Abstract
Background. T-cell responses have been described in seronegative patients who test negative for hepatitis C virus (HCV) RNA despite frequent HCV exposure. However, the cross-sectional design of those studies did not clarify whether T cells were indeed induced by low-level HCV exposure without seroconversion or whether they resulted from regular acute infection with subsequent antibody loss.
Methods. Over a 10-year period, our longitudinal study recruited 72 healthcare workers with documented HCV exposure. We studied viremia and antibody and T-cell responses longitudinally for 6 months.
Results. All healthcare workers remained negative for HCV RNA and antibodies. However, 48% developed proliferative T-cell response and 42% developed responses in interferon-gamma enzyme-linked immunosorbent spot assays, with 29 healthy HCV-unexposed controls used to define assay cutoffs. The response prevalence was associated with the transmission risk score. T-cell responses peaked at week 4 and returned to baseline by week 12 after exposure. They predominantly targeted nonstructural HCV proteins, which are not part of the HCV particle and thus must have been synthesized in infected cells.
Conclusions. Subclinical transmission of HCV occurs frequently, resulting in infection and synthesis of nonstructural proteins despite undetectable systemic viremia. T-cell responses are more sensitive indicators of this low-level HCV exposure than antibodies.
Keywords: exposure, needlestick, antibody, T cell, hepatitis, healthcare worker
Hepatitis C virus (HCV) infection constitutes a serious global health problem, with more than 120 million people chronically infected. Since the introduction of blood donor screening in the early 1990s, the epidemiology of HCV infection has changed. In the United States, transmission via blood transfusion is now effectively prevented via donor screening [1], and most cases of infection occur in injection drug users via HCV-contaminated needles [2, 3]. In addition, healthcare workers are at risk of infection due to sharp injuries.
Some of the largest studies on virus exposure in healthcare workers were conducted by the Centers for Disease Control and Prevention Needlestick Surveillance Group as multicenter case-control studies. In those studies, healthcare workers who developed antibodies to either HCV [4] or human immunodeficiency virus (HIV) [5] were classified as cases, whereas those who did not seroconvert were classified as controls. Likewise, a large surveillance study in Europe followed 245 healthcare workers for 5 years using seroconversion as evidence of exposure [6].
However, none of these prospective studies assessed HCV-specific T-cell responses. T-cell responses are relevant because the small percentage of patients who clear acute HCV infection spontaneously, approximately 20%, mount vigorous HCV-specific T-cell responses, and T-cell–mediated immune memory may protect humans [7] and chimpanzees [8, 9] upon reexposure. In contrast, antibodies are not required for HCV clearance as shown in hypogammaglobulinemic patients [10], and antibody titers decrease significantly within 10–20 years after HCV clearance [11].
HCV-specific T-cell responses were also described in cross-sectional studies on seronegative subjects such as injection drug users [4, 12–15], family members of HCV-infected patients [16–18], and healthcare workers [19] who test negative for HCV RNA despite an increased risk of HCV exposure. Based on these studies, it has often been suggested that years of low-dose HCV exposure may prime and maintain HCV-specific T cells that protect against systemic infection [13, 14, 17]. However, due to unknown exposure dates and lack of longitudinal immune response analysis, in particular with early time points after exposure, it cannot be excluded that the studied subjects had resolved a regular acute HCV infection in the distant past and subsequently lost HCV-specific antibodies [11].
In the current study, 72 healthcare workers were enrolled upon documented HCV exposure and studied frequently, that is, up to 5 times in the first 6 weeks and at 3 months and 6 months after exposure. In contrast to a previous study that was smaller and had less frequent and later sampling [20], we found a pattern of early transient T-cell responses that was associated with the transmission risk score. Our results support the hypothesis that HCV-specific T cells are more sensitive indicators of low-dose HCV exposure than antibodies, which is relevant for surveillance studies and for vaccine development.
METHODS
Study Cohort
Seventy-two healthcare workers with documented HCV exposure were followed prospectively at the National Institutes of Health (NIH; n = 31), Washington Hospital Center (n = 27), and Inova Fairfax Hospital (n = 14; Table 1). All gave written informed consent for research testing according to protocols approved by the participating hospitals’ institutional review boards (ClinicalTrials.gov NCT00006301). Needlestick injuries were assigned a transmission risk score of “low” (0–1 points), “medium” (2–3 points), or “high” (4–5 points) that we derived from epidemiologic studies on HIV and HCV transmission [5, 21]. This score was based on the transmission route (1 point, transmission from a blood vessel, ie, the needle had been inserted into a patient's blood vessel prior to the accidental needlestick; 0 points, no transmission from a blood vessel), exposure type (1 point, fresh blood; 0 points, old blood), depth of injury (1 point, deep; 0 points, superficial), and the needle itself (2 points, hollow-bore >18G; 1 point, hollow-bore <18G; 0 points, solid).
Table 1.
Characteristics of the Studied Healthcare Workers
| Characteristic | n (%) |
|---|---|
| Subjects | 72 |
| Male | 30 (41.6) |
| Type of Exposure | |
| Needlestick/Cut | 54 (75) |
| Risk score, lowa | 16 (22.2) |
| Risk score, medium | 25 (30.6) |
| Risk score, high | 13 (18.1) |
| Cutaneous | 7 (9.7) |
| Mucosal | 11 (15.3) |
| Serum HCV RNAb | 0 (0) |
| HCV antibodiesc | 0 (0) |
Abbreviation: HCV, hepatitis C virus.
a Risk score for HCV transmission as described in the Materials and Methods section.
b Determined by qualitative reverse-transcription polymerase chain reaction (COBAS Amplicor HCV Test 2.0) at all study time points.
Study time points for clinical visits and analysis of HCV RNA (Cobas Amplicor, HCV Test 2.0, Roche, Branchburg, NJ; with a lower detection limit of 100 IU/mL), HCV-specific antibodies (Abbott HCV EIA 2.0, Abbott, Princeton, NJ), and HCV-specific T-cell responses were the day of exposure (n = 21 subjects) and week 1 (n = 22), week 2 (n = 49), week 4 (n = 66), week 6 (n = 67), week 12–13 (n = 67), and week 25–26 (n = 53) thereafter. Because some of the healthcare workers visited slightly before or after the planned study time points, the average time after exposure was calculated for the cohort for each study time point. Samples from 13 healthcare workers were tested with T-cell proliferation assays at week 0 and week 26, and samples from 14 healthcare workers were tested with interferon-gamma (IFN-γ) enzyme-linked immunospot (ELISPOT) assays at week 0 and week 25. Because there were no significant differences in the strength of the responses at these 2 time points (Wilcoxon signed rank test), the week 25–26 time point was used as the baseline for all healthcare workers in this study.
Proliferation Assay
Proliferation Assay
Peripheral blood mononuclear cells (PBMCs) were separated from citrate dextrose–anticoagulated blood and stimulated with 1 μg/mL HCV core, NS3, NS4, NS5A, or NS5B proteins; buffer control (Mikrogen, Germany); and 1 μg/mL phytohemagglutinin (PHA-M; Invitrogen, Carlsbad, CA) as positive control as described [11]. Tetanus toxoid (5 μg/mL; Chiron Corporation, Emeryville, CA) was used as additional control for 36 subjects. The cutoff for a significant proliferative response was defined as stimulation index (SI; counts of incorporated 3H-thymidine in the presence of antigen/counts in the absence of antigen) greater than the mean SI plus 2 standard deviations (SDs) of proliferation assays performed with PBMCs from 29 anti-HCV–negative blood donors from the NIH Department of Transfusion Medicine.
IFN-γ Enzyme-Linked Immunospot
PBMCs were stimulated with pools of overlapping pentadecamer peptides spanning the HCV core, NS3, NS4A, and NS4B sequence (1 μg/mL of each peptide), 1 μg/mL PHA as positive control, or dimethyl sulfoxide (DMSO) as negative control as described [22]. To test whether other immune responses changed due to HCV exposure, tetanus toxoid and a pool of 15 Epstein-Barr viruses (EBVs) were used as additional controls for 18 subjects. A subject was classified as T-cell responder if there was a significant response to at least 2 HCV antigens in the proliferation assay (core, NS3, NS4, NS5A, or NS5B) or the ELISPOT assay (core, NS3, NS4A, or NS4B), respectively. A subject was classified as T-cell nonresponder if all assays were negative when tested on at least 3 of the 5 time points after exposure. If tested on fewer than 3 time points, the subject was excluded (n = 10). The cutoff for a significant HCV-specific response (spots with antigen minus spots without antigen) was defined as greater than the mean plus 2 SDs of the response of 29 anti-HCV–negative blood donors and more than 2-fold above the DMSO background.
Statistical Analysis
Fisher exact test, χ² test, D'Agostino and Pearson omnibus normality tests, and nonparametric Wilcoxon matched-pairs tests were performed with GraphPad Prism 5.0a (GraphPad Software, La Jolla, CA). Two-sided P values < .05 were considered significant.
Funding Source
The funding source did not influence the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication.
RESULTS
HCV-Specific T-Cell Responses in Exposed Healthcare Workers in the Absence of Systemic Viremia and Seroconversion
Seventy-two healthcare workers were studied prospectively after documented accidental exposure to HCV-contaminated blood via needlestick or cut (n = 54, 75%) or via a splash of blood onto skin or mucosa (n = 18, 25%). All study participants tested negative for HCV RNA at the sensitivity level of 100 IU/mL of the standard clinical assay and for HCV antibodies on all study dates. To determine whether HCV-specific T cells were induced despite the absence of detectable systemic viremia, we tested serial PBMC samples in proliferation and IFN-γ ELISPOT assays. Thirty of 63 tested healthcare workers (48%) demonstrated HCV-specific T-cell proliferation and 26 of 62 tested healthcare workers (42%) demonstrated HCV-specific IFN-γ responses against at least 2 HCV antigens. Fifty-three exposed healthcare workers were tested using both assays. Using both 13/53 (24%) showed both HCV-specific proliferative and IFN-γ T-cell responses, 21/53 (40%) showed neither, and 19/53 (36%) showed either proliferative or IFN-γ T-cell responses. Calculation of the needlestick transmission risk score for those exposed via needlestick did not reveal any significant difference among the groups.
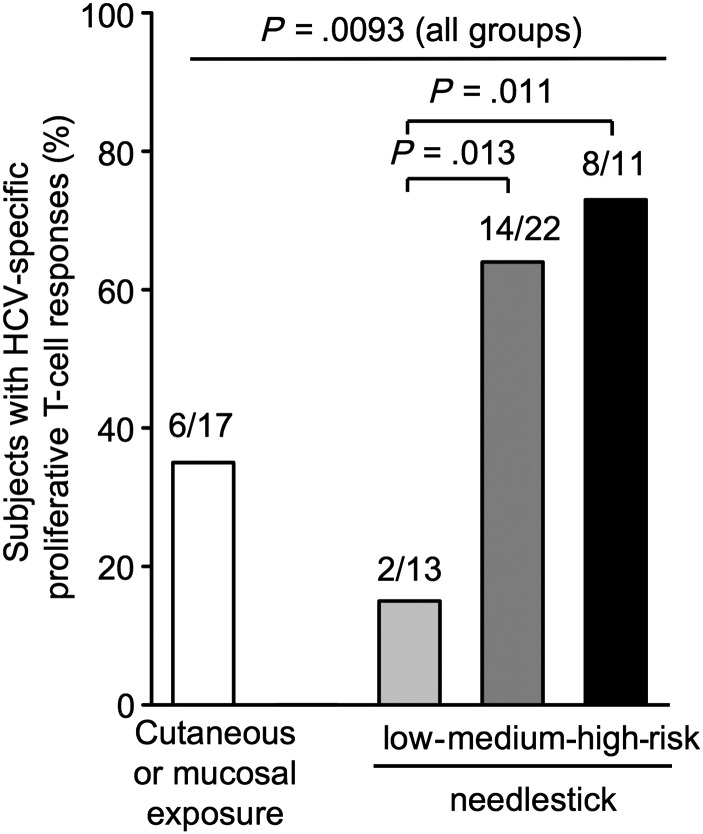
The prevalence of proliferative T-cell responses differed among groups with different types of exposure (P = .0093 comparing all groups, Figure 1). Furthermore, among healthcare workers with needlestick injuries, the prevalence of proliferative T-cell responses was significantly higher in those with a high-risk needlestick (transmission risk score of 4–5) than in those with a low-risk needlestick (score 0–1; 73% vs 15%, P = .011; Figure 1). In contrast, there was no difference in the prevalence of IFN-γ ELISPOT responses among these subgroups (data not shown).
Figure 1.
Prevalence of T-cell responses in groups of healthcare workers with different types of exposure. Percentage of healthcare workers with hepatitis C virus (HCV)-specific proliferative T-cell responses (n = 63). Cutaneous/mucosal exposure is defined as a splash of HCV-infected blood on skin or eye/mouth mucosa. Needlestick exposures are classified based on the transmission risk as low, medium, and high. Statistical analysis: χ² test to compare all groups and Fisher exact test to compare subgroups.
Timing of HCV-Specific T-Cell Responses After Low-Dose HCV Exposure
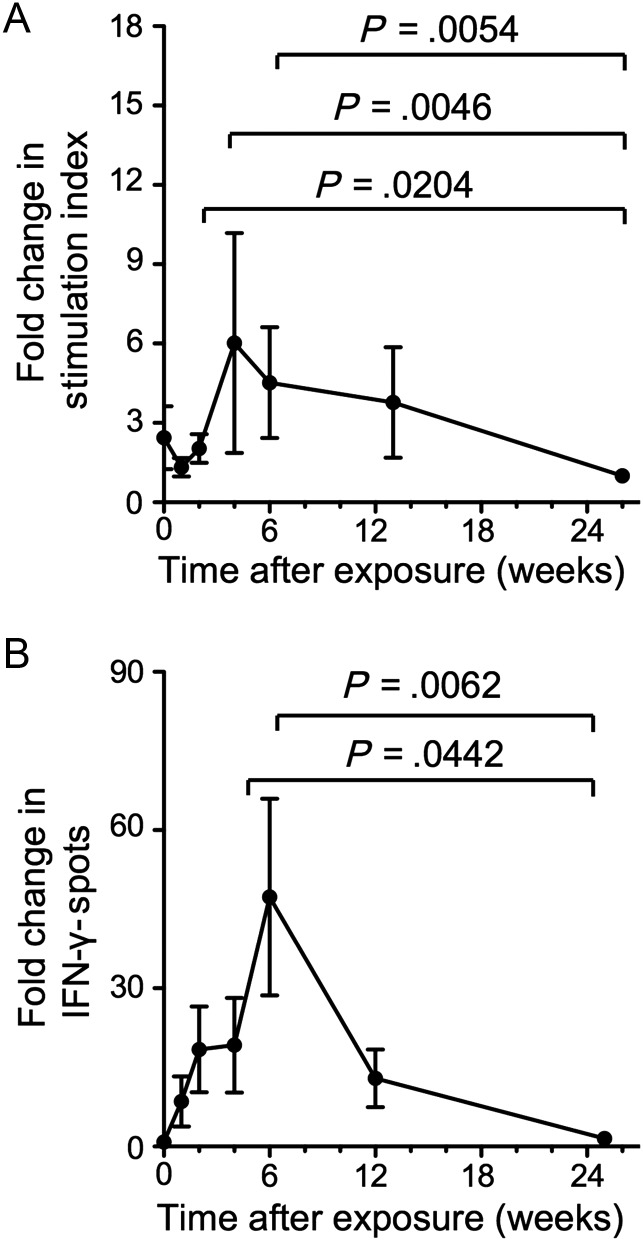
As shown in Figure 2, HCV-specific T-cell proliferation peaked at week 4 (6-fold over the week 26 baseline; paired analysis, P = .0046) and HCV-specific IFN-γ responses peaked at week 6 after exposure (32-fold over baseline; P = .0062). Week 25–26 was used as a baseline because more samples were available for week 25–26 than for week 0 and because the week 25–26 response did not differ from the week 0 response for those tested at both time points. Changes in T-cell responsiveness were HCV specific because there was no significant change in the magnitude of T-cell responses against tetanus toxoid and EBV peptides.
Figure 2.
Magnitude and kinetics of hepatitis C virus (HCV)-specific T-cell responses after HCV exposure. Fold-change in the magnitude of HCV-specific T-cell proliferation (A) and interferon-gamma (IFN-γ) enzyme-linked immunospot (ELISPOT) responses (B). Mean ± standard error of data from all subjects with a significant T-cell response are shown (n = 22 in panel A, n = 21 in panel B). For each healthcare worker, the sum of responses to all individual HCV antigens is normalized to the week 25–26 response. Week 25–26 was used as baseline because more samples were available for week 25–26 than for week 0 and because the week 25–26 response did not differ from the week 0 response for those tested with T-cell proliferation assays (n = 13) and IFN-γ ELISPOT assays (n = 14) at both time points. Because some healthcare workers did not visit on the exact date of the planned visit, the average time after exposure is indicated for the cohort at each study time point. Statistical analysis: Nonparametric Wilcoxon matched pairs tests comparing the magnitude of the HCV-specific T-cell response of each healthcare worker at different time points after exposure (paired analysis).
Breadth of the HCV-Specific T-Cell Response
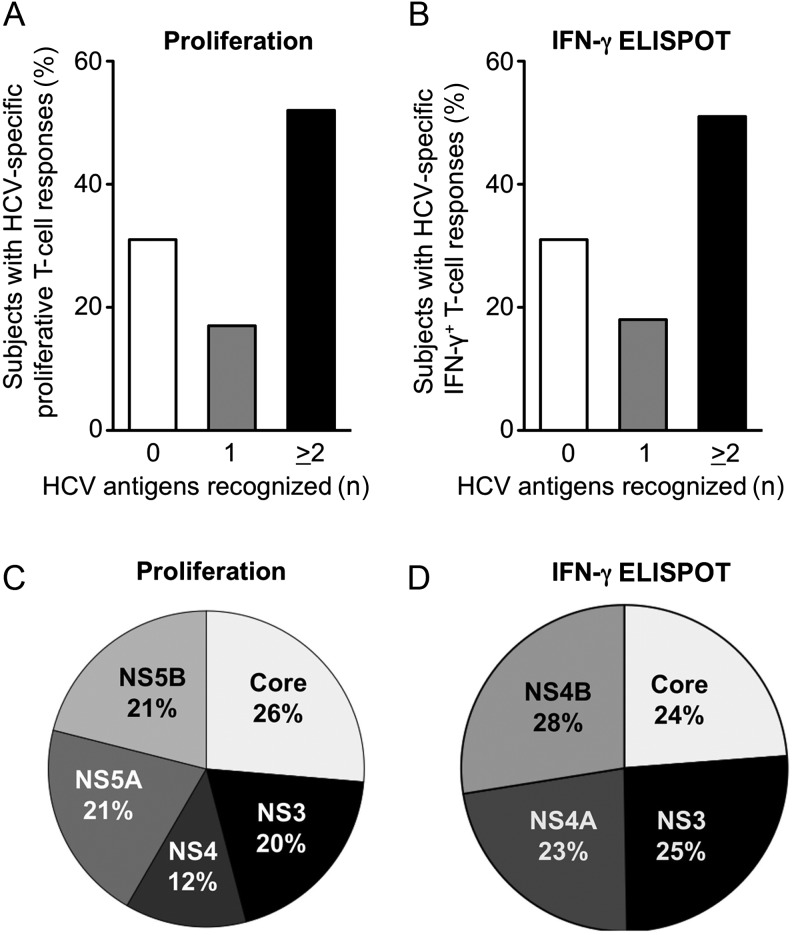
To analyze the breadth of the T-cell response, we determined the number of HCV antigens recognized by each healthcare worker and the frequency with which each antigen was recognized by the entire healthcare worker cohort (Figure 3). The majority of the healthcare workers recognized multiple HCV antigens in both proliferation (Figure 3A) and ELISPOT assays (Figure 3B), but only 7/63 (11%) subjects responded to all antigens in the proliferation assay and 5/62 (8%) subjects responded to all antigens in the ELISPOT assay (data not shown). Four of the 5 HCV proteins (core, NS3, NS5A, and NS5B) were recognized with almost equal frequency (20%–26%) in the proliferation assays, while NS4 was recognized somewhat less frequently (12%; Figure 3C). Likewise, the core-, NS3-, NS4A-, and NS4B-specific peptide pools were recognized with almost equal frequency (23%–28%) in the IFN-γ ELISPOT assays (Figure 3D). Overall, about three quarters of the HCV-specific T-cell responses of exposed healthcare workers targeted nonstructural HCV antigens even though these are not present as protein components of the HCV particle but encoded by viral RNA inside. However, relative to their respective amino acid length, all nonstructural antigens were less immunogenic than the core antigen, which is a structural component of the HCV particle.
Figure 3.
Breadth and specificity of the hepatitis C virus (HCV)-specific T-cell response. Number of HCV antigens recognized by individual patients in proliferation (A) and interferon-gamma (IFN-γ) enzyme-linked immunospot (ELISPOT) assays (B). Prevalence of HCV-specific T-cell responses against structural and nonstructural HCV antigens in proliferation assays (C) and IFN-γ ELISPOT assays (D). Only significant responses, that is, above the cutoff defined in the Material and Methods section, were evaluated.
DISCUSSION
This study demonstrates that T-cell responses are more sensitive indicators of low-dose HCV exposure than antibodies and that these responses can be the sole evidence for HCV exposure if viremia is below the detection limit of the standard clinical assay. T-cell responses against nonstructural HCV proteins provide evidence that transient and/or anatomically contained HCV infection must have occurred despite undetectable systemic viremia. This is because nonstructural HCV proteins are not part of the HCV particle and are expressed only when a virus has infected a cell and initiated RNA translation and potentially replication. Moreover, the infectious source in this current study is blood from chronic HCV patients with systemic viremia, which implies that viral RNA (with the capacity to directly prime T cells) as well as viral proteins (that would allow T-cell induction via cross-priming) are likely transmitted.
A brief and transient period of HCV replication below the detection limit of the standard virological assay used at the NIH may indeed be sufficient for T-cell induction because the magnitude and kinetics of the T-cell response to a given pathogen are determined upon exposure and do not require antigen persistence [23]. In contrast, the induction of antibodies, in particular neutralizing antibodies in HCV infection, depends on the continued presence of high antigen levels [11]. This is consistent with data from other infections where low-level exposure to HIV or woodchuck hepatitis virus (WHV) leads to the appearance of virus-specific T-cell response in the absence of virus-specific antibodies [24, 25]. Indeed, low-level viral DNA was detected in the WHV study [25]. While low or transient HCV RNA may be detectable in the plasma samples of the exposed subjects in our study, with more sensitive assays it might be possible that HCV is solely detectable in the PBMC or the liver compartment, as has been reported for cases with occult HCV infection [26–28]. Thus, healthcare workers who do not seroconvert should not be classified as nonexposed controls in HCV surveillance studies.
How do the observed T-cell responses in this exposed cohort, which tested HCV RNA nonreactive at the assay sensitivity of 100 IU/mL, differ from those of acutely infected patients with high levels of systemic viremia who later clear HCV or progress to chronic infection? Notably, HCV-specific T-cell proliferation peaked at week 4 and IFN-γ production peaked at week 6 in our study. This is 1 to 2 months earlier than described for acute HCV infection, where the virus frequently outpaces the adaptive immune response [29, 30]. A potential explanation is a boost of preexisting HCV-specific T-cell memory induced by previous exposures in the distant past. This is possible because most of the studied subjects had been healthcare workers for many years when this study was conducted. However, while HCV-specific T-cell proliferation was slightly increased at week 0 (2.44-fold over the week 26 baseline level), this did not reach significance and was not associated with increased IFN-γ ELISPOT responses. Moreover, HCV-specific T-cell responses were transient and returned to levels below the mean plus 2 SDs of the response of healthy unexposed controls in all but 2 healthcare workers within 12 weeks after exposure. They therefore differ from the strong memory responses that remain readily detectable in proliferation and IFN-γ ELISPOT assays for decades after recovery and resolution of HCV [11]. We favor the explanation that the rapidity of the T-cell response was due to the very small amount of transmitted virus; this is supported by an inverse correlation between the speed of appearance of HCV-specific T cells and the amount of transmitted virus that was recently demonstrated in a nonhuman primate model [3].
A second difference between our exposed cohort with undetectable viremia and a typical acutely infected cohort with high-level viremia and increased alanin aminotransferase values [22] is the strength of the T-cell response. Both proliferative T-cell responses and IFN-γ production were weaker in the current study than in a previous cohort with acute hepatitis that we studied using the same techniques [22]. Unfortunately, the weakness of the T-cell response rendered it technically impossible to study responses at the single peptide level and to use tetramers to further characterize the phenotype of the induced T cells. It is therefore an important confirmation that we observed similar kinetics and antigen specificity in proliferation and ELISPOT assays and when using HCV proteins and peptide pools.
The sole difference between proliferation assay and ELISPOT assay results in our study is the observation that proliferative T-cell responses, but not IFN-γ ELISPOT responses, differentiated between high-risk and low-risk needlestick injuries. This is consistent with reports in HIV-infected individuals that proliferation rather than ex vivo IFN-γ production of HIV-specific T cells is associated with control of viremia [31]. Likewise, we reported previously that T-cell proliferation rather than IFN-γ production peaked at time points of transient control of viremia in acute HCV infection [22, 24].
In conclusion, the results demonstrate that HCV-specific T-cell responses, as measured in particular with in vitro proliferation assays, are more sensitive biomarkers of HCV exposure than antibodies. Whether these T-cell responses reflect protective immunity or whether they are downstream events of protective innate immune responses or abortive replication of defective viral genomes requires further studies in suitable models.
Notes
Financial support. This study was supported by the National Institute for Diabetes, Digestive and Kidney Diseases, National Institute of Health Intramural Research Program, and by the Deutsche Forschungsgemeinschaft (DFG), Bonn, Germany (We-4675/1-1 to JMW).
Potential conflicts of interest. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–54. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGovern BH, Wurcel A, Kim AY, et al. Acute hepatitis C virus infection in incarcerated injection drug users. Clin Infect Dis. 2006;42:1663–70. doi: 10.1086/504327. [DOI] [PubMed] [Google Scholar]
- 3.Shata MT, Tricoche N, Perkus M, et al. Exposure to low infective doses of HCV induces cellular immune responses without consistently detectable viremia or seroconversion in chimpanzees. Virology. 2003;314:601–16. doi: 10.1016/s0042-6822(03)00461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yazdanpanah Y, De Carli G, Migueres B, et al. Risk factors for hepatitis C virus transmission to health care workers after occupational exposure: a European case-control study. Clin Infect Dis. 2005;41:1423–30. doi: 10.1086/497131. [DOI] [PubMed] [Google Scholar]
- 5.Cardo DM, Culver DH, Ciesielski CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485–90. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 6.Baldo V, Floreani A, Dal Vecchio L, et al. Occupational risk of blood-borne viruses in healthcare workers: a 5-year surveillance program. Infect Control Hosp Epidemiol. 2002;23:325–7. doi: 10.1086/502059. [DOI] [PubMed] [Google Scholar]
- 7.Osburn WO, Fisher BE, Dowd KA, et al. Spontaneous control of primary hepatitis C virus infection and immunity against persistent reinfection. Gastroenterology. 2010;138:315–24. doi: 10.1053/j.gastro.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grakoui A, Shoukry NH, Woollard DJ, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–62. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 9.Shoukry NH, Grakoui A, Houghton M, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–55. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semmo N, Lucas M, Krashias G, Lauer G, Chapel H, Klenerman P. Maintenance of HCV-specific T-cell responses in antibody-deficient patients a decade after early therapy. Blood. 2006;107:4570–1. doi: 10.1182/blood-2005-11-4522. [DOI] [PubMed] [Google Scholar]
- 11.Takaki A, Wiese M, Maertens G, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–82. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 12.Mizukoshi E, Eisenbach C, Edlin BR, et al. Hepatitis C virus (HCV)-specific immune responses of long-term injection drug users frequently exposed to HCV. J Infect Dis. 2008;198:203–12. doi: 10.1086/589510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zeremski M, Shu MA, Brown Q, et al. Hepatitis C virus-specific T-cell immune responses in seronegative injection drug users. J Viral Hepat. 2009;16:10–20. doi: 10.1111/j.1365-2893.2008.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman AJ, Ffrench RA, Post JJ, et al. Prevalence of production of virus-specific interferon-gamma among seronegative hepatitis C-resistant subjects reporting injection drug use. J Infect Dis. 2004;190:1093–7. doi: 10.1086/422605. [DOI] [PubMed] [Google Scholar]
- 15.Thurairajah PH, Hegazy D, Chokshi S, et al. Hepatitis C virus (HCV)–specific T cell responses in injection drug users with apparent resistance to HCV infection. J Infect Dis. 2008;198:1749–55. doi: 10.1086/593337. [DOI] [PubMed] [Google Scholar]
- 16.Al-Sherbiny M, Osman A, Mohamed N, et al. Exposure to hepatitis C virus induces cellular immune responses without detectable viremia or seroconversion. Am J Trop Med Hyg. 2005;73:44–9. [PubMed] [Google Scholar]
- 17.Scognamiglio P, Accapezzato D, Casciaro MA, et al. Presence of effector CD8+ T cells in hepatitis C virus-exposed healthy seronegative donors. J Immunol. 1999;162:6681–9. [PubMed] [Google Scholar]
- 18.Roque-Cuellar MC, Sanchez B, Garcia-Lozano JR, Praena-Fernandez JM, Nunez-Roldan A, Aguilar-Reina J. Cellular immune responses and occult infection in seronegative heterosexual partners of chronic hepatitis C patients. J Viral Hepat. 2011;18:e541–9. doi: 10.1111/j.1365-2893.2011.01464.x. [DOI] [PubMed] [Google Scholar]
- 19.Abdelwahab SF, Zakaria Z, Sobhy M, et al. Hepatitis C virus-multispecific T-cell responses without viremia or seroconversion among Egyptian health care workers at high risk of infection. Clin Vaccine Immunol. 2012;19:780–6. doi: 10.1128/CVI.00050-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kubitschke A, Bahr MJ, Aslan N, et al. Induction of hepatitis C virus (HCV)-specific T cells by needle stick injury in the absence of HCV-viraemia. Eur J Clin Invest. 2007;37:54–64. doi: 10.1111/j.1365-2362.2007.01753.x. [DOI] [PubMed] [Google Scholar]
- 21.Tomkins SE, Elford J, Nichols T, et al. Occupational transmission of hepatitis C in healthcare workers and factors associated with seroconversion: UK surveillance data. J Viral Hepat. 2012;19:199–204. doi: 10.1111/j.1365-2893.2011.01543.x. [DOI] [PubMed] [Google Scholar]
- 22.Rahman F, Heller T, Sobao Y, et al. Effects of antiviral therapy on the cellular immune response in acute hepatitis C. Hepatology. 2004;40:87–97. doi: 10.1002/hep.20253. [DOI] [PubMed] [Google Scholar]
- 23.Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–9. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 24.Promadej N, Costello C, Wernett MM, et al. Broad human immunodeficiency virus (HIV)-specific T cell responses to conserved HIV proteins in HIV-seronegative women highly exposed to a single HIV-infected partner. J Infect Dis. 2003;187:1053–63. doi: 10.1086/368127. [DOI] [PubMed] [Google Scholar]
- 25.Gujar SA, Mulrooney-Cousins PM, Michalak TI. Repeated exposure to trace amounts of woodchuck hepadnavirus induces molecularly evident infection and virus-specific T cell response in the absence of serological infection markers and hepatitis. J Virol. 2013;87:1035–48. doi: 10.1128/JVI.01363-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pham TN, MacParland SA, Mulrooney PM, Cooksley H, Naoumov NV, Michalak TI. Hepatitis C virus persistence after spontaneous or treatment-induced resolution of hepatitis C. J Virol. 2004;78:5867–74. doi: 10.1128/JVI.78.11.5867-5874.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartolome J, Lopez-Alcorocho JM, Castillo I, et al. Ultracentrifugation of serum samples allows detection of hepatitis C virus RNA in patients with occult hepatitis C. J Virol. 2007;81:7710–5. doi: 10.1128/JVI.02750-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Quiroga JA, Llorente S, Castillo I, Rodriguez-Inigo E, Pardo M, Carreno V. Cellular immune responses associated with occult hepatitis C virus infection of the liver. J Virol. 2006;80:10972–9. doi: 10.1128/JVI.00852-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thimme R, Oldach D, Chang KM, Steiger C, Ray SC, Chisari FV. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin EC, Park SH, Demino M, et al. Delayed induction, not impaired recruitment, of specific CD8(+) T cells causes the late onset of acute hepatitis C. Gastroenterology. 2011;141:686–95. doi: 10.1053/j.gastro.2011.05.006. , 695 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calarota SA, Foli A, Maserati R, et al. HIV-1-specific T cell precursors with high proliferative capacity correlate with low viremia and high CD4 counts in untreated individuals. J Immunol. 2008;180:5907–15. doi: 10.4049/jimmunol.180.9.5907. [DOI] [PubMed] [Google Scholar]