Abstract
Background. Antibodies induced by infection with any 1 of 4 dengue virus (DENV) serotypes (DENV-1–4) may influence the clinical outcome of subsequent heterologous infections. To quantify potential cross-protective effects, we estimated disease risk as a function of DENV infection, using data from longitudinal studies performed from September 2006 through February 2011 in Iquitos, Peru, during periods of DENV-3 and DENV-4 transmission.
Methods. DENV infections before and during the study period were determined by analysis of serial serum samples with virus neutralization tests. Third and fourth infections were classified as postsecondary infections. Dengue fever cases were detected by door-to-door surveillance for acute febrile illness.
Results. Among susceptible participants, 39% (420/1077) and 53% (1595/2997) seroconverted to DENV-3 and DENV-4, respectively. Disease was detected in 7% of DENV-3 infections and 10% of DENV-4 infections. Disease during postsecondary infections was reduced by 93% for DENV-3 and 64% for DENV-4, compared with primary and secondary infections. Despite lower disease rates, postsecondary infections constituted a significant proportion of apparent infections (14% [for DENV-3 infections], 45% [for DENV-4 infections]).
Conclusions. Preexisting heterotypic antibodies markedly reduced but did not eliminate the risk of disease in this study population. These results improve understanding of how preinfection history can be associated with dengue outcomes and DENV transmission dynamics.
Keywords: dengue, dengue fever, antibody cross-protection, seroepidemiology
Dengue virus (DENV) is the most prevalent arthropod-transmitted virus among humans, with conservative estimates placing half of the world's population at risk for infection [1, 2]. Dengue can be caused by any of 4 viral serotypes (DENV-1–4), and infection can lead to a range of outcomes, from subclinical infection to death [3]. Infection outcomes are determined by a suite of factors, including host susceptibility, virus genetics, cell-mediated immune response, and cross-reactive antibodies [4, 5]. Cross-reaction between preexisting DENV antibodies and virus of a heterotypic infecting serotype is due to the conservation of some viral envelope proteins across serotypes, which results in antibody binding to the virus particles without fully neutralizing them [6]. There is epidemiologic and in vitro evidence that these cross-reactive antibodies can enhance infection and disease severity during secondary infections (referred to as antibody-dependent enhancement [7–9]). It has also been inferred from hospital admissions data that severe disease is rare during third and fourth infections [10], suggesting that there is a protective effect conferred by cumulative cross-reactive antibodies. Despite the uncertainties, a general lesson from multiple-strain DENV mathematical models is that the effects of preexisting antibodies, either enhancing or protective, can significantly influence projected virus transmission and disease dynamics [11–15].
Because of the possibility for disease potentiation from subneutralizing antibodies, vaccine design efforts have aimed for a tetravalent formulation that simultaneously protects against all 4 DENV serotypes [16, 17]. Recent results from phase 2b trials of the leading vaccine candidate showed partial efficacy against DENV-1, DENV-3, and DENV-4 but did not protect against febrile illness associated with DENV-2 infection [18]. An important unanswered question concerns the effect that neutralizing antibody to ≥2 serotypes will have on disease burden and virus transmission. There is, therefore, a need for empirical data characterizing population-level outcomes of serial DENV infections.
In this study, we used data from a longitudinal cohort in Iquitos, Peru, where prospective seroepidemiology and febrile surveillance studies have been ongoing since 1999. For the same individuals, biannual serological results were used to determine the baseline serostatus of participants, and door-to-door febrile surveillance paired with laboratory confirmation was used to identify subsequent disease due to a DENV infection. Of special interest were disease outcomes during third and fourth infections, which were classified together as postsecondary infections. Our data indicate that the risk of developing febrile dengue illness was significantly reduced in individuals with a history of ≥2 prior DENV exposures, despite the absence of preexisting antibodies specific to the infecting serotype.
METHODS
Study Population
We used data from longitudinal cohort studies conducted in Iquitos from September 2006 through February 2011. Iquitos is a city of 380 000 inhabitants located in the Amazon Basin of northeastern Peru and has been described in detail elsewhere [19]. The city has experienced a well-defined pattern of serotype introductions, with each new serotype replacing the previous one: DENV-1 was introduced during the early 1990s [20], followed by American genotype DENV-2 in 1995 [21, 22], DENV-3 in 2001 [19], and DENV-4 in 2008 [23]. During our study interval, DENV-3 was in an interepidemic period. DENV-4 is known to have been introduced to the city by February 2008 and became the dominant serotype by October 2008 [23], although it may also have cocirculated at low levels with DENV-1 during 1990 [20]. Given very low seroprevalence (1.5%) of DENV-4 detected before 2008, we assumed that the study population was uniformly susceptible to that serotype before the 2008 introduction. Despite multiple serotype introductions, the incidence rate of reported severe disease remained extremely low throughout the region [24].
Longitudinal Cohorts
Beginning in September 2006, a baseline serum sample was collected from 2356 individuals (cohort A), with follow-up samples collected and assayed by plaque reduction neutralization test (PRNT) approximately every 6 months until October 2008. A second study with a similar structure (blood sample collection every 6 months) was initiated in September 2007, with baseline serum samples collected from 2445 individuals (cohort B). Persons aged ≥5 years were eligible to participate. Study protocols were approved by the institutional review boards of the University of California, Davis (cohort A: protocol 2006.14405/261811; cohort B: protocol 2007.15244/296683), and the Naval Medical Research Center (cohort A: protocol NMRCD2005.0009; cohort B: protocol NMRCD2007.0007), in compliance with regulations in the United States and Peru governing the protection of human subjects.
For both studies, participants’ homes were visited by technicians 3 times per week to inquire about febrile illness consistent with dengue [25]. Technicians were assigned specific city blocks. Dengue-like illness was classified as occurrence of fever, either by observation (oral temperature, ≥38°C; axillary temperature, ≥37.5°C) or subject self-report, plus at least 1 other symptom consistent with DENV infection, including headache, retro-orbital pain, or bone pain. There were 2 instances in which a classification was made without the presence of fever, given the presence of multiple other consistent symptoms (Table 1). Serum samples (10 mL) were collected at the time of acute fever presentation, with convalescent samples collected 2–4 weeks following collection of the initial acute-phase blood specimen.
Table 1.
Signs and Symptoms in >10% of Cases of Apparent Illness Due to Dengue Virus Serotype 3 (DENV-3) and DENV-4
| Sign or Symptom | DENV-3, Cases, No. (%) (n = 50) | DENV-4, Cases, No. (%) (n = 144) |
|---|---|---|
| Fever | 50 (100) | 142 (99) |
| Headache | 50 (100) | 139 (97) |
| Chills | 49 (98) | 137 (95) |
| Body pain | 48 (96) | 135 (94) |
| Loss of appetite | 38 (76) | 127 (88) |
| Arthralgia | 43 (86) | 117 (81) |
| Myalgia | 41 (82) | 116 (81) |
| Retro-ocular pain | 46 (92) | 113 (78) |
| Rash | 45 (90) | 104 (72) |
| Nausea | 34 (68) | 84 (58) |
| Vomiting | 16 (32) | 37 (26) |
| Diarrhea | 14 (28) | 45 (31) |
| Abdominal pain | 28 (56) | 71 (49) |
| Sore throat | 17 (34) | 43 (30) |
| Cough | 20 (40) | 40 (28) |
| Nasal congestion | 14 (28) | 34 (24) |
| Petechiae | 6 (12) | 18 (13) |
| Hematuria | … | 18 (13) |
Laboratory Methods
DENV neutralizing antibodies were measured by PRNT as described by Morrison et al and Comach et al [19, 26]. Test viruses used were DENV-1 strain 16007 (Thailand 1974), DENV-2 strain 16681 (Thailand 1974), DENV-3 strain IQT1728 (Peru 2001), and DENV-4 strain 1036 (Indonesia 1976). Neutralization was measured at 70% plaque reduction relative to negative control specimens (ie, DENV-naive normal human serum) at cutoffs of 1:60 for DENV-1, 1:80 for DENV-2, 1:60 for DENV-3, and 1:40 for DENV-4.
Acute serum samples from febrile cases were inoculated onto C6/36 cells for virus isolation, using an immunofluorescence assay (IFA) as previously described, and screened for DENV RNA by reverse transcription polymerase chain reaction (RT-PCR) [27, 28]. Acute and convalescent samples were assayed for DENV-specific immunoglobulin M (IgM) antibodies by an antibody-capture enzyme-linked immunosorbent assay [27].
Baseline Serostatus
Two study periods were identified: (1) a period of DENV-3 dominance, between late 2006 and mid-2008, when >99% of all DENV isolates were DENV-3 [27]; and (2) a period of DENV-4 dominance, between late 2008 and late 2010, when >96% of all DENV isolates were DENV-4 (Figure 1) [23]. We defined baseline serostatus by PRNT70 for each participant, using samples collected at the beginning of the 2 retrospective study periods (from August to October 2006 for DENV-3 and from November 2007 to August 2008 for DENV-4). On the basis of previous studies by our research group, we were able to reliably classify individuals’ DENV exposure histories during various distinct waves of DENV invasion and transmission [19, 22, 29, 30]. However, owing to the uncertainties often associated with PRNT following multiple DENV infections [31–34], we organized individuals into broad exposure categories: naive, monotypic, and multitypic. Participants without neutralizing antibodies against any of the 4 serotypes at the dilutions specified above were defined as DENV naive, participants with neutralizing antibodies against 1 serotype were defined as monotypic for that serotype and susceptible to the remaining 3, and participants with neutralizing antibodies against ≥2 serotypes were defined as multitypic. Serostatus and seroconversion were confirmed by PRNT70 with subsequent sequential serum samples, when available. Individuals with DENV-3– or DENV-4–specific antibodies at the start of the respective study periods (ie, participants with prior DENV-3 or DENV-4 exposure) were excluded from the analysis (see below).
Figure 1.
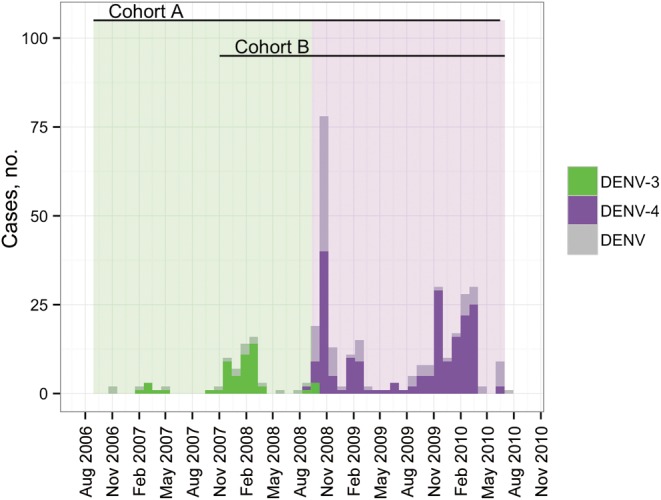
Monthly occurrence of diagnosed dengue fever cases in Iquitos, Peru, during 2006–2010. The period during which dengue virus serotype 3 (DENV-3) transmission was predominant is denoted by green bars, and the period during which DENV-4 transmission was predominant is denoted by purple bars. The timing of each longitudinal cohort (A and B) is shown at the top of the figure; during the DENV-4 period, they overlap. Cases were detected by active community-based surveillance for febrile illness and confirmed in the laboratory. When virus was isolated, the infecting serotype is shown; otherwise, cases positive for immunoglobulin M antibody are denoted as “DENV.”
Seroconversions
We estimated infection rates among the study population by identifying DENV-3– or DENV-4–susceptible individuals at baseline and comparing the proportion who seroconverted during the study period with those who continued to show no evidence of serotype-specific antibodies throughout the study period. Primary infections occurred in people classified as naive, secondary infections in people with prior monotypic exposure, and postsecondary infections in people with prior multitypic exposures. On the basis of the low seroprevalence of DENV-3 and DENV-4 within the study population before their introductions into the region, we estimated a high serotype specificity (>95%) for DENV-3 and DENV-4 PRNTs, despite the previous circulation of other DENV serotypes [30]. Using serial samples from confirmed cases, we observed high sensitivity (approximately 88%) for DENV-3 and moderate sensitivity (approximately 67%) for DENV-4 PRNT. In light of the absence of detected concurrent circulation of heterologous serotypes, we found that we could improve our ability to detect DENV-4 seroconversions (approximately 85% sensitivity). During the period of DENV-4 circulation, we assumed that all seroconversions were attributable to DENV-4 infection and validated this assumption against samples from participants with virologically confirmed DENV-4 infections.
Symptomatic Infections
We estimated the proportion of infections that resulted in disease by comparing participants with serotype-specific seroconversions at any point during the study period against febrile surveillance data to determine whether an individual had experienced a dengue-like illness during the intervening period. Disease was defined as an acute febrile illness identified by febrile surveillance (see above), with DENV infection confirmed by IFA, RT-PCR, or a 4-fold rise in serum IgM titer between collection of acute and convalescent blood specimens.
To estimate the proportion of symptomatic infections, we included participants who seroconverted at any point during the study period, because our door-to-door surveillance permitted us to detect febrile episodes during the intervening period. In contrast, to determine seroconversion rates, as described above, we only included participants who provided samples at the beginning and end of the study period, to avoid underestimating the number of seroconversions. Thus, the number of seroconversions used in the numerator of the seroconversion rate differed from the number of seroconversions used in the denominator of the symptomatic infection rate.
Analyses
The effects of preinfection serostatus on disease risk were estimated with mixed-effects general linear models with the assumption of a binomial error distribution and logit link function. Age was included as a fixed effect to account for age-dependent effects on disease outcomes. City block was included as a random effect to account for variation in surveillance efficiency due to differences among technicians and neighborhoods. We used Akaike's information criterion with small sample size correction and log-likelihood ratio tests (for nested models) to compare models.
Because of the positive correlation between age and number of DENV exposures, we performed a matched-pair case-control analyses to isolate the effect of infection history on disease rate for both DENV-3 and DENV-4. Individuals with disease were matched by sex and age with controls who did not report disease. To avoid introducing systematic biases, controls were blinded for exposure history before being matched on the basis of sex and date of birth (typically within 1 month). The control with the closest birth date was chosen, and if there were 2 potential controls with the same birth date, the first on the list was used. These data were then analyzed with the McNemar test.
Initial mixed-effects generalized linear models included all infection histories, to consider specific interserotype interactions. We subsequently collapsed these into exposure categories based on preinfection serostatus: naive, monotypic, and multitypic. Broad categories were used in the final analyses, to provide a parsimonious representation of the data and to reduce uncertainties arising from the difficulty of interpreting DENV serological results after a secondary infection.
Statistical analysis was performed using R, version 2.14.1 [35]. Statistical significance was assessed at α = 0.05.
RESULTS
DENV-3
Transmission and Case Capture
Of 1077 cohort participants fulfilling our inclusion criteria for estimating infection rates, 420 (39%) seroconverted to DENV-3 between September 2006 and October 2008. Seroconversion rates varied with infection history and were highest in the group of individuals with ≥2 previous exposures (Table 2).
Table 2.
Observed Outcomes for Dengue Virus Serotype 3 (DENV-3) and DENV-4 Infection and Disease
| Baseline Serostatus | Infection |
Disease |
||||
|---|---|---|---|---|---|---|
| Infections/ Total Sampled, No. | Proportion Seroconverted (95% CI) | Febrile Cases/Total Infections, No. | Proportion Symptomatic (95% CI) | Febrile Case/All Symptomatic Cases, No. (%) | Apparent-to-Inapparent Ratio | |
| DENV-3 | ||||||
| Naive | 64/218 | 0.29 (.24–.36) | 28/109 | 0.26 (.18–.35) | 28/51 (55) | 1:3 |
| Monotypic | 63/206 | 0.31 (.24–.37) | 16/107 | 0.15 (.090–.23) | 16/51 (31) | 1:6 |
| Multitypica | 293/653 | 0.45 (.41–.49) | 7/475 | 0.015 (.0065–.031) | 7/51 (14) | 1:67 |
| Total | 420/1077 | 0.39 (.36–.42) | 51/691 | 0.074 (.056–.097) | … | 1:13 |
| DENV-4 | ||||||
| Naive | 168/345 | 0.49 (.43–.54) | 35/168 | 0.21 (.15–.28) | 35/161 (22) | 1:4 |
| Monotypic | 320/599 | 0.53 (.49–.57) | 54/320 | 0.17 (.13–.22) | 54/161 (33) | 1:5 |
| Multitypica | 1107/2053 | 0.54 (.52–.56) | 72/1107 | 0.065 (.052–.082) | 72/161 (45) | 1:14 |
| Total | 1595/2997 | 0.53 (.51–.55) | 161/1595 | 0.10 (.087–.12) | … | 1:9 |
Abbreviation: CI, confidence interval.
a Defined as having neutralizing antibody to ≥2 serotypes, as measured by a plaque reduction neutralization test.
There were 51 cases of apparent illness detected by activ surveillance among the 691 participants with evidence of seroconversion at any point during the study period (ie, not restricted to those with samples from both the first and last 6-month sample periods; ratio of apparent to inapparent infections, 1:13). Signs and symptoms were recorded (Table 1). No cases required hospitalization. Ninety percent of the disease cases were confirmed by IFA or PCR, and 10% were confirmed by IgM analysis. Disease rates were lower among individuals with ≥2 prior exposures relative to individuals who were never previously exposed to DENV or with only a single prior exposure (Table 2). Still, postsecondary infections accounted for 14% of apparent dengue cases.
Estimates of Disease Risk
Relative to naive individuals, the odds of developing disease was significantly reduced in the presence of multitypic heterologous antibodies (odds ratio [OR], 0.048; 95% confidence interval [CI], .018–.1; Table 3 and Supplementary Tables 1 and 2) after control for age. Prior exposure to DENV-1 (either in a monotypic or multitypic profile) significantly reduced the odds of disease from DENV-3 exposure, whereas DENV-2 alone did not (Supplementary Table 2). A multitypic antibody profile (in this case, DENV-1/DENV-2 infection history) conferred significantly more protection from disease then DENV-1 alone (P < .001, by the Wald test). Variation in surveillance effort (city block) was minimal and did not significantly modify model fit. Although neither sex nor age were significantly associated with disease outcome in any model for DENV-3, we ran a paired case-control analysis, matching on these variables. There was a highly significant relationship between infection history and disease (P < .001, by the McNemar test,) independent of age and sex, with prior exposure to ≥2 DENV serotypes associated with reduced disease during heterotypic DENV-3 infection (OR, 0.08; 95% CI, .0092–.32).
Table 3.
Parameter Estimates for Dengue Virus Serotype 3 (DENV-3) and DENV-4 Maximum Likelihood Models
| Serotype, Parameter | OR (95% CI) | P |
|---|---|---|
| DENV-3a | ||
| Naive | 1.00 (reference) | |
| Monotypic | 0.55 (.26–1.11) | .098 |
| Multitypicb | 0.048 (.018–.11) | <.001 |
| Age | 0.99 (.97–1.01) | .35 |
| DENV-4c | ||
| Naive | 1.00 (reference) | |
| Prior exposure to DENV-1 | 0.45 (.21–.91) | .031 |
| Prior exposure to DENV-2 or -3 | 0.97 (.57–1.63) | .90 |
| Multitypicb | 0.22 (.13–.38) | <.001 |
| Age | 1.07 (1.02–1.12) | .0039 |
| Age squared | 0.9989 (.998–.9996) | .0014 |
Abbreviations: CI, confidence interval; OR, odds ratio.
a Approximate R2, 22.41%.
b Defined as having neutralizing antibody to ≥2 serotypes, as measured by a plaque reduction neutralization test.
c Approximate R2, 6.94%
DENV-4
Transmission and Case Capture
We observed a 53% overall seroconversion rate due to DENV-4 infection among 2997 participants who fulfilled the inclusion criteria (Table 2). We observed minimal variation in seroconversion rates across exposure histories (Table 2). There was no significant association between infection history and the risk of seroconversion when data were analyzed using logistic regression, although having ≥2 prior infections did approach statistical significance as a risk factor (OR, 1.23; 95% CI, .98–1.55; P = .072). Of the 1595 participants that seroconverted to DENV-4, active surveillance detected 161 cases of fever with at least 1 additional DENV-associated symptom (Table 1). No participants were hospitalized with DENV infection. Cases were confirmed as dengue by either PCR or IFA (82% of cases) or by a 4-fold rise in IgM titer between acute and convalescent samples (18%). Although symptomatic illness was more common with naive or monotypic serostatus than with multitypic serostatus (Table 2), the latter accounted for 45% of all detected cases of disease due to DENV-4. Overall, the ratio of apparent to inapparent DENV-4 infections was 1:9.
Estimates of Disease Risk
In a multivariable model, infection history and participant age were significantly associated with risk of disease due to DENV-4 infection (Table 3 and Supplementary Tables 1 and 2). Monotypic DENV-1 antibodies were associated with reduced odds of disease, whereas monotypic DENV-2 or DENV-3 antibodies were not (Table 3). Disease risk was significantly reduced for individuals with multitypic serostatus (OR, 0.22; 95% CI, .13–.38), with additional protective effects beyond that of DENV-1 antibodies alone (P = .0069 by the Wald test). Disease risk appeared to vary nonlinearly with age (Figure 2), with the highest risk between 25 and 30 years of age. As with DENV-3, when city block was included as a random effect, no impact on the relationship between age, serostatus, and disease was observed. Matched-pairs case-control analysis also showed that the presence of antibodies to ≥2 DENV serotypes was associated with reduced disease, independent of age and sex (OR, 0.2; 95% CI, .086–.41).
Figure 2.
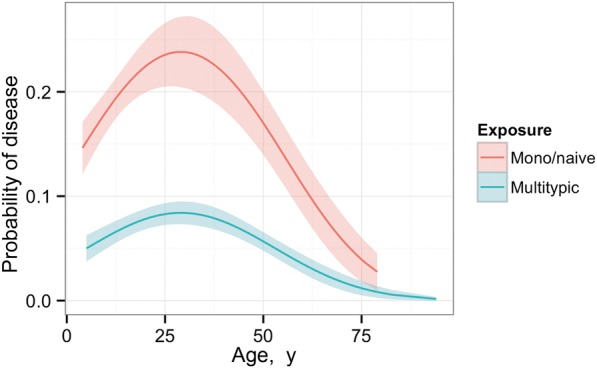
Predicted risk of disease due to infection with dengue virus serotype 4 (DENV-4), as a function of age and serostatus. Naive and monotypic exposures were combined because they were statistically indistinguishable (P > .05). Curves were estimated from the best fit model (Table 3) and plotted with standard error of the mean.
DISCUSSION
Disease in Iquitos was significantly reduced among individuals with postsecondary DENV-3 and DENV-4 infections. Relative to average disease rates during primary and secondary infections, the incidence among postsecondary infections was reduced by 93% for DENV-3 and 64% for DENV-4, even though infection rates were not reduced among people with prior exposures to DENV. To our knowledge, this is the first population-based evidence quantifying a cumulative protective effect of heterologous DENV neutralizing antibodies against disease, which has been hypothesized for >40 years [36].
Previous studies investigating cross-protection in sequential infections have provided evidence for a short period of protection against classic dengue fever [37] and long-term protection against severe disease in third and fourth infections [10]. Although we did not attempt to estimate a short-term effect and we did not observe any cases of severe dengue, we were able to show that the effect of heterologous antibody was cumulative, resulting in a reduced incidence of disease during postsecondary infection with DENV-3 or DENV-4.
Cross-protection was not uniform across serotypes. People with DENV-1 neutralizing antibodies were less likely to develop disease, pointing to an epidemiologically important role of the sequence of infecting serotypes in determining clinical outcome [9, 29, 38–40]. This finding, however, is discordant with the observation made in Cuba that infections with DENV-3 were more pathogenic in the presence of preexisting DENV-1 antibody than with DENV-2 antibody [41], which underscores the need to exercise caution in generalizing epidemiological observations between different populations. Previously, cross-reactive DENV-1 antibodies were hypothesized to protect against severe disease during secondary infection with American genotype DENV-2 [29]. Together, these data indicate that, in the context of the study population in Iquitos, DENV-1 antibodies may be broadly cross-protective but that antibodies to American DENV-2 and genotype III of DENV-3 are not, although they do contribute to a cumulative effect of reduced disease.
In contrast to reduced rates of disease, infection rates were higher among people with neutralizing antibody to ≥2 DENV serotypes. Studies in Cuba of 1981 and 1997 dengue epidemics also found higher infection rates in individuals with prior DENV exposure [42, 43], which the authors hypothesized was due to household-level heterogeneities in mosquito populations. In Iquitos, we have observed consistent spatial variation in Aedes aegypti population densities [44] that correlates well with patterns of seroprevalence on broad spatial scales [19, 30] and supports the premise that seroconversion rates are attributable at least in part to variation in individual exposure to mosquito bites. Our observation that a substantial proportion (approximately 70%) of the study population that seroconverted during 2006–2011 had preexisting antibodies to ≥2 serotypes leads us to predict that people experiencing postsecondary infections constitute a subpopulation that contributes disproportionately more to DENV transmission. A key unanswered question is whether the individuals with asymptomatic or mild ambulatory infections were also infectious to mosquitoes.
We observed a nonlinear, age-specific variation in the risk of disease due to DENV-4, with risk peaking around 25 years of age. Possible biological explanations are that individuals aged 15–25 years are more likely to be bitten by mosquitoes [45] and/or that an immunological response related to age is present [46]. We found, however, that age was not a significant factor in DENV-3 infection outcomes. A matched-pairs case-control analysis showed that reduction in both DENV-3 and DENV-4 disease resulting from ≥2 prior DENV exposures is independent of the effect of age. Together, these findings indicate that, within the context of the Iquitos study population, age was not a significant driver of infection outcomes.
The goal of leading DENV vaccine candidates is to induce a protective response against all 4 serotypes, as measured by neutralizing antibody titers and reductions in disease incidence [17, 47, 48]. Should a vaccine provide incomplete protection (ie, to only 2 or 3 serotypes) because of immunological interference [49] or inconsistency in delivering the complete series of inoculations, our analyses indicate that in a population where dengue is endemic, there may be a reduction in disease without a corresponding reduction in human infection and virus transmission to mosquitoes. Given our results that postsecondary infections accounted for approximately 70% of total seroconversions to DENV-3 and DENV-4 (Table 2), it may be necessary to reevaluate critical immunization thresholds by using revised basic reproduction numbers that take into consideration infection of people with preexisting exposure to ≥2 DENV serotypes [50].
A central issue in the interpretation of data from our study was the necessary reliance on in vitro virus neutralization assays to characterize individual DENV exposure history. PRNT is considered the criterion standard for measuring serotype-specific DENV neutralizing antibodies and is thought to distinguish among naive, monotypic, and multitypic antibody profiles. We grouped individuals with neutralizing antibody to ≥2 DENV serotypes into 1 category (multitypic) because of limitations in differentiating between third and fourth infections. In our study, interpretation was facilitated by the sequential dominance of single serotypes in Iquitos. DENV-3 and DENV-4 constituted >99% of all isolates during the first and second halves, respectively. DENV-3 seroconversion was assessed in a previous study and found to be highly reliable [19], and we recently estimated a specificity of approximately 96% for our DENV-4 PRNT, regardless of infection history. PRNT is the method currently used to assess whether a vaccine induces a humoral response, and thus our results are relevant despite uncertainties inherent in the assay and its interpretation (ie, disease was reduced among individuals with elevated antibody titers to ≥2 DENV serotypes and may have been sequence/serotype specific, but infection risk was not).
The distinct sequence of serotype introductions and accompanying well-defined serological characteristics of the Iquitos cohort population constitute a unique opportunity to quantitatively assess the role of preexisting antibodies in DENV infection outcome. In our study, preexisting cross-neutralizing antibodies were strongly associated with a reduced risk of disease in postsecondary infections. The effect was not perfectly protective against disease and did not offer detectable protection against infection. This underscores the need to account for and better understand the impact of DENV postsecondary infections on disease burden and virus transmission dynamics.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the residents of Iquitos, for welcoming us into their homes and permitting these studies to be conducted; the Loreto Regional Health Department, including Hugo Rodríguez Ferruchi, Carmen Montelvan, Cristiam Carey, Carlos Manrique de Lara Estrada, Carlos Coral, Yuri Alegre, and Ernesto Curto, who facilitate our work in Iquitos; Dr Moises Sihuincha at the Hospital Apoyo de Iquitos, for the instrumental role in initiating these studies; our Peruvian field team, composed of Claudio Rocha, Helvio Astete Vega, Gabriela Vásquez La Torre, Isabel Bazán Arista, Rosa Regina Fernandez Montano, Rebeca Carrión, Wieslawa Álava Flores, Wilder Carrasco Huamán, Esther Jennifer Ríos López, Shirley Maribel Guédez Gonzales, Wendy Lorena Quiroz Flores, Diana Bazaán Ferrando, Llerme Armas Pisco, Sadith Jovita Ricopa Manuyama, María Edith Juárez Baldera, Acela Rosario Mafaldo García, Jenny Fílida Gonzáles Sangama, Rosa Tamani Babilonia, Karina Chuquipiondo Vásquez, Rina Gonzales Jaba, Yolanda Torres Arévalo, Naida Rocío del Río Chávez, Luz Angélica Galvez Huayllahua, Leslye Angulo, Patricia del Carmen Barrera Bardales, Lupe Flore, Alex Vásquez, Jorge Vásquez Belchoir, Alan Lozano, John Ramirez, Angelo Mitidieri, Rommel Vásquez Álvez, Jimmy Espinoza Benevides, Jimmy Maykol Castillo Pizango, Rusbel Huiñapi Tamani, Juan Luiz Sifuentes Rios, Néstor José Nonato Lancha, Federico Reátegui Viena, Victor Eléspuru Hidalgo, Edson Pilco Mermao, Abner Enrique Varzallo Lachi, Fernando Chota Ruíz, Ángel Puertas Lozano, Guillermo Iñapi Huamán, Fernando Espinoza Benevides, and Manuel Ruiz Rioja, for their enthusiastic and dedicated assistance in our efforts to study the movements of residents of Iquitos and the epidemiology of dengue; Dr Greg Martin, Dr John Sanders, and Roxana Lescano of the US Naval Medical Research Center in Lima, Peru, for their instrumental role in facilitating these studies; Carolina Guevara, Zonia Ríos, and Angélica Espinoza, for providing laboratory support in Lima; Leslie Sandberg and Yui Yin Chu, for administrative support; and Kelly Liebman and Kanya Long, for providing valuable feedback over the course of the study.
Disclaimers. The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the US government.
Authors Eric S. Halsey and Tadeusz J. Kochel are military servicemembers and Claudio Rocha and Stalin Vilcarromero are employees of the U.S. Government. This work was prepared as part of their official duties. Title 17 U.S.C. § 105 provides that ‘Copyright protection under this title is not available for any work of the United States Government’. Title 17 U.S.C. § 101 defines a U.S. Government work as a work prepared by a military service members or employees of the U.S. Government as part of those person's official duties.
Financial support. This work was supported by the National Institutes of Health (grant R01 AI06934) and the US Military Infectious Disease Research Program (grants S0147_07_LI, S0216_09_LI, and S0263_10_LI); the Research and Policy in Infectious Disease Dynamics program of the Science and Technology Directorate, Department of Homeland Security (to T. W. S.); and the Fogarty International Center, National Institutes of Health (to T. W. S.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Dengue and dengue hemorrhagic fever. Geneva: World Health Organization; 2012. Fact sheet 117. [Google Scholar]
- 2.Brady OJ, Gething PW, Bhatt S, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis. 2012;6:e1760. doi: 10.1371/journal.pntd.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simmons CP, Farrar JJ, Chau NV, Wills B. Dengue. N Engl J Med. 2012;366:1423–32. doi: 10.1056/NEJMra1110265. [DOI] [PubMed] [Google Scholar]
- 4.Rothman AL. Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nature Rev Immunol. 2011;11:532–43. doi: 10.1038/nri3014. [DOI] [PubMed] [Google Scholar]
- 5.Whitehorn J, Simmons CP. The pathogenesis of dengue. Vaccine. 2011;29:7221–8. doi: 10.1016/j.vaccine.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 6.Midgley CM, Flanagan A, Tran HB, et al. Structural analysis of a dengue cross-reactive antibody complexed with envelope domain III reveals the molecular basis of cross-reactivity. J Immunol. 2012;188:4971–9. doi: 10.4049/jimmunol.1200227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dejnirattisai W, Jumnainsong A, Onsirisakul N, et al. Cross-reacting antibodies enhance dengue virus infection in humans. Science. 2010;328:745–8. doi: 10.1126/science.1185181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halstead SB, O'Rourke EJ. Dengue viruses and mononuclear phagocytes. I. Infection enhancement by non-neutralizing antibody. J Exp Med. 1977;146:201–17. doi: 10.1084/jem.146.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181:2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons RV, Kalanarooj S, Jarman RG, et al. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am J Trop Med Hyg. 2007;77:910–3. [PubMed] [Google Scholar]
- 11.Adams B, Holmes EC, Zhang C, et al. Cross-protective immunity can account for the alternating epidemic pattern of dengue virus serotypes circulating in Bangkok. Proc Natl Acad Sci U S A. 2006;103:14234–9. doi: 10.1073/pnas.0602768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cummings DA, Schwartz IB, Billings L, Shaw LB, Burke DS. Dynamic effects of antibody-dependent enhancement on the fitness of viruses. Proc Natl Acad Sci U S A. 2005;102:15259–64. doi: 10.1073/pnas.0507320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferguson N, Anderson R, Gupta S. The effect of antibody-dependent enhancement on the transmission dynamics and persistence of multiple-strain pathogens. Proc Natl Acad Sci U S A. 1999;96:790–4. doi: 10.1073/pnas.96.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Recker M, Blyuss KB, Simmons CP, et al. Immunological serotype interactions and their effect on the epidemiological pattern of dengue. Proc Biol Sci. 2009;276:2541–8. doi: 10.1098/rspb.2009.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wearing HJ, Rohani P. Ecological and immunological determinants of dengue epidemics. Proc Natl Acad Sci U S A. 2006;103:11802–7. doi: 10.1073/pnas.0602960103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas SJ, Endy TP. Critical issues in dengue vaccine development. Curr Op Inf Dis. 2011;24:442–50. doi: 10.1097/QCO.0b013e32834a1b0b. [DOI] [PubMed] [Google Scholar]
- 17.Durbin AP, Whitehead SS. Dengue vaccine candidates in development. Curr Top Microbiol Immunol. 2010;338:129–43. doi: 10.1007/978-3-642-02215-9_10. [DOI] [PubMed] [Google Scholar]
- 18.Sabchareon A, Wallace D, Sirivichayakul C, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380:1559–1567. doi: 10.1016/S0140-6736(12)61428-7. [DOI] [PubMed] [Google Scholar]
- 19.Morrison AC, Minnick SL, Rocha C, et al. Epidemiology of dengue virus in Iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl Trop Dis. 2010;4:e670. doi: 10.1371/journal.pntd.0000670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips I, Need J, Escamilla J, et al. First documented outbreak of dengue in the Peruvian Amazon region. Bull Pan Am Health Organ. 1992;26:201–7. [PubMed] [Google Scholar]
- 21.Hayes CG, Phillips IA, Callahan JD, et al. The epidemiology of dengue virus infection among urban, jungle, and rural populations in the Amazon region of Peru. Am J Trop Med Hyg. 1996;55:459–63. doi: 10.4269/ajtmh.1996.55.459. [DOI] [PubMed] [Google Scholar]
- 22.Watts DM, Porter KR, Putvatana P, et al. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet. 1999;354:1431–4. doi: 10.1016/S0140-6736(99)04015-5. [DOI] [PubMed] [Google Scholar]
- 23.Forshey BM, Morrison AC, Cruz C, et al. Dengue virus serotype 4, northeastern Peru, 2008. Emerg Infect Dis. 2009;15:1815–8. doi: 10.3201/eid1511.090663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.PAHO. Dengue: statistics and maps. http://new.paho.org/hq/index.php?option=com_content&task=blogcategory&id=1221&Itemid=2481&lang=en. Accessed 12 February 2013.
- 25.Rocha C, Morrison AC, Forshey BM, et al. Comparison of two active surveillance programs for the detection of clinical dengue cases in Iquitos, Peru. Am J Trop Med Hyg. 2009;80:656–60. [PubMed] [Google Scholar]
- 26.Comach G, Blair PJ, Sierra G, et al. Dengue virus infections in a cohort of schoolchildren from Maracay, Venezuela: a 2-year prospective study. Vector Borne Zoonotic Dis. 2009;9:87–92. doi: 10.1089/vbz.2007.0213. [DOI] [PubMed] [Google Scholar]
- 27.Forshey BM, Guevara C, Laguna-Torres VA, et al. Arboviral etiologies of acute febrile illnesses in Western South America, 2000-2007. PLoS Negl Trop Dis. 2010;4:e787. doi: 10.1371/journal.pntd.0000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanciotti RS, Calisher CH, Gubler DJ, Chang GJ, Vorndam AV. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J Clin Microbiol. 1992;30:545–51. doi: 10.1128/jcm.30.3.545-551.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kochel TJ, Watts DM, Halstead SB, et al. Effect of dengue-1 antibodies on American dengue-2 viral infection and dengue haemorrhagic fever. Lancet. 2002;360:310. doi: 10.1016/S0140-6736(02)09522-3. [DOI] [PubMed] [Google Scholar]
- 30.Liebman KA, Stoddard ST, Morrison AC, et al. Spatial dimensions of dengue virus transmission across interepidemic and epidemic periods in Iquitos, Peru (1999–2003) PLoS Negl Trop Dis. 2012;6:e1472. doi: 10.1371/journal.pntd.0001472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuno G, Gubler DJ, Oliver A. Use of “original antigenic sin” theory to determine the serotypes of previous dengue infections. Trans R Soc Trop Med Hyg. 1993;87:103–5. doi: 10.1016/0035-9203(93)90444-u. [DOI] [PubMed] [Google Scholar]
- 32.van Panhuis WG, Gibbons RV, Endy TP, et al. Inferring the serotype associated with dengue virus infections on the basis of pre- and postinfection neutralizing antibody titers. J Infect Dis. 2010;202:1002–10. doi: 10.1086/656141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roehrig JT, Hombach J, Barrett AD. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol. 2008;21:123–32. doi: 10.1089/vim.2008.0007. [DOI] [PubMed] [Google Scholar]
- 34.Thomas SJ, Nisalak A, Anderson KB, et al. Dengue plaque reduction neutralization test (PRNT) in primary and secondary dengue virus infections: How alterations in assay conditions impact performance. Am J Trop Med Hyg. 2009;81:825–33. doi: 10.4269/ajtmh.2009.08-0625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org/ . Accessed 22 December 2011. [Google Scholar]
- 36.Halstead SB. Observations related to pathogensis of dengue hemorrhagic fever. VI. Hypotheses and discussion. Yale J Biol Med. 1970;42:350–62. [PMC free article] [PubMed] [Google Scholar]
- 37.Sabin AB. Research on Dengue during World War II. Am J Trop Med Hyg. 1952;1:30–50. doi: 10.4269/ajtmh.1952.1.30. [DOI] [PubMed] [Google Scholar]
- 38.Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annual Rev Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 39.Halstead SB. Dengue in the Americas and Southeast Asia: do they differ? Rev Panam Salud Publica. 2006;20:407–15. doi: 10.1590/s1020-49892006001100007. [DOI] [PubMed] [Google Scholar]
- 40.OhAinle M, Balmaseda A, Macalalad AR, et al. Dynamics of dengue disease severity determined by the interplay between viral genetics and serotype-specific immunity. Sci Transl Med. 2011;3:114ra128. doi: 10.1126/scitranslmed.3003084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guzman MG, Alvarez A, Vazquez S, Alvarez M. Epidemiological studies on dengue virus type 3 in Playa municipality, Havana, Cuba, 2001–2002. Int J Infect Dis. 2012;16:e198–203. doi: 10.1016/j.ijid.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 42.Guzman MG, Kouri GP, Bravo J, Soler M, Vazquez S, Morier L. Dengue hemorrhagic fever in Cuba, 1981: A retrospective seroepidemiologic study. Am J Trop Med Hyg. 1990;42:179–184. doi: 10.4269/ajtmh.1990.42.179. [DOI] [PubMed] [Google Scholar]
- 43.Guzman MG, Kouri GP, Valdes L, et al. Epidemiologic studies on dengue in Santiago de Cuba, 1997. Am J Epidemiol. 2000;152:793–9. doi: 10.1093/aje/152.9.793. [DOI] [PubMed] [Google Scholar]
- 44.Morrison AC, Gray K, Getis A, et al. Temporal and geographic patterns of Aedes aegypti (Diptera: Culicidae) production in Iquitos, Peru. J Med Entomol. 2004;41:1123–42. doi: 10.1603/0022-2585-41.6.1123. [DOI] [PubMed] [Google Scholar]
- 45.De Benedictis J, Chow-Shaffer E, Costero A, Clark GG, Edman JD, Scott TW. Identification of the people from whom engorged Aedes aegypti took blood meals in Florida, Puerto Rico, using polymerase chain reaction-based DNA profiling. Am J Trop Med Hyg. 2003;68:437–46. [PubMed] [Google Scholar]
- 46.Egger JR, Coleman PG. Age and clinical dengue illness. Emerg Infect Dis. 2007;13:924–5. doi: 10.3201/eid1306.070008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Edelman R. Dengue vaccines approach the finish line. Clin Infect Dis. 2007;45(Suppl 1):S56–60. doi: 10.1086/518148. [DOI] [PubMed] [Google Scholar]
- 48.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe. 2008;4:229–38. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson KB, Gibbons RV, Edelman R, et al. Interference and facilitation between dengue serotypes in a tetravalent live dengue virus vaccine candidate. J Infect Dis. 2011;204:442–50. doi: 10.1093/infdis/jir279. [DOI] [PubMed] [Google Scholar]
- 50.Johansson MA, Hombach J, Cummings DAT. Models of the impact of dengue vaccines: A review of current research and potential approaches. Vaccine. 2011;29:5860–8. doi: 10.1016/j.vaccine.2011.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


