Abstract
Gastric linitis plastica is a diffuse type of cancer which is characterised by a thickening and rigidity of the stomach wall. It is notorious for its failure to cause early symptoms, and patients with symptoms generally have a more advanced form of the disease. We report our 18-month-long experience of managing gastric linitis plastica at Barnsley District General Hospital, UK. In our series of 8 patients, only one patient was offered surgery; the rest were offered palliative or supportive treatment. The findings in our series were consistent with the available evidence that curative treatment is not an option for the majority of cases with linitis plastica.
Keywords: Gastric Cancer, Linitis Plastica, Symptoms, Disease Management, Case Reports, Great Britain
Linitis plastica denotes a diffuse type of carcinoma which accounts for 3–19% of gastric adenocarcinomas.1 It is characterised by a rigidity of a major portion, or all of the stomach, with the absence of a filling defect or extensive ulceration. Gastric carcinoma is notorious for its failure to cause early symptoms so that patients do not present themselves for diagnosis until late in the course of the disease. Because of the rich lymphatic supply, the cancer rapidly disseminates beyond the reach of surgical resection. Consequently, the patients with symptoms generally have far-advanced malignancy.2
We report our experience of managing patients who were diagnosed with linitis plastica over a period of 18 months in 2011–2012 at Barnsley District General Hospital, UK. Patients diagnosed with linitis plastica were identified from the cancer database, and their clinicopathological findings were retrieved from medical case notes. All cases were discussed in multidisciplinary meetings at a tertiary referral centre, and their clinical progress and outcomes were noted.
Case Series
In this series, 8 patients diagnosed with linitis plastica are discussed. Their demographic details, symptoms, endoscopic and computed tomography (CT) findings, treatment details, and survival statistics are given in Table 1. The average age upon presentation was 75 years (range 59–87 years). All patients were symptomatic at presentation. One patient (Case 7) was scoped 3 times as the first endoscopy showed diffuse mucosal inflammation, and biopsies showed only chronic inflammation with no evidence of dysplasia. Her CT scan was suspicious for linitis plastica but, in the absence of histological diagnosis, she underwent a repeat endoscopy and had negative biopsies. A diagnostic laparoscopy did not show serosal or peritoneal disease and she remained under close observation. However, 10 months after presentation, she started having dyspepsia despite being on proton-pump inhibitors. A repeat endoscopy and biopsy showed an intramucosal adenocarcinoma. Four patients with metastatic disease and two patients with extensive local disease were not offered surgery. One patient had local lymph node involvement and was not known to have any major comorbidities but, because of poor functional status, he was deemed unfit for surgery. Only one patient was offered surgery in this cohort, but she declined the treatment.
Table 1:
Charateristics of each of the 8 linitis plastica cases
| Age | Symptoms | Comorbidities | Endoscopic findings | CT scan findings | Treatment offered | Treatment received | Outcome | Time since diagnosis |
|---|---|---|---|---|---|---|---|---|
| 70 | Dyspepsia; dysphagia | HTN | Prominent gastric folds; non-distending stomach [Figure 1]. | Thick-walled stomach with extensive local disease. | Palliative chemotherapy | None | Alive | 6 months |
| 83 | Upper abdominal discomfort, Postprandial vomiting, Weight loss | HTN, IHD, PVD, CKD | Thickened area of irregular gastric mucosa at greater curvature. | Diffusely thickened gastric wall affecting the whole stomach [Figure 2]; suspicious lung nodules. | Supportive care | Supportive care | Dead | 3 months |
| 87 | Early satiety; weight loss | None | Circumferential thickening of proximal gastric mucosa. | Local lymph-adenopathy; lung nodules; enlarged mediastinal nodes. | Supportive care | Supportive care | Dead | 3 months |
| 69 | Early satiety; dyspepsia; dysphagia | Dementia, IHD, PVD | Thickened gastric mucosa; polypoidal growth in proximal stomach. | Enlarged local lymph nodes; liver metastases. | Palliative radiotherapy | Palliative radiotherapy | Alive | 4 months |
| 76 | Vomiting; weight loss | DM, IHD | Fungating lesion extending from OG junction to antrum [Figure 3]. | Enlarged local lymph nodes; liver metastases. | Supportive care | Supportive care | Dead | 5 months |
| 59 | Dysphagia | None | Suspicious lesion at the cardia; thick mucosal folds; non-distending stomach. | Thick-walled stomach; perigastric stranding; extensive local involvement. | Palliative chemotherapy | None | Dead | 4 months |
| 80 | Dyspepsia; weight loss | None | Diffuse gastric mucosal inflammation with normal biopsy. Repeat endoscopic biopsy showed intramucosal carcinoma. | Thick-walled stomach. | Surgery | None | Dead | 5 months |
| 78 | Dyspepsia; vomiting | None | Prominent and rigid gastric folds. | Diffuse gastric wall thickening; perigastric stranding; local lymph node involvement [Figure 4]. | Palliative chemotherapy | None | Alive | 6 weeks |
CT = computed tomography; HTN = hypertension; IHD = ischaemic heart disease; PVD = peripheral vascular disease; CKD = chronic kidney disease; DM = diabetes mellitus; OG = oesophagogastric.
Out of 8 patients, 5 patients died within 5 months of follow-up. The maximum survival recorded so far in this cohort of patients has been 6 months.
Discussion
Gastric linitis plastica is a diffuse type of cancer in which the cells invade throughout the stomach, resulting in the thickening and rigidity of the stomach wall. Most of the patients presenting with symptoms have an advanced form of the disease, with the reason being the limited sensory qualities of the stomach. All the patients in these series were symptomatic. Dyspepsia was the commonest feature of presentation (55%), followed by dysphagia (33%), vomiting (33%) and weight loss (33%). The infiltration of malignant cells reduces the volume of the stomach and interferes with peristalsis so that the stomach acts as a funnel between the oesophagus and duodenum. As a result, food is easily regurgitated into the oesophagus. This was the commonest presentation in these series.
Linitis plastica generally arises from the lower third of the mucosa without destroying the architecture of the stomach wall. The mucosa is often spared malignant infiltration, making endoscopic diagnosis extremely difficult. Since macroscopic features do not permit the distinction between benign and malignant lesions, multiple endoscopic biopsies are required. The characteristic stroma reaction of the tissue is especially apparent in the submucosa, although it can also be noted in the muscle layer and subserosa.3 In most typical cases, the cells appear in a signet-ring form. The standard endoscopic biopsy specimens which contain only mucosa may result in a negative yield. One patient in these series had a negative first biopsy. In such patients with endoscopic suspicion, a diathermic snare can be used which will allow the acquisition of larger and deeper tissue, but this procedure has a higher risk of perforation. The diagnostic yield can also be increased by taking multiple forceps biopsies from the same site.
The preoperative work-up involves an assessment of the spread of the disease. A CT scan of the abdomen may reveal thickening of the stomach wall with poor definition of the plane between the stomach and adjacent organs, or the involvement of surrounding nodes. Sometimes an endoscopic ultrasound is useful to establish the diagnosis and in guiding treatment.
The treatment for linitis plastica is a controversial issue. Some authors have proposed using more radical multimodality treatments such as systemic and/or intraperitoneal chemotherapy in addition to radical surgery, whereas others suggest that these patients should be treated with primary chemotherapy even in the absence of unfavourable parameters, as the overall survival rate has been reported to be low in patients undergoing curative surgery.4–6 Aranha et al. reported a slightly improved survival rate, with an average of 11 months, in patients who received palliative chemotherapy or radiotherapy when compared to those patients who did not receive any treatment.7
During their 21-year experience, Moreaux et al. reported a 5-year survival rate of 11% after curative resection, but this was only possible in a small number of cases.8 In the series reported by Schauer et al., only one-third of their cases had complete resection despite undergoing multivisceral resection and metatsatectomy.9 Although a survival advantage was reported in this series, the median survival only increased from 8 to 17 months with complete resection. In contrast, Kodera et al. reported an improved survival from 7.8 months in patients who did not receive any treatment to 30.2 months with complete resection, with no survival advantage in those undergoing palliative resection.10 In our series, the patients with locally advanced disease had poor physiological or functional status and were deemed unfit for major local resection. Our findings are limited by the small number of patients in the series, the majority of whom were elderly and unfit for extensive local resections.
Conclusion
In conclusion, gastric linitis plastica is one of the forms of adenocarcinoma which usually presents at a later stage, where curative treatment is not an option for the majority of cases. The prognosis may be ameliorated with complete resection. Surgery should only be offered where complete resection is anticipated. In the era of modern medicine, we are increasingly being faced with an ageing population where surgery may not be a viable option.
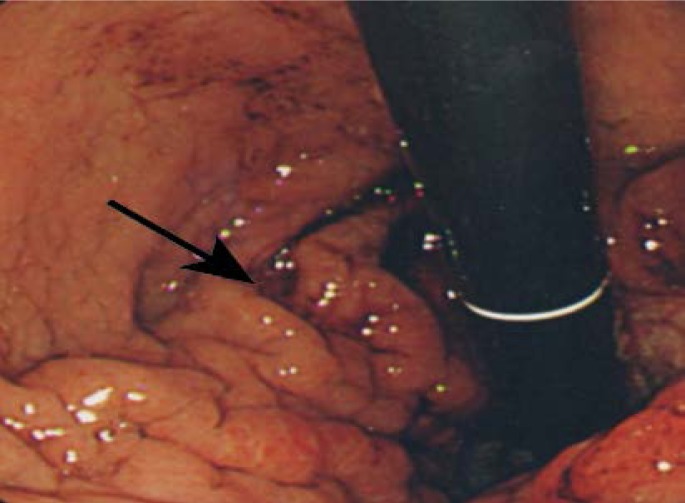
Figure 1:
Endoscopy showing thickened gastric folds.
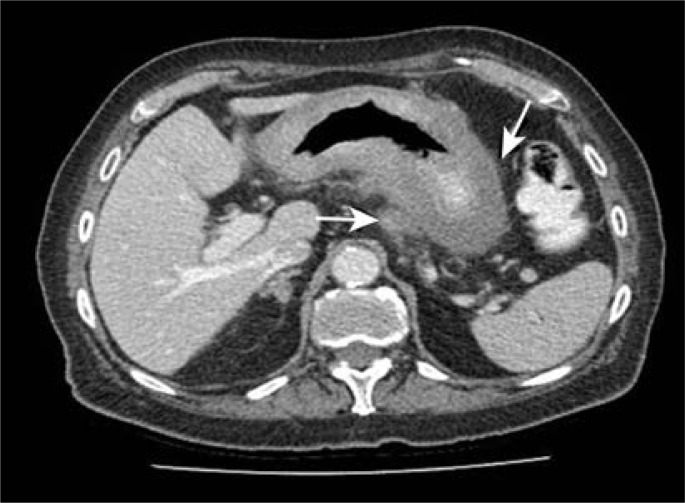
Figure 2:
Computed tomography scan showing the grossly thickened wall (oblique arrow) and the perigastric extension of tumour (horizontal arrow).
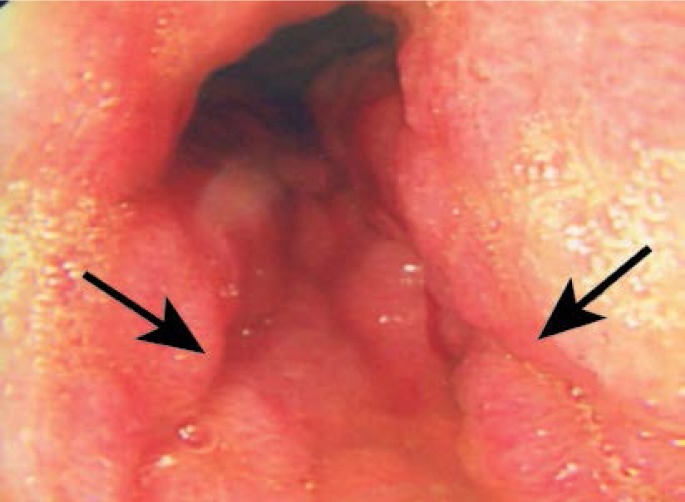
Figure 3:
Endoscopy showing a circumferential tumour with gross stomach contraction.
Figure 4:
Computed tomography scan showing grossly thickened gastric wall (white arrow) and early perigastric involvement (arrowhead).
References
- 1.Sah BK, Zhu Jg, Chen MM, Yan M, Yin HR, Zhen LY. Gastric cancer surgery and its hazards: Postoperative infection is the most common complication. Hepatogastroenterology. 2008;55:2259–63. [PubMed] [Google Scholar]
- 2.Yu J, Yang D, Wei F, Sui Y, Li H, Shao F, et al. The staging system of metastatic lymph node ratio in gastric carcinoma. Hepatogastroenterology. 2008;55:2287–90. [PubMed] [Google Scholar]
- 3.Karila-Cohen P, Petit T, Aparicio T, Teissier J, Merran S. Linitis plastica. J Radiol. 2005;86:37–40. doi: 10.1016/s0221-0363(05)81320-4. [DOI] [PubMed] [Google Scholar]
- 4.Komorowski RA, Caya JG, Geenen JE. The morphological spectrum of large gastric folds: Utility of snare biopsy. Gastrointest Endosc. 1986;32:190–2. doi: 10.1016/s0016-5107(86)71802-6. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmerman GN, et al. Chemotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. New Engl J Med. 2001;345:725–30. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 5.Yonemura Y, de Aretxabala X, Fujimura T, Fushida S, Katayama K, Bandou E, et al. Intraoperative chemo hyperthermic peritoneal perfusion as an adjuvant to gastric cancer: Final results of a randomised controlled study. Hepatogastroenterology. 2001;48:1776–82. [PubMed] [Google Scholar]
- 6.Sasaki T, Koizumi W, Tanabe S, Higuchi K, Nakayama N, Saigenji K. TS-1 as first line therapy for gastric linitis plastica: Historical control study. Anti-Cancer Drugs. 2006;17:581–6. doi: 10.1097/00001813-200606000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Aranha GV, Georgen R. Gastric linitis plastica is not a surgical disease. Surgery. 1989;106:758–62. [PubMed] [Google Scholar]
- 8.Moreaux J, Barratt F, Msika S. Linitis plastica of stomach: Study of 102 cases surgically treated. Results of the surgical treatment. Chirurgie. 1986;112:485–92. [PubMed] [Google Scholar]
- 9.Schauer M, Peiper M, Theisen J, Knoefel W. Prognostic factors in patients with diffuse type gastric cancer (Linitis Plastica) after operative treatment. Eur J Med Res. 2011;16:29–33. doi: 10.1186/2047-783X-16-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodera Y, Seiji I, Yoshinari M, Yoshitaka Y, Kazunari M, Norifumi O, et al. The number of metastatic lymph nodes is a significant risk factor for bone metastasis and poor outcome after surgery for linitis plastic-type gastric carcinoma. World J Surg. 2008;32:2015–20. doi: 10.1007/s00268-008-9672-z. [DOI] [PubMed] [Google Scholar]