Abstract
Objectives:
This study aimed to describe the demographic and virological characteristics of chronic hepatitis B virus (HBV) infection in a sample of Omani patients, and indirectly assess the efficacy of hepatitis B vaccination programmes and catch-up strategies.
Methods:
A retrospective study was undertaken of all patients with chronic HBV infections evaluated and followed-up at the Hepatology Clinic of the Armed Forces Hospital (AFH), Muscat, Oman, between January 2009 and April 2011.
Results:
A total of 154 patients met the inclusion criteria. The mean age of infected patients was 33 years with 72.7% being over 27 years. Females constituted 47.7% of the patients. Half of the cohort was referred either from the AFH’s Obstetric Clinic (29.2%) or its Blood Bank (22.1%). A family history of chronic HBV infection was present in 70% of patients. A total of 95% had positive hepatitis B surface antigens, while only 5% had isolated total hepatitis B core antibodies. Most patients (96%) were hepatitis B e-antigen-negative. The majority (77.9%) had low HBV dioribonucleic acid levels of <2,000 IU/ml. Radiological features of liver cirrhosis were observed in 5%. Patients requiring treatment were in the minority (9%).
Conclusion:
Almost 50% of the infected patients were female, the majority being of childbearing age. Medical authorities in Oman should consider enforcing a screening policy for all pregnant women using complete hepatitis B serological testing.
Keywords: Hepatitis B, Chronic; Hepatitis B Vaccines; Hepatitis B, prevention and control; Blood Donors; Oman
Advances in Knowledge
- Hepatitis B vaccination has led to a lower prevalence of hepatitis B infection in the younger Omani population as compared to older age groups. The postulated vaccination failure rate determined by this study (4%) is in keeping with internationally-documented figures.
- Almost 50% of the infected patients in this study were female, and the majority was of childbearing age. This could lead to a higher number of infected children in the future if these potential mothers are not identified by screening and then managed appropriately.
- Most patients had chronic inactive hepatitis B virus infections while a minority had active infection requiring therapy.
Application to Patient Care
- This study recommends that prevention of hepatitis B virus (HBV) infection be enforced by obligatory antenatal screening of all pregnant women, regardless of a history of previous testing or vaccinations, using a complete hepatitis B serology (including hepatitis B core antibodies) as the standard of care.
- As 70% of the patients had a positive family history of HBV infection, and bearing in mind that HBV can be transmitted horizontally, unvaccinated or non-immune family members should also be vaccinated.
- Patients should be educated that the traditional medical practice of cautery may increase the rate of virus transmission.
Despite the introduction of effective vaccinations in 1981, hepatitis B virus (HBV) infection is still a major health problem with approximately 350 to 400 million people infected worldwide.1 A total of 75% of those infected live in Asia.2,3 Chronic HBV infection is responsible for 60–80% of the cases of liver cancer around the world.4 In addition, 25–40% of infected patients are expected to die from HBV-related complications such as decompensated cirrhosis and hepatocellular carcinoma (HCC). This means that approximately one million people around the globe die each year from HBV infections and related complications. This high rate of mortality makes chronic HBV infection the ninth leading cause of death worldwide.5
The estimated prevalence of chronic HBV infection in Oman is considered to be intermediate at 2–7%;6 however, there are no true prevalence data to substantiate this figure. Only a few studies have been published concerning the prevalence of HBV infection in Omani patients—those with sickle cell disease and renal disorders requiring dialysis or renal transplant—and these studies had small sample sizes.7,8 The prevalence of hepatitis B among pregnant Omani women in 2006 was found to be 7.1%, with 0.5% being hepatitis B e-antigen (e-Ag)-positive.9
In Oman, hepatitis B vaccinations were carried out for all children born in the period from 1984 to 1990, with a procedure of three serial doses at 0, 1 and 6 months. The goal of this programme was to ensure that by the year 2005 everyone under 21 years of age would have been vaccinated against HBV.10 In addition to the above measures, blood bank screening for HBV, using hepatitis B surface antigens (HBsAg) as the only marker, was started in 1990. In 2009, the screening of all blood units using HBsAg as well as hepatitis B core antibodies (anti-HBc) was implemented. To date, however, routine screening of pregnant women is not yet the standard of care in Ministry of Health (MoH) institutions.10
The primary aim of this study was to record the descriptive, demographic and virological characteristics of chronic HBV mono-infection among Omani patients. The secondary aim was an indirect assessment of the efficacy of the hepatitis B vaccination programmes and the catch-up strategies in reducing the prevalence of chronic HBV within the age group targeted for vaccination.
Methods
This was a retrospective study based on a review of the chart and computer data of all patients with chronic HBV infection, who attended and had regular follow-up at the Hepatology Clinic of the Armed Forces Hospital (AFH), Oman, between January 2009 and April 2011. The AFH is one of three tertiary hospitals located in the area of the capital city, Muscat. Almost one third of the Omani population is entitled to receive medical services from the AFH. Entitled patients are referred from all over Oman, and this study’s sample is therefore representative of the Omani population. Any demographic data missing from the charts and computer data review were obtained by telephone. Ethical approval for the study was obtained from the AFH administration.
The inclusion criteria for the study were all Omani patients in the above-specified time period who were over 13 years old and had chronic HBV infection. Non-Omani patients and patients with missing data were excluded, as well as patients co-infected with hepatitis B and other viruses, such as hepatitis C or human immunodeficiency virus (HIV). These latter were excluded because co-infection with HIV and hepatitis C is known to change the natural history of hepatitis B. Patients co-infected with HIV tend to have a higher rate of chronic hepatitis B, a high rate of replication and a low rate of HBsAg and e-Ag seroconversion.
A total of 154 patients met the inclusion criteria. The following data were collected: patient demographic data [Table 1], HBV serology using the ARCHITECT i2000SR (Abbott Diagnostics, Lake Forest, Illinois, USA) and molecular markers using the COBAS® TaqMan® Analyzer (Roche Diagnostics International Ltd., Rotkreuz, Switzerland) for amplification and detection. It has a lower limit of detection of 20 IU/mL and a linear dynamic range, with an upper limit of 1.7 × 108 IU/mL [Table 2]. The markers of liver injury (liver enzymes and function) were detected using a COBAS® 6000 (C501 Module) Clinical Chemistry Analyzer (Roche Diagnostics International Ltd., Rotkreuz, Switzerland) and with liver radiological investigations. Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS) Version 16 (IBM Corp., Chicago, Illinois, USA). Data are presented as a mean or, when indicated, as an absolute number and a percentage.
Table 1:
Demographic data of the studied patients
| Demographic Characteristic | N = 154 n (%) |
|---|---|
| Age (mean) | 33 years |
| Gender | |
| Female | 73 (47.4) |
| Male | 81 (52.6) |
| Referred from | |
| Obstetric clinics | 45 (29.2) |
| Blood bank | 34 (22.1) |
| History of | |
| Family history of hepatitis B | 108 (70) |
| Traditional cautery (wasm) | 100 (65) |
| Body piercing(s) | 62 (40) |
| Blood transfusion(s) | 7 (4.5) |
| Surgery(ies) | 28 (18.2) |
Wasm = traditional medicine technique where the tip of a small hot rod is applied to certain parts of the body, particularly the abdomen and the arms, as a method of treating jaundice.
Table 2:
Viral, serological and molecular markers of hepatitis B infection
| Marker | N = 154 n (%) |
|---|---|
| HBsAg and anti-HBc | 146 (94.8) |
| Isolated anti-HBc (OBI) | 8 (5.2) |
| e-Ag | 6 (3.9) |
| anti-e-Ag | 145 (94.2) |
| HBV DNA levels | |
| <2000 IU/ml | 120 (77.9) |
| 2000–20,000 IU/ml | 23 (14.9) |
| >20,000 IU/ml | 11 (7.1) |
HBsAg = hepatitis B surface antigen; anti-HBc = hepatitis B core antibodies; OBI = occult hepatitis B infection; e-Ag = hepatitis B e-antigen; anti-e-Ag = hepatitis B e-antigen antibody; HBV DNA = hepatitis B virus dioribonucleic acid.
Results
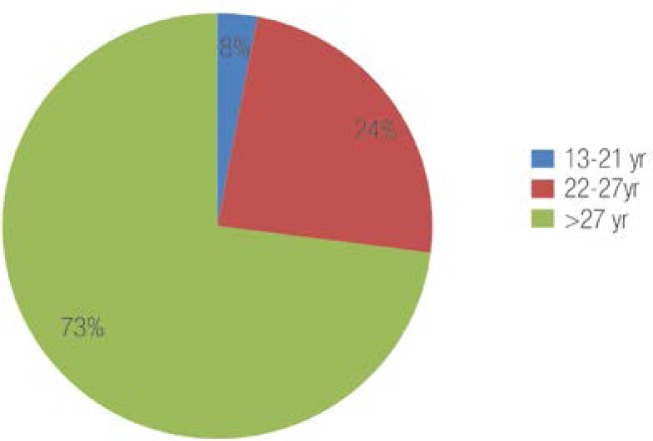
A total of 170 patients were initially included in the study, with 16 patients subsequently excluded for the following reasons: one died, two were co-infected with hepatitis C and 13 patients had missing data. There were therefore 154 patients included in the final analysis. The mean age of the patients was 33 years, ranging from 13–78 years old. A total of 5 patients (3.2%) were 13–21 years old, 37 patients (24.1%) were 22–27 years old, and 112 patients (72.7%) were over 27 years old [Figure 1]. Almost half of the patients (47.4%) were female. Half of the cohort was referred from obstetric clinics and blood banks, reflecting the efficiency of screening certain high-risk groups in identifying HBV-infected patients. A family history of chronic hepatitis B was present in 70% of the patients, with most having more than one family member infected with HBV. A total of 65% had undergone traditional therapy in the form of cautery (wasm in Arabic), which is used by Omani healers to treat patients with jaundice. This therapy entails applying the tip of a small hot rod to certain parts of the body, particularly the abdomen and the arms. A total of 40% of the females had ear and/or nose piercings that had been performed at home or by local dealers using non-sterile needles. A history of blood transfusions and surgeries was present in 15% and 6% of the patients, respectively. A total of 95% had positive HBsAg while only 5% had isolated total anti-HBc. All of the isolated anti-HBc patients (negative HBsAg and hepatitis B surface antibodies [anti-HBs]) had a low to undetectable viral load. A total of 5 patients had a repeatedly undetectable viral load (i.e. <20 IU/ml) and did not exhibit any rise in HBsAg after one dose of the hepatitis B vaccination. One patient had a viral load of 80 IU/ml and two patients had viral loads from 30–75 IU/ml. The age of these patients ranged from 30–60 years old.
Figure 1:
The age distribution of the hepatitis B-infected Omani patients.
Most of our patients (96%) were e-Ag-negative. The hepatitis B dioribonucleic acid (HBV DNA) level was >20,000 IU/ml in 7%, between 2,000 IU/ml and 20,000 IU/ml in 15%, and the rest had low DNA levels of <2,000 IU/ml. Elevated liver enzymes were observed in all DNA level categories and radiological features of liver cirrhosis were seen in 5% of the patients. A minority of our patients (9%) required treatment based on international criteria and guidelines.
Discussion
The estimated prevalence of HBV infection in Oman is between 2–7%.11,12 The most common source of HBV transmission in intermediate- and high-prevalence areas is vertical transmutation.13–16 The majority of the patients in this study were HB e-Ag-negative and had a family history of HBV-infected members. This illustrates that the majority of the infections were acquired during the early years of life through vertical, peri- or postnatal transmission.
The introduction of HBV vaccinations in Taiwan in 1984 reduced the rate of HBV infection among children from 10% in 1984 to only 1.3% in 1994.17 The reduction in HBV infection was also associated with a reduction in the incidence of HCC.17–19
The introduction of HBV vaccinations in Oman in August 1990, using a procedure of three serial doses (at birth, one month and 6 months) as well as a catch-up strategy of school campaigns from 2001 to 2005, has undoubtedly reduced the number of new cases of HBV infection in the country.10,20 This study revealed that the prevalence of HBV infection among those born after 1991 is low (around 4%), which is in keeping with the 5–10% expected failure rate of HBV vaccinations.21,22 However, the rate of infection among those born before the introduction of the vaccination, but within the period of the catch-up campaigns, is high. This can be partly explained by the fact that many of the people targeted by the catch-up campaigns received the vaccination without being tested to determine if they were infected. The other major factor contributing to the high rate of infection among this group is the length of time the catch-up campaigns took to cover the entire country, thus increasing the risk of unvaccinated children contracting the infection from infected family members.
The MoH in Oman does not currently support a policy of screening pregnant women for viral hepatitis.10 However, a substantial percentage of our patients (29.2%) were referred from obstetric clinics whereas 22.1% were referred from blood banks. Jonas et al. demonstrated that if screening were only applied to high-risk pregnant women, 47% of infected women would not have been identified.23 Cowan et al. confirmed this and showed that screening pregnant women for HBV in a low-prevalence area based on identifiable risk factors may miss up to 50% of infected pregnant women.24 The USA Centers for Disease Control and Prevention recommend that the screening of pregnant women for hepatitis B be the standard of care regardless of previous testing or vaccinations.25 This screening will identify those patients who might need treatment. The benefit of screening is not limited to pregnant women, but also helps to identify infants who require prophylaxis, and other individuals who are in contact with infected people and who would also benefit from testing, counselling, vaccination and, if indicated, therapy. Therefore, we believe that the screening of pregnant women in Oman is a necessity.
The prevalence of occult HBV infection (OBI)—which is defined as the presence of HBV DNA in the liver of individuals testing HBsAg-negative with currently available assays—varies from one country to another. It has been reported to be as low as 0.56% among blood donors in the UK, and as high as 76% among blood donors in Ghana.26 Kaminski et al. reported a prevalence of isolated anti-HBc (negative HBsAg and negative anti-HBs) to be 2.8% among 200 random HBsAg-negative Omani blood donor samples.27
The current study revealed that 5% of patients had OBI [Table 2]. The presence of isolated anti-HBc in a low-endemic area can be due to false-positive test results.28 However, an isolated anti-HBc in intermediate to high prevalence areas can be due to long-standing infections with a low level of replication.29 Several possibilities have been suggested to explain the mechanisms of OBI. These include, 1) a surface gene ‘escape’ mutation leading to decreased reactivity of the HBsAg detection assay;30,31 2) a mutation within the polymerase domain that results in a lower production of HBV DNA and HBsAg expression;32 3) the formation of an HBV-containing immune complex;33 4) altered host immune response,34 and 5) co-infection with other viruses such as hepatitis C and the hepatitis delta virus that may decrease production of HBV DNA and increase HBsAg clearance.35–39
Due to the high reported prevalence of OBI in intermediate- to high-prevalence areas, the screening of high-risk groups should not be restricted to HBsAg alone but include total anti-HBc as well. In 2009, the Central Blood Bank of Oman introduced screening for anti-HBc as part of the routine screening of blood units for hepatitis B. This concept of combining HBsAg with anti-HBc adds another protective measure against the accidental transfusion of infected blood units.
The majority of the patients in this study were positive for HBsAg and anti-e-Ag with a low viral load, indicating a chronic inactive infection most likely acquired during the early years of life. The minority had an active HBV infection requiring treatment (9%). More than 50% of the patients had tried the traditional therapy of wasm before seeking medical attention. The belief by patients and community in the curative action of this traditional therapy has many serious implications. Most of the patients who had tried this form of traditional therapy believed they were cured and had not sought further medical attention until the formal screening. Individuals who undergo wasm and do not seek further medical advice may present later with HBV-related complications such as cirrhosis or HCC, and will additionally remain a continuous source of HBV infection.
Conclusion
Almost 50% of this study cohort was female and the most likely mode of transmission was vertical. It is therefore suggested that medical authorities in Oman enforce a policy of screening all pregnant women, regardless of previous testing or vaccinations, using a complete hepatitis B serology (including anti-HBc) as the standard of care. Transmission through horizontal infection is also illustrated in this study, and therefore newborn and non-immune family members in contact with an infected person must also be vaccinated.
References
- 1.Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395–403. doi: 10.1016/s1473-3099(02)00315-8. [DOI] [PubMed] [Google Scholar]
- 2.Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337:1733–45. doi: 10.1056/NEJM199712113372406. [DOI] [PubMed] [Google Scholar]
- 4.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45–55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 5.Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529–38. doi: 10.1016/j.jhep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 6.Custer B, Sullivan SD, Hazlet K, Iloeje U, Veenstra DL, Kowdley KV. Global epidemiology of hepatitis B virus. J Clin Gastroenterol. 2004;38:S158–68. doi: 10.1097/00004836-200411003-00008. [DOI] [PubMed] [Google Scholar]
- 7.Al-Dhahry SS, Aghanashinikar PN, Al-Marhuby HA, Buhl MR, Daar AS, Al-Hasani MK. Hepatitis B, delta and human immunodeficiency virus infections among Omani patients with renal diseases: A seroprevalence study. Ann Saudi Med. 1994;14:312–5. doi: 10.5144/0256-4947.1994.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soliman AT, Bassiouny MR, Elbanna NA. Study of hepatic functions and prevalence of hepatitis-B surface antigenaemia in Omani children with sickle cell disease. J Trop Pediatr. 1995;41:174–6. doi: 10.1093/tropej/41.3.174. [DOI] [PubMed] [Google Scholar]
- 9.Al Awaidy S, Abu-Elyazeed R, Al Hosani H, Al Mulla A, Al Busaiedy S, Al Amiry A, et al. Sero-epidemiology of hepatitis B infection in pregnant women in Oman, Qatar and the United Arab Emirates. J Infect. 2006;52:202–6. doi: 10.1016/j.jinf.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Ministry of Health, Oman. Oman Viral Hepatitis Survey . Community Health & Disease Surveillance Newsletter. Muscat: Ministry of Health; 2005. From: www.moh.gov.om/en/reports/publications/Newsletter14-2.pdf Accessed: Jul 2012. [Google Scholar]
- 11.Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol. 2005;34:1329–39. doi: 10.1093/ije/dyi206. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention, USA . Travelers’ health, Yellow Book 2008. Atlanta: CDC; 2008. [Google Scholar]
- 13.Ghendon Y. Perinatal transmission of hepatitis B virus in high-incidence countries. J Virol Methods. 1987;17:69–79. doi: 10.1016/0166-0934(87)90070-x. [DOI] [PubMed] [Google Scholar]
- 14.Schweitzer IL, Dunn AE, Peters RL, Spears RL. Viral hepatitis B in neonates and infants. Am J Med. 1973;55:762–71. doi: 10.1016/0002-9343(73)90257-x. [DOI] [PubMed] [Google Scholar]
- 15.Xu DZ, Yan YP, Choi BC, Xu JQ, Men K, Zhang JX, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67:20–6. doi: 10.1002/jmv.2187. [DOI] [PubMed] [Google Scholar]
- 16.Lin HH, Lee TY, Chen DS, Sung JL, Ohto H, Kawana T, et al. Transplacental leakage of HBeAg-positive maternal blood as the most likely route in causing intrauterine infection with hepatitis B virus. J Pediatr. 1987;111:877–81. doi: 10.1016/s0022-3476(87)80210-x. [DOI] [PubMed] [Google Scholar]
- 17.Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J of Med. 1997;336:1855–9. doi: 10.1056/NEJM199706263362602. [DOI] [PubMed] [Google Scholar]
- 18.Chang MH, Chen TH, Hsu HM, Wu TC, Kong MS, Liang DC, et al. Prevention of hepatocellular carcinoma by universal vaccination against hepatitis B virus: the effect and problems. Clin Cancer Res. 2005;11:7953–7. doi: 10.1158/1078-0432.CCR-05-1095. [DOI] [PubMed] [Google Scholar]
- 19.Ni YH, Chen DS. Hepatitis B vaccination in children: the Taiwan experience. Pathol Biol. 2010;58:296–300. doi: 10.1016/j.patbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Bhat SK, Sachdeva VN, Saleem HI. Profile of viral hepatitis patients in Dakhliya, Oman. Saudi Med J. 2005;26:819–23. [PubMed] [Google Scholar]
- 21.Dienstag JL, Werner BG, Polk BF, Snydman DR, Craven DE, Platt R, et al. Hepatitis B vaccine in health care personnel: Safety, immunogenicity, and indicators of efficacy. Ann Intern Med. 1984;101:34–40. doi: 10.7326/0003-4819-101-1-34. [DOI] [PubMed] [Google Scholar]
- 22.Craven DE, Awdeh ZL, Kunches LM, Yunis EJ, Dienstag JL, Werner BG, et al. Nonresponsiveness to hepatitis B vaccine in health care workers. Results of revaccination and genetic typings. Ann Intern Med. 1986;105:356–60. doi: 10.7326/0003-4819-105-3-356. [DOI] [PubMed] [Google Scholar]
- 23.Jonas MM, Schiff ER, O’Sullivan MJ, de Medina M, Reddy KR, Jeffers LJ, et al. Failure of the Centers for Disease Control criteria to identify hepatitis B infection in a large municipal obstetrical population. Ann Intern Med. 1987;107:335–7. doi: 10.7326/0003-4819-107-2-335. [DOI] [PubMed] [Google Scholar]
- 24.Cowan SA, Bagdonaite J, Qureshi K. Universal hepatitis B screening of pregnant women in Denmark ascertains substantial additional infections: results from the first five months. Euro Surveill. 2006;11:E060608.3. doi: 10.2807/esw.11.23.02969-en. [DOI] [PubMed] [Google Scholar]
- 25.Mast EE, Margolis HS, Flore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1–31. [PubMed] [Google Scholar]
- 26.Allain JP, Candotti D, Soldan K, Sarkodie F, Phelps B, Giachetti C, et al. The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood. 2003;101:2419–25. doi: 10.1182/blood-2002-04-1084. [DOI] [PubMed] [Google Scholar]
- 27.Kaminski G, Alnaqdy A, Al-Belushi I, Nograles J, Al-Dhahry SH. Evidence of occult hepatitis B virus infection among Omani blood donors: a preliminary study. Med Princ Pract. 2006;15:368–72. doi: 10.1159/000094271. [DOI] [PubMed] [Google Scholar]
- 28.Lok AS, Lai CL, Wu PC. Prevalence of isolated antibody to hepatitis B-core antigen in an area endemic for hepatitis B virus infection: Implications in hepatitis B vaccination programs. Hepatology. 1988;8:766–70. doi: 10.1002/hep.1840080411. [DOI] [PubMed] [Google Scholar]
- 29.Allain JP. Occult hepatitis B virus infection. Transfus Clin Biol. 2004;11:18–25. doi: 10.1016/j.tracli.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 30.El Chaar M, Candotti D, Crowther RA, Allain JP. Impact of hepatitis B virus surface protein mutations on the diagnosis of occult hepatitis B virus infection. Hepatology. 2010;52:1600–10. doi: 10.1002/hep.23886. [DOI] [PubMed] [Google Scholar]
- 31.Gerlich WH, Bremer C, Saniewski M, Schüttler CG, Wend UC, Willems WR, et al. Occult hepatitis B virus infection: Detection and significance. Dig Dis. 2010;28:116–25. doi: 10.1159/000282074. [DOI] [PubMed] [Google Scholar]
- 32.Gutiérrez C, Devesa M, Loureiro CL, León G, Liprandi F, Pujol FH. Molecular and serological evaluation of surface antigen negative hepatitis B virus infection in blood donors from Venezuela. J Med Virol. 2004;73:200–7. doi: 10.1002/jmv.20076. [DOI] [PubMed] [Google Scholar]
- 33.Zhang JM, Xu Y, Wang XY, Yin YK, Wu XH, Weng XH, et al. Coexistence of hepatitis B surface antigen (HBsAg) and heterologous subtype-specific antibodies to HBsAg among patients with chronic hepatitis B virus infection. Clin Infect Dis. 2007;44:1161–9. doi: 10.1086/513200. [DOI] [PubMed] [Google Scholar]
- 34.Rehermann B, Ferrari C, Pasquinelli C, Chisari FV. The hepatitis B virus persists for decades after patients’ recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nat Med. 1996;2:1104–8. doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- 35.Sheen IS, Liaw YF, Lin DY, Chu CM. Role of hepatitis C and delta viruses in the termination of chronic hepatitis B surface antigen carrier state: a multivariate analysis in a longitudinal follow-up study. J Infect Dis. 1994;170:358–61. doi: 10.1093/infdis/170.2.358. [DOI] [PubMed] [Google Scholar]
- 36.Guido M, Thung SN, Fattovich G, Cusinato R, Leandro G, Cecchetto A, et al. Intrahepatic expression of hepatitis B virus antigens: Effect of hepatitis C virus infection. Mod Pathol. 1999;12:599–603. [PubMed] [Google Scholar]
- 37.Sagnelli E, Coppola N, Scolastico C, Filippini P, Santantonio T, Stroffolini T, et al. Virologic and clinical expressions of reciprocal inhibitory effect of hepatitis B, C, and delta viruses in patients with chronic hepatitis. Hepatology. 2000;32:1106–10. doi: 10.1053/jhep.2000.19288. [DOI] [PubMed] [Google Scholar]
- 38.Chu CJ, Lee SD. Hepatitis B virus/hepatitis C virus coinfection: Epidemiology, clinical features, viral interactions and treatment. J Gastroenterol Hepatol. 2008;23:512–20. doi: 10.1111/j.1440-1746.2008.05384.x. [DOI] [PubMed] [Google Scholar]
- 39.Alavian SM, Miri SM, Hollinger FB, Jazayeri SM. Occult Hepatitis B (OHB) in Clinical Settings. Hepat Mon. 2012;12:e6126. doi: 10.5812/hepatmon.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]