Abstract
Colonization surface antigens (CSs) represent key virulence-associated factors of enterotoxigenic Escherichia coli (ETEC) strains. They are required for gut colonization, the first step of the diarrhoeal disease process induced by these bacteria. One of the most prevalent CSs is CS21, or longus, a type IV pili associated with bacterial self-aggregation, protection against environmental stresses, biofilm formation and adherence to epithelial cell lines. The objectives of this study were to assess the role of CS21 in adherence to primary intestinal epithelial cells and to determine if CS21 contributes to the pathogenesis of ETEC infection in vivo. We evaluated adherence of a CS21-expressing wild-type ETEC strain and an isogenic CS21-mutant strain to pig-derived intestinal cell lines. To determine the role of CS21 in pathogenesis we used the above ETEC strains in a neonatal mice challenge infection model to assess mortality. Quantitative adherence assays confirmed that ETEC adheres to primary intestinal epithelial cells lines in a CS21-dependent manner. In addition, the CS21-mediated ETEC adherence to cells was specific as purified LngA protein, the CS21 major subunit, competed for binding with the CS21-expressing ETEC while specific anti-LngA antibodies blocked adhesion to intestinal cells. Neonatal DBA/2 mice died after intra-stomach administration of CS21-expressing strains while lack of CS21 expression drastically reduced the virulence of the wild-type ETEC strain in this animal model. Collectively these results further support the role of CS21 during ETEC infection and add new evidence on its in vivo relevance in pathogenesis.
Introduction
Enterotoxigenic Escherichia coli (ETEC) strains are leading causes of infant diarrhoea in developing countries, traveller’s diarrhoea and re-emergent diarrhoeal pathogens in the United States (Beatty et al., 2006; Black et al., 2010; Guerrant et al., 2002; Gupta et al., 2008; Jain et al., 2008; Steffen et al., 2003; Wennerås & Erling, 2004). ETEC adheres to the microvilli of small intestinal epithelial cells by pili and non-pili colonization surface antigens (CSs), and they deliver enterotoxins that act locally on enterocytes (Gaastra & Svennerholm, 1996; Qadri et al., 2005). Twenty-two human ETEC CSs have been reported to date. The frequency of CSs among ETEC clinical isolates varies widely according to geographical areas, seasons and patients (Qadri et al., 2005; Blackburn et al., 2009). CS21 is one of the most frequent CSs worldwide among ETEC clinical isolates with prevalence ranging between 12 and 36.5 % (Clavijo et al., 2010; Girón et al., 1997; Isidean et al., 2011; Nishimura et al., 2002; Pichel et al., 2002; Qadri et al., 2000).
CS21 is a plasmid-encoded type IV pilus that requires at least 14 genes within a 14 kb cluster (Gomez-Duarte et al., 2007). The CS21 major structural subunit, designated LngA, is a 22 kDa protein that shares homology with other type IV pili major subunits, including the toxin-coregulated pilin of Vibrio cholerae and the bundle-forming pilin of enteropathogenic E. coli (Gómez-Duarte et al., 1999, 2007). Phenotypes associated with CS21 expression include self-aggregation, protection against environmental stress factors, biofilm formation and cell adhesion (Clavijo et al., 2010; Mazariego-Espinosa et al., 2010). A CS21-expressing wild-type ETEC strain adhered to transformed epithelial cell lines 22–32 % better than the CS21-mutant ETEC strain (Mazariego-Espinosa et al., 2010). It is unclear if the lower CS21-mediated ETEC adherence to transformed cell lines is a result of the lack of specific CS21 receptors on undifferentiated epithelial cell lines or marginal CS21 binding properties. One hypothesis to explain low CS21-mediated adherence to transformed epithelial cell lines such as HT-29, T84, Caco-2 and HeLa cells is that expression of CS21-specific surface receptors on undifferentiated cell lines is lower than on differentiated pig-derived primary intestinal cells lines.
Pig-derived primary intestinal cell lines are used to study adherence properties of human and animal ETEC strains. The two types of small intestinal cells available for ETEC adherence studies are the IPEC-1 and IPEC-J2 cell lines. These unique cells derived from 1-day-old piglets, either from the small intestine (IPEC-1) or specifically from the jejunum (IPEC-J2), have provided valuable insights on ETEC interactions with the human intestinal mucosa (Brown et al., 2007; Geens & Niewold, 2010; Johnson et al., 2009; Koh et al., 2008; Schierack et al., 2006). Pig intestinal cells closely resemble human intestinal cells in physiology and may express receptors for ETEC CSs, including CS21. Similar to human intestinal cells, pig intestinal cells become polarized, express glycocalix, and form tight junctions and microvilli (Schierack et al., 2006). Evidence for the expression of ETEC pili receptors on IPEC-J2 cells is demonstrated by the ability of animal and human ETEC pili to adhere to and to invade these cells, and to induce pro-inflammatory cytokine responses in them (Hermes et al., 2011; Koh et al., 2008; Pavlova et al., 2008; Rasschaert et al., 2010; Sargeant et al., 2011). Enteropathogenic E. coli clinical isolates also adhere to IPEC-J2 cells and induce classical attaching and effacing lesions originally observed in human-derived epithelial cell lines (Schierack et al., 2006). Adhesion of CS21+ ETEC to intestinal cell lines may indicate that differentiated intestinal cells express more CS21 receptors than undifferentiated cells.
Nonetheless, studies of ETEC pathogenesis under in vivo conditions have been limited by the lack of an appropriate animal model capable of mimicking the human disease. As an in vivo surrogate for ETEC pathogenesis, a neonate mouse infection model has been successfully applied to demonstrate the pathogenicity of ETEC strains (Duchet-Suchaux, 1988; Duchet-Suchaux et al., 1990). In this model, animal-derived ETEC strains killed newborn mice after oral administration. An adaptation of the ETEC challenge model allowed the testing of human-derived ETEC strains and the evaluation of the protective immunity passively transferred by vaccinated dams after intra-stomach inoculation of virulent ETEC strains (Lásaro et al., 2004; Luiz et al., 2008). In this experimental model, lethality was positively related to the expression levels of enterotoxins and CSs as specifically demonstrated with CFA/I-expressing strains (Lásaro et al., 2004; Luiz et al., 2008).
The aim of the present study was to obtain experimental evidence that the CS21 pilus has a role on ETEC pathogenesis. Our hypothesis is that CS21-expressing ETEC adhere to pig-derived intestinal cell lines, and contribute to lethality in a neonatal model. Experiments comparing the CS21-expressing ETEC strain, an isogenic CS21-mutant derivative and the corresponding complemented CS21-mutant strain clearly showed that CS21 is directly involved in lethality to neonatal mice by unknown mechanisms. In addition, CS21 is involved in binding to primary intestinal cell lines in vitro. Together, these results further support the relevant role of CS21 in ETEC pathogenesis.
Methods
Strains.
The E9034A wild-type ETEC strain (wild-type ETEC), originally isolated from an outbreak of diarrhoea in the Caribbean, expresses O8 : H9 serotype, LT and ST toxins, and CS21 and CS3 CSs. Based on single PCR DNA amplification testing, we found that E9034A was positive for tibA, irp2, etpA and etpB non-classical virulence genes, and it was negative for tia, leoA, eatA and fyuA. The PCR testing method for detection of non-classical virulence factors was as described by Del Canto et al. (2011). The E9034AΔlngA mutant (CS21-mutant) and E9034AΔlngA(pLngA) complemented mutant (complemented CS21-mutant) were cultured on Luria broth (LB) agar plates or in terrific broth (TB) overnight at 37 °C as previously described (Clavijo et al., 2010). These strains were electroporated with pGFPuv plasmid (Clonetech) and the resulting E9034A(pGFPuv), E9034AΔlngA(pGFPuv) and E9034AΔlngA(pLngA,pGFPuv) strains expressed green fluorescence protein and ampicillin resistance.
Cell lines.
Jejunum-derived IPEC-J2 and small intestine-derived IPEC-1 intestinal epithelial cells from 1-day-old piglets were cultured in IPEC media as described previously (Koh et al., 2008). Cells grown to 70–80 % confluence were trypsinized, resuspended in IPEC media and seeded as a 150 µl suspension on air–liquid interface Millicell culture plate inserts (PIHA 012 50; Millipore), previously coated with type VI human placental collagen (Sigma-Aldrich). Then, 500 µl IPEC media was added baso-laterally to the Millicell culture, and cells were incubated for 24 h. After 24 h, the medium was replaced with 500 µl fresh Ultroser G serum substitute USG medium (BioSepra). The cultures were incubated at 37 °C in 5 % CO2 for 10−15 days with USG media exchanges every 2 days to optimize differentiation (Karp et al., 2002).
Adherence assays.
Evaluation of ETEC adherence to epithelial cells by quantitative adherence assays as well as confocal and electron microscopy were performed as described by Mazariego-Espinosa et al. (2010) and Scaletsky et al. (1984). In brief, epithelial cells grown on 24-well plates at 70–80 % confluence were infected with 5 µl (~1.0×107 c.f.u. µl–1) of ETEC bacterial suspension from an overnight TB liquid culture. Infected cells were incubated for 3 h with tissue culture media alone or with media containing 50 % TB. The tissue culture medium was supplemented with 1 % mannose to block non-specific type I pili-mediated binding. Arabinose at 0.02 % was also added to the media to induce lngA expression of the complemented CS21-mutant as described previously (Clavijo et al., 2010). After 3 h infection, cells were washed three times with PBS to remove unbound bacteria. For quantitative analysis, cells were lysed with 250 µl 1 % Triton X-100 in PBS for 10 min, serially diluted and plated onto LB plates for further c.f.u. counts. Adherence assays were performed in duplicate and repeated several times.
Inhibition adherence assays.
Quantitative adherence assays with IPEC-1 and IPEC-J2 intestinal cell lines incubated in IPEC tissue culture media supplemented with 50 % TB were used for all inhibition assays. Cells incubated as described above were supplemented with putative CS21 adherence inhibitors added immediately before infection at increasing concentrations. The inhibitors tested included anti-LngA monoclonal antibody ICA39 (Qadri et al., 2000), with anti-tubulin (Thermo Scientific) used as a negative control, purified LngA protein (GenScript), neuraminidase from V. cholerae (type II) (Sigma) and N-acetyl neuraminic acid (Sigma). Anti-LngA monoclonal ICA39 recognizes specifically CS21 from ETEC strains and does not cross-react with other type IV pili-expressing bacteria, diarrhoeagenic E. coli not expressing CS21 or non-E. coli enteric bacteria (Qadri et al., 2000). Neuraminidase treatment was used to evaluate adherence inhibition, which consisted of neuraminidase pretreatment of cells for 1 h followed by a single wash with PBS before infection with ETEC strains. Neuraminidase-treated cells and cells containing inhibitors were infected with 5 µl ETEC bacterial suspension for 3 h, treated as described above and processed for c.f.u. quantification.
Microscopy.
Transmission electron microscopy (TEM) was used to evaluate the adherence of ETEC to IPEC1 and to IPEC-J2 cells at high magnification. Infected cells on air–liquid interface membranes were sequentially fixed with 2.5 % glutaraldehyde, 1 % osmium tetroxide and 1 % uranyl acetate. Samples were dehydrated in a graded series of ethanol (25, 50, 75, 100 %) before embedding with EPON812 (Electron Microscopy Sciences) using standard procedures (Clavijo et al., 2010). The samples were sectioned to 0.5 mm thickness with a diamond knife, and stained with toluidine blue for light microscopy examination before TEM visualization (model JEM-1230; Jeol) at high magnification. TEM images were recorded as TIFF computer files.
For immunofluorescence assays cells were fixed with 4 % paraformaldehyde for 15 min, washed three times with PBS and permeabilized with 0.2 % Triton X-100 for 8 min at room temperature, followed by three washes with PBS. Cells were subsequently stained with Alexa Phalloidin 568 (Life Technologies) at a 1 : 40 dilution, incubated for 1 h at room temperature in the dark, washed three times with PBS and mounted with Vectashield with DAPI (Vector Laboratories). Confocal fluorescence imaging was performed using a Zeiss 510 META laser scanning confocal microscope under a 63× oil immersion lens (numerical aperture 1.40). Images were recorded as TIFF computer files for further analysis.
Animal studies.
DBA/2 mice were supplied by the Isogenic Mouse Breeding Facility at the Department of Immunology, Biomedical Sciences Institute (ICB), University of São Paulo (USP), and all procedures were done in accordance with the principles of the Brazilian code for the use of laboratory animals. The neonatal mouse challenge model was performed as previously reported (Lásaro et al., 2004, 2005; Luiz et al., 2008). DBA2 mice were used preferentially, due to their susceptibility to ETEC strains and the non-aggressive behaviour of dams with regard to offspring after manipulation. Wild-type ETEC strain, CS21-mutant, and the complemented CS21-mutant strain were cultured at late exponential growth phase (A600nm = 0.8), harvested, washed once with PBS and resuspended in 0.1 M sodium bicarbonate at optical densities corresponding to different bacterial cell concentrations. Each neonate mouse was challenged with a total volume of 0.2 ml of the bacterial suspension. After the challenge, the actual number of bacteria injected into each mouse was confirmed by serial dilutions and plating on LB medium agar plates. One- to 2-day-old neonatal mice were inoculated directly into milk-filled stomachs with disposable syringes using ultrathin needles (12.7×0.33 mm). Newborn mice that died between 24 h after inoculation and up to 7 days after challenge were recorded to determine the number of survivors. The experiments involved 8–12 newborn mice for each bacterial strain and concentration tested. A group of 38 newborn mice were treated with PBS as inocula without any recorded death during the observation period.
Statistical analysis.
Results were calculated using VassarStats, 2009. Differences in adherence among ETEC E9034A wild-type strains and recombinant strains were determined by anova. Values are the mean of at least three separate experiments.
Mouse survival represented by Kaplan–Meier curves was compared using the Cox–Mantel test using the Prism software (Graph Pad). Statistically significant P-values were set at ≤0.05.
Results
CS21-mediated adherence to differentiated epithelial cells requires proficient LngA-expression conditions
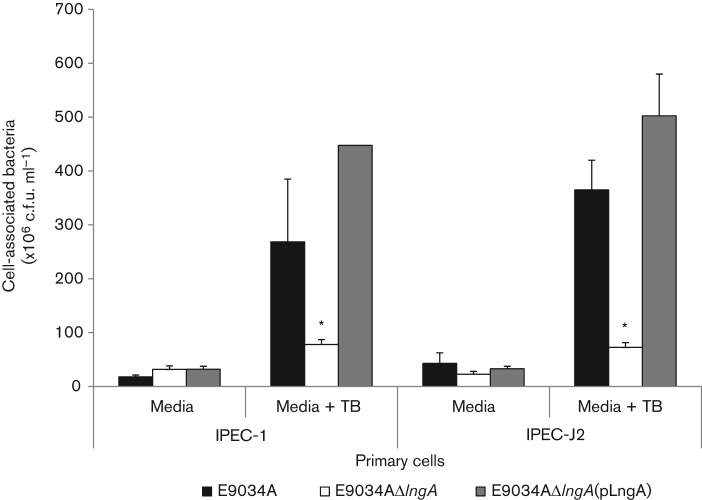
The CS21-expressing ETEC strain adheres better to human intestine-derived epithelial cells in vitro than the CS21-mutant strain (Mazariego-Espinosa et al., 2010). The adherence level between the E9034A ETEC and the CS21-mutant strain is, however, low (22–32 % difference). We hypothesized that CS21-mediated ETEC adherence to epithelial cells in vitro and in vivo depends upon optimal conditions for CS21 expression and the presence of CS21-specific receptors on epithelial cells. To test this hypothesis, we used two different tissue culture media conditions during quantitative adherence assays. One condition used tissue culture media alone and the other used tissue culture media supplemented with 50 % TB medium, which is known to induce CS21 expression (Clavijo et al., 2010). In addition, we used primary intestinal cell lines IPEC-1 and IPEC-J2 that after differentiation express microvilli, and surface molecules, and may express CS21 receptors. As shown in Fig. 1, the adherence assays using tissue culture media supplemented with 50 % TB resulted in 5- to 7-fold higher CS21-mediated adherence. The CS21-expressing E9034A wild-type ETEC strain adhered to IPEC-1, and IPEC-J2 cells 5- to 7-fold higher than the CS21-mutant ETEC strain. The complemented CS21 mutant adhered at the same level as the wild-type ETEC strain. These data indicate that under proficient conditions of CS21 expression, CS21 mediates ETEC adherence to primary intestinal cell lines.
Fig. 1.
CS21-mediated ETEC adherence to epithelial cells. Adherence values correspond to the number of cell-associated bacteria after 3 h infection. E9034A is a wild-type ETEC strain, E9034AΔlngA is the CS21 isogenic mutant and E9034AΔlngA(pLngA) is the complemented CS21 mutant. IPEC-1 and IPEC-J2 are pig-derived primary intestinal cell lines grown in IPEC tissue culture media or IPEC media supplemented with 50 % TB. Absolute c.f.u. values are as millions of c.f.u. ml–1. Error bars represent ±1 sd. Asterisks represent statistically significant differences in adherence to cells (P≤0.05) between wild-type ETEC (or completed CS21-mutant) and the CS21-mutant strain.
No difference in adherence was observed in HeLa cells between wild-type and CS21-mutant regardless of TB supplement (data not shown). This suggests that primary cell lines, IPEC-1 and IPEC-J2, may express CS21-specific receptors not present in undifferentiated cell lines such as HeLa cells.
CS21 mediates ETEC adherence to intestinal cells
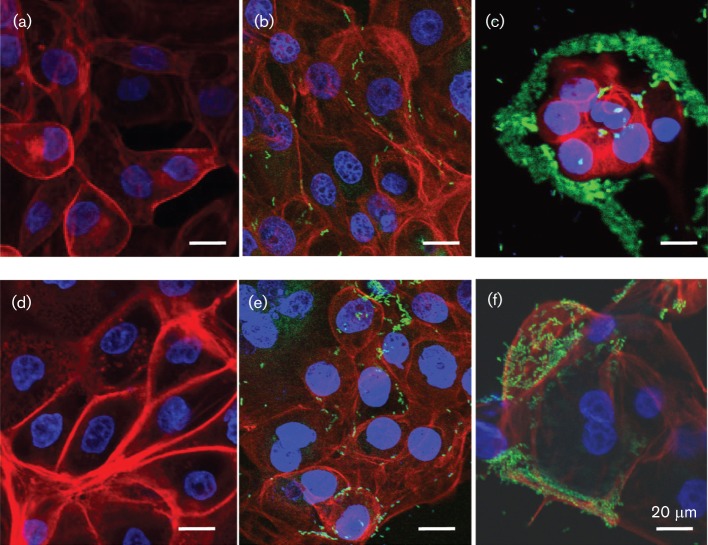
To evaluate CS21-mediated ETEC adherence to epithelial cells, IPEC-1 and IPEC-J2 intestinal cells lines were infected with ETEC strains and evaluated under confocal microscopy. Adherence assays were conducted using IPEC tissue culture media supplemented with 50 % TB to induce CS21 expression as described above. As shown in Fig. 2, CS21-expressing wild-type ETEC adhered at a higher level to IPEC-1 and IPEC-J2 as compared with the CS21-mutant strain. The wild-type ETEC strain tended to form clusters at specific locations on the surface of the epithelial cells. In contrast to the wild-type, fewer CS21-mutant bacteria adhered to cells and did not cluster around the epithelial cell membrane, which confirms that the self-aggregation phenotype is mediated by CS21 pili (Fig. 2b, e). The wild-type ETEC adherence pattern observed with IPEC-1 was also observed with IPEC-J2 cells (data not shown). The actin cytoskeleton fibre pattern observed in uninfected cells (Fig. 2a, d) was altered in IPEC-1 and IPEC-J2 cells after infection with either wild-type or CS21-mutant strains. The actin fibres in infected cells lost definition, and cells became rounded. These changes were more prominent in cells infected with the wild-type ETEC.
Fig. 2.
Confocal micrographs of CS21-mediated ETEC adherence to IPEC-1 cells (a–c) and IPEC-J2 cells (d–f). (a, d) Non-infected control cells; (b, e) cells infected with CS21-mutant strains [E9034AΔlngA(pGFPuv)]; (c, f) cells infected with wild-type ETEC strain [E9034A(pGFPuv)]. Control cells and cells infected with ETEC strains were cultivated in IPEC media supplemented with 50 % TB. Bars, 20 μm. Green fluorescence corresponds to GFP expressed by ETEC strains.
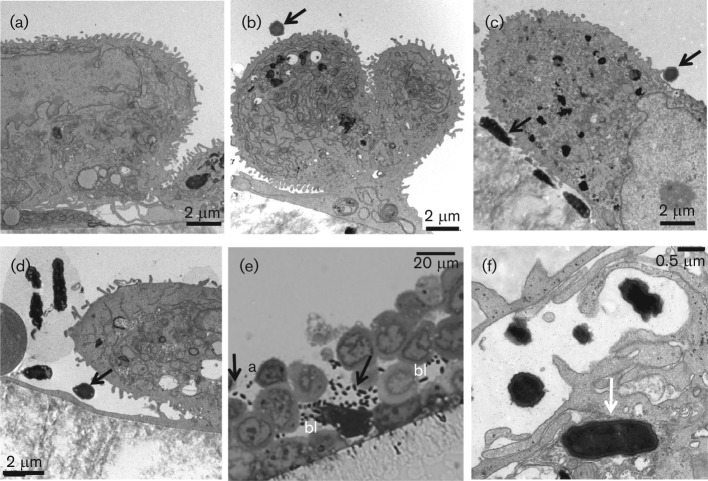
Intestinal cells examined by TEM expressed microvilli-like structures similar to in vivo intestinal mucosa microvilli (Nossol et al., 2011). The wild-type ETEC strain adhered to cells individually or as clusters (Fig. 3c, e). The wild-type ETEC bacteria were in close contact with the cell membrane or the microvilli-like structures (Fig. 3c, f). Adherent bacteria were observed on apical and basolateral cell surfaces (Fig. 3e) and occasional wild-type ETECs were also observed invading intestinal cells (Fig. 3f). The complemented CS21-mutant strain also adhered to cells by making contact with membrane and micovilli-like structures (Fig. 3c). No difference in the adherence pattern was observed between wild-type ETEC and complemented CS21-mutant strains infecting IPEC-J2 intestinal cells (Fig. 3c, d).
Fig. 3.
Adherence of CS21-expressing ETEC infection of IPEC-2 cells. (a) IPEC-J2 non-infected control cells; (b) cells infected with E9034AΔlngA CS2-mutant strain; (c, e, f) cells infected with E9034A wild-type ETEC strain; (d) cells infected with E9034AΔlngA(pLngA) complemented CS21-mutant strain. (a–d, f) Transmission electron micrographs; (e) thick cross-section of infected cells stained with toluidine blue and examined under light microscopy. Adherence assays were performed using IPEC tissue culture media supplemented with 50 % TB. Black arrows indicate bacteria adhering to the cell plasma membrane. White arrows indicate bacteria inside epithelial cell. a, Apical side; bl, basolateral side.
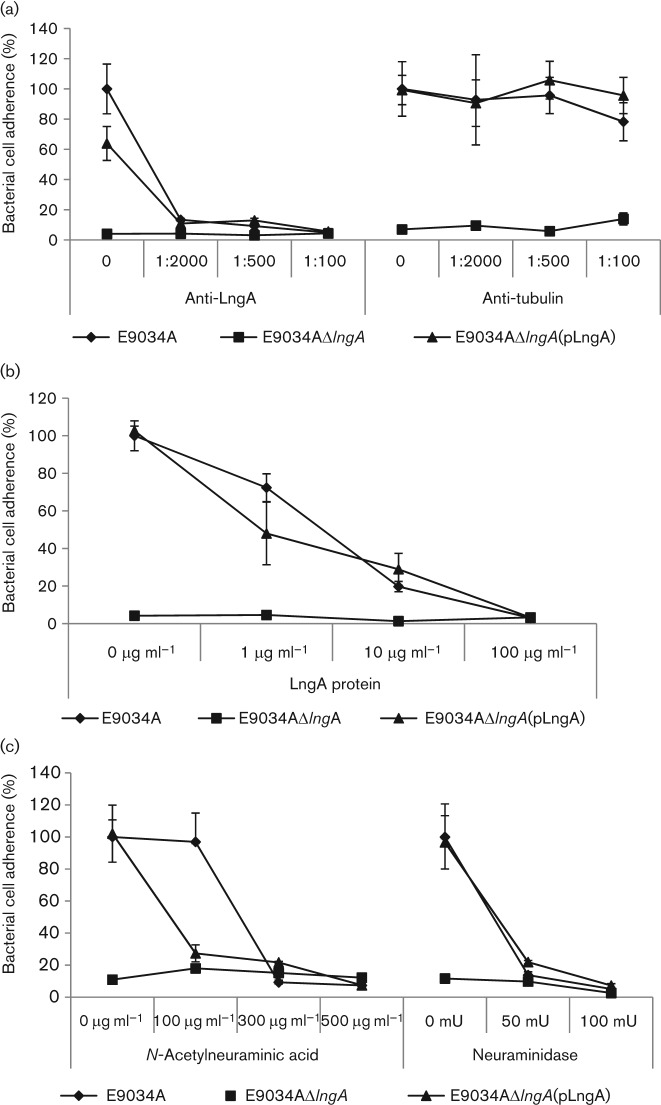
Anti-LngA antibodies and soluble LngA purified protein inhibited CS21-mediated ETEC adherence to intestinal cells
To evaluate the specificity of CS21 adherence of ETEC to intestinal cells we incorporated soluble anti-LngA antibody or purified LngA protein into the cell adherence assays as specific CS21-adherence inhibitors. Intestinal cells infected with wild-type ETEC, CS21-mutant or complemented CS21-mutant strains were evaluated for adherence in the absence or presence of increasing concentrations of anti-LngA mouse monoclonal antibodies. To optimize CS21 ETEC expression, IPEC tissue culture medium was supplemented with 50 % TB. Anti-tubulin monoclonal antibody was used as a negative control. As shown in Fig. 4(a), only specific anti-LngA monoclonal antibody decreased adherence of the wild-type ETEC strain to IPEC-J2 intestinal cells to the same background adherence level of the CS21-mutant strain. No effect on adherence was observed with the wild-type, the CS21-mutant or the complemented CS21-mutant when the anti-tubulin antibody control was used at identical dilutions. Identical results were obtained with IPEC-1 cells (data not shown).
Fig. 4.
Inhibition adherence assays in the presence of CS21 inhibitors. IPEC-J2 cells were infected with E9034A wild-type ETEC, E9034AΔlngA CS21-mutant and E9034AΔlngA(pLngA) complemented CS21-mutant strains and incubated for 3 h before c.f.u. quantification. IPEC tissue culture medium supplemented with 50 % TB was used for all inhibition adherence assays. (a) Anti-LngA monoclonal antibody inhibition assay. Increasing concentrations of anti-LngA monoclonal antibody or anti-tubulin monoclonal antibody were added to infected cells at the time of infection. The anti-tubulin antibody was used as a negative control. Antibody concentrations are represented as dilutions. (b) Purified LngA protein inhibition assay. LngA protein inhibitor was added to infected cells at time zero. (c) Neuraminidase and neuraminic acid inhibition assays. Infected cells were treated with increasing concentrations of N-acetylneuraminic acid at the time of the infection. In contrast, neuraminidase was added to cells 1 h prior to infection with ETEC strains. Values represent percentage of cell-associated bacteria. Error bars represent ±1 sd from three experiments.
Increasing concentrations of LngA purified protein specifically competed with CS21-expressing ETEC cells for binding to intestinal cells, while adherence of the CS21-mutant strain was unaffected (Fig. 4b). The data indicate that CS21 is involved in ETEC adherence to intestinal cells given that anti-LngA antibodies and purified LngA protein inhibited only the adherence of CS21-expressing bacteria. Overall, these data indicate that CS21-mediated ETEC adherence to intestinal cells is specific.
Neuraminic acid and neuraminidase treatments inhibit CS21-mediated ETEC adherence to intestinal cells
Bacterial pili binding to specific sugar structures on glycoprotein receptors at the epithelial cell surface are involved in virulence (Humphries et al., 2010; Mulvey, 2002). We have examined the role of monosaccharides in CS21-mediated adherence and have found that the addition of simple sugars such as mannose, arabinose and galactose did not inhibit CS21-mediated ETEC adherence to intestinal epithelial cells (data not shown). We hypothesize that CS21-mediated ETEC adherence to cells is inhibited by neuraminic (sialic) acid because glycoconjugate receptors on the surface of epithelial cells contain neuraminic acid residues. Structures containing neuraminic acid residues and unique glycoprotein substitutions may result in highly specific CS21 receptors on differentiated intestinal cell plasma membranes. These structures may be absent on transformed undifferentiated cells. To test this hypothesis, we conducted adherence inhibition assays in which increasing concentrations of neuraminic acid were added to ETEC-infected cells. To optimize CS21 expression by ETEC, IPEC tissue culture medium was supplemented with 50 % TB on all inhibition adherence assays. As shown in Fig. 4(c), increasing concentrations of N-acetyl-neuraminic acid significantly inhibited wild-type ETEC and complemented CS21-mutant strains adherence to intestinal cells to the level of the CS21-mutant. In contrast, none of the N-acetylneuraminic acid concentrations affected the low adherence level of the CS21-mutant ETEC strain. To demonstrate whether neuraminic acid residues that inhibited CS21-mediated adherence were on the surface of intestinal cell membranes, IPEC-1 and IPEC-J2 cells were treated with neuraminidase prior to infection with ETEC strains. As shown in Fig. 4(c), increasing concentrations of neuraminidase prior to infection inhibited CS21-mediated adherence of wild-type ETEC and complemented CS21-mutant strains. The CS21-mutant adherence level did not change at any concentration of neuraminidase, indicating that in the absence of CS21, the putative CS21 receptors were not participating in ETEC binding. These results also suggest that N-acetylneuraminic acid residues participate in the CS21–host cell receptor interactions between CS21-expressing ETEC and differentiated intestinal cells.
CS21 deletion drastically reduces the lethality of inoculated neonatal mice
Studies on ETEC pathogenesis have been limited by the lack of animal models capable of mimicking human disease. Nonetheless, alternative experimental models can be used as correlates for the in vivo pathogenesis of ETEC strains. Intra-stomach administration of ETEC strains causes lethality of neonatal mice and requires the expression of both enterotoxins and CSs, such as CFA/I (Lasaro et al., 2004, 2005; Luiz et al., 2008). To evaluate the role of CS21 in the pathogenesis of the E9034A wild-type ETEC, we measured the impact of CS21 deletion on the survival of inoculated neonatal mice using both the CS21-mutant strain and the complemented CS21-mutant strain. As shown in Fig. 5(a), administration of 6×105 c.f.u. of the E9034A strain caused 100 % lethality of the inoculated mice (LD50 between 4×102 and 8×103 c.f.u.). Mice inoculated with the CS21-mutant at the same concentration survived. In contrast, mice inoculated with the CS21-mutant ETEC strain showed 100 % lethality after administration of 6×108 c.f.u. (LD50 between 1×107 and 6×107 c.f.u.) (Fig. 5b). In addition, neonatal mice inoculated with the CS21-mutant strain complemented with plasmid-encoded recombinant CS21 resulted in lethality levels similar to those determined for the E9034A wild-type strain (Fig. 5). These data indicate that CS21 contributes to lethality of the E9034A ETEC strain in this animal model.
Fig. 5.
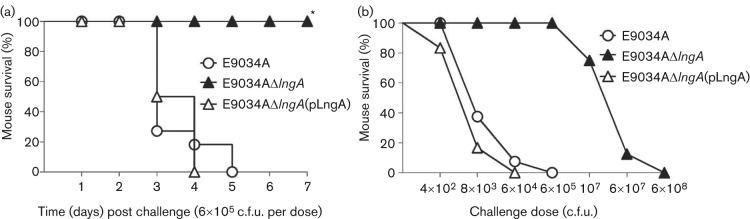
CS21-dependent lethality caused by ETEC strains using a neonate mouse challenge model. (a) CS21 contributes to the lethality of neonatal mice inoculated with the wild-type E9034A strain. Groups of DBA/2 neonatal mice were percutaneously inoculated into the milk-filled stomachs with 6×105 c.f.u. of strains E9034A, E9034AΔlngA or E9034AΔlngA(pLngA). Survival was followed for a period 7 days. (b) Challenge doses capable of killing neonatal mice inoculated with different ETEC strains. Groups of neonatal mice were challenged with different c.f.u. of strains E9034A, E9034AΔlngA or E9034AΔlngA(pLngA). Values on the y-axis correspond to the per cent of survivors after an observation period of 7 days. Values on the x-axis correspond to the number (c.f.u.) of bacteria injected into each neonatal mouse. The total numbers of pups used for testing of each bacterial strain were: 50 for the group inoculated with the E9034A strain, 56 for the E9034AΔlngA group and 58 for the E9034AΔlngA(pLngA) group. Data in (a) are presented as a Kaplan–Meir survival curve and statistically significant differences were determined with the Cox–Mantel test when comparing mice challenged with the E9034A strain with respect to mice challenged with E9034AΔlngA. *P<0.0001 when comparing wild-type ETEC E9034A strain (and complemented CS21-mutant) and CS21-mutant strain.
Discussion
The ability of E. coli intestinal pathogens to adhere to host epithelial cells and colonize the host intestine is regarded as a prerequisite for the development of diarrhoeal disease (Bieber et al., 1998; Torres et al., 2005). CS21 type IV pili, a common CS in ETEC, induce microcolony formation, protection against stress environmental factors, twitching motility and adherence to cells (Gomez-Duarte et al., 2007; Isidean et al., 2011). In this study we show that the CS21-expressing wild-type ETEC strain adhered to IPEC-1 and IPEC-J2 primary intestinal cell lines at levels 5- to 7-fold greater than the CS21-mutant strain. CS21-mediated adherence is specific as anti-LngA monoclonal antibody, as well as LngA purified protein, inhibit adherence to cells. Also, CS21-expressing wild-type ETEC and complemented CS21-mutant strains killed newborn mice after intra-stomach inoculation. In contrast, the CS21-mutant strain caused a reduction of four orders of magnitude in LD50. These results are significant as CS21 pili are one of the most prevalent CSs among human-derived ETEC strains worldwide (Girón et al., 1995; Isidean et al., 2011; Nishimura et al., 2002; Pichel et al., 2002).
CS21 is believed to contribute to ETEC colonization of the human gut presumably by binding specifically to intestinal cells. This study shows that CS21 mediates ETEC adherence to primary small intestinal cell lines. In contrast to transformed epithelial cell lines, primary intestinal cell lines express microvilli, mucus and presumably CS21-specific receptors. Evidence for the expression of ETEC pili receptors on IPEC-J2 cells is demonstrated by the ability of animal and human ETEC pili to adhere to these cells and to induce a pro-inflammatory cytokine response on them (Hermes et al., 2011; Koh et al., 2008; Pavlova et al., 2008; Sargeant et al., 2011). CS21-expressing ETEC bacteria adhered to intestinal cell surfaces including microvilli-like structures on both IPEC-1 and IPEC-J2 cells, suggesting that CS21 receptors may be present along the entire plasma membrane.
While CS21 receptors on epithelial cells are currently unknown, we believe that CS21 receptors are expressed on the apical region of polarized IPEC-1 and IPEC-J2 once tight junctions are formed and transepithelial resistance is increased. It is likely that CS21 receptors may also be expressed on the basolateral surface. Evaluation of CS-mediated ETEC adherence to these cells should include the measurement of transepithelial resistance as a marker of cell polarization and differentiation. Further research on the identification of CS21 receptors on intestinal cells is necessary to better understand the specific mechanisms involved in CS21-mediated adherence.
CS21-mediated ETEC adherence may be the result of interactions between the LngA structural subunit and specific receptors on the plasma membrane of intestinal cells. No information is available on the nature of the CS21 receptor. This study shows, however, that N-acetylneuraminic acid on the surface of intestinal cells may be involved in CS21-mediated adherence to cells as neuraminic acid and neuraminidase pretreatment of cells before infection also abrogated adherence. Neuraminic acid derivatives, collectively known as sialic acids, are present as glycoconjugates in tissues of all mammals and bacterial cell surfaces and play an essential role in molecular recognition (Sakarya et al., 2003; Sohanpal et al., 2004). Glycan modifies pili expressed by Gram-negative bacteria. Glycan modification of type IV pili of Neisseria gonorrhoeae is critical in adherence to epithelial cells by allowing pili to specifically bind to the I-domain region of complement receptor 3 (Jennings et al., 2011).
Neuraminic acid, ubiquitously expressed in glycoconjugates on the mammalian cell surface, binds to membrane proteins to create molecules with critical binding specificity. The neuraminic acid-containing host membrane proteins may promote specific interactions with Gram-negative micro-organism structures such as pili and non-pili adhesins (Sakarya et al., 2003, 2010). Accordingly, sialic acid bound to specific membrane proteins on primary intestinal cell lines may result in specific binding to CS21. The presence of sialic acid in the absence of differentially expressed specific membrane proteins on HeLa cells, or other transformed cells, may explain, at least in part, the marginal CS21-mediated adherence to these cells.
Our data also indicate that tissue culture conditions that optimize CS21 expression are essential for CS21-mediated ETEC adherence to intestinal cells and that similar conditions may be present in the host intestinal lumen. Quantitative adherence assays and microscopy showed that the CS21 mutant still binds to cells at significant levels. It is likely that other adhesins expressed by the wild-type E9034A ETEC strain and its isogenic CS21 mutant may contribute to adherence. Among them, CS3 pili have been described to be expressed in the wild-type E9034A ETEC strain (Levine et al., 1984). CS3 is known to bind to epithelial cells and to rabbit intestinal glycoproteins (Wennerås et al., 1995). CS21-expressing ETEC was seldom observed inside IPEC-1 and IPEC-J2 cells, and the CS21 mutant was not observed at all. This suggests that CS21 together with invasion-associated proteins such as TibA may contribute to cell invasion (Fleckenstein et al., 1996). We believe that ETEC adherence to cells is a complex and organized process of host–parasite interactions that involves multiple bacterial adhesins and host cell receptors leading to efficient gut colonization and severe disease.
Lethality of neonatal DBA/2 mice inoculated with human-derived ETEC strains was dependent on the expression of the CFA/I pilus and enterotoxins (Lásaro et al., 2004, 2005; Luiz et al., 2008). The present results demonstrate that expression of CS21 affects the ability of the E9034A strain to kill neonatal mice after intra-stomach administration of live bacteria. Although the precise mechanisms leading to the lethality caused by the ETEC strains are not fully understood, the present evidence demonstrates that CS21 plays an important role in the in vivo pathogenicity of the E9034A strain, probably reflecting a specific role in gut colonization. Nonetheless, similar to other CS-expressing ETEC strains, lethality is limited by age of the animals as only pups up to 1 week old are sensitive to ETEC lethality (our unpublished observations). Further experiments will be required to characterize the fate of ETEC cells inoculated into newborn mice and the mechanism leading to death. Our results demonstrate that the lethal challenge model based on the intra-stomach administration to neonatal mice represents a useful tool for the evaluation of ETEC pathogenicity and may find broader application in the study of the in vivo relevance of virulence-associated factors of ETEC.
Acknowledgements
Research support to O. G. G.-D was received from: NIAID-NIH Mentored Research Award (K08) no. KAI079410A and the Robert Wood Johnson Foundation award no. 65879 through the Harold Amos Faculty Development Program. The work carried out at the University of São Paulo was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). We are grateful to Dr Ricardo Peña and Ms Maria Tamborski for critical evaluation and helpful recommendations.
Abbreviations:
- CSs
colonization surface antigens
- ETEC
enterotoxigenic Escherichia coli
- TEM
transmission electron microscopy
References
- Beatty M. E., Adcock P. M., Smith S. W., Quinlan K., Kamimoto L. A., Rowe S. Y., Scott K., Conover C., Varchmin T. & other authors (2006). Epidemic diarrhea due to enterotoxigenic Escherichia coli. Clin Infect Dis 42, 329–334. 10.1086/499246 [DOI] [PubMed] [Google Scholar]
- Bieber D., Ramer S. W., Wu C. Y., Murray W. J., Tobe T., Fernandez R., Schoolnik G. K. (1998). Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli. Science 280, 2114–2118. 10.1126/science.280.5372.2114 [DOI] [PubMed] [Google Scholar]
- Black R. E., Cousens S., Johnson H. L., Lawn J. E., Rudan I., Bassani D. G., Jha P., Campbell H., Walker C. F. & other authors (2010). Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375, 1969–1987. 10.1016/S0140-6736(10)60549-1 [DOI] [PubMed] [Google Scholar]
- Blackburn D., Husband A., Saldaña Z., Nada R. A., Klena J., Qadri F., Girón J. A. (2009). Distribution of the Escherichia coli common pilus among diverse strains of human enterotoxigenic E. coli. J Clin Microbiol 47, 1781–1784. 10.1128/JCM.00260-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. A., Kaushik R. S., Hardwidge P. R. (2007). Susceptibility of human enterotoxigenic Escherichia coli isolates to growth inhibition by porcine intestinal epithelial cells. FEMS Microbiol Lett 274, 95–101. 10.1111/j.1574-6968.2007.00814.x [DOI] [PubMed] [Google Scholar]
- Clavijo A. P., Bai J., Gómez-Duarte O. G. (2010). The Longus type IV pilus of enterotoxigenic Escherichia coli (ETEC) mediates bacterial self-aggregation and protection from antimicrobial agents. Microb Pathog 48, 230–238. 10.1016/j.micpath.2010.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Canto F., Valenzuela P., Cantero L., Bronstein J., Blanco J. E., Blanco J., Prado V., Levine M., Nataro J. & other authors (2011). Distribution of classical and nonclassical virulence genes in enterotoxigenic Escherichia coli isolates from Chilean children and tRNA gene screening for putative insertion sites for genomic islands. J Clin Microbiol 49, 3198–3203. 10.1128/JCM.02473-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchet-Suchaux M. (1988). Protective antigens against enterotoxigenic Escherichia coli O101 : K99,F41 in the infant mouse diarrhea model. Infect Immun 56, 1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchet-Suchaux M., Le Maitre C., Bertin A. (1990). Differences in susceptibility of inbred and outbred infant mice to enterotoxigenic Escherichia coli of bovine, porcine and human origin. J Med Microbiol 31, 185–190. 10.1099/00222615-31-3-185 [DOI] [PubMed] [Google Scholar]
- Fleckenstein J. M., Kopecko D. J., Warren R. L., Elsinghorst E. A. (1996). Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect Immun 64, 2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., Svennerholm A. M. (1996). Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol 4, 444–452. 10.1016/0966-842X(96)10068-8 [DOI] [PubMed] [Google Scholar]
- Geens M. M., Niewold T. A. (2010). Preliminary characterization of the transcriptional response of the porcine intestinal cell line IPEC-J2 to enterotoxigenic Escherichia coli, Escherichia coli, and E. coli lipopolysaccharide. Comp Funct Genomics 2010, 469583. 10.1155/2010/469583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón J. A., Viboud G. I., Sperandio V., Gómez-Duarte O. G., Maneval D. R., Albert M. J., Levine M. M., Kaper J. B. (1995). Prevalence and association of the longus pilus structural gene (lngA) with colonization factor antigens, enterotoxin types, and serotypes of enterotoxigenic Escherichia coli. Infect Immun 63, 4195–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girón J. A., Gómez-Duarte O. G., Jarvis K. G., Kaper J. B. (1997). Longus pilus of enterotoxigenic Escherichia coli and its relatedness to other type-4 pili–a minireview. Gene 192, 39–43. 10.1016/S0378-1119(97)00039-5 [DOI] [PubMed] [Google Scholar]
- Gómez-Duarte O. G., Ruiz-Tagle A., Gómez D. C., Viboud G. I., Jarvis K. G., Kaper J. B., Girón J. A. (1999). Identification of lngA, the structural gene of longus type IV pilus of enterotoxigenic Escherichia coli. Microbiology 145, 1809–1816. 10.1099/13500872-145-7-1809 [DOI] [PubMed] [Google Scholar]
- Gomez-Duarte O. G., Chattopadhyay S., Weissman S. J., Giron J. A., Kaper J. B., Sokurenko E. V. (2007). Genetic diversity of the gene cluster encoding longus, a type IV pilus of enterotoxigenic Escherichia coli. J Bacteriol 189, 9145–9149. 10.1128/JB.00722-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Kosek M., Moore S., Lorntz B., Brantley R., Lima A. A. M. (2002). Magnitude and impact of diarrheal diseases. Arch Med Res 33, 351–355. 10.1016/S0188-4409(02)00379-X [DOI] [PubMed] [Google Scholar]
- Gupta S. K., Keck J., Ram P. K., Crump J. A., Miller M. A., Mintz E. D. (2008). Part III. Analysis of data gaps pertaining to enterotoxigenic Escherichia coli infections in low and medium human development index countries, 1984–2005. Epidemiol Infect 136, 721–738. 10.1017/S095026880700934X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermes R. G., Manzanilla E. G., Martín-Orúe S. M., Pérez J. F., Klasing K. C. (2011). Influence of dietary ingredients on in vitro inflammatory response of intestinal porcine epithelial cells challenged by an enterotoxigenic Escherichia coli (K88). Comp Immunol Microbiol Infect Dis 34, 479–488. 10.1016/j.cimid.2011.08.006 [DOI] [PubMed] [Google Scholar]
- Humphries R. M., Griener T. P., Vogt S. L., Mulvey G. L., Raivio T., Donnenberg M. S., Kitov P. I., Surette M., Armstrong G. D. (2010). N-Acetyllactosamine-induced retraction of bundle-forming pili regulates virulence-associated gene expression in enteropathogenic Escherichia coli. Mol Microbiol 76, 1111–1126. 10.1111/j.1365-2958.2010.07192.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidean S. D., Riddle M. S., Savarino S. J., Porter C. K. (2011). A systematic review of ETEC epidemiology focusing on colonization factor and toxin expression. Vaccine 29, 6167–6178. 10.1016/j.vaccine.2011.06.084 [DOI] [PubMed] [Google Scholar]
- Jain S., Chen L., Dechet A., Hertz A. T., Brus D. L., Hanley K., Wilson B., Frank J., Greene K. D. & other authors (2008). An outbreak of enterotoxigenic Escherichia coli associated with sushi restaurants in Nevada, 2004. Clin Infect Dis 47, 1–7. 10.1086/588666 [DOI] [PubMed] [Google Scholar]
- Jennings M. P., Jen F. E.-C., Roddam L. F., Apicella M. A., Edwards J. L. (2011). Neisseria gonorrhoeae pilin glycan contributes to CR3 activation during challenge of primary cervical epithelial cells. Cell Microbiol 13, 885–896. 10.1111/j.1462-5822.2011.01586.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. M., Kaushik R. S., Francis D. H., Fleckenstein J. M., Hardwidge P. R. (2009). Heat-labile enterotoxin promotes Escherichia coli adherence to intestinal epithelial cells. J Bacteriol 191, 178–186. 10.1128/JB.00822-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karp P. H., Moninger T. O., Weber S. P., Nesselhauf T. S., Launspach J. L., Zabner J., Welsh M. J. (2002). An in vitro model of differentiated human airway epithelia. Methods for establishing primary cultures. Methods Mol Biol 188, 115–137. [DOI] [PubMed] [Google Scholar]
- Koh S. Y., George S., Brözel V., Moxley R., Francis D., Kaushik R. S. (2008). Porcine intestinal epithelial cell lines as a new in vitro model for studying adherence and pathogenesis of enterotoxigenic Escherichia coli. Vet Microbiol 130, 191–197. 10.1016/j.vetmic.2007.12.018 [DOI] [PubMed] [Google Scholar]
- Lásaro M. O., Luiz W. B., Sbrogio-Almeida M. E., Nishimura L. S., Guth B. E. C., Ferreira L. C. S. (2004). Combined vaccine regimen based on parenteral priming with a DNA vaccine and administration of an oral booster consisting of a recombinant Salmonella enterica serovar Typhimurium vaccine strain for immunization against infection with human-derived enterotoxigenic Escherichia coli strains. Infect Immun 72, 6480–6491. 10.1128/IAI.72.11.6480-6491.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasaro M. O., Luiz W. B., Sbrogio-Almeida M. E., Ferreira L. C. S. (2005). Prime-boost vaccine regimen confers protective immunity to human-derived enterotoxigenic Escherichia coli. Vaccine 23, 2430–2438. 10.1016/j.vaccine.2004.11.026 [DOI] [PubMed] [Google Scholar]
- Levine M. M., Ristaino P., Marley G., Smyth C., Knutton S., Boedeker E., Black R., Young C., Clements M. L. & other authors (1984). Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect Immun 44, 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luiz W. B., Cavalcante R. C. M., Paccez J. D., Souza R. D., Sbrogio-Almeida M. E., Ferreira R. C. C., Ferreira L. C. S. (2008). Boosting systemic and secreted antibody responses in mice orally immunized with recombinant Bacillus subtilis strains following parenteral priming with a DNA vaccine encoding the enterotoxigenic Escherichia coli (ETEC) CFA/I fimbriae B subunit. Vaccine 26, 3998–4005. 10.1016/j.vaccine.2008.05.030 [DOI] [PubMed] [Google Scholar]
- Mazariego-Espinosa K., Cruz A., Ledesma M. A., Ochoa S. A., Xicohtencatl-Cortes J. (2010). Longus, a type IV pilus of enterotoxigenic Escherichia coli, is involved in adherence to intestinal epithelial cells. J Bacteriol 192, 2791–2800. 10.1128/JB.01595-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvey M. A. (2002). Adhesion and entry of uropathogenic Escherichia coli. Cell Microbiol 4, 257–271. 10.1046/j.1462-5822.2002.00193.x [DOI] [PubMed] [Google Scholar]
- Nishimura L. S., Girón J. A., Nunes S. L., Guth B. E. C. (2002). Prevalence of enterotoxigenic Escherichia coli strains harboring the longus pilus gene in Brazil. J Clin Microbiol 40, 2606–2608. 10.1128/JCM.40.7.2606-2608.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossol C., Diesing A.-K., Walk N., Faber-Zuschratter H., Hartig R., Post A., Kluess J., Rothkötter H.-J., Kahlert S. (2011). Air–liquid interface cultures enhance the oxygen supply and trigger the structural and functional differentiation of intestinal porcine epithelial cells (IPEC). Histochem Cell Biol 136, 103–115. 10.1007/s00418-011-0826-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova B., Volf J., Alexa P., Rychlik I., Matiasovic J., Faldyna M. (2008). Cytokine mRNA expression in porcine cell lines stimulated by enterotoxigenic Escherichia coli. Vet Microbiol 132, 105–110. 10.1016/j.vetmic.2008.04.024 [DOI] [PubMed] [Google Scholar]
- Pichel M. G., Binsztein N., Qadri F., Girón J. A. (2002). Type IV longus pilus of enterotoxigenic Escherichia coli: occurrence and association with toxin types and colonization factors among strains isolated in Argentina. J Clin Microbiol 40, 694–697. 10.1128/JCM.40.2.694-697.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qadri F., Girón J. A., Helander A., Begum Y. A., Asaduzzaman M., Xicohténcatl-Cortes J., Negrete E., Albert M. J. (2000). Human antibody response to longus type IV pilus and study of its prevalence among enterotoxigenic Escherichia coli in Bangladesh by using monoclonal antibodies. J Infect Dis 181, 2071–2074. 10.1086/315507 [DOI] [PubMed] [Google Scholar]
- Qadri F., Svennerholm A.-M., Faruque A. S. G., Sack R. B. (2005). Enterotoxigenic Escherichia coli in developing countries: epidemiology, microbiology, clinical features, treatment, and prevention. Clin Microbiol Rev 18, 465–483. 10.1128/CMR.18.3.465-483.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasschaert K., Devriendt B., Favoreel H., Goddeeris B. M., Cox E. (2010). Clathrin-mediated endocytosis and transcytosis of enterotoxigenic Escherichia coli F4 fimbriae in porcine intestinal epithelial cells. Vet Immunol Immunopathol 137, 243–250. 10.1016/j.vetimm.2010.05.016 [DOI] [PubMed] [Google Scholar]
- Sakarya S., Ertem G. T., Oncu S., Kocak I., Erol N., Oncu S. (2003). Escherichia coli bind to urinary bladder epithelium through nonspecific sialic acid mediated adherence. FEMS Immunol Med Microbiol 39, 45–50. 10.1016/S0928-8244(03)00185-8 [DOI] [PubMed] [Google Scholar]
- Sakarya S., Göktürk C., Oztürk T., Ertugrul M. B. (2010). Sialic acid is required for nonspecific adherence of Salmonella enterica ssp. enterica serovar Typhi on Caco-2 cells. FEMS Immunol Med Microbiol 58, 330–335. [DOI] [PubMed] [Google Scholar]
- Sargeant H. R., Miller H. M., Shaw M.-A. (2011). Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E. coli infection is modulated by zinc supplementation. Mol Immunol 48, 2113–2121. 10.1016/j.molimm.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Scaletsky I. C., Silva M. L., Trabulsi L. R. (1984). Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun 45, 534–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierack P., Nordhoff M., Pollmann M., Weyrauch K. D., Amasheh S., Lodemann U., Jores J., Tachu B., Kleta S. & other authors (2006). Characterization of a porcine intestinal epithelial cell line for in vitro studies of microbial pathogenesis in swine. Histochem Cell Biol 125, 293–305. 10.1007/s00418-005-0067-z [DOI] [PubMed] [Google Scholar]
- Sohanpal B. K., El-Labany S., Lahooti M., Plumbridge J. A., Blomfield I. C. (2004). Integrated regulatory responses of fimB to N-acetylneuraminic (sialic) acid and GlcNAc in Escherichia coli K-12. Proc Natl Acad Sci U S A 101, 16322–16327. 10.1073/pnas.0405821101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen R., Kollaritsch H., Fleischer K. (2003). Travelers’ diarrhea in the new millennium: consensus among experts from German-speaking countries. J Travel Med 10, 38–45. 10.2310/7060.2003.30672 [DOI] [PubMed] [Google Scholar]
- Torres A. G., Zhou X., Kaper J. B. (2005). Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect Immun 73, 18–29. 10.1128/IAI.73.1.18-29.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerås C., Erling V. (2004). Prevalence of enterotoxigenic Escherichia coli-associated diarrhoea and carrier state in the developing world. J Health Popul Nutr 22, 370–382. [PubMed] [Google Scholar]
- Wennerås C., Neeser J. R., Svennerholm A. M. (1995). Binding of the fibrillar CS3 adhesin of enterotoxigenic Escherichia coli to rabbit intestinal glycoproteins is competitively prevented by GalNAcβ1-4Gal-containing glycoconjugates. Infect Immun 63, 640–646. [DOI] [PMC free article] [PubMed] [Google Scholar]