilization studies in chicks that synovial joint development is impaired in the absence of physiologic loads18. For example, the menisci of the tibiofemoral joint and the plantar tarsal sesamoid of the tibiotarsal joint fail to form, suggesting the inability of tendinous structures to form properly in the absence of mechanical loading and the importance of mechanical stress for proper musculoskeletal development18.
It is postulated that embryonic and early postnatal growth of tendon relies on the generation of two types of stresses: rapid muscular activity and slow, growth-related elongation of bone21. Early in tendon development, the collagen fibril diameter is characterized as a homogeneous distribution of small fibrils (ranging from approximately 40 to 75 nm). Through tendon maturation and force generation during early postnatal growth, the tendon develops a distribution of both large (approximately 100 to 150 nm) and small fibrils (approximately 40 to 75 nm)23,24. The formation of large fibrils provides the majority of resistance to tensile strength, while the small fibrils negate creep and support improved interfibrillar binding24. Using an in vitro model of embryonic tendon formation, Kalson et al. found that applying a slow, steady strain rate to embryonic chick metatarsal tendon cells produced an increase in collagen fibril diameter and fibril volume fraction, improved cell elongation, and led to increases in both Young’s modulus and ultimate tensile stress compared with unstretched controls21.
Tendon Homeostasis
Normal, physiologic loads are required to maintain tendon homeostasis and prevent excessive degradation of the extracellular matrix25-27. Nabeshima et al. found that culturing nontensioned rabbit patellar tendon explants in the presence of collagenase over a period of twenty hours significantly decreased linear stiffness (p < 0.0001), elongation to failure (p < 0.002), and maximum failure force (p < 0.002) by 80% compared with explants tensioned with constant 4% strain26. Further, Flynn et al. found that reconstituted type-I collagen micronetworks, strained between micropipettes, degraded significantly slower (p < 0.05) than unloaded controls when exposed to mammalian collagenase matrix metalloproteinase 827. These experiments support the beneficial effect of mechanical load and the need for its incorporation in both clinical postoperative rehabilitation protocols and in tissue-engineering applications27.
It is hypothesized that the cell population of a tendon responds to the application of mechanical stress and modulation of the extracellular matrix through the activation and/or effects of a number of growth factors, including transforming growth factor-β1 (TGF-β1), connective tissue growth factor, and interleuken-6 (IL-6)28-30. Type-I collagen synthesis and deposition have been linked to increasing expression levels of TGF-β1 both in human Achilles tendon studies and studies investigating the effects of cyclic loading on TGF-β1 expression in human tendon fibroblasts29,30.
Scleraxis has been identified as a DNA-binding transcription factor critical to embryonic limb tendon formation31,32. It is the primary regulator of tenocyte differentiation and overall tendon phenotype, while also modulating type-I collagen synthesis in response to the application of stress33-37. Scott et al. found that applying cyclic load to a bioartificial tendon over a three-week period produced higher scleraxis and type-I collagen expression compared with the nonstimulated control35. Mendias et al. subjected reporter mice that expressed green fluorescent protein under the control of a scleraxis promoter to a six-week treadmill-training program and showed an upregulation of scleraxis and type-I collagen expression for the exercised mice compared with the controls36. Mechanical stress is key in promoting and maintaining a tendon-specific phenotype, and scleraxis may play a role in the adaptation of tendon to physiologic loading37.
Mechanisms of Tendon Injury, Natural Healing, and Repair
Mechanisms of Tendon Injury
The type and prevalence of tendon injuries are dependent on a number of factors including sex, age, normal daily activity levels, overall health, and the circumstances contributing to the injury38-41. Tendon impairments typically result from an internal tensile overloading event, with acute injuries occurring after one isolated, overloading event and chronic injuries occurring over time through repetitive, excessive loading events39,41. In contrast, others have suggested that acute injuries are indicative of an underlying chronic impairment that contributed to the injury41.
Overuse tendon injuries, commonly involving the patellar tendon, Achilles tendon, and the origin of the extensor carpi radialis brevis (tennis elbow), account for 7% (66,575 of 889,980 office visits in 2002) of the musculoskeletal disorders in the United States42. Tendinopathy is characterized by a loss of normal tendon architecture, changes to normal tenocyte morphology and apoptosis, alterations in the collagen fibril distribution profile, and neovascularization43-47. Chronic tendon impairments are frequently attributed to repetitive motion and/or overuse. Excessive loading events lead to microtear formation in the tendon48, which, if not repaired properly, may lead to the initiation of inflammatory and degenerative responses. This results in an overall weakened structure and increased propensity for tendon rupture48-50. In an in vivo tendinopathy model, Nakama et al. found that when the New Zealand White rabbit flexor digitorum profundus muscle was stimulated repetitively for eighty hours, microtears were found in all tendon regions and were significantly greater (p < 0.0001) in the loaded limb compared with the unloaded limb50. Other investigators have claimed that underuse of a damaged segment of a tendon may be the source of the chronic impairment49. Egerbacher et al. found that a loss of homeostatic tension following stress deprivation correlated with increased cell apoptosis in a rat tail tendon model49. Ultimately, tendinopathy may result from a combination of overuse and underuse mechanisms, with overloading creating microtears leading to decreased loading of the cell population resident on the damaged collagen fibers.
Natural Healing
On the basis of studies on horses, rabbits, and rats39,40,51-56, given that obtaining human biopsy samples can be difficult, it has been shown that following tendon injury, the natural healing process forms scar tissue via a three-stage process: inflammation, matrix production, and remodeling and maturation39,40. The inflammatory stage initiates the response to injury, typically throughout the first week39. The process is characterized by the development of a fibrin clot to stabilize the site; hemostasis; migration of neutrophils, macrophages, and erythrocytes; and subsequent neovascularization39. The matrix production stage initiates as matrix-producing fibroblasts localized to the injury site begin synthesizing collagen and other extracellular matrix proteins throughout approximately one to four weeks following injury39,40. Substantial cellular proliferation and matrix production occur; however, the collagen produced is highly disorganized40. The final stage, remodeling and maturation, begins approximately four weeks following injury and continues until the tissue is repaired through scar formation39. During this phase, the extracellular matrix is remodeled to create a more organized structure through collagen turnover, realignment, and formation of collagen cross-links39. Cell density and vascularity decrease as the tissue further repairs40.
Studies have shown that natural healing leads to tendon biomechanical properties that fail to match normal levels at up to eight weeks, twenty-six weeks, and twelve months following injury in a murine and rabbit central patellar tendon model, and sheep Achilles tendon model, respectively51-53. Investigators have suggested the poor mechanical properties result from predominantly small fibrils in the resulting repair tissue, compared with the normal distribution of both large and small fibrils observed in normal adult human tendon54,55, but conflicting results exist. Matthew and Moore showed that, following an extensor digitorum longus tendon transection in a rat model, the collagen fibril diameter distribution remained at approximately 40 nm up to 240 days following injury55. In contrast, Lavagnino et al. found that there was no difference between control and stress-deprived rat tail tendons in the number of fibrils per tendon counted, mean fibril diameter, density, or size distribution when cultured in vitro56. This may imply that the collagen fibril diameter distribution is not solely responsible, although more work investigating the mechanisms contributing to the ultimate decrease in mechanical properties is needed.
Repair
After tendon injury, operative intervention is often necessary to restore function. Previous work has shown inferior healing when operative repair is delayed following injury57-59. While current therapies produce functional outcomes in the short term, long-term repair outcome varies with respect to type of injury, injury location, and severity38,40. A large number of tendon injuries result from ruptures at the tendon-to-bone insertion site. This complex, zonal interface is characterized by the integration of a tendon’s collagen fibers transitioning through a fibrocartilaginous region into the mineralized bone. The differences in material properties of the soft and hard tissue lead to high stress concentrations at this site, contributing to injury60-64. In an effort to improve tendon-to-bone healing, the application of static or cyclic loading at the insertion site may be necessary to restore the zonal phenotype60,65-67. Additionally, the repair tension, the amount of tension placed on a tendon to reattach it to bone, is important in recreating the insertion site59. Stasiak et al. developed a knee joint fixation system to study tendon and ligament-to-bone healing in a rat model of anterior cruciate ligament reconstruction65. The system applies a cyclic stimulus to the knee joint while monitoring the forces generated across the joint. The system has been validated in a preclinical experimental setting but has yet to be implemented clinically65. Brophy et al. investigated the effect of cyclic, axial displacement of the femur and tibia on an anterior cruciate ligament reconstruction and found no healing impairment with the application of cyclic load, although the inflammatory response increased in comparison with nonstimulated controls66. Further work is needed to elucidate the effects of loading on insertion site repair prior to implementation in a clinical setting.
Clinical Applications
Normal tendon development and homeostasis is closely linked to the degree and pattern of mechanical loading to which the tissue is exposed47,67. Likewise, tendon injury and degeneration may be related to alterations in the physiologic loading profile14,47,68-70. Manipulation of the mechanical environment of healing tendon may exert a biologic effect through the mechanotransduction mechanism and holds promise for promoting a repair process that restores normal tendon structure and function. Clinical applications of mechanobiological principles following tendon injury form the basis of rehabilitation protocols71-73. Programs emphasizing tendon loading may be applied for the reversal of age and disuse-related tendon dysfunction, rehabilitation following tendon overuse injury, and in the development of postoperative physical therapy regimens that optimize healing and function following surgical repair.
Disuse muscle atrophy and weakness are causes of impaired function in older individuals74-78. While age-related sarcopenia has been repeatedly documented, the effect of aging on tendon biomechanical properties is inconclusive74,79-83. Methodological differences make study comparisons difficult, although the majority of investigators have suggested that collagen loss occurs in older individuals84-86. A substantial component of age-related strength loss may be the result of inactivity and can be modified by an appropriate exercise program87-92. Studies have shown that collagen and elastin production, along with collagen fibril diameter and collagen cross-linking, decrease as a result of age82. Consequently, tendon tensile strength and stiffness decrease, contributing to injury. Others have shown that while aging does result in decreased tendon mechanical properties, collagen fibril morphology, packing fraction, and collagen cross-linking remain relatively constant over time93,94. An in vivo study comparing the patellar tendons of twenty-seven and sixty-five- year-old men found decreased collagen content with increased age, yet there was no difference in the degree of collagen cross-linking between age groups, indicating that alterations in mechanical properties may be a result of other factors, such as reduction in glycoprotein and proteoglycan levels or the inability of the tissue to interact with water appropriately93,94.
Disuse following immobilization has been associated with decreased levels of extracellular matrix protein expression, alterations in tenocyte morphology, and loss of normal extracellular matrix architecture, resulting in impaired function and healing capacity14,95-98. Exercise improves the mechanical properties following age and disuse changes99-101. The ideal exercise program seeks to avoid injury while providing a biologic stimulus to maintain tendon homeostasis and function. Resistance training utilizing 80% of the five-repetition maximum three times per week for fourteen weeks in elderly individuals was shown to result in a 65% to 70% increase in tendon stiffness102. Similarly, resistance training following a ninety-day period of simulated weightlessness resulted in improved tendon mechanical properties, although this regimen did not fully restore function to the levels before the period of weightlessness95. These data suggest that to ameliorate the loss of tendon strength due to aging and/or disuse, incorporating resistance training into exercise regimens may be beneficial95.
Eccentric strengthening exercise programs have been advocated as effective treatments for tendon overuse injuries and prevention of reinjury100-108. Arampatzis et al. demonstrated that exercises involving high tendon strain (mean and standard deviation, 4.72% ± 1.08%) were more effective than those producing low tendon strain (mean, 2.97% ± 0.47%) in triggering an adaptive response in human Achilles tendon100. Stanish et al. suggested that eccentric exercises prepare patients for return to functional, sports-related activities better than those that emphasize concentric muscle strengthening108. Multiple studies have demonstrated eccentric exercise regimens to be effective for the treatment of tendinopathy103,109-115. Ohberg et al. found decreased Achilles tendon thickness and normal tendon structure in response to a twelve-week eccentric training program in patients with chronic Achilles tendinosis103.
While the mechanism of action of eccentric exercise is poorly understood, it is theorized that eccentric exercise loads the tendon to a greater magnitude compared with concentric contraction, thereby stimulating a more effective repair response108,110. Further, eccentric exercise may facilitate remodeling by increasing the number of collagen cross-linkages109. However, more recently, a study comparing concentric and eccentric exercises for Achilles tendinopathy found no differences in the magnitude of peak tendon load or tendon length change between the regimens116. The authors described the presence of high-frequency oscillations in tendon force during eccentric contractions, which are rare with concentric exercises. These oscillations may modulate the therapeutic effects attributed to eccentric exercise regimen116. While many have advocated eccentric exercise programs, others have suggested that understanding the mechanisms involved in these regimens is necessary to support the efficacy of these programs for treatment117,118. The optimal load, speed of movement, number of repetitions, and duration of contraction remain to be determined and require further investigation109,116.
While tendon overuse rehabilitation protocols still require optimization, current therapeutic protocols often employ primarily eccentric exercises. However, for the treatment of lateral elbow tendinopathy (tennis elbow), one proposed muscle-strengthening regimen incorporates a combination of both eccentric and concentric contraction exercises119. In this regimen, the patient undergoes a series of daily, ten-repetition maximum exercises using a light weight (1 to 2-lb [0.45 to 0.91-kg] dumbbell) to strengthen the wrist extensor muscles119. Combining eccentric contractions, in which the muscle group lengthens with wrist palmar flexion, and concentric contractions, in which the muscle group shortens with wrist dorsiflexion, results in a protocol that may decrease muscle tension, leading to a reduction in muscle and tendon soreness119.
Joint mobilization with protection of the newly repaired tendon is a mainstay of rehabilitation following tendon repair120-122. Depending on injury type and location, controlling joint range of motion restricts the amount of loading possible, thereby preventing reinjury. Joint contractures following flexor tendon repair are a major potential source of functional impairment. A canine model of joint mobilization following tendon repair found that early motion resulted in fewer adhesions with improved tendon gliding and superior tensile strength compared with postoperative immobilization120-122. Multiple rehabilitation protocols ranging from those emphasizing passive motion, those that incorporate active finger flexion, and those that incorporate combinations of the two, have been utilized postoperatively and are effective in restoring joint motion123,124. Kitis et al. found improved grip strength, range of motion, and hand function (Disabilities of the Arm, Shoulder and Hand score125) when patients were treated with active mobilization with a dynamic splinting protocol compared with those treated with a controlled passive movement regimen126. A 2004 Cochrane review failed to detect outcome-based differences when the various controlled motion and/or loading rehabilitation protocols were compared, finding that all produced acceptable and comparable outcomes127. While the optimal rehabilitative protocol after repair has yet to be determined, it is clear that controlled tendon loading and movement within a synovial sheath provide a mechanical environment that is beneficial to flexor tendon repair and results in improved functional restoration.
Early mobilization protocols with controlled weight-bearing have been employed following operative and nonoperative treatment of Achilles tendon rupture. This approach is supported by several animal models, in which early motion accelerates the repair process and results in superior tissue quality128. Twaddle and Poon found comparable functional results and no significant difference in rerupture rate when controlled early motion was instituted following immobilization for ten days in patients treated with or without surgery129. These findings have been investigated in other studies in which accelerated rehabilitation protocols have resulted in improved functional recovery and low rerupture rates130-133.
Mechanical Loading in Tissue-Engineering Applications
Tissue engineers are incorporating mechanical stimulation to enhance tendon tissue augmentations and replacements134-146. By mechanically preconditioning the tissue-engineered construct cell population prior to in vivo implantation, the cells may be better equipped to enhance the repair since they have been exposed to the appropriate mechanical environment134,135. Further elucidating the cellular processes involved in the response to the normal and abnormal mechanical environments will improve tissue engineering therapies.
Given the importance of physiological loading to maintain tendon homeostasis, investigators have shown that applying load promotes a tendon-like phenotype in both two and three-dimensional culture conditions139-146. Ralphs et al. showed that when tendon cells are cultured on a two-dimensional substrate and subjected to biaxial strain at 1 Hz for eight hours per day for a total of ninety-six hours, cells link together using actin adherens junctions along the principal line of strain to monitor tensile load142. Garvin et al. found, after seven days of loading, improved biomechanical properties (mean and standard deviation, 327.65 ± 172.03 MPa versus 112.20 ± 6.07 MPa), gene expression results that trended toward normal tendon tissue, and improved cellular alignment and contraction for the loaded bioartificial tendon compared with unstimulated controls146.
Others have sought to improve tendon repair by creating tissue-engineered constructs using autologous progenitor cells derived from the bone marrow of New Zealand White rabbits seeded on a bovine-derived type-I collagen sponge and incorporating a mechanical stimulation profile at a frequency of 1 Hz, for eight hours per day, at a peak strain of 2.4% for a total of twelve days134. The tissue-engineered constructs were implanted into defects in the central third of rabbit patellar tendons, and repair tissues were harvested at twelve weeks following surgery. The results showed that mechanical stimulation improved repair tissue maximum force, linear stiffness, maximum stress, and linear modulus to 70%, 85%, 70%, and 50%, respectively, of the values in the normal, uninjured central third of patellar tendons134. Mechanical stimulation increased collagen type-I and type-III gene expression three and four times greater than in nonstimulated controls, respectively135. Additionally, the stimulated tissue-engineered constructs were 2.5 times stiffer than nonstimulated controls135.
Overview
Tendons are dynamic tissues composed of a cell population capable of responding to mechanical cues by altering the extracellular matrix. While it is known that loading and tension play a large role in overall tendon function, it is still necessary to determine the most suitable methods of incorporating these findings toward improving tendon repair. How does a tendon naturally heal and when is the most effective phase to implement repair procedures and/or intervention? When is the optimal time for incorporating loading regimens in patient rehabilitation protocols? How much and how often should loading regimens be implemented in a clinical setting? Should it be based on type of injury and/or location? How can loading parameters be incorporated when creating tissue-engineered constructs that are best primed for in vivo tendon repair? To answer these questions, further investigation with a focus on improving clinical outcomes is needed.
Supplementary Material
Disclosure of Potential Conflicts of Interest
Footnotes
Disclosure: None of the authors received payments or services, either directly or indirectly (i.e., via his or her institution), from a third party in support of any aspect of this work. One or more of the authors, or his or her institution, has had a financial relationship, in the thirty-six months prior to submission of this work, with an entity in the biomedical arena that could be perceived to influence or have the potential to influence what is written in this work. No author has had any other relationships, or has engaged in any other activities, that could be perceived to influence or have the potential to influence what is written in this work. The complete Disclosures of Potential Conflicts of Interest submitted by authors are always provided with the online version of the article.
References
- 1.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6(1):11-23 [DOI] [PubMed] [Google Scholar]
- 2.Zhang G. Evaluating the viscoelastic properties of biological tissues in a new way. J Musculoskelet Neuronal Interact. 2005 Mar;5(1):85-90 [PubMed] [Google Scholar]
- 3.Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003 Nov 28;55(12):1531-46, doi:10.1016/j.addr.2003.08.002 [DOI] [PubMed] [Google Scholar]
- 4.Malaviya P, Butler DL, Korvick DL, Proch FS. In vivo tendon forces correlate with activity level and remain bounded: evidence in a rabbit flexor tendon model. J Biomech. 1998 Nov;31(11):1043-9 [DOI] [PubMed] [Google Scholar]
- 5.Defrate LE, Nha KW, Papannagari R, Moses JM, Gill TJ, Li G. The biomechanical function of the patellar tendon during in-vivo weight-bearing flexion. J Biomech. 2007;40(8):1716-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maganaris CN. Tensile properties of in vivo human tendinous tissue. J Biomech. 2002 Aug;35(8):1019-27 [DOI] [PubMed] [Google Scholar]
- 7.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999 Oct;18(5):417-26 [DOI] [PubMed] [Google Scholar]
- 8.Rigozzi S, Müller R, Snedeker JG. Local strain measurement reveals a varied regional dependence of tensile tendon mechanics on glycosaminoglycan content. J Biomech. 2009 Jul 22;42(10):1547-52, doi:10.1016/j.jbiomech.2009.03.031 [DOI] [PubMed] [Google Scholar]
- 9.Anderson JC, Jackson DS. The isolation of glycoproteins from bovine achilles tendon and their interaction with collagen. Biochem J. 1972 Mar;127(1):179-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pins GD, Christiansen DL, Patel R, Silver FH. Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J. 1997 Oct;73(4):2164-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang G, Ezura Y, Chervoneva I, Robinson PS, Beason DP, Carine ET, Soslowsky LJ, Iozzo RV, Birk DE. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006 Aug 15;98(6):1436-49, doi:10.1002/jcb.20776 [DOI] [PubMed] [Google Scholar]
- 12.Douglas T, Heinemann S, Bierbaum S, Scharnweber D, Worch H. Fibrillogenesis of collagen types I, II, and III with small leucine-rich proteoglycans decorin and biglycan. Biomacromolecules. 2006 Aug;7(8):2388-93 [DOI] [PubMed] [Google Scholar]
- 13.Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE. Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact. 2005 Mar;5(1):5-21 [PubMed] [Google Scholar]
- 14.Wang JHC. Mechanobiology of tendon. J Biomech. 2006;39(9):1563-82 [DOI] [PubMed] [Google Scholar]
- 15.Chiquet M, Renedo AS, Huber F, Flück M. How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biol. 2003 Mar;22(1):73-80 [DOI] [PubMed] [Google Scholar]
- 16.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011 Mar-Apr;19(2):134-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson JH, Carter DR. Mechanical induction in limb morphogenesis: the role of growth-generated strains and pressures. Bone. 2002 Dec;31(6):645-53 [DOI] [PubMed] [Google Scholar]
- 18.Mikic B, Johnson TL, Chhabra AB, Schalet BJ, Wong M, Hunziker EB. Differential effects of embryonic immobilization on the development of fibrocartilaginous skeletal elements. J Rehabil Res Dev. 2000 Mar-Apr;37(2):127-33 [PubMed] [Google Scholar]
- 19.Wozniak MA, Chen CS. Mechanotransduction in development: a growing role for contractility. Nat Rev Mol Cell Biol. 2009 Jan;10(1):34-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nowlan NC, Murphy P, Prendergast PJ. Mechanobiology of embryonic limb development. Ann N Y Acad Sci. 2007 Apr;1101:389-411 [DOI] [PubMed] [Google Scholar]
- 21.Kalson NS, Holmes DF, Herchenhan A, Lu Y, Starborg T, Kadler KE. Slow stretching that mimics embryonic growth rate stimulates structural and mechanical development of tendon-like tissue in vitro. Dev Dyn. 2011 Nov;240(11):2520-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalson NS, Holmes DF, Kapacee Z, Otermin I, Lu Y, Ennos RA, Canty-Laird EG, Kadler KE. An experimental model for studying the biomechanics of embryonic tendon: Evidence that the development of mechanical properties depends on the actinomyosin machinery. Matrix Biol. 2010 Oct;29(8):678-89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ansorge HL, Adams S, Birk DE, Soslowsky LJ. Mechanical, compositional, and structural properties of the post-natal mouse Achilles tendon. Ann Biomed Eng. 2011 Jul;39(7):1904-13 Epub 2011 Mar 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ansorge HL, Hsu JE, Edelstein L, Adams S, Birk DE, Soslowsky LJ. Recapitulation of the Achilles tendon mechanical properties during neonatal development in a mouse model. J Orthop Res. 2012 Mar;30(3):448-56 Epub 2011 Aug 24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhole AP, Flynn BP, Liles M, Saeidi N, Dimarzio CA, Ruberti JW. Mechanical strain enhances survivability of collagen micronetworks in the presence of collagenase: implications for load-bearing matrix growth and stability. Philos Transact A Math Phys Eng Sci. 2009 Sep 13;367(1902):3339-62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nabeshima Y, Grood ES, Sakurai A, Herman JH. Uniaxial tension inhibits tendon collagen degradation by collagenase in vitro. J Orthop Res. 1996 Jan;14(1):123-30 [DOI] [PubMed] [Google Scholar]
- 27.Flynn BP, Bhole AP, Saeidi N, Liles M, DiMarzio CA, Rubeti JW. Mechanical strain stabilizes reconstituted collagen fibrils against enzymatic degradation by mammalian collagenase matrix metalloproteinase 8 (MMP-8). PLoS ONE. 2010;5(8):e12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skutek M, van Griensven M, Zeichen J, Brauer N, Bosch U. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur J Appl Physiol. 2001 Nov;86(1):48-52 [DOI] [PubMed] [Google Scholar]
- 29.Heinemeier K, Langberg H, Olesen JL, Kjaer M. Role of TGF-β1 in relation to exercise-induced type I collagen synthesis in human tendinous tissue. J Appl Physiol. 2003 Dec;95(6):2390-7 [DOI] [PubMed] [Google Scholar]
- 30.Heinemeier KM, Kjaer M. In vivo investigation of tendon responses to mechanical loading. J Musculoskelet Neuronal Interact. 2011 Jun;11(2):115-23 [PubMed] [Google Scholar]
- 31.Murchison ND, Price BA, Conner DA, Keene DR, Olson EN, Tabin CJ, Schweitzer R. Regulation of tendon differentiation by scleraxis distinguishes force-transmitting tendons from muscle-anchoring tendons. Development. 2007 Jul;134(14):2697-708 [DOI] [PubMed] [Google Scholar]
- 32.Schweitzer R, Zelzer E, Volk T. Connecting muscles to tendons: tendons and musculoskeletal development in flies and vertebrates. Development. 2010 Sep 1;137(17):2807-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001 Oct;128(19):3855-66 [DOI] [PubMed] [Google Scholar]
- 34.Léjard V, Brideau G, Blais F, Salingcarnboriboon R, Wagner G, Roehrl MH, Noda M, Duprez D, Houillier P, Rossert J. Scleraxis and NFATc regulate the expression of the pro-α1(I) collagen gene in tendon fibroblasts. J Biol Chem. 2007 Jun 15;282(24):17665-75 [DOI] [PubMed] [Google Scholar]
- 35.Scott A, Danielson P, Abraham T, Fong G, Sampaio AV, Underhill TM. Mechanical force modulates scleraxis expression in bioartificial tendons. J Musculoskelet Neuronal Interact. 2011 Jun;11(2):124-32 [PubMed] [Google Scholar]
- 36.Mendias CL, Gumucio JP, Bakhurin KI, Lynch EB, Brooks SV. Physiological loading of tendons induces scleraxis expression in epitenon fibroblasts. J Orthop Res. 2012 Apr;30(4):606-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eliasson P, Andersson T, Aspenberg P. Rat Achilles tendon healing: mechanical loading and gene expression. J Appl Physiol. 2009 Aug;107(2):399-407 [DOI] [PubMed] [Google Scholar]
- 38.Lin TW, Cardenas L, Soslowsky LJ. Biomechanics of tendon injury and repair. J Biomech. 2004 Jun;37(6):865-77 [DOI] [PubMed] [Google Scholar]
- 39.Sandrey MA. Acute and chronic tendon injuries: factors affecting the healing response and treatment. J Sport Rehabil. 2003;12:70-91 [Google Scholar]
- 40.Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am. 2005 Jan;87(1):187-202 [DOI] [PubMed] [Google Scholar]
- 41.Maganaris CN, Narici MV, Almekinders LC, Maffulli N. Biomechanics and pathophysiology of overuse tendon injuries: ideas on insertional tendinopathy. Sports Med. 2004;34(14):1005-17 [DOI] [PubMed] [Google Scholar]
- 42.Rutland M, O’Connell D, Brismée JM, Sizer P, Apte G, O’Connell J. Evidence-supported rehabilitation of patellar tendinopathy. N Am J Sports Phys Ther. 2010 Sep;5(3):166-78 [PMC free article] [PubMed] [Google Scholar]
- 43.Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998 Nov-Dec;14(8):840-3 [DOI] [PubMed] [Google Scholar]
- 44.Maffulli N, Longo UG, Franceschi F, Rabitti C, Denaro V. Movin and Bonar scores assess the same characteristics of tendon histology. Clin Orthop Relat Res. 2008 Jul;466(7):1605-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohberg L, Lorentzon R, Alfredson H. Neovascularisation in Achilles tendons with painful tendinosis but not in normal tendons: an ultrasonographic investigation. Knee Surg Sports Traumatol Arthrosc. 2001 Jul;9(4):233-8 [DOI] [PubMed] [Google Scholar]
- 46.Perry SM, McIlhenny SE, Hoffman MC, Soslowsky LJ. Inflammatory and angiogenic mRNA levels are altered in a supraspinatus tendon overuse animal model. J Shoulder Elbow Surg. 2005 Jan-Feb;14(1)(Suppl S):79S-83S, doi:10.1016/j.jse.2005.09.020 [DOI] [PubMed] [Google Scholar]
- 47.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007 Aug;88(4):217-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fung DT, Wang VM, Andarawis-Puri N, Basta-Pljakic J, Li Y, Laudier DM, Sun HB, Jepsen KJ, Schaffler MB, Flatow EL. Early response to tendon fatigue damage accumulation in a novel in vivo model. J Biomech. 2010 Jan 19;43(2):274-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Egerbacher M, Arnoczky SP, Caballero O, Lavagnino M, Gardner KL. Loss of homeostatic tension induces apoptosis in tendon cells: an in vitro study. Clin Orthop Relat Res. 2008 Jul;466(7):1562-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakama LH, King KB, Abrahamsson S, Rempel DM. Evidence of tendon microtears due to cyclical loading in an in vivo tendinopathy model. J Orthop Res. 2005 Sep;23(5):1199-205 [DOI] [PubMed] [Google Scholar]
- 51.Dyment NA, Kazemi N, Aschbacher-Smith LE, Barthelery NJ, Kenter K, Gooch C, Shearn JT, Wylie C, Butler DL. The relationships among spatiotemporal collagen gene expression, histology, and biomechanics following full-length injury in the murine patellar tendon. J Orthop Res. 2012 Jan;30(1):28-36 Epub 2011 Jun 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Awad HA, Boivin GP, Dressler MR, Smith FN, Young RG, Butler DL. Repair of patellar tendon injuries using a cell-collagen composite. J Orthop Res. 2003 May;21(3):420-31 [DOI] [PubMed] [Google Scholar]
- 53.Bruns J, Kampen J, Kahrs J, Plitz W. Achilles tendon rupture: experimental results on spontaneous repair in a sheep-model. Knee Surg Sports Traumatol Arthrosc. 2000;8(6):364-9 [DOI] [PubMed] [Google Scholar]
- 54.Williams IF, Craig AS, Parry DAD, Goodship AE, Shah J, Silver IA. Development of collagen fibril organization and collagen crimp patterns during tendon healing. Int J Biol Macromol. 1985;7(5):275-82 [Google Scholar]
- 55.Matthew CA, Moore MJ. Collagen fibril morphometry in transected rat extensor tendons. J Anat. 1991 Apr;175:263-8 [PMC free article] [PubMed] [Google Scholar]
- 56.Lavagnino M, Arnoczky SP, Frank K, Tian T. Collagen fibril diameter distribution does not reflect changes in the mechanical properties of in vitro stress-deprived tendons. J Biomech. 2005 Jan;38(1):69-75, doi:10.1016/j.jbiomech.2004.03.035 [DOI] [PubMed] [Google Scholar]
- 57.Thomopoulos S, Genin GM, Galatz LM. The development and morphogenesis of the tendon-to-bone insertion - what development can teach us about healing -. J Musculoskelet Neuronal Interact. 2010 Mar;10(1):35-45 [PMC free article] [PubMed] [Google Scholar]
- 58.Boyer MI, Goldfarb CA, Gelberman RH. Recent progress in flexor tendon healing. The modulation of tendon healing with rehabilitation variables. J Hand Ther. 2005 Apr-Jun;18(2):80-5, quiz :86 [DOI] [PubMed] [Google Scholar]
- 59.Galatz LM, Rothermich SY, Zaegel M, Silva MJ, Havlioglu N, Thomopoulos S. Delayed repair of tendon to bone injuries leads to decreased biomechanical properties and bone loss. J Orthop Res. 2005 Nov;23(6):1441-7 [DOI] [PubMed] [Google Scholar]
- 60.Bedi A, Kovacevic D, Fox AJS, Imhauser CW, Stasiak M, Packer J, Brophy RH, Deng XH, Rodeo SA. Effect of early and delayed mechanical loading on tendon-to-bone healing after anterior cruciate ligament reconstruction. J Bone Joint Surg Am. 2010 Oct 20;92(14):2387-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galatz LM, Charlton N, Das R, Kim HM, Havlioglu N, Thomopoulos S. Complete removal of load is detrimental to rotator cuff healing. J Shoulder Elbow Surg. 2009 Sep-Oct;18(5):669-75 [DOI] [PubMed] [Google Scholar]
- 62.Gimbel JA, Van Kleunen JP, Lake SP, Williams GR, Soslowsky LJ. The role of repair tension on tendon to bone healing in an animal model of chronic rotator cuff tears. J Biomech. 2007;40(3):561-8 [DOI] [PubMed] [Google Scholar]
- 63.Hettrich CM, Rodeo SA, Hannafin JA, Ehteshami J, Shubin Stein BE. The effect of muscle paralysis using Botox on the healing of tendon to bone in a rat model. J Shoulder Elbow Surg. 2011 Jul;20(5):688-97 [DOI] [PubMed] [Google Scholar]
- 64.Thomopoulos S, Zampiakis E, Das R, Silva MJ, Gelberman RH. The effect of muscle loading on flexor tendon-to-bone healing in a canine model. J Orthop Res. 2008 Dec;26(12):1611-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stasiak M, Imhauser C, Packer J, Bedi A, Brophy R, Kovacevic D, Jackson K, Deng XH, Rodeo S, Torzilli P. A novel in vivo joint loading system to investigate the effect of daily mechanical load on a healing anterior cruciate ligament reconstruction. J Med Device. 2010;4(1):15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brophy RH, Kovacevic D, Imhauser CW, Stasiak M, Bedi A, Fox AJS, Deng XH, Rodeo SA. Effect of short-duration low-magnitude cyclic loading versus immobilization on tendon-bone healing after ACL reconstruction in a rat model. J Bone Joint Surg Am. 2011 Feb 16;93(4):381-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gelberman RH, Woo SL, Lothringer K, Akeson WH, Amiel D. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg Am. 1982 Mar;7(2):170-5 [DOI] [PubMed] [Google Scholar]
- 68.Jozsa LG, Kannus P. Overuse injuries of tendons. Human tendons: anatomy, physiology, and pathology. Champaign: Human Kinetics; 1997. p 164-253 [Google Scholar]
- 69.Jones GC, Corps AN, Pennington CJ, Clark IM, Edwards DR, Bradley MM, Hazleman BL, Riley GP. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum. 2006 Mar;54(3):832-42 [DOI] [PubMed] [Google Scholar]
- 70.Archambault JM, Wiley JP, Bray RC. Exercise loading of tendons and the development of overuse injuries. A review of current literature. Sports Med. 1995 Aug;20(2):77-89 [DOI] [PubMed] [Google Scholar]
- 71.Pettengill KM. The evolution of early mobilization of the repaired flexor tendon. J Hand Ther. 2005 Apr-Jun;18(2):157-68 [DOI] [PubMed] [Google Scholar]
- 72.Strickland JW. The scientific basis for advances in flexor tendon surgery. J Hand Ther. 2005 Apr-Jun;18(2):94-110, quiz :111 [DOI] [PubMed] [Google Scholar]
- 73.Grewal R, Chan Saw SS, Varitimidus S, Bastidas JA, Sotereanos DG, Fischer KJ. Evaluation of passive and active rehabilitation and of tendon repair for partial tendon lacerations after three weeks of healing in canines. Clin Biomech (Bristol, Avon). 2006 Oct;21(8):804-9 Epub 2006 Jun 27 [DOI] [PubMed] [Google Scholar]
- 74.Koopman R, van Loon LJ. Aging, exercise, and muscle protein metabolism. J Appl Physiol. 2009 Jun;106(6):2040-8 [DOI] [PubMed] [Google Scholar]
- 75.Narici MV, Maganaris C, Reeves N. Myotendinous alterations and effects of resistive loading in old age. Scand J Med Sci Sports. 2005 Dec;15(6):392-401 [DOI] [PubMed] [Google Scholar]
- 76.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998 Apr 15;147(8):755-63 [DOI] [PubMed] [Google Scholar]
- 77.Melton LJ, 3rd, Khosla S, Crowson CS, O’Connor MK, O’Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc. 2000 Jun;48(6):625-30 [PubMed] [Google Scholar]
- 78.Wolfson L, Judge J, Whipple R, King M. Strength is a major factor in balance, gait, and the occurrence of falls. J Gerontol A Biol Sci Med Sci. 1995 Nov;50(Spec No):64-7 [DOI] [PubMed] [Google Scholar]
- 79.Haut RC, Lancaster RL, DeCamp CE. Mechanical properties of the canine patellar tendon: some correlations with age and the content of collagen. J Biomech. 1992 Feb;25(2):163-73 [DOI] [PubMed] [Google Scholar]
- 80.Nielsen HM, Skalicky M, Viidik A. Influence of physical exercise on aging rats. III. Life-long exercise modifies the aging changes of the mechanical properties of limb muscle tendons. Mech Ageing Dev. 1998 Feb 16;100(3):243-60 [DOI] [PubMed] [Google Scholar]
- 81.Shadwick RE. Elastic energy storage in tendons: mechanical differences related to function and age. J Appl Physiol. 1990 Mar;68(3):1033-40 [DOI] [PubMed] [Google Scholar]
- 82.Dressler MR, Butler DL, Boivin GP. Age-related changes in the biomechanics of healing patellar tendon. J Biomech. 2006;39(12):2205-12 [DOI] [PubMed] [Google Scholar]
- 83.Vogel HG. Influence of maturation and age on mechanical and biochemical parameters of connective tissue of various organs in the rat. Connect Tissue Res. 1978;6(3):161-6 [DOI] [PubMed] [Google Scholar]
- 84.Vogel HG. Species differences of elastic and collagenous tissue—influence of maturation and age. Mech Ageing Dev. 1991 Jan;57(1):15-24 [DOI] [PubMed] [Google Scholar]
- 85.Vilarta R, Vidal BdeC. Anisotropic and biomechanical properties of tendons modified by exercise and denervation: aggregation and macromolecular order in collagen bundles. Matrix. 1989 Jan;9(1):55-61 [DOI] [PubMed] [Google Scholar]
- 86.Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res. 1994 Nov;12(6):796-803 [DOI] [PubMed] [Google Scholar]
- 87.Petrella RJ, Chudyk A. Exercise prescription in the older athlete as it applies to muscle, tendon, and arthroplasty. Clin J Sport Med. 2008 Nov;18(6):522-30 [DOI] [PubMed] [Google Scholar]
- 88.Ferri A, Scaglioni G, Pousson M, Capodaglio P, Van Hoecke J, Narici MV. Strength and power changes of the human plantar flexors and knee extensors in response to resistance training in old age. Acta Physiol Scand. 2003 Jan;177(1):69-78 [DOI] [PubMed] [Google Scholar]
- 89.Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA. 1990 Jun 13;263(22):3029-34 [PubMed] [Google Scholar]
- 90.Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. J Appl Physiol. 1988 Mar;64(3):1038-44 [DOI] [PubMed] [Google Scholar]
- 91.Galloway MT, Jokl P. Aging successfully: the importance of physical activity in maintaining health and function. J Am Acad Orthop Surg. 2000 Jan-Feb;8(1):37-44 [DOI] [PubMed] [Google Scholar]
- 92.Galloway MT, Kadoko R, Jokl P. Effect of aging on male and female master athletes’ performance in strength versus endurance activities. Am J Orthop (Belle Mead NJ). 2002 Feb;31(2):93-8 [PubMed] [Google Scholar]
- 93.Wood LK, Arruda EM, Brooks SV. Regional stiffening with aging in tibialis anterior tendons of mice occurs independent of changes in collagen fibril morphology. J Appl Physiol. 2011 Oct;111(4):999-1006, doi:10.1152/japplphysol.00460.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Couppé C, Hansen P, Kongsgaard M, Kovanen V, Suetta C, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties and collagen cross-linking of the patellar tendon in old and young men. J Appl Physiol. 2009 Sep;107(3):880-6 [DOI] [PubMed] [Google Scholar]
- 95.Reeves ND, Maganaris CN, Ferretti G, Narici MV. Influence of 90-day simulated microgravity on human tendon mechanical properties and the effect of resistive countermeasures. J Appl Physiol. 2005 Jun;98(6):2278-86 [DOI] [PubMed] [Google Scholar]
- 96.Yasuda T, Kinoshita M, Abe M, Shibayama Y. Unfavorable effect of knee immobilization on Achilles tendon healing in rabbits. Acta Orthop Scand. 2000 Feb;71(1):69-73 [DOI] [PubMed] [Google Scholar]
- 97.Yamamoto N, Ohno K, Hayashi K, Kuriyama H, Yasuda K, Kaneda K. Effects of stress shielding on the mechanical properties of rabbit patellar tendon. J Biomech Eng. 1993 Feb;115(1):23-8 [DOI] [PubMed] [Google Scholar]
- 98.Maganaris CN, Paul JP. In vivo human tendinous tissue stretch upon maximum muscle force generation. J Biomech. 2000a Nov;33(11):1453-9 [DOI] [PubMed] [Google Scholar]
- 99.Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. J Anat. 2006 Apr;208(4):433-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Arampatzis A, Peper A, Bierbaum S, Albracht K. Plasticity of human Achilles tendon mechanical and morphological properties in response to cyclic strain. J Biomech. 2010 Dec 1;43(16):3073-9 [DOI] [PubMed] [Google Scholar]
- 101.Magnusson SP, Narici MV, Maganaris CN, Kjaer M. Human tendon behaviour and adaptation, in vivo. J Physiol. 2008 Jan 1;586(1):71-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol. 2003 May 1;548(Pt 3):971-81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ohberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up. Br J Sports Med. 2004 Feb;38(1):8-11, discussion :11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Giffin JR, Stanish WD. Overuse tendonitis and rehabilitation. Can Fam Physician. 1993 Aug;39:1762-9 [PMC free article] [PubMed] [Google Scholar]
- 105.Alfredson H, Pietilä T, Jonsson P, Lorentzon R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998 May-Jun;26(3):360-6 [DOI] [PubMed] [Google Scholar]
- 106.Woo SL, Gomez MA, Woo YK, Akeson WH. Mechanical properties of tendons and ligaments. II. The relationships of immobilization and exercise on tissue remodeling. Biorheology. 1982;19(3):397-408 [DOI] [PubMed] [Google Scholar]
- 107.Silbernagel KG, Thomeé R, Thomeé P, Karlsson J. Eccentric overload training for patients with chronic Achilles tendon pain—a randomised controlled study with reliability testing of the evaluation methods. Scand J Med Sci Sports. 2001 Aug;11(4):197-206 [DOI] [PubMed] [Google Scholar]
- 108.Stanish WD, Rubinovich RM, Curwin S. Eccentric exercise in chronic tendinitis. Clin Orthop Relat Res. 1986 Jul;(208):65-8 [PubMed] [Google Scholar]
- 109.Maffulli N, Longo UG, Denaro V. Novel approaches for the management of tendinopathy. J Bone Joint Surg Am. 2010 Nov 3;92(15):2604-13, doi:10.2106/JBJS.I.01744 [DOI] [PubMed] [Google Scholar]
- 110.Fyfe I, Stanish WD. The use of eccentric training and stretching in the treatment and prevention of tendon injuries. Clin Sports Med. 1992 Jul;11(3):601-24 [PubMed] [Google Scholar]
- 111.Sayana MK, Maffulli N. Eccentric calf muscle training in non-athletic patients with Achilles tendinopathy. J Sci Med Sport. 2007 Feb;10(1):52-8 [DOI] [PubMed] [Google Scholar]
- 112.Roos EM, Engström M, Lagerquist A, Söderberg B. Clinical improvement after 6 weeks of eccentric exercise in patients with mid-portion Achilles tendinopathy — a randomized trial with 1-year follow-up. Scand J Med Sci Sports. 2004 Oct;14(5):286-95 [DOI] [PubMed] [Google Scholar]
- 113.Rompe JD, Nafe B, Furia JP, Maffulli N. Eccentric loading, shock-wave treatment, or a wait-and-see policy for tendinopathy of the main body of tendo Achillis: a randomized controlled trial. Am J Sports Med. 2007 Mar;35(3):374-83 [DOI] [PubMed] [Google Scholar]
- 114.Alfredson H, Pietilä T, Jonsson P, Lorentzon R. Heavy-load eccentric calf muscle training for the treatment of chronic Achilles tendinosis. Am J Sports Med. 1998 May-Jun;26(3):360-6 [DOI] [PubMed] [Google Scholar]
- 115.Croisier JL, Foidart-Dessalle M, Tinant F, Crielaard JM, Forthomme B. An isokinetic eccentric programme for the management of chronic lateral epicondylar tendinopathy [ads]. Br J Sports Med. 2007 Apr;41(4):269-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rees JD, Lichtwark GA, Wolman RL, Wilson AM. The mechanism for efficacy of eccentric loading in Achilles tendon injury; an in vivo study in humans. Rheumatology (Oxford). 2008 Oct;47(10):1493-7, doi:10.1093/rheumatology/ken262 [DOI] [PubMed] [Google Scholar]
- 117.Andres BM, Murrell GA. Treatment of tendinopathy: what works, what does not, and what is on the horizon. Clin Orthop Relat Res. 2008 Jul;466(7):1539-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Woodley BL, Newsham-West RJ, Baxter GD. Chronic tendinopathy: effectiveness of eccentric exercise. Br J Sports Med. 2007 Apr;41(4):188-98, discussion :199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Finestone HM, Rabinovitch DL. Tennis elbow no more: practical eccentric and concentric exercises to heal the pain. Can Fam Physician. 2008 Aug;54(8):1115-6 [PMC free article] [PubMed] [Google Scholar]
- 120.Takai S, Woo SL, Horibe S, Tung DK, Gelberman RH. The effects of frequency and duration of controlled passive mobilization on tendon healing. J Orthop Res. 1991 Sep;9(5):705-13 [DOI] [PubMed] [Google Scholar]
- 121.Gelberman RH, Vande Berg JS, Lundborg GN, Akeson WH. Flexor tendon healing and restoration of the gliding surface. An ultrastructural study in dogs. J Bone Joint Surg Am. 1983 Jan;65(1):70-80 [PubMed] [Google Scholar]
- 122.Woo SL, Gelberman RH, Cobb NG, Amiel D, Lothringer K, Akeson WH. The importance of controlled passive mobilization on flexor tendon healing. A biomechanical study. Acta Orthop Scand. 1981 Dec;52(6):615-22 [DOI] [PubMed] [Google Scholar]
- 123.Chesney A, Chauhan A, Kattan A, Farrokhyar F, Thoma A. Systematic review of flexor tendon rehabilitation protocols in zone II of the hand. Plast Reconstr Surg. 2011 Apr;127(4):1583-92 [DOI] [PubMed] [Google Scholar]
- 124.Trumble TE, Vedder NB, Seiler JG, 3rd, Hanel DP, Diao E, Pettrone S. Zone-II flexor tendon repair: a randomized prospective trial of active place-and-hold therapy compared with passive motion therapy. J Bone Joint Surg Am. 2010 Jun;92(6):1381-9, doi:10.2106/JBJS.H.00927 [DOI] [PubMed] [Google Scholar]
- 125.De Smet L. The DASH questionnaire and score in the evaluation of hand and wrist disorders. Acta Orthop Belg. 2008 Oct;74(5):575-81 [PubMed] [Google Scholar]
- 126.Kitis PT, Buker N, Kara IG. Comparison of two methods of controlled mobilisation of repaired flexor tendons in zone 2. Scand J Plast Reconstr Surg Hand Surg. 2009;43(3):160-5 [DOI] [PubMed] [Google Scholar]
- 127.Thien TB, Becker JH, Theis JC. Rehabilitation after surgery for flexor tendon injuries in the hand. Cochrane Database Syst Rev. 2004 Oct 18;(4):CD003979. [DOI] [PubMed] [Google Scholar]
- 128.Palmes D, Spiegel HU, Schneider TO, Langer M, Stratmann U, Budny T, Probst A. Achilles tendon healing: long-term biomechanical effects of postoperative mobilization and immobilization in a new mouse model. J Orthop Res. 2002 Sep;20(5):939-46 [DOI] [PubMed] [Google Scholar]
- 129.Twaddle BC, Poon P. Early motion for Achilles tendon ruptures: is surgery important? A randomized, prospective study. Am J Sports Med. 2007 Dec;35(12):2033-8 [DOI] [PubMed] [Google Scholar]
- 130.Weber M, Niemann M, Lanz R, Müller T. Nonoperative treatment of acute rupture of the achilles tendon: results of a new protocol and comparison with operative treatment. Am J Sports Med. 2003 Sep-Oct;31(5):685-91 [DOI] [PubMed] [Google Scholar]
- 131.Speck M, Klaue K. Early full weightbearing and functional treatment after surgical repair of acute achilles tendon rupture. Am J Sports Med. 1998 Nov-Dec;26(6):789-93 [DOI] [PubMed] [Google Scholar]
- 132.Suchak AA, Bostick GP, Beaupré LA, Durand DC, Jomha NM. The influence of early weight-bearing compared with non-weight-bearing after surgical repair of the Achilles tendon. J Bone Joint Surg Am. 2008 Sep;90(9):1876-83 [DOI] [PubMed] [Google Scholar]
- 133.Kangas J, Pajala A, Siira P, Hämäläinen M, Leppilahti J. Early functional treatment versus early immobilization in tension of the musculotendinous unit after Achilles rupture repair: a prospective, randomized, clinical study. J Trauma. 2003 Jun;54(6):1171-80, discussion :1180-1 [DOI] [PubMed] [Google Scholar]
- 134.Juncosa-Melvin N, Shearn JT, Boivin GP, Gooch C, Galloway MT, West JR, Nirmalanandhan VS, Bradica G, Butler DL. Effects of mechanical stimulation on the biomechanics and histology of stem cell-collagen sponge constructs for rabbit patellar tendon repair. Tissue Eng. 2006 Aug;12(8):2291-300 [DOI] [PubMed] [Google Scholar]
- 135.Juncosa-Melvin N, Matlin KS, Holdcraft RW, Nirmalanandhan VS, Butler DL. Mechanical stimulation increases collagen type I and collagen type III gene expression of stem cell-collagen sponge constructs for patellar tendon repair. Tissue Eng. 2007 Jun;13(6):1219-26 [DOI] [PubMed] [Google Scholar]
- 136.Nirmalanandhan VS, Rao M, Shearn JT, Juncosa-Melvin N, Gooch C, Butler DL. Effect of scaffold material, construct length and mechanical stimulation on the in vitro stiffness of the engineered tendon construct. J Biomech. 2008;41(4):822-8 [DOI] [PubMed] [Google Scholar]
- 137.Shearn JT, Juncosa-Melvin N, Boivin GP, Galloway MT, Goodwin W, Gooch C, Dunn MG, Butler DL. Mechanical stimulation of tendon tissue engineered constructs: effects on construct stiffness, repair biomechanics, and their correlation. J Biomech Eng. 2007 Dec;129(6):848-54 [DOI] [PubMed] [Google Scholar]
- 138.Chokalingam K, Juncosa-Melvin N, Hunter SA, Gooch C, Frede C, Florert J, Bradica G, Wenstrup R, Butler DL. Tensile stimulation of murine stem cell-collagen sponge constructs increases collagen type I gene expression and linear stiffness. Tissue Eng Part A. 2009 Sep;15(9):2561-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Butler DL, Juncosa-Melvin N, Boivin GP, Galloway MT, Shearn JT, Gooch C, Awad H. Functional tissue engineering for tendon repair: A multidisciplinary strategy using mesenchymal stem cells, bioscaffolds, and mechanical stimulation. J Orthop Res. 2008 Jan;26(1):1-9 [DOI] [PubMed] [Google Scholar]
- 140.Saber S, Zhang AY, Ki SH, Lindsey DP, Smith RL, Riboh J, Pham H, Chang J. Flexor tendon tissue engineering: bioreactor cyclic strain increases construct strength. Tissue Eng Part A. 2010 Jun;16(6):2085-90 [DOI] [PubMed] [Google Scholar]
- 141.Zhang J, Wang JHC. Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. J Orthop Res. 2010 May;28(5):639-43 [DOI] [PubMed] [Google Scholar]
- 142.Ralphs JR, Waggett AD, Benjamin M. Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 2002 Jan;21(1):67-74 [DOI] [PubMed] [Google Scholar]
- 143.Woon CYL, Kraus A, Raghavan SS, Pridgen BC, Megerle K, Pham H, Chang J. Three-dimensional-construct bioreactor conditioning in human tendon tissue engineering. Tissue Eng Part A. 2011 Oct;17(19-20):2561-72 [DOI] [PubMed] [Google Scholar]
- 144.Zeichen J, van Griensven M, Bosch U. The proliferative response of isolated human tendon fibroblasts to cyclic biaxial mechanical strain. Am J Sports Med. 2000 Nov-Dec;28(6):888-92 [DOI] [PubMed] [Google Scholar]
- 145.Joshi SD, Webb K. Variation of cyclic strain parameters regulates development of elastic modulus in fibroblast/substrate constructs. J Orthop Res. 2008 Aug;26(8):1105-13 [DOI] [PubMed] [Google Scholar]
- 146.Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003 Oct;9(5):967-79 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosure of Potential Conflicts of Interest


