Abstract
Borrelia burgdorferi (Bb) sensu lato, the etiologic agent of Lyme borreliosis, adapts to distinct environments in the mammalian host and the tick vector by differential gene expression. As a result, infected mice are not exposed to and rarely make antibodies to the set of antigens that are preferentially expressed in the tick, including outer surface protein A (OspA), Borrelia iron and copper-binding protein A (BicA), and OspD. Surprisingly, however, antibodies to OspA and BicA have been noted in American patients with Lyme arthritis. Here, we examined serum samples from 210 American patients and 66 European patients with a range of early or late manifestations of Lyme borreliosis and found that only American patients with Lyme arthritis commonly had antibody responses to OspA, BicA, and OspD. This suggests that infection with American but not European Borrelia strains often leads to concerted upregulation or derepression of tick-specific spirochetal antigens in these patients.
Keywords: Lyme borreliosis, Borrelia burgdorferi, antibody response, tick, OspA, OspD, BicA
Lyme borreliosis (LB), the most common tick-borne disease in temperate regions of the northern hemisphere, is caused by infection with spirochetes belonging to the Borrelia burgdorferi sensu lato (Bbsl) complex [1, 2]. Only B. burgdorferi sensu stricto strains (hereafter referred to as Bb) have been found in North America, whereas the most common cause of the illness in Europe is Borrelia afzelii (Ba) followed by Borrelia garinii (Bg) and then Bb. The basic course of the disease is similar worldwide, but there are regional variations, primarily between the illness in America and Europe.
In both locations, the disease usually begins with a characteristic skin lesion termed erythema migrans (EM), and after weeks, patients may develop acute Lyme neuroborreliosis (LNB) [1, 2]. The major differences are in late manifestations of the disease. In untreated patients in the northeastern United States, arthritis with intense joint inflammation is a frequent late manifestation of the disease [3]. Moreover, a small percentage of these patients have persistent proliferative synovitis in joints for months to years after treatment with 2–3 months of oral and intravenous antibiotic therapy, termed antibiotic-refractory arthritis [4]. In contrast, arthritis occurs infrequently in Europe and, when present, it is often associated with minimal joint swelling [2]. Acrodermatitis chronica atrophicans (ACA), which is usually caused by chronic Ba infection of the skin, is the most common late manifestation of the disease in Europe [5]. However, this feature of the illness has not been found definitively in America [1]. In some patients, arthritis or ACA is the presenting manifestation of the infection, and early stages of the illness are asymptomatic.
Lyme borreliae are maintained in nature in an enzootic life cycle that involves a vertebrate host and an Ixodes tick vector [6]. Differential expression of genes involved in tick and mammalian host colonization is a hallmark of virulence regulation in these pathogens [7, 8]. A deficiency in critical components of this regulatory pathway renders the spirochetes noninfectious in mammals [7, 8]. Genes required for tick colonization are often turned off during mammalian infection, and vice versa [7, 8]. For example, outer surface protein A (OspA) is required for spirochetal survival in ticks but not for infectivity in mammals [9, 10]. Thus, the expression of this protein is markedly downregulated in response to mammalian host-specific signals [11, 12]. As a result, mice infected with Bb through tick infestation, either naturally or experimentally, are not exposed to OspA and do not make antibodies to this protein [13, 14].
In America, antibodies to OspA, although found rarely in patients with EM, are frequently present in patients with Lyme arthritis (LA) [15–17]. About 60%–70% of American patients with LA develop antibody responses to OspA, and these responses are often higher in patients with antibiotic-refractory arthritis [18]. Because of the low spirochetal burden in joints, even prior to antibiotic therapy, and because synovial tissue has only been available after treatment [19], spirochetal gene or protein expression in joints cannot be measured directly and must be implied from the host immune response. Therefore, it is assumed that the spirochetes in LA patients who have antibody responses to OspA must express this tick-specific protein in inflamed joints. However, it has been unclear whether OpsA is expressed in patients with other manifestations of LB, whether other tick-specific Bb proteins may be upregulated as well, or whether this happens with infection with all Bbsl genospecies or only with Bb genotypes found in the northeastern United States.
To answer these questions, we analyzed serum samples from American and European patients with a range of LB manifestations for antibody responses to OspA, Borrelia iron- and copper-binding protein A (BicA) [20], and OspD. These antigens are representative of tick-specific Bb antigens because their functions are not required during mammalian infection and their expression is significantly repressed in the mammal [10–12, 21, 22]. Like OspA, BicA (encoded by chromosomal gene bb0690 and also known as NapA for neutrophil-activating peptide A) has been shown to play an important role in spirochetal survival in the tick [22]. Similar to the situation with OspA, antibodies to BicA were noted in 13 (48%) of 27 American patients with LA [23]. OspD was included in our analysis because its expression is the most significantly repressed in the mammal of all tick-specific proteins [11], even though this protein is not present in all Bb strains and appears to play only a marginal role in tick colonization [21]. We found that American patients with LA in the northeastern United States, regardless of the infecting Bb genotype, commonly developed antibody responses to these tick-specific Bb antigens. However, these responses were detected only at low levels in a few European patients with late disease manifestations and rarely in patients with early disease in either location.
METHODS
Patient Samples
The study patients from America, all of whom came from the northeastern United States, met the criteria of the Centers for Disease Control and Prevention for the diagnosis of Lyme disease [24]. The human investigations committees at Tufts Medical Center (1987–2002) and Massachusetts General Hospital (MGH; 2002–2008) approved the protocols, and study participants provided written informed consent.
In this study, we included serum samples from 63 patients with antibiotic-responsive arthritis and 62 patients with antibiotic-refractory arthritis who were seen from 1987 to 2008, the year that we began to treat patients with antibiotics according to guidelines now recommended by the Infectious Diseases Society of America [25]. Patients with responsive or refractory arthritis had joint inflammation for a median duration of 1 month after the onset of arthritis. Serum samples from patients with responsive arthritis were obtained prior to or near the initiation of antibiotic therapy, whereas those from patients with refractory arthritis who were usually referred because of lack of response to 1 or more courses of antibiotics were obtained a median duration of 3 months after the start of antibiotics.
For comparison, convalescent serum samples from 25 culture-positive patients with EM, acute serum samples from 15 patients with LNB, and serum samples from 30 healthy donors at the MGH blood bank were also tested. In an effort to correlate OspA antibody reactivity with Bb genotype, serum samples from 45 patients with LA seen from 1976 through 2006 in whom it was possible to determine the genotype of the infecting Bb strain by polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis were also analyzed [26].
For comparison with European LB, serum samples were obtained from patients seen in the Outpatient Lyme Borreliosis Clinic at the University Medical Center, Ljubljana, Slovenia. The Medical Ethics Committee of the Ministry of Health of the Republic of Slovenia approved the approach. Acute-phase serum samples from 20 patients with EM, 18 patients with LNB, 20 patients with LA, and 20 patients with ACA were tested. These patients met European criteria for LB [27]. Borrelia culture were performed routinely in patients with EM, ACA, or LNB [28] but not in patients with LA.
Bb Antigens
The whole-cell lysate was prepared using Bb type strain B31 (infectious clone 5A11) [22], which was cultured in the BSK-H complete media (Sigma-Aldrich) to early stationary phase. Recombinant OspA, BicA, and OspD proteins were expressed and purified using the glutathione S-transferase (GST) Gene Fusion System (GE Healthcare), as described previously [21, 22]. DNA fragments encoding OspA, BicA, and OspD were PCR-amplified from genomic DNA of Bb type strain B31. Expression and purification of the GST fusion proteins and enzymatic removal of the GST tag were performed according to the manufacturer's instructions. Protein concentrations of all antigen preparations were determined using the Bradford reagent (Bio-Rad).
Enzyme-Linked Immunosorbent Assay
Enzyme-linked immunosorbent assay was performed in 96-well flat-bottom Immulon 1B plates (Thermo Scientific) according to a standard protocol [29]. Briefly, the plates were coated with antigens at a concentration of 2 μg/mL. Human serum samples were assayed at a 1:400 dilution. The secondary antibody (goat anti-human immunoglobulin (Ig)G conjugated with horseradish peroxidase; Kirkegaard & Perry Laboratories) was used at a 1:1000 dilution. On each plate, a series of 2-fold dilutions of a positive human serum sample with known titer was included as a positive control, and the same 8 samples from healthy subjects were included as negative controls. The mean value plus 3 standard deviations of the group of 8 normal controls was set as the cutoff absorbance (the zero on each graph).
Data Analysis and Statistics
The software GraphPad Prism 5 for Windows (version 5.01) was used for statistical and graphical analyses. All reported P values are 2-tailed. P values < .05 are considered statistically significant. The statistical test for each P value is indicated in the text.
RESULTS
Antibody Responses to Bb Lysates and Tick-Specific Bb Antigens in LB Patients
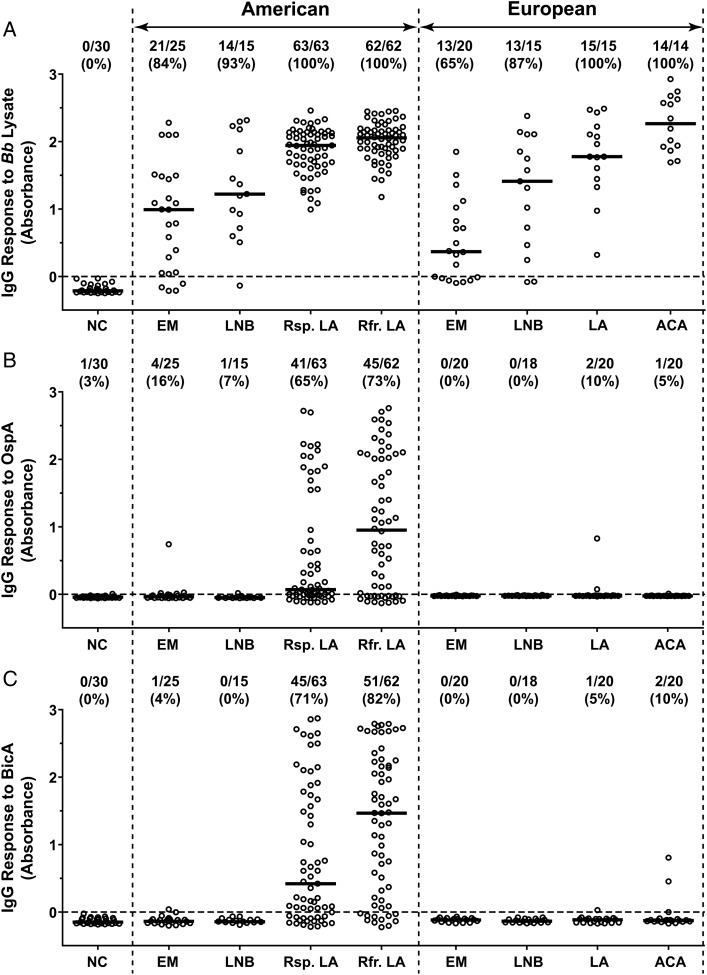
In LB patients from the northeastern United States and in those from south-central Europe, IgG antibody levels to Bb lysates and the percentage of patients with positive responses increased with the duration of infection (Figure 1A). In both locations, patients with EM had the lowest percentage of positive responses and the lowest median responses; reactivity was greater in those with LNB, and the highest responses were seen in those with late manifestations of the disease, LA or ACA. With each manifestation of the infection, the antibody levels to Bb lysates and the percentages with positive responses were similar in patients from America and Europe. However, American patients with antibiotic-refractory arthritis had significantly higher levels than those with antibiotic-responsive arthritis (P = .02, Mann-Whitney test).
Figure 1.
Differential antibody responses to outer surface protein A (OspA) and Borrelia iron and copper-binding protein A (BicA) in American and European Lyme borreliosis patients. Serum samples from normal controls (NC) or patients with erythema migrans (EM), Lyme neuroborreliosis (LNB), Lyme arthritis (LA), or acrodermatitis chronica atrophicans (ACA) were analyzed for immunoglobulin (Ig)G responses to Borrelia burgdorferi (Bb) lysate (A), OspA (B), and BicA (C). The American LA patients were subdivided into 2 groups based on whether the arthritis was responsive (Rsp.) or refractory (Rfr.) to antibiotic therapy. The circles represent individual serum samples and the bars represent the medians. The ratio of positive/total number of samples tested and the percentage of positive samples are shown for each group at the top.
In contrast with the responses to Bb lysates, antibody responses to OspA, a spirochetal protein expressed primarily in the tick, were found almost exclusively in American patients with LA (Figure 1B). Of the 125 patients tested, 65% with antibiotic-responsive arthritis and 73% with antibiotic-refractory arthritis had IgG antibody reactivity with OspA, and the levels were significantly greater in those with refractory arthritis (P = .03, Mann-Whitney test). In contrast, only 1 American patient with EM had low-level reactivity and only 2 European patients with LA had low-level responses to this protein.
A similar picture was observed with the antibody responses to BicA, another spirochetal protein preferentially expressed in the tick (Figure 1C). Among American patients with LA, 71% with antibiotic-responsive arthritis and 82% with antibiotic-refractory arthritis had IgG antibody responses to BicA, and the levels were significantly greater in those with antibiotic-refractory arthritis (P = .007, Mann-Whitney test). In contrast, only 2 European patients with ACA had low-level responses to this protein, and reactivity was not found in patients with early manifestations of the disease in either America or Europe.
An IgG antibody response to OspD, the remaining tick-specific Bb antigen tested, was only detected in 7 of 63 (11%) American patients with antibiotic-responsive arthritis and in 14 of 62 (22%) patients with antibiotic-refractory arthritis, a trend of possible significance (P = .1, Fisher exact test). Given the low percentage of positivity seen in American patients with LA, we did not determine whether American patients with early manifestations of the disease or European patients had responses to OspD.
Among the 125 American patients with LA, the responses to the tick-specific Bb antigens often occurred together. There was a direct correlation between antibody responses to OspA and BicA (Spearman r = 0.42, P < .001), to OspA and OspD (Spearman r = 0.21, P = .02), and to BicA and OspD (Spearman r = 0.25, P = .004). Furthermore, 18 of the 21 (86%) serum samples that were positive for OspD antibodies were also positive for both OspA and BicA antibodies, whereas only 54 of the 104 (52%) serum samples that were negative for OspD were positive for both OspA and BicA antibodies (P = .004, Fisher exact test).
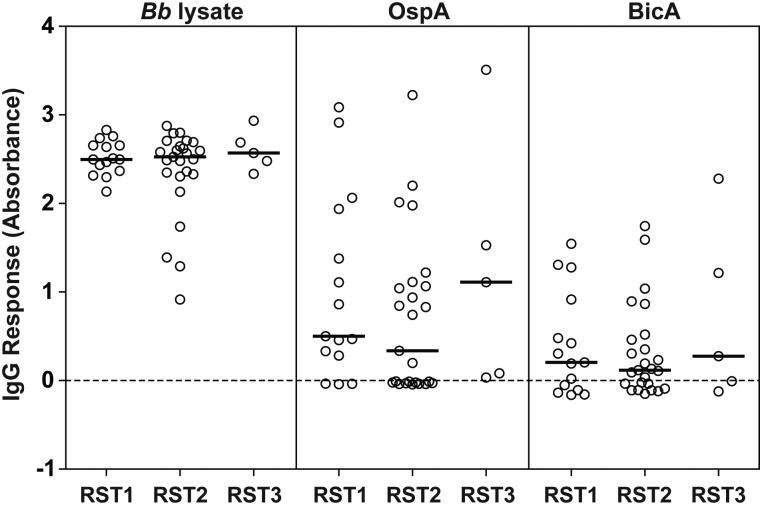
Lack of Correlation Between Antibody Responses and Bb Genotype
There is emerging evidence that certain Bb genotypes found in American LB patients are more pathogenic than others [26, 30–32]. Therefore, to investigate whether antibody responses to tick-specific proteins occurred more often with particular Bb genotypes, we tested serum samples from 45 American LA patients in whom the genotype of the infecting Bb strain had been previously determined [26]. Among the 45 patients, 15 were infected with ribosomal RNA intergenic spacer type 1 (RST1) strains, 25 with RST2 strains, and 5 with RST3 strains. However, the levels of antibody to Bb lysate, OspA, or BicA were similar among the 3 Bb genotypes (Figure 2). These data suggest that regardless of their genotypes, the Bb strains in the northeastern United States are able to upregulate or derepress OspA and BicA expression.
Figure 2.
Lack of correlation between antibody responses to Borrelia burgdorferi (Bb) lysate, outer surface protein A (OspA), or Borrelia iron and copper-binding protein A (BicA) and the genotype of the infecting Bb strain in American Lyme arthritis patients. The circles represent individual serum samples and the bars represent the medians. Abbreviation: IgG, immunoglobulin G.
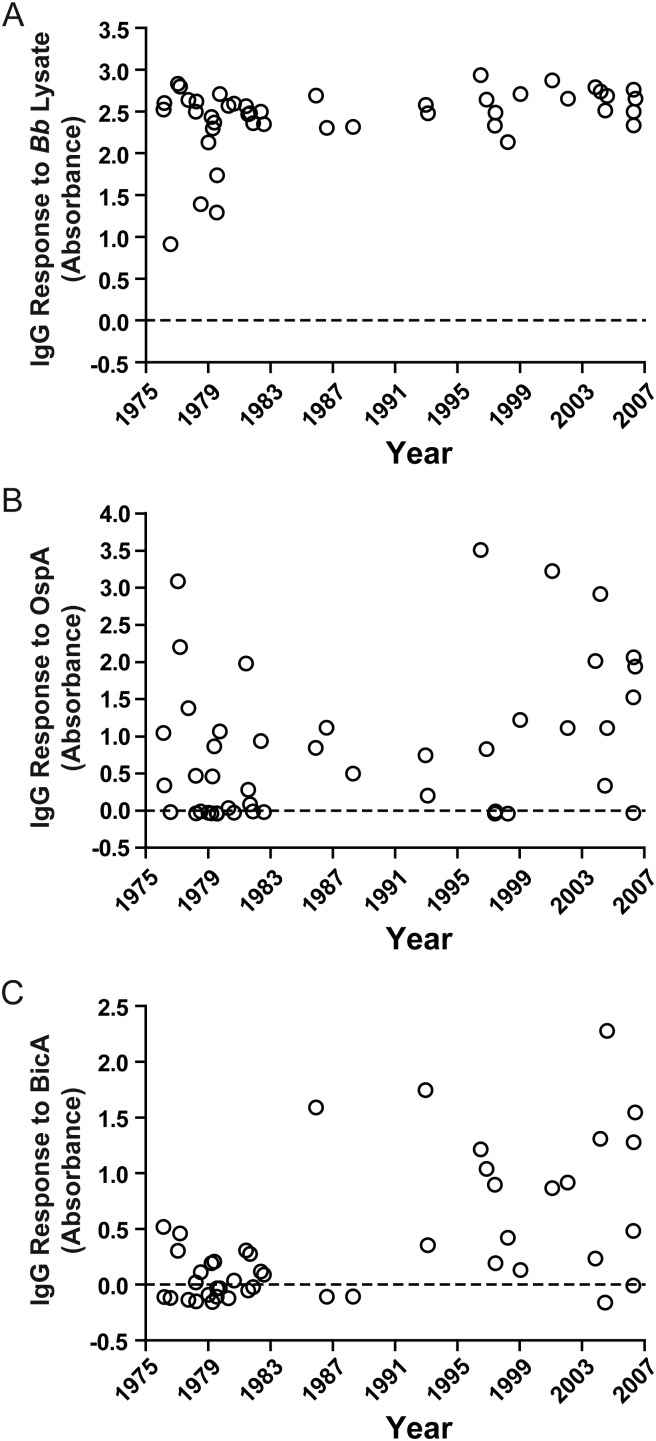
Increased BicA Antibody Response in American LA Patients After Antibiotic Therapy
Serum samples from the 45 patients were collected over a 30-year period from 1976 to 2006 [26]. During this period, treatment advanced as knowledge about the disease increased. None of the 24 LA patients seen prior to 1983 received antibiotic therapy prior to the time of serum collection. In contrast, 17 of the 21 patients seen after that time received antibiotic therapy prior to sample collection.
There was a statistically significant increase over time in the antibody response to BicA (Spearman r = 0.5, P = .0005; Figure 3). Although there was a similar trend for the responses to Bb lysate (Spearman r = 0.25, P = .09) and OspA (Spearman r = 0.25, P = .1), the differences were not statistically significant. Moreover, the antibody levels to BicA were much higher after 1983 than before that time (median: 0.8661 vs −0.0004; P = .0003, Mann-Whitney test), which was not the case for Bb lysate or OspA. Thus, there appeared to be differential factors in the induction of OspA or BicA expression in American LA patients.
Figure 3.
Increased antibody response to Borrelia iron and copper-binding protein A (BicA) in American Lyme arthritis patients after the introduction of antibiotic therapy for Lyme borreliosis in 1983. Antibody responses to Borrelia burgdorferi (Bb) lysate (A), outer surface protein A (OspA) (B) or BicA (C) were plotted against the time when samples were collected. Abbreviation: IgG, immunoglobulin G.
DISCUSSION
Consistent with previous analyses [18, 23], we show that 65%–82% of American LA patients from the northeastern United States had IgG antibody responses to OspA and BicA, Bb antigens that are expressed primarily in the tick but not in the mammalian host. In contrast, only a few European patients with LA or ACA had low-level responses to either of these antigens. In addition, 11%–22% of American LA patients also developed antibody reactivity with OspD. The infrequent response to OspD may be attributed, in part, to the fact that not all Bb isolates have the ospD gene or the lp38 plasmid carrying this gene [33, 34]. Nevertheless, in American LA patients, we found that antibody responses to these 3 representative tick-specific Bb antigens often occurred together.
Since testing was done with recombinant proteins based on the sequence of Bb type strain B31, an American isolate, we considered whether the near lack of responses to OspA or BicA in European patients may be due to sequence variations in these proteins. However, the amino acid sequence of BicA is 99% identical among different Bb strains and is 88%–92% identical and 93%–94% homologous between Bb and Bg or Ba [35–38]. The amino acid sequence of OspA is 99% identical among different Bb strains and is 77%–80% identical and 86%–90% homologous between Bb and Bg or Ba [35–38]. Although sequence variations in OspA can be distinguished by monoclonal antibodies, human patients develop polyclonal antibody responses to multiple epitopes of OspA [17]. Therefore, these small differences in sequences are unlikely to explain the marked differences in OspA reactivity between American and European patients.
There are several possible explanations for antibody responses to tick-specific Bb antigens in LB. First, we have previously shown that antibody responses to OspA and OspB, which are closely related proteins, are the last to develop [16, 39], suggesting that spirochetes may express these proteins only after prolonged infection. However, in the current study, European patients with ACA, which also occurs months to years after the onset of infection, rarely had antibody responses to these tick-specific antigens, implying that it is not simply the duration of the infection that leads to spirochetal expression of these proteins. Since American patients in our study were almost exclusively of European heritage, it is likely that genetic variations in spirochetes, rather than host factors, account for these differences in antibody reactivity.
In an effort to address this issue, we examined a subset of American LA patients in whom the Bb subtype of the infecting strain had been previously determined [26]. However, in these patients, the OspA or BicA antibody responses were not significantly different according to the infecting Bb strain. This suggests that derepression of OspA or BicA expression occurs commonly in most or all of the Bb genotypes found in the northeastern United States. In Slovenia, Ba most commonly causes EM and ACA, Bg most frequently causes LNB, and Bb accounts for only 1%–4% of these infections [28]. While the causative Borrelia species were not identified in Slovenian LA patients, each of the 3 pathogenic Bbsl species has been found in European LA patients [40], although Bb may be relatively more common with this disease manifestation. Regardless, it would appear that the European Bbsl species as a whole rarely upregulate tick-specific proteins during infection.
In its enzootic life cycle, the spirochete upregulates OspA when preparing for entry into an engorging tick [41]. The host neuroendocrine stress hormones, epinephrine and norepinephrine, may play a role in stimulating OspA expression during tick feeding [42]. However, this mechanism would not explain why OspA antibody responses in humans are found almost exclusively in American LA patients. Rather, we think that the marked joint inflammation associated with Bb infection in the northeastern United States is likely to be the major reason for derepression of OspA. Zymosan, a potent inflammatory reagent, induces OspA expression in Bb spirochetes cultivated in dialysis membrane chambers implanted in mouse peritoneal cavities [43]. Moreover, cell culture studies have shown that Bb strains from the northeastern United States have greater inflammatory potential than Ba or Bg strains from Europe [44].
Genetic differences between Bb and Ba or Bg may also influence how the spirochetes respond to signals that induce or derepress OspA/BicA expression. Pathways for transcriptional regulation of ospA and bicA appear to converge on Borrelia oxidative stress regulator (BosR), a homologue of ferric uptake regulator (Fur). OspA repression in the mammal requires the alternative sigma factor RpoS, and BosR has been shown to bind directly to promoters for rpoS and bicA [7, 8]. The Fur family of DNA-binding proteins is known to sense metals [45]. It will be important to learn whether BosR function in the spirochete is regulated by transition metals and whether BosR regulation varies among Bbsl genospecies.
In addition, there may be mechanistic differences in production of antibodies to OspA and BicA in American LA patients. Over the 30-year period during which serum samples were collected, there was a significant increase in antibody responses to BicA after the introduction of antibiotic therapy for the treatment of LA in 1983, which was not the case with the responses to Bb lysates or OspA. Thus, antibody to OspA, an outer-membrane lipoprotein, was found both in antibiotic-treated and non–antibiotic-treated LA patients, whereas BicA, an intracellular metal-binding protein [20], was found primarily in antibiotic-treated patients. Perhaps antibiotic-mediated killing of spirochetes helps expose cytosolic BicA to host immune recognition. Therefore, while marked inflammation may derepress OspA and BicA, antibiotic treatment seems to be important in further stimulating BicA antibody responses.
Among American patients with LA, the antibiotic-refractory group had higher antibody responses to OspA, BicA, and Bb lysate than the antibiotic-responsive group. Patients in the responsive group were usually seen prior to antibiotic therapy, whereas those in the refractory group were often seen several months after the start of antibiotic therapy. Since antibody responses to Bb typically decline after treatment, these differences in timing would not explain the higher antibody responses in the refractory group. Rather, these high antibody responses are consistent with the exceptionally high Th1 responses observed in the joints of these patients [46]. Host factors, such as a TLR1 polymorphism [47] and certain HLA-DR alleles [48], have been implicated in these inflammatory responses. For example, as suggested in a transgenic mouse model [49], the processing of OspA during Bb infection in genetically susceptible human patients, such as those with the HLA-DRB1*0401 allele, may stimulate a particularly strong Th1 response [50]. Thus, spirochetal genetics appear to be critical for the expression of tick-specific proteins, which occurs in patients with both responsive or refractory arthritis; however, host factors may lead to excessive immune and inflammatory responses in the refractory group.
In summary, American LA patients from the northeastern United States often had robust antibody responses not only to OspA but also to other tick-specific Bb proteins. In contrast, these responses were rarely found in patients with early manifestations of the infection, and low-level responses to these proteins were observed in only a few European patients with late manifestations of the disease. We hypothesize that it is primarily differences in the spirochetes that lead to upregulation of the tick transcriptome in LA patients in the northeastern United States. Uncovering the genetic elements in spirochetes that lead to these responses will be important in gaining an understanding of why Bb genotypes in the northeastern United States are particularly arthritogenic.
Notes
Financial support. This work was supported in part by the National Institutes of Health (AI-110175 and AR-20358 to A. C. S.; AR-062098 to K. S.); Centers for Disease Control and Prevention (CCU110 291 to A. C. S); the Arthritis Foundation (Arthritis Investigator Award to X. L.; Postdoctoral Fellowship to K. S.); the English, Bonter, Mitchell Foundation (A. C. S.); the Eshe Fund (A. C. S.); Massachusetts General Hospital (Lyme Disease and Arthritis Research Fund to A. C. S.; Claflin Award to X. L.); and the Slovenian Research Agency (P3-0296 to F. S.).
Potential conflicts of interest. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Steere AC. Lyme disease. N Engl J Med. 2001;345:115–25. doi: 10.1056/NEJM200107123450207. [DOI] [PubMed] [Google Scholar]
- 2.Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–73. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 3.Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–31. doi: 10.7326/0003-4819-107-5-725. [DOI] [PubMed] [Google Scholar]
- 4.Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–86. doi: 10.1002/art.22131. [DOI] [PubMed] [Google Scholar]
- 5.Mullegger RR. Dermatological manifestations of Lyme borreliosis. Eur J Dermatol. 2004;14:296–309. [PubMed] [Google Scholar]
- 6.Piesman J, Gern L. Lyme borreliosis in Europe and North America. Parasitology. 2004;129(Suppl):S191–220. doi: 10.1017/s0031182003004694. [DOI] [PubMed] [Google Scholar]
- 7.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuels DS. Gene regulation in Borrelia burgdorferi. Annu Rev Microbiol. 2011;65:479–99. doi: 10.1146/annurev.micro.112408.134040. [DOI] [PubMed] [Google Scholar]
- 9.Battisti JM, Bono JL, Rosa PA, Schrumpf ME, Schwan TG, Policastro PF. Outer surface protein A protects Lyme disease spirochetes from acquired host immunity in the tick vector. Infect Immun. 2008;76:5228–37. doi: 10.1128/IAI.00410-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XF, Pal U, Alani SM, Fikrig E, Norgard MV. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J Exp Med. 2004;199:641–8. doi: 10.1084/jem.20031960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks CS, Hefty PS, Jolliff SE, Akins DR. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect Immun. 2003;71:3371–83. doi: 10.1128/IAI.71.6.3371-3383.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Revel AT, Talaat AM, Norgard MV. DNA microarray analysis of differential gene expression in Borrelia burgdorferi, the Lyme disease spirochete. Proc Natl Acad Sci U S A. 2002;99:1562–7. doi: 10.1073/pnas.032667699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunet LR, Sellitto C, Spielman A, Telford SR., 3rd Antibody response of the mouse reservoir of Borrelia burgdorferi in nature. Infect Immun. 1995;63:3030–6. doi: 10.1128/iai.63.8.3030-3036.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golde WT, Kappel KJ, Dequesne G, et al. Tick transmission of Borrelia burgdorferi to inbred strains of mice induces an antibody response to P39 but not to outer surface protein A. Infect Immun. 1994;62:2625–7. doi: 10.1128/iai.62.6.2625-2627.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fikrig E, Huguenel ED, Berland R, Rahn DW, Hardin JA, Flavell RA. Serologic diagnosis of Lyme disease using recombinant outer surface proteins A and B and flagellin. J Infect Dis. 1992;165:1127–32. doi: 10.1093/infdis/165.6.1127. [DOI] [PubMed] [Google Scholar]
- 16.Kalish RA, Leong JM, Steere AC. Association of treatment-resistant chronic Lyme arthritis with HLA-DR4 and antibody reactivity to OspA and OspB of Borrelia burgdorferi. Infect Immun. 1993;61:2774–9. doi: 10.1128/iai.61.7.2774-2779.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalish RA, Leong JM, Steere AC. Early and late antibody responses to full-length and truncated constructs of outer surface protein A of Borrelia burgdorferi in Lyme disease. Infect Immun. 1995;63:2228–35. doi: 10.1128/iai.63.6.2228-2235.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kannian P, McHugh G, Johnson BJ, Bacon RM, Glickstein LJ, Steere AC. Antibody responses to Borrelia burgdorferi in patients with antibiotic-refractory, antibiotic-responsive, or non-antibiotic-treated Lyme arthritis. Arthritis Rheum. 2007;56:4216–25. doi: 10.1002/art.23135. [DOI] [PubMed] [Google Scholar]
- 19.Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, Steere AC. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum. 2011;63:2238–47. doi: 10.1002/art.30384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang P, Lutton A, Olesik J, Vali H, Li X. A novel iron- and copper-binding protein in the Lyme disease spirochete. Mol Microbiol. 2012;86:1441–51. doi: 10.1111/mmi.12068. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Neelakanta G, Liu X, et al. Role of outer surface protein D in the Borrelia burgdorferi life cycle. Infect Immun. 2007;75:4237–44. doi: 10.1128/IAI.00632-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Pal U, Ramamoorthi N, et al. The Lyme disease agent Borrelia burgdorferi requires BB0690, a Dps homologue, to persist within ticks. Mol Microbiol. 2007;63:694–710. doi: 10.1111/j.1365-2958.2006.05550.x. [DOI] [PubMed] [Google Scholar]
- 23.Codolo G, Amedei A, Steere AC, et al. Borrelia burgdorferi NapA-driven Th17 cell inflammation in Lyme arthritis. Arthritis Rheum. 2008;58:3609–17. doi: 10.1002/art.23972. [DOI] [PubMed] [Google Scholar]
- 24.Case definitions for infectious conditions under public health surveillance. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1997;46:1–55. [PubMed] [Google Scholar]
- 25.Wormser GP, Dattwyler RJ, Shapiro ED, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 26.Jones KL, McHugh GA, Glickstein LJ, Steere AC. Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: High frequency of ribosomal RNA intergenic spacer type 1 strains in antibiotic-refractory arthritis. Arthritis Rheum. 2009;60:2174–82. doi: 10.1002/art.24812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanek G, Fingerle V, Hunfeld KP, et al. Lyme borreliosis: clinical case definitions for diagnosis and management in Europe. Clin Microbiol Infect. 2011;17:69–79. doi: 10.1111/j.1469-0691.2010.03175.x. [DOI] [PubMed] [Google Scholar]
- 28.Ruzic-Sabljic E, Maraspin V, Lotric-Furlan S, et al. Characterization of Borrelia burgdorferi sensu lato strains isolated from human material in Slovenia. Wien Klin Wochenschr. 2002;114:544–50. [PubMed] [Google Scholar]
- 29.Hornbeck P, Winston SE, Fuller SA. Enzyme-linked immunosorbent assays (ELISA) Curr Protoc Mol Biol. 2001 doi: 10.1002/0471142727.mb1102s15. Chapter 11:Unit11.2. [DOI] [PubMed] [Google Scholar]
- 30.Jones KL, Glickstein LJ, Damle N, Sikand VK, McHugh G, Steere AC. Borrelia burgdorferi genetic markers and disseminated disease in patients with early Lyme disease. J Clin Microbiol. 2006;44:4407–13. doi: 10.1128/JCM.01077-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strle K, Jones KL, Drouin EE, Li X, Steere AC. Borrelia burgdorferi RST1 (OspC Type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol. 2011;178:2726–39. doi: 10.1016/j.ajpath.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wormser GP, Brisson D, Liveris D, et al. Borrelia burgdorferi genotype predicts the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis. 2008;198:1358–64. doi: 10.1086/592279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marconi RT, Samuels DS, Landry RK, Garon CF. Analysis of the distribution and molecular heterogeneity of the ospD gene among the Lyme disease spirochetes: evidence for lateral gene exchange. J Bacteriol. 1994;176:4572–82. doi: 10.1128/jb.176.15.4572-4582.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casjens SR, Mongodin EF, Qiu WG, et al. Genome stability of Lyme disease spirochetes: comparative genomics of Borrelia burgdorferi plasmids. PLoS One. 2012;7:e33280. doi: 10.1371/journal.pone.0033280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fraser CM, Casjens S, Huang WM, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–6. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 36.Glockner G, Lehmann R, Romualdi A, et al. Comparative analysis of the Borrelia garinii genome. Nucleic Acids Res. 2004;32:6038–46. doi: 10.1093/nar/gkh953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schutzer SE, Fraser-Liggett CM, Casjens SR, et al. Whole genome sequences of thirteen isolates of Borrelia burgdorferi. J Bacteriol. 2011;193:1018–20. doi: 10.1128/JB.01158-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Casjens SR, Mongodin EF, Qiu WG, et al. Whole-genome sequences of two Borrelia afzelii and two Borrelia garinii Lyme disease agent isolates. J Bacteriol. 2011;193:6995–6. doi: 10.1128/JB.05951-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akin E, McHugh GL, Flavell RA, Fikrig E, Steere AC. The immunoglobulin (IgG) antibody response to OspA and OspB correlates with severe and prolonged Lyme arthritis and the IgG response to P35 correlates with mild and brief arthritis. Infect Immun. 1999;67:173–81. doi: 10.1128/iai.67.1.173-181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasiliu V, Herzer P, Rossler D, Lehnert G, Wilske B. Heterogeneity of Borrelia burgdorferi sensu lato demonstrated by an ospA-type-specific PCR in synovial fluid from patients with Lyme arthritis. Med Microbiol Immunol. 1998;187:97–102. doi: 10.1007/s004300050079. [DOI] [PubMed] [Google Scholar]
- 41.Schwan TG, Piesman J. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol. 2000;38:382–8. doi: 10.1128/jcm.38.1.382-388.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheckelhoff MR, Telford SR, Wesley M, Hu LT. Borrelia burgdorferi intercepts host hormonal signals to regulate expression of outer surface protein A. Proc Natl Acad Sci U S A. 2007;104:7247–52. doi: 10.1073/pnas.0607263104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowley H, Huber BT. Host-adapted Borrelia burgdorferi in mice expresses OspA during inflammation. Infect Immun. 2003;71:4003–10. doi: 10.1128/IAI.71.7.4003-4010.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strle K, Drouin EE, Shen S, et al. Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J Infect Dis. 2009;200:1936–43. doi: 10.1086/648091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JW, Helmann JD. Functional specialization within the Fur family of metalloregulators. Biometals. 2007;20:485–99. doi: 10.1007/s10534-006-9070-7. [DOI] [PubMed] [Google Scholar]
- 46.Shin JJ, Glickstein LJ, Steere AC. High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2007;56:1325–35. doi: 10.1002/art.22441. [DOI] [PubMed] [Google Scholar]
- 47.Strle K, Shin JJ, Glickstein LJ, Steere AC. Association of a toll-like receptor 1 polymorphism with heightened Th1 inflammatory responses and antibiotic-refractory Lyme arthritis. Arthritis Rheum. 2012;64:1497–507. doi: 10.1002/art.34383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steere AC, Klitz W, Drouin EE, et al. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203:961–71. doi: 10.1084/jem.20052471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iliopoulou BP, Guerau-de-Arellano M, Huber BT. HLA-DR alleles determine responsiveness to Borrelia burgdorferi antigens in a mouse model of self-perpetuating arthritis. Arthritis Rheum. 2009;60:3831–40. doi: 10.1002/art.25005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steere AC, Drouin EE, Glickstein LJ. Relationship between immunity to Borrelia burgdorferi outer-surface protein A (OspA) and Lyme arthritis. Clin Infect Dis. 2011;52(Suppl 3):s259–65. doi: 10.1093/cid/ciq117. [DOI] [PMC free article] [PubMed] [Google Scholar]