Abstract
Background. Cryptococcosis-associated immune reconstitution inflammatory syndrome (C-IRIS) may be driven by aberrant T-cell responses against cryptococci. We investigated this in human immunodeficiency virus (HIV)–infected patients with treated cryptococcal meningitis (CM) commencing combination antiretroviral therapy (cART).
Methods. Mitogen- and cryptococcal mannoprotein (CMP)–activated (CD25+CD134+) CD4+ T cells and -induced production of interferon-gamma (IFN-γ), IL-10, and CXCL10 were assessed in whole blood cultures in a prospective study of 106 HIV–CM coinfected patients.
Results. Patients with paradoxical C-IRIS (n = 27), compared with patients with no neurological deterioration (no ND; n = 63), had lower CMP-induced IFN-γ production in 24-hour cultures pre-cART and 4 weeks post-cART (P = .0437 and .0257, respectively) and lower CMP-activated CD4+ T-cell counts pre-cART (P = .0178). Patients surviving to 24 weeks had higher proportions of mitogen-activated CD4+ T cells and higher CMP-induced CXCL10 and IL-10 production in 24-hour cultures pre-cART than patients not surviving (P = .0053, .0436 and .0319, respectively). C-IRIS was not associated with higher CMP-specific T-cell responses before or during cART.
Conclusion. Greater preservation of T-cell function and higher CMP-induced IL-10 and CXCL10 production before cART are associated with improved survival while on cART. Lower CMP-induced IFN-γ production pre-cART, but not higher CMP-specific T-cell responses after cART, were risk factors for C-IRIS.
Keywords: antigen-specific T cells, cryptococcosis-associated immune reconstitution inflammatory syndrome, cryptococcal meningitis, interferon-gamma, whole blood assay, cryptococcal mannoprotein
Cryptococcal meningitis (CM) is the leading cause of adult meningitis in southern and central Africa and it particularly affects persons infected with human immunodeficiency virus (HIV), accounting for 13%–44% of HIV-related deaths [1]. Morbidity and mortality associated with this condition are affected by cryptococcosis-associated immune reconstitution inflammatory syndrome (C-IRIS), which has been reported in up to 50% of HIV-infected patients with treated CM who commence combination antiretroviral therapy (cART) [2]. Like tuberculosis-IRIS, the immunopathogenesis of C-IRIS is poorly understood; both are thought to be due, in part, to an aberrant interferon-gamma (IFN-γ) driven Th1 response to pathogen-specific antigens [3]. A better understanding of C-IRIS pathogenesis is necessary to recognize high-risk patients and develop diagnostic and treatment strategies.
Currently, the diagnosis of C-IRIS remains a diagnosis of exclusion [4]. No definitive diagnostic marker or predictive signature has been adopted into clinical practice, outside of research settings [5]. Distinguishing C-IRIS from other causes of neurological deterioration (ND) after commencing cART is crucial as management and prognosis are vastly different. Analysis of T-cell responses to cryptococcal antigens may be informative.
Whole blood assays for detecting pathogen-specific T cells bear potential as a “field-based assay” that would be particularly attractive in resource-limited settings. Readouts may include expression of activation markers on CD4+ T cells and production of cytokines and chemokines by antigen-activated T cells and other cells activated by these T cells. Accordingly, coexpression of CD25 (interleukin-2 receptor-alpha chain) and CD134 (OX40) on CD4+ T cells in whole blood incubated with antigens has been suggested as an alternative measure of activated and proliferating antigen-specific memory CD4+ T cells [6, 7].
Cryptococcal mannoproteins (CMP) are a group of heterogenous T-cell antigenic determinants isolated from Cryptococcus neoformans [8]. CMPs stimulate lymphoproliferative responses and cytokine production in patients recovering from cryptococcosis [9, 10] and have been shown experimentally to induce the production of tumor necrosis factor-alpha, IL-12, and IFN-γ in human peripheral blood monouclear cells (PBMCs) and murine macrophages cocultured with T cells [8, 11–13].
We explored the use of a whole blood assay to detect CMP-reactive T cells that expressed CD25 and CD134 or produced IFN-γ as a potential field-based assay for the prediction and diagnosis of paradoxical central nervous system (CNS) C-IRIS. CXCL10 (also known as IFN-γ–inducible protein 10 [IP-10]) and IL-10 were also assayed in plasma from the whole blood cultures as the former is induced by IFN-γ and the latter is an antiinflammatory and regulatory cytokine.
METHODS
Patients
Patients with paradoxical CNS C-IRIS (n = 27) and patients with no ND (n = 63) were recruited in Durban, KwaZulu-Natal, South Africa, from a prospective study of adult, HIV-infected, cART-naive patients (n = 106) experiencing their first episode of CM (positive cerebrospinal fluid [CSF] cryptococcal antigen or India ink test) who were treated with standard antifungal therapy. Patients demonstrated a good response to antifungal therapy before starting on cART and were followed for 24 weeks for episodes of ND such as recurrence of headaches, new seizures, or new neurological deficits [14]. Patients were excluded from enrollment if they were concurrently pregnant, had a severe coagulopathy (international normalised ratio >2, platelets <20 × 109/L), had a previous history of CM or previous use of cART within the last 6 months (excluding cART taken as part of prevention for mother-to-child transmission), or were deemed likely to be unwilling to comply with study protocol. All patients provided written informed consent, and the protocol was approved by the ethics committees of the University of KwaZulu-Natal (BF053/09), Monash University (2009001224), and the University of Western Australia (RA/4/1/2541).
Reagents
R10 medium was made with 500 mL RPMI 1640 without L-glutamine (Lonza Walkersville Inc., Walkersville, Maryland) and supplemented with 10% fetal calf serum (PAA Laboratories, Pasching, Austria), 10 mM L-glutamine, 50 mM HEPES buffer, and 50 000 unit/50 000 unit/125 μg Pen/Strep/Fungizone (all from Lonza Walkersville Inc.). Red cell lysis buffer was made by adding 7.7 g ammonium chloride and 0.84 g sodium bicarbonate to 1 L of distilled water. Lyophilized CMP was purified from culture supernatants of C. neoformans acapsular strain Cap 67 as described [8] and reconstituted with distilled water to a final concentration of 1 mg/mL.
Whole Blood Assay of Mitogen- and CMP-Specific T-cell Responses
Mitogen (phytohemagglutinin [PHA]) or CMP-antigen–reactive T cells were detected in whole blood cultures. Two hundred-fifty microlitre of lithium heparin-anticoagulated freshly collected whole blood was mixed with 250 microlitre R10 in the presence of no additive (unstimulated control), polyclonal mitogen Gibco PHA-M 1.5% (Life Technologies, Johannesburg, South Africa), or CMP 12.5 μg and cultured for 24 hours at 37°C in 5% CO2. At 24 hours, 100 microlitre each of unstimulated, mitogen-stimulated, and CMP-stimulated whole blood were lysed using a red cell lysis buffer and stained with CD3 PE/Cy7, CD4 PerCP-Cy5.5, CD25 APC, and CD134 PE (BD Biosciences, Franklin Lakes, New Jersey) based on the method of Zaunders et al [7]. Data acquisition was performed on an LSRII flow cytometer using FACSDiva software (both, BD Biosciences) and analyzed with FlowJo (TreeStar Inc., Ashland, Oregon). Corrected expression of CD25+CD134+ was calculated by subtracting the background expression on unstimulated cells from antigen-stimulated cells. To obtain an estimate of the CMP-specific CD4+ T-cell count, the proportion of CD4+ T cells that were CD25+CD134+ in CMP-stimulated whole blood was multiplied by the absolute CD4+ T-cell count.
In addition to the analysis of CD4+ T-cell activation by flow cytometry, cultured plasma was collected from the set of 24-hour whole blood cultures, here termed “24h-Unstim,” “24h-mitogen,” and “24h-CMP.” Also, 5-day cultures of 100 microlitre of whole blood mixed with 400 microlitre of R10 in the presence of no additive (unstimulated control), mitogen 1%, or CMP 5 μg, here termed “5d-Unstim,” “5d-mitogen,” and “5d-CMP,” were also undertaken. Cultured plasma samples were cryopreserved at −80°C.
Assay of Cytokines and Chemokines in Plasma from Whole Blood Cultures
Thawed plasma samples from culture supernatants were assayed for IFN-γ, CXCL10, and IL-10 by customized multiplex Bio-Plex Pro Assays (Bio-Rad, Gladesville, Australia) using the Bio-Plex 200 suspension array system and Bio-Plex Manager 5.0 software. The lower limits of detection for the cytokine assays were derived by extending the standard curve (after consultation with the manufacturer) and set at 7, 6, and 5 pg/mL for IL-10, IFN-γ, and CXCL10, respectively, ensuring these were above values for blank wells. Plasma from unstimulated mitogen- and CMP-stimulated tubes was assayed on the same plate. Corrected cytokine or chemokine levels were calculated by subtracting the value for unstimulated blood from the value for mitogen- or CMP-stimulated blood. Negative corrected values were reported as zero, represented on log-axis graphs as 0.01.
Statistical Analyses
Continuous variables were assessed for skew and summarized using mean and standard error or median and interquartile range (IQR) as appropriate and analyzed using either a t test or a Wilcoxon rank sum test. Predictors of time to C-IRIS were analyzed using univariable and multivariable Cox proportional hazards regression model. Reported P values are 2 tailed; in these analyses, P < .05 was considered significant.
Overall differences for each variable were tested using Kruskal-Wallis test. If a significant difference was seen, each pair of time points was tested separately using a Wilcoxon rank sum test with Bonferroni adjustment of the P-level cutoff for multiple comparisons, where P values <.0083 were considered significant. Curve comparison of survival analysis to 24 weeks was performed with log rank (Mantel-Cox) test.
All analyses were performed using Stata v.12 (StataCorp, College Station, Texas) and GraphPad Prism v5.
RESULTS
Summary of Baseline Patient Demographics
Data on the entire patient cohort were analyzed (n = 106) as well as subsets of patients experiencing C-IRIS (n = 27) or no ND (n = 63) and survivors (n = 85) or nonsurvivors (n = 21) at 24 weeks post-cART [15]. The C-IRIS group, when compared with the no-ND group, had lower CD4+ T-cell counts pre-cART (median 16 [IQR 6–53] vs 36 [16–83] cells/μL, P = .015), but there was no significant difference in age (median 34 vs 33 years, P = .704), HIV viral load (5.1 vs 5.3 log10 copies/mL, P = .322), and CSF or serum cryptococcal antigen (P = .947 and .756, respectively) [15].
Mitogen-Reactive CD4+ T Cells Increased Over 24 Weeks of cART but not CMP-reactive CD4+ T cells
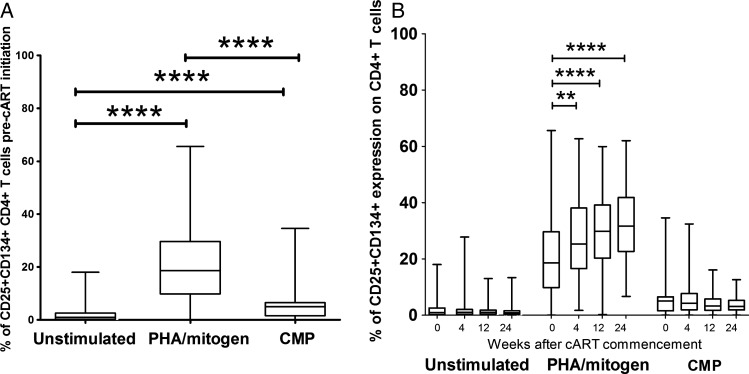
Blood collected prior to cART commencement had significantly higher proportions of activated (CD25+CD134+) T cells after stimulation with mitogen (P < .0001) and CMP (P < .0001) compared with unstimulated tubes (Figure 1A). Over 24 weeks of cART, the proportion of activated CD4+ T cells remained low in the unstimulated tubes, while the proportion of mitogen-activated CD4+ T cells increased significantly (P < .0001). In contrast, the proportion of CMP-activated CD4+ T cells did not change over 24 weeks of cART (Figure 1B).
Figure 1.
Percentage of CD4+ T cells that express CD25+ and CD134+ in unstimulated cultures or following stimulation with phytohemagglutinin/mitogen or cryptococcal mannoprotein in whole blood collected from patients pre-combination antiretroviral therapy (cART) (A) and over 24 weeks of cART (B). Data are shown as a box-and-whisker plot with the median and 25th and 75th percentiles shown as the middle, lower, and upper lines of the box, respectively. P values are indicated as *P < .0500, **P < .0100, ***P < .0010, and ****P < .0001.
Mitogen- and CMP-Induced Cytokine and Chemokine Production Before and After cART
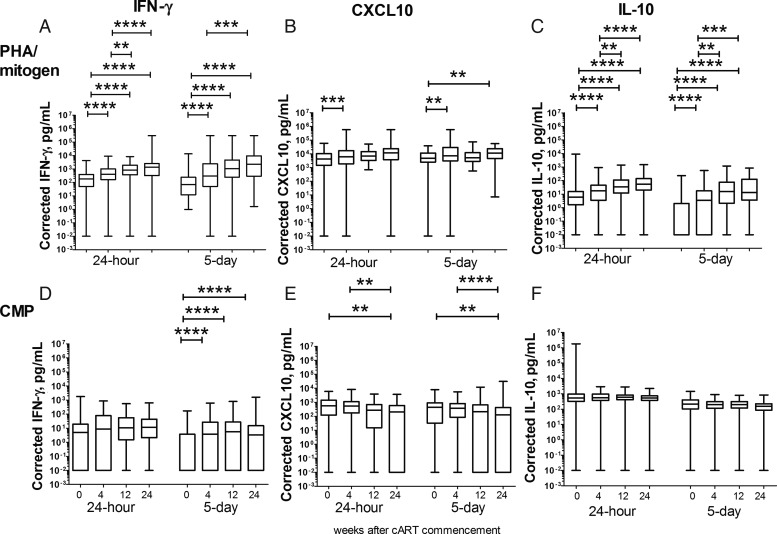
In blood collected pre-cART, CMP-induced IFN-γ was undetectable in 32.7% and 54.8% of the entire patient cohort after 24 hours and 5 days of stimulation, respectively. Levels of mitogen-induced IFN-γ and IL-10 production in plasma after 24 hours and 5 days of stimulation increased on cART (all P ≤ .0001; Figure 2A and 2C), while levels of mitogen-induced CXCL10 increased following 5 days (P = .0008) but not 24 hours of stimulation (Figure 2B). CMP-induced IFN-γ production increased on cART following 5 days of stimulation (P = .0001; Figure 2D), and CMP-induced CXCL10 production decreased on cART following both 24 hours and 5 days of stimulation (P = .0038 and .0023, respectively; Figure 2E). There were no significant changes in CMP-induced IL-10 production over 24 weeks of cART (Figure 2F).
Figure 2.
Changes in mitogen- and cryptococcal mannoprotein (CMP)–induced cytokine and chemokine production before and after combination antiretroviral therapy (cART). Whole blood collected from patients prior to cART and over 24 weeks of cART was incubated with either mitogen (upper panel) or CMP (lower panel) for either 24 hours or 5 days. Interferon-gamma, CXCL10, and interleukin-10 were assayed in plasma from the whole blood cultures. Corrected values (after subtraction of values for unstimulated cultures) are shown as a box-and-whisker plot with the median and 25th and 75th percentiles shown as the middle, lower, and upper lines of the box, respectively. P values are indicated as *P < .0500, **P < .0100, ***P < .0010, and ****P < .0001.
C-IRIS was Associated With Lower CMP-Induced IFN-γ Production Pre-cART but Not Increased CMP-Specific T-Cell Responses During cART
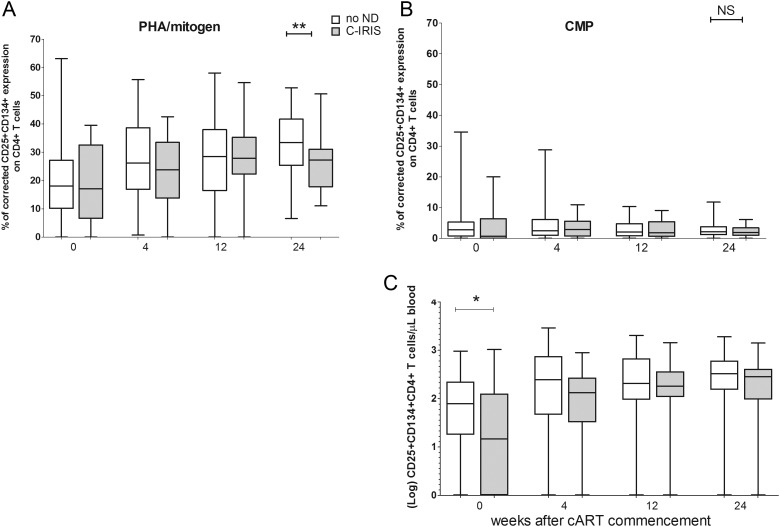
Having established the validity of the whole blood assay methods by examining the entire patient cohort, we compared T-cell responses in patients who experienced C-IRIS or no ND. Proportions of mitogen-activated (CD25+CD134+) CD4+ T cells did not differ between the groups before cART but were lower in the C-IRIS group after 24 weeks of cART (P = .0077; Figure 3A). Proportions of CMP-activated CD4+ T cells did not differ between groups before or during cART (Figure 3B), though CMP-activated CD4+ T-cell counts were lower in the C-IRIS group pre-cART (P = .0178) but not during cART (Figure 3C).
Figure 3.
Cryptococcal mannoprotein (CMP)- and mitogen-activated T cells detected in whole blood from patients with cryptococcosis-associated immune reconstitution inflammatory syndrome (C-IRIS) or neurological deterioration (ND) using flow cytometry. Percentage of CD25+CD134+CD4+ T cells following stimulation with mitogen (A) or CMP (B) in whole blood from patients with C-IRIS (grey box) and no ND (open box) prior to and during 24 weeks of combination antiretroviral therapy. Absolute numbers of CMP-activated CD25+CD134+CD4+ T cells per microliter blood are also shown (C). The corrected data (after subtraction of values in unstimulated cultures) are shown as a box-and-whisker plot with the median and 25th and 75th percentiles shown as the middle, lower, and upper lines of the box, respectively. P values are indicated as *P < .0500, **P < .0100, ***P < .0010, and ****P < .0001.
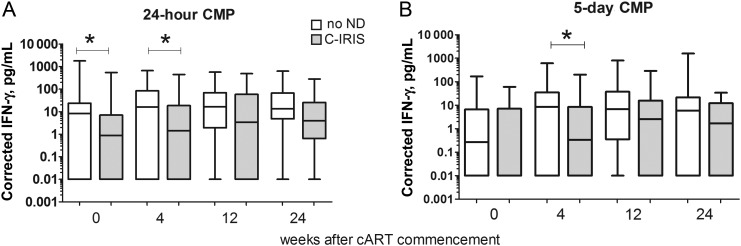
In whole blood cultures, the C-IRIS group when compared with the no-ND group had lower CMP-induced IFN-γ production after stimulation for 24 hours pre-cART (P = .0437; Figure 4A) and after stimulation for 24 hours (P = .0257) and 5 days (P = .0261) at 4 weeks after cART initiation (Figure 4A and 4B). There were no differences in CMP-induced IL-10 or CXCL10 levels between these groups before and during cART.
Figure 4.
Cryptococcal mannoprotein (CMP)–induced interferon-gamma (IFN-γ) production in whole blood cultures from patients with cryptococcosis-associated immune reconstitution inflammatory syndrome (C-IRIS) or no neurological deterioration (ND) before and during combination antiretroviral therapy. IFN-γ levels in plasma after 24 hours (A) or 5 days (B) of culture are shown for patients with C-IRIS (grey box) or no ND (white box). The corrected data (after subtraction of values in unstimulated cultures) are shown as a box-and-whisker plot with the median and 25th and 75th percentiles shown as the middle, lower, and upper lines of the box, respectively. P values are indicated as *P < .0500, **P < .0100, ***P < .0010, and ****P < .0001.
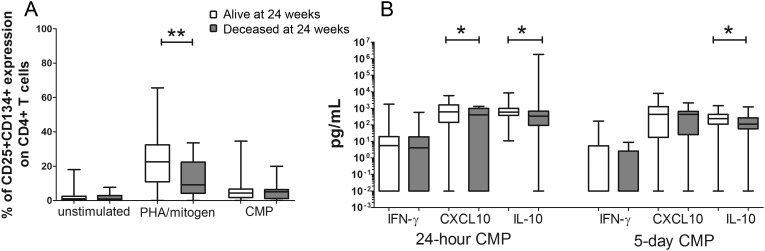
Higher Proportions of Mitogen-Activated CD4+ T Cells and Greater Production of CXCL10 and IL-10 Pre-cART Were Associated With Improved 24-week Survival
Next, we explored whether CMP- and mitogen-induced T-cell and cytokine and chemokine responses in whole blood cultures before cART were associated with patient survival during 24 weeks of cART. The proportion of mitogen-activated (CD25+ CD134+) CD4+ T cells in blood collected pre-cART was higher in survivors than nonsurvivors (median 22.5 [IQR 10.9–32.5] vs 9.2 [4.2–22.4] %; P = .0053; Figure 5A). In CMP-stimulated cultures, survivors had higher pre-cART CXCL10 production in 24-hour cultures (median 611.3 [143.4–1598.0] vs 399.4 [0.01–987.8] pg/mL; P = .0436) and higher IL-10 production in both 24-hour and 5-day cultures (median 588.2 [373.0–984.3] vs 341.2 [91.9–675.7] pg/mL; P = .0319; and 239.0 [109.1–427.7] vs 109.2 [57.9–267.4] pg/mL; P = .0444, respectively; Figure 5B). There was no difference in CMP-induced IFN-γ production between these groups.
Figure 5.
Pre-combination antiretroviral therapy (cART) cryptococcal mannoprotein (CMP)–specific T-cell and cytokine/chemokine responses in patients who did (open box) or did not (grey box) survive the first 24 weeks of cART. (A) Percentage of CD4+ T cells that expressed CD25+ and CD134+ when unstimulated or following stimulation with mitogen or CMP. (B) Levels of CMP-induced interferon-gamma, CXCL10, or IL-10 in plasma from 24-hour (left panel) or 5-day (right panel) whole blood cultures. The corrected data (after subtraction of values for unstimulated cultures) are shown as a box-and-whisker plot with the median and 25th and 75th percentiles shown as the middle, lower, and upper lines of the box, respectively. P values are indicated as *P < .0500, **P < .0100, ***P < .0010, and ****P < .0001.
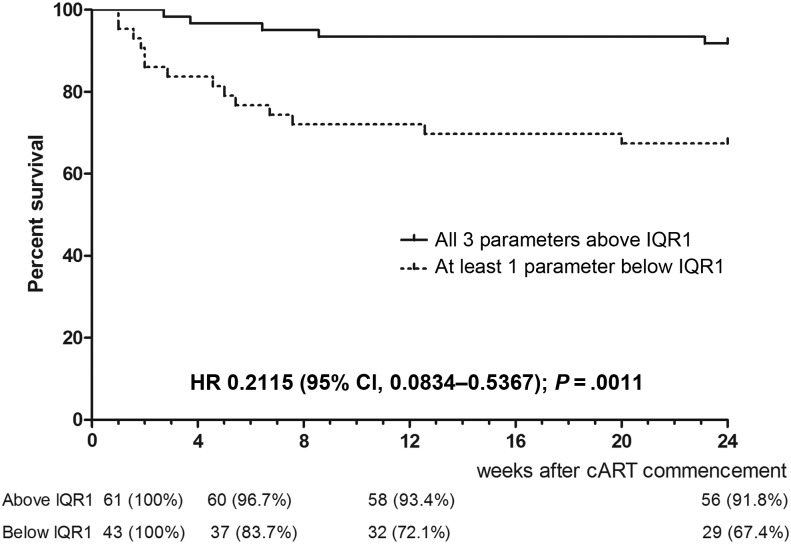
Using survival analysis, patients in the lowest quartile for proportion of mitogen-activated (CD25+ CD134+) CD4+ T cells pre-cART had a reduced 24-week survival compared with other patients (hazard ratio [HR] 0.1386; 95% confidence interval [CI], .0454–.4229; P = .0005). Patients in the lowest quartile for 24-hour CMP-induced CXCL10 and IL-10 production also had decreased survival (HR 0.2553; 95% CI, .0878–.7426; P = .0122 and HR 0.1837 95% CI, .0422–.7994; P = .0239). When we analyzed these 3 parameters together, patients who had at least 1 of these parameters in the lowest quartile were less likely to survive to 24 weeks than those with all 3 parameters measured in the upper 3 quartiles (HR 0.2115; 95% CI, .0834–.5367; P = .0011; Figure 6). There were not enough events to undertake a multivariable analysis of the relationship between pre-cART values for these parameters and survival.
Figure 6.
A 24-week survival analysis for 3 immunological parameters measured in whole blood cultures prior to combination antiretroviral therapy initiation. Survival was compared for patients who demonstrated all 3 parameters in the upper 3 quartiles (solid line) compared with patients with at least 1 parameter in the lower quartile (broken line).
DISCUSSION
We have undertaken the first large-scale analysis of cryptococcal-specific T-cell responses in HIV-infected patients with treated CM before and during the first 24 weeks of cART using assay methods with the potential for translation into routine clinical settings. We established the validity of our assay methods by demonstrating a clear improvement in T-cell function in the entire cohort of patients during the first 6 months of cART, as evidenced by a graduated increase in the proportion of mitogen-activated (CD25+CD134+) CD4+ T cells and a rise in mitogen-induced IFN-γ, CXCL10, and IL-10 production in whole blood cultures. This is consistent with previous studies demonstrating increased proliferative responses to mitogens and common antigens as a surrogate of immune recovery [16–18]. In addition, we demonstrated that a higher proportion of mitogen-activated CD4+ T cells and higher CMP-induced production of CXCL10 and IL-10 in whole blood cultures pre-cART were associated with improved patient survival after 24 weeks of cART. While we did not detect higher CMP-activated CD4+ T cells after cART in the C-IRIS group, these patients exhibited lower CMP-activated CD4+ T-cell count pre-cART, likely reflecting the lower total CD4+ T-cell count in this group. Patients with greater CD4+ T-cell depletion are known to have poorer antigen-specific proliferation [16].
The increase in mitogen-induced IFN-γ and CXCL10 production on cART was associated with a decline in CMP-induced CXCL10 production, perhaps reflecting a declining effector memory T-cell response to a lower pathogen load. However, CMP-induced IFN-γ responses in 5-day cultures increased during cART. The explanation for this discrepancy is unclear; however, it is possible that the relationship between CXCL10 and IFN-γ is different in 5-day cultures.
More than 30% of patients in our study had undetectable CMP-induced IFN-γ in whole blood cultures pre-cART. Notably, the C-IRIS group showed significantly lower CMP-induced production of IFN-γ pre-cART and after 4 weeks of cART. It is well known that IFN-γ plays a critical role in the control of cryptococcosis. In a murine model of pulmonary cryptococcosis, an increase in early IFN-γ secretion from lung-associated lymph nodes was observed in cryptococcal-resistant mice compared with cryptococcal-sensitive mice, and both an anti–IFN-γ and anti–IL-12 antibody resulted in reduced cryptococcal clearance [19, 20]. Furthermore, mice lacking the IFN-γ receptor gene in a model of pulmonary cryptococcosis demonstrated uncontrolled fungal burden and disseminated disease despite enhanced pulmonary leucocytosis [21]. Higher CSF IFN-γ levels in patients with CM were associated with improved rates of CSF cryptococcal clearance and survival [22]. The reduced CMP-induced IFN-γ production in our patients may have contributed to the poorer cryptococcal clearance seen in the C-IRIS group [14, 15], therefore, predisposing them to C-IRIS. In support of this, a higher proportion of IFN-γ+ CD4+ T cells in HIV-infected patients with CM was associated with a lower fungal burden and a better survival rate at 2 weeks after cART [23]. In our study, CMP-induced IFN-γ production in blood collected pre-cART was not associated with improved survival at 24 weeks; however, production of the IFN-γ–inducible protein CXCL10 was. The discrepant association between IFN-γ and survival may reflect the use of whole blood culture assays in our study compared with intracellular staining of T cells in PBMCs in the study by Jarvis et al [23].
Two human studies have explored the promising role of adjunctive, short-course exogenous IFN-γ therapy in HIV-infected patients with CM, with the more recent study showing significantly improved rates of CSF cryptococcal clearance [24, 25] and a suggestion that the impact of exogenous IFN-γ may have been greatest in patients with poor baseline cryptococcal-specific CD4+ T-cell memory responses [23]. Taken together, these findings suggest that patients with low IFN-γ responses to CMP have a higher risk of developing C-IRIS and may be the group that would most benefit from adjunctive exogenous IFN-γ therapy. Equally, those patients at risk of developing C-IRIS–related early mortality may potentially be detected by low or absent IFN-γ or CXCL10 production using this simple assay of CMP-stimulated whole blood cultures.
In our study, the proportion of CMP-activated (CD25+CD134+) CD4+ T cells did not distinguish C-IRIS patients from no-ND patients either before or after cART. This finding has several possible explanations. First, T-cell responses to CMP may not be involved in the immunopathology of all cases of C-IRIS. In this study, we only examined patients with paradoxical C-IRIS, whereas increased CMP-specific T-cell responses have mainly been reported in patients with “unmasking” C-IRIS [3]. Second, C-IRIS events occurring in the CNS may have immune responses compartmentalized to the CNS and may not be detectable in blood. Third, whole blood assay methods may not be sensitive enough to detect CMP-reactive T cells in patients with “paradoxical” C-IRIS, as appears to be the case in paradoxical tuberculosis-IRIS [26, 27]. Importantly, Jarvis et al [23] also did not demonstrate an increase in CMP-reactive CD4+ T cells after 4 weeks of cART using polychromatic flow cytometry. Finally, it is possible the CMP purified from the Cap67 acapsular Cryptococcus spp. mutant may not induce a robust T-cell response in patients infected with cryptococcal strains found in South Africa. Different cryptococcal genotypes have been shown to induce different cytokine profiles [28].
IL-10 is a critical immunoregulatory molecule and is able to downregulate both innate and adaptive immunity, leading to increased susceptibility, to intracellular pathogens, including Cryptococcus spp., and microbial persistence [29]. On the other hand, IL-10 may also protect hosts from exaggerated inflammatory and immune reactions and tissue injuries secondary to infections [29]. IL-10 is known to reduce IFN-γ production in Th1 cells, thereby amplifying deactivation of antigen-presenting cells [30], and is able to suppress all functions of monocytes/macrophages by interfering with antigen presentation, release of immune mediators, and phagocytosis. The poorer survival rates seen in patients with lower CMP-induced IL-10 responses pre-cART support its role in containing the inflammation and tissue injury associated with CM.
Higher proportions of mitogen-activated CD4+T cells in blood from patients pre-cART were also associated with improved survival at 24 weeks, suggesting that preservation of mitogenic response associated with CD4+ T-cell preservation pre-cART is important for survival in HIV-CM coinfected patients. Pre-cART measurement of these 3 parameters found to be associated with survival might identify those at risk for increased mortality and requiring enhanced clinical surveillance. Other previously reported laboratory test predictors of C-IRIS and death have included perturbations of serum levels of several cytokines [31]. A direct comparison of all biomarkers is warranted.
Our study has some limitations. Our C-IRIS and no-ND groups were not matched for CD4+ T-cell count pre-cART, and this disparity may have contributed to the difference between our finding of a reduced IFN-γ level in our C-IRIS patients compared with other studies. We chose to analyze our data collected strictly at scheduled time points prospectively, rather than reverse-matching at C-IRIS event for an equivalent time control. However, we believe our longitudinal follow-up of the cohort without selecting for equivalence in CD4+ T-cell count or reverse time-matching lends credence to our findings.
In conclusion, using whole blood assays of T-cell responses to CMP, we have shown that HIV-infected patients with treated CM who exhibit lower CMP-induced IFN-γ production pre-cART are at increased risk of C-IRIS. The effect of adjunctive exogenous IFN-γ should be trialed in patients who have low CMP-induced IFN-γ responses as a means of enhancing cryptococcal clearance and potentially preventing C-IRIS. Further, preserved T-cell function, as demonstrated by mitogen-activated CD4+ T cells, and increased IL-10 and CXCL10 responses in CMP-stimulated whole blood cultures pre-cART were associated with better survival at 24 weeks post-cART. This assay might be incorporated into a risk-stratification algorithm in the management of HIV-CM coinfected patients.
Notes
Acknowledgments. We acknowledge the patients and their families and staff at the HIV Pathogenesis Programme, King Edward VIII Hospital, and the Burnet Institute.
Authors’ contribution. M. F., A. L., C. C., and S. R. L; designed the experiments. C. C., T. S., M. F., and S. R. L. analyzed the data. C. C., M. F., S. R. L., and S. M. L. wrote the manuscript. C. C. and S. O. performed the immunological assays. S. M. L. provided the cryptococcal mannoproteins. C. C. and B. G. conducted the clinical study. M. F., S. R. L., C. C., A. L., T. N., M.-Y. S. M., W. C., and J. E. were members of the protocol steering committee. All read and approved the final manuscript.
Financial support. This work was supported by the REACH Initiative (Research and Education in HIV/AIDS for Resource Poor Countries) and Pfizer Neuroscience research grant. C. C. C. was supported by an Australian Commonwealth Government Postgraduate Award 2009 and the Australian National Health and Medical Research Council (NHMRC) Postgraduate Scholarship 2010–2012. S. M. L. was supported by National Institutes of Health grant AI025780. T. N. was supported in part by an international Early Career Scientist grant from the Howard Hughes Medical Institute and by the South African Department of Science and Technology/National Research Foundation Research Chairs Initiative. S. R. L. is a NHMRC practitioner fellow. M. A. F. was supported by NHMRC grant 510448.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS. 2009;23:525–30. doi: 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- 2.Muller M, Wandel S, Colebunders R, Attia S, Furrer H, Egger M. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:251–61. doi: 10.1016/S1473-3099(10)70026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tan DB, Yong YK, Tan HY, et al. Immunological profiles of immune restoration disease presenting as mycobacterial lymphadenitis and cryptococcal meningitis. HIV Med. 2008;9:307–16. doi: 10.1111/j.1468-1293.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- 4.Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10:791–802. doi: 10.1016/S1473-3099(10)70170-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boulware DR, Bonham SC, Meya DB, et al. Paucity of initial cerebrospinal fluid inflammation in cryptococcal meningitis is associated with subsequent immune reconstitution inflammatory syndrome. J Infect Dis. 2010;202:962–70. doi: 10.1086/655785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keoshkerian E, Helbig K, Beard M, et al. A novel assay for detection of hepatitis C virus-specific effector CD4(+) T cells via co-expression of CD25 and CD134. J Immunol Methods. 2012;375:148–58. doi: 10.1016/j.jim.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Zaunders JJ, Munier ML, Seddiki N, et al. High levels of human antigen-specific CD4+ T cells in peripheral blood revealed by stimulated coexpression of CD25 and CD134 (OX40) J Immunol. 2009;183:2827–36. doi: 10.4049/jimmunol.0803548. [DOI] [PubMed] [Google Scholar]
- 8.Wozniak KL, Levitz SM. Isolation and purification of antigenic components of cryptococcus. Host-pathogen interactions. Totowa, NJ: Humana Press; 2008. pp. 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levitz SM, North EA. Lymphoproliferation and cytokine profiles in human peripheral blood mononuclear cells stimulated by Cryptococcus neoformans. J Med Vet Mycol. 1997;35:229–36. doi: 10.1080/02681219780001201. [DOI] [PubMed] [Google Scholar]
- 10.Hoy JF, Murphy JW, Miller GG. T cell response to soluble cryptococcal antigens after recovery from cryptococcal infection. J Infect Dis. 1989;159:116–9. doi: 10.1093/infdis/159.1.116. [DOI] [PubMed] [Google Scholar]
- 11.Pietrella D, Cherniak R, Strappini C, et al. Role of mannoprotein in induction and regulation of immunity to Cryptococcus neoformans. Infect Immun. 2001;69:2808–14. doi: 10.1128/IAI.69.5.2808-2814.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitzurra L, Cherniak R, Giammarioli M, Perito S, Bistoni F, Vecchiarelli A. Early induction of interleukin-12 by human monocytes exposed to Cryptococcus neoformans mannoproteins. Infect Immun. 2000;68:558–63. doi: 10.1128/iai.68.2.558-563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaka W, Verheul AF, Vaishnav VV, et al. Cryptococcus neoformans and cryptococcal glucuronoxylomannan, galactoxylomannan, and mannoprotein induce different levels of tumor necrosis factor alpha in human peripheral blood mononuclear cells. Infect Immun. 1997;65:272–8. doi: 10.1128/iai.65.1.272-278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang CC, Dorasamy AA, Elliott JH, et al. HIV-infected patients with cryptococcal meningitis who attain CSF sterility pre-cART commencement experience improved outcomes in the first 24 weeks. 2012 Conference on Retroviruses and Opportunistic Infections (CROI), Seattle, Washington. March 2012. [Google Scholar]
- 15.Chang CC, Dorasamy AA, Gosnell BI, et al. Clinical and mycological predictors of cryptococcosis-associated Immune reconstitution inflammatory syndrome (C-IRIS) AIDS. 2013 doi: 10.1097/QAD.0b013e3283614a8d. Mar 21. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 16.Wendland T, Furrer H, Vernazza PL, et al. HAART in HIV-infected patients: restoration of antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viraemia. AIDS. 1999;13:1857–62. doi: 10.1097/00002030-199910010-00007. [DOI] [PubMed] [Google Scholar]
- 17.Autran B, Carcelain G. AIDS. Boosting immunity to HIV–can the virus help? Science. 2000;290:946–9. doi: 10.1126/science.290.5493.946. [DOI] [PubMed] [Google Scholar]
- 18.Li TS, Tubiana R, Katlama C, Calvez V, Ait MH, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351:1682–6. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 19.Hoag KA, Lipscomb MF, Izzo AA, Street NE. IL-12 and IFN-gamma are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–9. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 20.Hoag KA, Street NE, Huffnagle GB, Lipscomb MF. Early cytokine production in pulmonary Cryptococcus neoformans infections distinguishes susceptible and resistant mice. Am J Respir Cell Mol Biol. 1995;13:487–95. doi: 10.1165/ajrcmb.13.4.7546779. [DOI] [PubMed] [Google Scholar]
- 21.Chen GH, McDonald RA, Wells JC, Huffnagle GB, Lukacs NW, Toews GB. The gamma interferon receptor is required for the protective pulmonary inflammatory response to Cryptococcus neoformans. Infect Immun. 2005;73:1788–96. doi: 10.1128/IAI.73.3.1788-1796.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siddiqui AA, Brouwer AE, Wuthiekanun V, et al. IFN-gamma at the site of infection determines rate of clearance of infection in cryptococcal meningitis. J Immunol. 2005;174:1746–50. doi: 10.4049/jimmunol.174.3.1746. [DOI] [PubMed] [Google Scholar]
- 23.Jarvis JN, Casazza JP, Stone HH, et al. The phenotype of the Cryptococcus-specific CD4+ memory T-cell response is associated with disease severity and outcome in HIV-associated cryptococcal meningitis. J Infect Dis. 2013;207:1817–28. doi: 10.1093/infdis/jit099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pappas PG, Bustamante B, Ticona E, et al. Recombinant interferon-gamma 1b as adjunctive therapy for AIDS-related acute cryptococcal meningitis. J Infect Dis. 2004;189:2185–91. doi: 10.1086/420829. [DOI] [PubMed] [Google Scholar]
- 25.Jarvis JN, Meintjes G, Rebe K, et al. Adjunctive interferon-gamma immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS. 2012;26:1105–13. doi: 10.1097/QAD.0b013e3283536a93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliott JH, Vohith K, Saramony S, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis. 2009;200:1736–45. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 27.Tieu HV, Ananworanich J, Avihingsanon A, et al. Immunologic markers as predictors of tuberculosis-associated immune reconstitution inflammatory syndrome in HIV and tuberculosis coinfected persons in Thailand. AIDS Res Hum Retroviruses. 2009;25:1083–9. doi: 10.1089/aid.2009.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiesner DL, Moskalenko O, Corcoran JM, et al. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. MBio. 2012;3:e00196–12. doi: 10.1128/mBio.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mege JL, Meghari S, Honstettre A, Capo C, Raoult D. The two faces of interleukin 10 in human infectious diseases. Lancet Infect Dis. 2006;6:557–69. doi: 10.1016/S1473-3099(06)70577-1. [DOI] [PubMed] [Google Scholar]
- 30.Sabat R, Grutz G, Warszawska K, et al. Biology of interleukin-10. Cytokine Growth Factor Rev. 2010;21:331–44. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7:e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]