Abstract
Wnt signaling pathways play a role in a variety of cellular processes including development, cell proliferation, cell fate, and motility. The Wnt/β-catenin pathway is among the most studied of the Wnt pathways and is highly conserved throughout evolution. Recent in vitro and slice physiology experiments have shown that this pathway also functions in synaptic transmission and activity-dependent synaptic plasticity. Since it has now been shown that many components of this signaling pathway are found in the adult brain, Wnt/β-catenin signaling may be important for maintaining and protecting neural connections throughout the lifespan. Here we summarize the role of Wnt/β-catenin signaling in the postnatal brain and discuss recent studies suggesting that deregulated Wnt signaling can result in altered behavior and cognitive disorders.
Keywords: Fear, Amygdala, Synaptic plasticity, PTSD, Gene, Anxiety
Overview of Wnt/β-catenin signaling
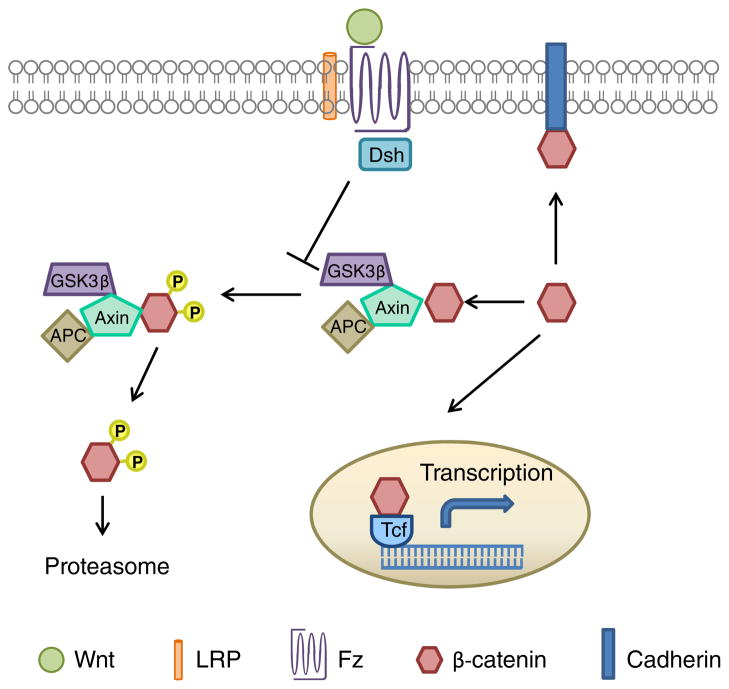
The Wnt/β-catenin pathway is involved in a variety of cellular processes including development, cell proliferation, cell survival, and motility (Moon et al. 2004; Inestrosa and Arenas, 2010). Wnts are highly conserved glycoproteins that bind to several distinct receptors, activating different signaling pathways. When Wnts bind to the Frizzled (Fz) and low-density lipoprotein-related protein (LRP) receptors, the cytoplasmic protein Dishevelled (Dvl) is recruited to the membrane. Activation of Dvl by Fz leads to the inhibition of glycogen synthase kinase-3 (GSK-3), a kinase which phosphorylates β-catenin, marking it for degradation by the proteasome pathway. The inhibition of GSK-3 by Fz, leads to the stabilization of β-catenin, allowing the protein to translocate to the nucleus, bind to the T-cell factor/lymphoid enhancer factor (TCF/LEF) family of transcription factors, and regulate the expression of Wnt target genes (Logan and Nusse 2004; Moon et al. 2004; Nelson and Nusse 2004; Gordon and Nusse 2006) (Fig. 1).
Fig. 1.
Schematic diagram of the Wnt/β-catenin signaling pathway
In addition to its role in Wnt signal transduction, β-catenin also plays a role in cadherin-mediated cell-cell adhesion. β-catenin associates with the cytoplasmic domain of cadherin and directly links to the actin cytoskeleton via α-catenin (Gumbiner 1996). This cadherin-catenin complex is localized in synaptic junctions, and alterations in this complex are thought to influence synapse strength and connectivity (Murase et al. 2002; Takeichi and Abe 2005).
There continues to be debate as to whether there is crosstalk between Wnt signaling and cadherin-mediated cell adhesion through β-catenin. Some studies have provided evidence that the same pool of β-catenin is involved in both cell adhesion at the plasma membrane and signal transduction in the nucleus (Reiss et al. 2005). However, others argue that there are different pools of β-catenin. These include a monomeric form that is generated by Wnt signaling and binds only to TCF transcription complexes and a second pool that forms a heterodimer with α-catenin and binds to cadherins (Gottardi and Gumbiner 2004). Although the details regarding the interaction between these two pathways remains unknown, there is clear evidence that β-catenin plays a role in neuronal synapse formation and plasticity.
Synapse assembly
The Wnt/β-catenin signaling pathway has been implicated in neuronal synapse formation and remodeling. Both Wnt7a and Wnt3 have been shown to increase growth cone size and axon branching (Hall et al. 2000; Purro et al. 2008). In addition, Wnts have been shown to play a role in synapse formation, by promoting the recruitment of pre- and post-synaptic components. For example, the addition of Wnt7 to cultured cerebellar granule cells and hippocampal neurons has been shown to promote presynaptic assembly and synaptic vesicle accumulation (Lucas and Salinas 1997; Ahmad-Annuar et al. 2006), while Wnt5a has been shown to stimulate postsynaptic assembly (Farias et al. 2009; 2010). There has also been evidence suggesting that β-catenin may act in both pre- and post- synaptic regions to modulate synapse assembly (Bamji et al. 2003; Gao et al., 2007; Salinas and Price, 2005; Sun et al., 2009; Yu and Malenka 2003).
β-catenin is expressed in the developing and adult CNS and can be found in both pre- and postsynaptic cells. In the presynaptic compartment, β-catenin is important for controlling the size and localization of vesicle clusters. When β-catenin is deleted from hippocampal pyramidal neurons, the number of synaptic vesicles per synapse decreases and the vesicles diffuse along the synapse (Bamji et al. 2003, 2006). Similar results can be observed when β-catenin is phosphorylated at tyrosine 654 (Y654). An increase in the phosphorylation of β-catenin at Y654 by the application of brain-derived neurotrophic factor (BDNF) or depletion of Fer in cultured hippocampal neurons increases synaptic vesicle mobility (Bamji et al. 2006; Lee et al. 2008). Thus, it appears that β-catenin acts presynaptically to control synaptic vesicle localization.
The role of β-catenin in postsynaptic structure and function has also been studied. It has been shown that β-catenin is important in regulating spine shape and size. Deletion of postsynaptic β-catenin alters spine morphology, as shown by an increase in immature, thin, elongated spines, and a decrease in mature, mushroom-like spines (Okuda et al. 2007). In addition to alterations in spine morphology, β-catenin also influences dendritic growth and arborization. Decreasing endogenous β-catenin in hippocampal neuronal cultures prevents dendritic morphogenesis while overexpressing β-catenin increases dendritic growth and arborization (Peng et al. 2009; Yu and Malenka 2003). The result obtained from overexpressing β-catenin in culture is very similar to the effect observed following treatment with high potassium. Since elevated potassium increases Wnt secretion (Yu and Malenka 2003), thereby leading to an increase in intracellular β-catenin, this may explain how both manipulations may produce similar enhancements in dendritic arborization.
Postsynaptic strength also changes with alterations in β-catenin expression. Loss of β-catenin in hippocampal neurons decreases the amplitude of miniature excitatory post-synaptic currents (mEPSCs), without affecting their frequency, suggesting that β-catenin plays a role in AMPA-mediated synaptic currents (Okuda et al. 2007). Interestingly, overexpression of β-catenin also reduces mEPSC amplitudes, and coincides with a decrease in AMPA receptor density without affecting synapse density (Peng et al. 2009). This finding may be explained by an observed increase in NMDAR/AMPAR ratio, which is indicative of silent, or inactive, synapses. The observation that these physiological changes co-occur with alterations in morphological changes following neural activity, suggests that the two phenomena may work together to regulate synaptic scaling. Since β-catenin plays a role in both postsynaptic structure and function, it may be important in the coupling process as well.
Activity-dependent synaptic plasticity
Neural activity induces changes in the synapse that may be important for neuronal circuitry formation and function. There is evidence that these changes may correspond to alterations in Wnt/β-catenin signaling. Following induction of long-term potentiation (LTP) in a hippocampal slice preparation, a microarray analysis revealed the activation of multiple Wnt signaling components (Chen et al. 2006). In addition, the release of Wnt-3a, which was found to be co-localized with the postsynaptic protein PSD-95 in the dentate gyrus, was increased following LTP-induced stimulation (Chen et al. 2006). Furthermore, LTP can be reduced or increased with suppression or activation of Wnt signaling, respectively (Chen et al. 2006).
In addition to changes in overall transcription of Wnt target genes, neural activity has also been shown to alter the localization of Wnt signaling components. Following depolarization of hippocampal neurons with a high concentration of KCl, β-catenin redistributes from dendritic shafts to spines. This redistribution is NMDA-dependent and can be mimicked or prevented by application of a tyrosine kinase or phosphatase inhibitor, respectively (Murase et al. 2002). Similarly, point mutations that prevent phosphorylation of β-catenin at the Y654 site cause a redistribution of β-catenin to the spine, and subsequent alterations in synaptic size and strength (Murase et al. 2002). These results suggest that activity-induced changes in the localization of β-catenin at the synapse may be important for synaptic regulation.
β-catenin regulation is also important for the transcription of genes in response to synaptic activity. Following NMDAR-dependent activation of calpain, β-catenin is cleaved at the N terminus, which then prevents GSK-3β mediated degradation (Abe and Takeichi 2007). As a result, the stabilized form of β-catenin increases, translocates to the nucleus, and regulates the transcription of Wnt target genes. One gene that is upregulated in response to NMDAR activation, Fosl1, has also been shown to be upregulated in the substantia nigraventral tegmental area complex (SNc/VTA) and hippocampus of rats following training to an instrumental task (Faure et al. 2006). Therefore, Wnt/β-catenin signaling may also be important for activity-dependent gene expression.
Wnt signaling in postnatal brain plasticity
Several components of the Wnt signaling pathway, along with Wnt ligands themselves, have been found to be expressed in the adult brain (Chacon et al. 2008; Shimogori et al. 2004; Cerpa et al. 2008; Maguschak and Ressler 2008; Maguschak and Ressler 2011). Consequently, evidence has been accumulating examining the role of Wnt signaling in postnatal/adult brain plasticity.
Neurogenesis is one active process that takes place in the adult brain. During this process, new neurons are created, which then differentiate, mature, and integrate into existing neural circuitry. Although there is still much to be determined about these new neurons, it is believed that they originate from multipotent adult neural stem cells. It has been suggested that the generation and further development of these neurons may be modulated by Wnt signaling due to its role in the proliferation of neural stem cells as well as the differentiation of stem cells into neurons (Ding et al. 2003; Michaelidis and Lie 2008; Lie et al. 2005).
In the adult mammalian brain, the subgranular zone (SGZ) of the hippocampal dentate gyrus and the subventricular zone (SVZ) of the lateral ventricles are the two regions that have been shown to undergo this process. There is evidence suggesting that Wnt signaling modulates neurogenesis in both regions. Lentiviral expression of a dominant-negative Wnt in the dentate gyrus, as well as deletion of NEUROD1, a pro-neurogenic transcription factor downstream of Wnt, reduce neurogenesis in the hippocampus (Gao et al. 2009; Kuwabara et al. 2009). Meanwhile, retrovirus-mediated expression of a stabilized β-catenin or treatment with a GSK-3β inhibitor enhances the proliferation of progenitor cells and increases the number of new neurons in the adult SVZ (Adachi et al. 2007). Conversely, expression of Dickkopf1 (Dkk1), an inhibitor of Wnt signaling, reduces proliferation of progenitor cells (Adachi et al. 2007). Together, these results indicate that Wnt/β-catenin signaling plays an important role in adult neurogenesis.
Interaction of DISC1, Wnt, and β-Catenin signaling and neurogenesis
Another gene that has been implicated in neuronal progenitor proliferation is the Disrupted in Schizophrenia 1 (DISC1) gene. A chromosomal translocation disrupting the DISC1 gene segregates with a high incidence of schizophrenia and bipolar disorder in a large Scottish pedigree (Blackwood et al. 2001). Although it has been shown that DISC1 functions in neurodevelopment, cytoskeletal function, and cAMP signaling, more recent data has identified a role for DISC1 in neural progenitor proliferation (Bradshaw and Porteous, 2012; Sawamura and Sawa, 2006).
DISC1 is expressed in the adult dentate gyrus and olfactory bulb, two regions displaying ongoing adult neurogenesis (Ma et al. 2002). Knockdown of DISC1 in adult hippocampal progenitors decreased cell proliferation, BrdU labeling, and mitotic index, along with an increase in Ser33/37 and Thr41 phosphorylation and ubiquitination of β-catenin (Mao et al. 2009). In contrast, overexpression of DISC1 resulted in the opposite effects. These data suggest that DISC1 facilitates the increased stability of β-catenin. These results also agree with previous reports showing an increase in proliferation following overexpression of β-catenin (Chenn and Walsh 2002; Zechner et al. 2003), suggesting that the effects of DISC1 and β-catenin manipulation on neurogenesis may be related. Indeed, Wnt-dependent progenitor proliferation, along with LEF/TCF activation is blocked by knockdown of DISC1 (Mao et al. 2009).
GSK-3β phosphorylates β-catenin at sites Ser33/37 and Thr41 marking it for degradation by the proteasome. The activity of GSK-3β is dependent on autophosphorylation at Tyr216 (Lochhead et al. 2006). Mao et al. (2009) found that DISC1 knockdown increased Tyr216 phosphorylation, while DISC1 overexpression reduced Tyr216 phosphorylation, providing support for the role of DISC1 in regulating GSK-3β activity. Furthermore, inhibiting GSK-3β rescued the negative effects of DISC1 knockdown on proliferation. In summary, these data strongly suggest that DISC1 regulates progenitor proliferation by inhibiting GSK-3β.
Wnt signaling in mood disorders
As discussed below, there are now numerous studies showing correlations between activation of the Wnt signaling pathways with a variety of neuropsychiatric disorders. Additionally molecular manipulations of the Wnt/β-catenin pathway which have used both inhibition (Table 1) or activation (Table 2) approaches have demonstrated a causal role for activation/inhibition of this pathway in the dynamic regulation of memory formation and affect regulation. Some of these studies are reviewed below.
Table 1.
Inhibition of Wnt signaling
| Manipulation | Effect | Reference | |
|---|---|---|---|
| Mania | |||
| GSK-3 | GOF | Increased hyperactivity | Prickaerts et al. 2006 |
| Depression | |||
| Dvl | LOF | Decreased sucrose consumption | Wilkinson et al. 2011 |
| Dvl | LOF | Increased immobility in FST | Wilkinson et al. 2011 |
| Dvl | LOF | Increased social avoidance | Wilkinson et al. 2011 |
| GSK-3 | GOF | Decreased sucrose consumption | Wilkinson et al. 2011 |
| GSK-3 | GOF | Decreased immobility in FST | Prickaerts et al. 2006 |
| GSK-3 | GOF | Increased immobility in FST | Wilkinson et al. 2011 |
| GSK-3 | GOF | Increased social avoidance | Wilkinson et al. 2011 |
| β-cat | LOF | Increased immobility in TST | Gould et al. 2008 |
| Memory | |||
| Wnt | LOF | Decreased long-term retention of spatial memory | Jessberger et al. 2009 |
| Wnt | LOF | Decreased performance in object recognition test | Jessberger et al. 2009 |
| Wnt | LOF | Decreased long-term fear memory consolidation | Maguschak and Ressler 2011 |
| Fzd | LOF | Decreased visuospatial memory | Zhao et al. 2005a,b |
| GSK-3 | GOF | Decreased spatial memory | Hernandez et al. 2002; Liu et al. 2003 |
| β-cat | LOF | Decreased long-term fear memory consolidation | Maguschak and Ressler 2008 |
Table 2.
Activation of Wnt signaling
| Manipulation | Effect | Reference | |
|---|---|---|---|
| Mania | |||
| GSK-3 | LOF | Decreased amphetamine-induced hyperlocomotion | Beaulieu et al. 2004; Gould et al. 2004a, b |
| β-cat | GOF | Decreased amphetamine-induced hyperlocomotion | Gould et al. 2007a, b |
| Depression | |||
| Wnt2 | GOF | Decreased latency to feed in NSF | Okamoto et al. 2010 |
| Wnt2 | GOF | Decreased number of escape failures in LH | Okamoto et al. 2010 |
| Wnt2 | GOF | Increased sucrose consumption | Okamoto et al. 2010 |
| GSK-3 | LOF | Decreased immobility in FST | O’Brien et al. 2004; Gould et al. 2004a, b; Kaidanovich-Beilin et al. 2004 |
| GSK-3 | LOF | Decreased CMS-induced increase in immobility time in FST | Silva et al. 2008 |
| β-cat Memory | GOF | Decreased immobility in FST | Gould et al. 2007a,b |
| Wnt | GOF | Decreased long-term fear memory consolidation | Maguschak and Ressler 2011 |
| GSK-3 | LOF | Decreased spatial memory deficits | De Ferrari et al. 2003; Toledo and Inestrosa 2010; Arrazola et al. 2009; Dash et al. 2011; Liu et al. 2010; Thotala et al. 2008 |
Mania
Impairments in neurogenesis have been linked to the development of psychiatric disorders including schizophrenia and depression. Knockdown of DISC1 not only produced deficits in neurogenesis, but it also produced schizophrenia- and depressive-like behaviors, including increased locomotion in a novel environment and increased immobility in the forced swim test (Mao et al. 2009). Such behaviors were reversed by treatment with a GSK-3β inhibitor. These results add support to the previous studies implicating GSK-3β and β-catenin in affective disorders (Gould et al. 2007a).
Manic-like behavior in rodents is a difficult phenotype to capture, but it is generally assessed by measuring locomotor activity in a home cage or novel environment and by hyper-activity in response to amphetamine treatment (Jope 2011). The behaviors observed can be alleviated by mood stabilizers (Machado-Vieira et al. 2004; Einat 2007). One of the most effective mood stabilizers is lithium, and lithium treatment has been used to manipulate locomotor activity in rodents. It has also been receiving wide acceptance as a modulator of β-catenin due to its inhibitory effects on GSK-3β (De Ferrari et al. 2003; Gould et al. 2004a, b; O’Brien et al. 2004). Consequently, the GSK-3β/β-catenin pathway has been strongly implicated in the pathophysiology of manic-like behavior.
There is evidence that the behavioral effects observed following lithium treatment, or other mood stabilizers, may be due to the activity of GSK-3. Pharmacological or genetic inhibition of GSK-3 reduces amphetamine-induced hyper-activity (Beaulieu et al. 2004). In contrast, overexpression of GSK-3β produces hyperactivity (Prickaerts et al. 2006). Due to the role of GSK-3 in Wnt/β-catenin signaling, studies have also examined the role of β-catenin in regulating the behavioral effects of lithium. Similar to the results obtained with genetic inhibition of GSK-3, overexpression of β-catenin reduced amphetamine-induced hyperactivity, once again recapitulating the behavioral effects of lithium (Gould et al. 2007b). In addition, Black Swiss mice, which have been used to model manic-like behavior in animals, have lower levels of β-catenin in the hippocampus (Hannah-Poquette et al. 2011). Together, these results suggest that the GSK-3/β-catenin pathway may be an important pathway to target for the treatment of mania.
It is important to note that GSK-3β activity can be regulated by many neuromodulators previously implicated in mood disorders. For example, brain-derived neurotrophic factor (BDNF), serotonin, and dopamine have all been shown to alter the phosphorylation and thus activity of GSK-3β (see Li and Jope 2010). Therefore, it is possible that the role of GSK-3β in mood disorders may be due to Wnt-independent pathways or molecules.
Depression
The Wnt/β-catenin pathway also appears to play a role in depressive symptoms. Depression is a difficult disorder to model in rodents, and as a result, many studies rely on measurements of behavior as opposed to mood to study the cause and treatments for the disorder. A number of studies have implicated Wnt signaling in the expression of depressive-like behavior, as well as the response to antidepressant treatment.
Many different classes of antidepressant treatments including serotonin-selective and mixed serotonin/norepinephrine reuptake inhibitors such as citalopram, fluoxetine, venlafaxine, and atomoxetine, have been shown to increase Wnt2 expression in the hippocampus of rats (Okamoto et al. 2010). Similar results have also been found with electroconvulsive shock, which remains the most robust treatment for refractory depression (Okamoto et al. 2010; Madsen et al. 2003). Increasing Wnt2 in the hippocampus produces a variety of antidepressant-like responses, including decreased number of escape failures in the learned helplessness (LH) paradigm, decreased latency to feed in novelty suppressed feeding (NSF) and increased sucrose consumption in the sucrose preference test (SPT). However, no difference was seen in the forced swim test (FST) (Okamoto et al. 2010).
Alterations in Wnt signaling components have also been observed in mouse models of depression. Recently, Wilkinson et al. (2011) identified Disheveled (DVL)-2 as one of the genes downregulated in the nucleus accumbens (NAc) of mice susceptible to social defeat stress. Blockade of DVL either with a dominant-negative mutant, or pharmacological inhibitor made mice more susceptible to social defeat and depressive-like behavior. Downregulation of DVL increases GSK-3β activity, and results from this same study showed that overexpressing GSK-3β induced depressive-like behavior. Likewise, inhibition of GSK-3β with a dominant-negative mutant promoted resilience (Wilkinson et al. 2011). Therefore, the Wnt/DVL/GSK-3β signaling cascade may regulate susceptibility to depression.
Another commonly used model for inducing depressive-like behavior in rodents is the chronic mild stress (CMS) paradigm. This model has been shown to increase GSK-3β in the hippocampus of rats (Silva et al. 2008). Treatment with lithium during the stress exposure reduced depressive-like symptoms as measured by immobility in the FST and prevented the CMS-induced increase in GSK-3β. These results are consistent with previous results showing the effect of GSK-3 modulation on depressive-like behavior. Mice heterozygous for GSK-3β or mice injected with GSK-3 inhibitors exhibit reduced immobility in the FST, consistent with the effects of antidepressant treatment (O’Brien et al. 2004; Gould et al. 2004a, b; Kaidanovich-Beilin et al. 2004). Furthermore, the decreased immobility time resulting from GSK-3 inhibition was associated with an in increase in β-catenin levels (Kaidanovich-Beilin et al. 2004; O’Brien et al. 2004).
Chronic electroconvulsive seizure (ECS), a treatment for severe depression, has also been shown to increase the expression of β-catenin in the rat hippocampus (Madsen et al. 2003). Furthermore, manipulation of the levels of β-catenin in the mouse brain produces changes in depressive-like behavior. Overexpression of β-catenin in mice decreased immobility time in the FST, which is thought to represent an anti-depressant effect. These results were similar to those obtained following chronic lithium treatment (Gould et al. 2007b). In contrast, forebrain specific knockout of β-catenin resulted in decreased struggling in the tail suspension test (TST), a depressive-like phenotype. However, no differences were observed in other tests of mood-related behaviors, including the FST and LH paradigms (Gould et al. 2008). Altogether, the above studies examining both manic-like and depression-like behaviors provide substantial evidence implicating Wnt/β-catenin signaling in mood regulation.
Alzheimer’s disease
The discovery of Wnt proteins in the adult brain along with their function in synapse formation, regulation, and neurogenesis, points toward a potential role for Wnt signaling in maintaining neural circuits throughout the lifespan. There is now evidence implicating Wnt/β-catenin signaling in pathological states of neurotoxicity such as Alzheimer’s disease (AD). Alzheimer’s Disease (AD) is a neurodegenerative disease characterized by progressive memory loss and cognitive impairment. It is also marked by the presence of neurofibrillary tangles and senile plaques which are comprised of the insoluble β-amyloid peptide (Aβ) (Hardy 2006).
Studies have shown that β-catenin levels are reduced in AD patients carrying presenilin-1 (PS1) mutations (Zhang et al. 1998), a mutation that accounts for the majority of cases of familial AD. Presenilins are crucial components of the multiprotein γ-secretase complex, which cleaves the amyloid precursor protein (APP), producing Aβ peptides (Selkoe and Wolfe 2007; Steiner et al. 2008). Mutations of PS1 are associated with the overproduction and aggregation of Aβ peptide (Hardy and Selkoe 2002), which then leads to the formation of amyloid plaques.
Furthermore, it has been shown that PS proteins form complexes with β-catenin (Kang et al. 2002; Zhou et al. 1997) and that lower levels of cytoplasmic β-catenin are associated with Aβ-induced neurotoxicity (De Ferrari and Inestrosa 2000). Aβ-induced neurotoxicity also induces the activation of GSK-3β, which increases the hyperphosphorylation of tau proteins, the main component of neurofibrillary tangles (De Ferrari et al. 2003). Interestingly, lithium, which inhibits GSK-3β and enhances β-catenin stability, has been shown to protect rat neurons from Aβ-induced damage (De Ferrari and Inestrosa 2000; Inestrosa et al. 2000). Lithium has also been shown to decrease tau phosphorylation in tau transgenic models with advanced neurofibrillary pathology (Leroy et al. 2010).
Behavioral impairments have been reported with in vivo models of the disease and activation of Wnt signaling has been shown to alter these impairments. Rats injected with preformed Aβ fibrils show deficits in spatial learning, as assessed by the Morris water maze paradigm. Activation of Wnt signaling with lithium improves their performance (De Ferrari et al. 2003). Treatment with lithium or Rosiglitazon, another activator of Wnt signaling, has also been shown to reduce impairments in spatial memory in a double transgenic mouse model of Alzheimer’s disease (Toledo and Inestrosa 2010; Arrazola et al. 2009). Thus, aberrant Wnt/β-catenin signaling may play a critical role in functional memory decline and the pathogenesis of AD.
Notably, studies have suggested that changes in cognitive functioning can be detected ten years or more prior to the clinical diagnosis of probable AD (Elias et al. 2000). Therefore, understanding how abnormalities in β-catenin function affect learning may provide insight into the functional deficits underlying the cognitive impairments associated with AD. If the initial learning and memory deficits, which predate gross neuropathology, can be detected and treated at the earliest stages, the vulnerability of neurons to the formation of neurofibrillary tangles and amyloid plaques may be decreased. It is possible that future drugs which stabilize β-catenin could be helpful both with early memory impairment and in decreasing later neuropathology.
Wnt signaling and learning and memory in the adult brain
The evidence linking Wnt signaling to AD, along with the role of this pathway in neuronal synapse regulation and plasticity, suggests a role for Wnt/β-catenin signaling in adult learning and memory processes. Additionally, although they may play a clear role in ongoing neurogenesis as described above, members of the Wnt/β-catenin pathway are also expressed in many cortical and subcortical areas involved in synaptic plasticity and learning and memory that are not undergoing neurogenesis (Fig. 2). Over the years, a number of studies have implicated GSK-3β in memory formation. Contextual and cued fear memory increases the phosphorylation of GSK-3β at Ser9 in the hippocampus and amygdala (Fujio et al. 2007; Maguschak and Ressler 2008). Additionally, mice that have been exposed to a rodent model of posttraumatic stress disorder (PTSD), which induces persistent and exaggerated trauma related fear, show increased levels of phosphorylated GSK-3β in the amygdala (Dahlhoff et al. 2010). Phosphorylation at this site inhibits GSK-3β activity (Zhao et al. 2005b), indicating that memory formation may require lower levels of GSK-3β.
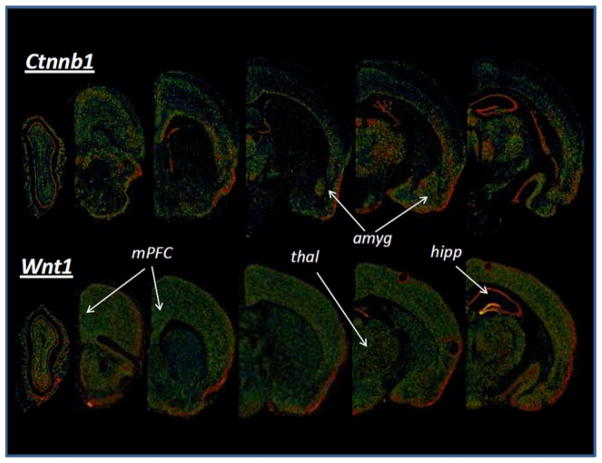
Fig. 2.
mRNA expression of Ctnnb1 and Wnt1 genes throughout the adult brain. A montage of in situ hybridization images from the Allen Brain Atlas (http://mouse.brain-map.org) is shown, demonstrating moderate to high levels of gene expression of β-catenin (ctnnb1) and wnt1 in many regions of the adult mouse brain that are involved in learning, memory, and behavior. Some regions that are clearly involved in behavior are noted, including medial prefrontal cortex (mPFC), amygdala (amyg), hippocampus (hipp), and thalamus (thal). In this digitized image, ‘hot’ colors (e.g. yellow-white) denote the highest levels of mRNA expression whereas ‘cool’ colors (e.g. blue to black) denote little to no expression
Increased levels of GSK-3β have been shown to produce opposite effects. Transgenic mice overexpressing GSK-3β in the brain display deficits in spatial learning as measured in the Morris water maze (Hernandez et al. 2002). Overactivation of GSK-3 by inhibition of PI3 kinase and PKC also produce deficits in spatial memory formation. The impairments observed were prevented by treatment with lithium (Liu et al. 2003). Lithium treatment has also been shown to improve learning and memory deficits including those resulting from traumatic brain injury, Sevoflurane treatment, and cranial irradiation (Dash et al. 2011; Liu et al. 2010; Thotala et al. 2008).
Recent evidence has suggested that Wnt signaling upstream and downstream of GSK-3 may also play a role in adult learning and memory. However, there have been only a few studies exploring this possibility. We have shown that many genes involved in Wnt-mediated signaling are significantly and robustly downregulated immediately following fear learning (Maguschak & Ressler 2011). Furthermore, inhibition of Wnt signaling in the amygdala with Dickkopf-1 (Dkk-1) impaired the consolidation, but not acquisition, of fear memory. Administration of Dkk-1 did not alter baseline locomotion or anxiety-like behavior (Maguschak & Ressler, 2010). Impairment in memory formation resulting from decreased Wnt function has also been reported previously. Jessberger et al. (2009) showed that expression of a dominant-negative Wnt in the dentate gyrus of rats produced impairments in long-term retention of spatial memory in the water maze, as well as impairments in a hippocampal-dependent object recognition task.
Interestingly, increasing Wnt signaling in the amygdala with a Wnt1 peptide, also impaired long-term fear memory consolidation, without affecting baseline locomotion or anxiety-like behaviors (Maguschak & Ressler 2011). These data suggest that counteracting the normal rapid decrease in Wnt prevents fear memory formation. We examined biochemical changes that occur during consolidation at baseline and in Wnt1 and Dkk-1 treated animals and found that both peptides had opposite effects on β-catenin/cadherin interactions: Wnt1 increased the β-catenin/cadherin interaction while Dkk-1 decreased the interaction. Together these data provide evidence that dynamic regulation of Wnt signaling is required for long-term memory formation, possibly through transient destabilization followed by restabilization of synaptic structures during memory consolidation.
There is also evidence that manipulation of Wnt receptors themselves can interfere with learning and memory. Frizzled 9 is expressed selectively in the hippocampus (Kim et al. 2001; Zhao and Pleasure 2004) and is thought to act as a Wnt receptor in the Wnt/β-catenin pathway (Karasawa et al. 2002). Like the effects observed following inhibition of Wnt signaling, deletion of frizzled produces deficits in hippocampal-dependent memory. Notably, the visuospatial deficits observed in these experiments persisted well into adulthood (Zhao et al. 2005a).
The studies reviewed thus far show that Wnt signaling can regulate memory formation. Work from our laboratory has identified a role for β-catenin in amygdala-dependent learning and memory (Maguschak and Ressler 2008). We first observed that β-catenin mRNA is increased in the amygdala following fear learning. Amygdala-specific deletion of β-catenin produced impairments in the consolidation, but not acquisition of the fear memory. However, neither locomotor nor anxiety-related behaviors were affected by the deletion.
We also found that β-catenin was regulated at the post-translational level with fear learning. Specifically, we showed that the phosphorylation state of β-catenin at Y654 is dynamically regulated during fear consolidation (Maguschak and Ressler 2008). Phosphorylation of β-catenin at Y654 has been shown to decrease the affinity of β-catenin for cadherin (Roura et al. 1999; Piedra et al. 2001). In our study, we showed that there is an increase in the phosphorylation of β-catenin immediately following learning which coincides with a decrease in the interaction between β-catenin and cadherin. Following a period of β-catenin-cadherin destabilization, we then observed a decrease in the phosphorylation of β-catenin and an increase in the β-catenin-cadherin interaction (Maguschak and Ressler 2008). Based on these results, along with our results showing the role of Wnt signaling in amygdala-dependent learning and memory, we have proposed a model of how Wnt/β-catenin signaling may function in memory formation (Fig. 3) (Maguschak and Ressler 2011).
Fig. 3.
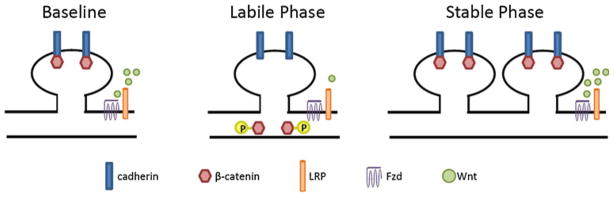
Schematic diagram of the role of Wnt/β-catenin signaling in producing the labile and stable phases of memory formation. a β-catenin is located in a complex with cadherin at the plasma membrane of the synapse. b Phosphorylation of β-catenin at Y654, along with a transient decrease in Wnt signaling causes a dissociation of β-catenin from the cadherin, allowing synapse rearrangement to occur. c A decrease in the phosphorylation of β-catenin accompanied by the normalization of Wnt signaling reforms the β-catenin/cadherin complex stabilizing newly formed synapses
We propose that Wnt signaling is reduced during the acquisition of fear memory and for a brief period immediately afterward. The reduction in Wnt signaling may allow for the phosphorylation of β-catenin at Y654 and the subsequent destabilization in the β-catenin-cadherin complex. The decrease in the interaction between β-catenin and cadherin may be required to weaken the synapse and allow synaptic remodeling to take place. Then, once the synapses have been modified and consolidation has occurred, Wnt signaling is normalized, the β-catenin Y654 site is dephosphorylated, and the β-catenin/cadherin complex is reformed. The effect of Wnt signaling on the β-catenin/cadherin complex, coupled with transient synapse destabilization and restabilization during memory consolidation, may provide for a structural mechanism underlying long-term memory formation.
Conclusions
Wnt/β-catenin signaling is important for normal neuronal functioning, from development into adulthood. There is now evidence suggesting that Wnt signaling is not only important for synapse formation, but also for the remodeling of synapses in response to activity. Several studies have now shown that perturbations in Wnt signaling can produce detrimental effects throughout the lifespan. Understanding how such alterations interfere with homeostasis of the healthy adult brain may provide insight into the etiology of psychiatric and neurodegenerative disorders.
Acknowledgments
This work was primarily supported by National Institutes Health (MH071537 and DA019624), the Burroughs Wellcome Fund, and the Yerkes Research Center (NIH 2P51RR000165-51).
Footnotes
Conflict of Interest The authors do not declare any conflicts of interest related to this manuscript.
Financial Disclosure Statement There were no commercial sponsors or commercial relationships related to the current work. Within the last 3 years, Dr. Ressler has received awards and/or funding support related to other studies from Burroughs Wellcome Foundation, NARSAD, NIMH, NIDA, and is a cofounder of Extinction Pharmaceuticals for use of NMDA-based therapeutics with psychotherapy.
Contributor Information
Kimberly A. Maguschak, Massachusetts Institute of Technology, Cambridge, MA, USA
Kerry J. Ressler, Email: kressle@emory.edu, Howard Hughes Medical Institute, Chevy Chase, MA, USA. Department of Psychiatry and Behavioral Sciences, Yerkes National Primate Research Center, Emory University School of Medicine, 954 Gatewood Dr, Atlanta, GA 30329, USA
References
- Abe K, Takeichi M. NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron. 2007;53:387–397. doi: 10.1016/j.neuron.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Adachi K, Mirzadeh Z, Sakaguchi M, Yamashita T, Nikolcheva T, Gotoh Y, Peltz G, Gong L, Kawase T, Alvarez-Buylla A, et al. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- Ahmad-Annuar A, Ciani L, Simeonidis I, Herreros J, Fredj NB, Rosso SB, Hall A, Brickley S, Salinas PC. Signaling across the synapse: a role for Wnt and Dishevelled in presynaptic assembly and neurotransmitter release. J Cell Biol. 2006;174:127–139. doi: 10.1083/jcb.200511054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrazola MS, Varela-Nallar L, Colombres M, Toledo EM, Cruzat F, Pavez L, Assar R, Aravena A, Gonzalez M, Montecino M, et al. Calcium/calmodulin-dependent protein kinase type IV is a target gene of the Wnt/beta-catenin signaling pathway. J Cell Physiol. 2009;221:658–667. doi: 10.1002/jcp.21902. [DOI] [PubMed] [Google Scholar]
- Bamji SX, Rico B, Kimes N, Reichardt LF. BDNF mobilizes synaptic vesicles and enhances synapse formation by disrupting cadherin-beta-catenin interactions. J Cell Biol. 2006;174:289–299. doi: 10.1083/jcb.200601087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamji SX, Shimazu K, Kimes N, Huelsken J, Birchmeier W, Lu B, Reichardt LF. Role of beta-catenin in synaptic vesicle localization and presynaptic assembly. Neuron. 2003;40:719–731. doi: 10.1016/s0896-6273(03)00718-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci U S A. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood DH, Fordyce A, Walker MT, St Clair DM, Porteous DJ, Muir WJ. Schizophrenia and affective disorders—cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am J Hum Genet. 2001;69:428–433. doi: 10.1086/321969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw NJ, Porteous DJ. DISC1-binding proteins in neural development, signalling and schizophrenia. Neuropharmacology. 2012;62:1230–1241. doi: 10.1016/j.neuropharm.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerpa W, Godoy JA, Alfaro I, Farias GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- Chacon MA, Varela-Nallar L, Inestrosa NC. Frizzled-1 is involved in the neuroprotective effect of Wnt3a against Abeta oligomers. J Cell Physiol. 2008;217:215–227. doi: 10.1002/jcp.21497. [DOI] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Chenn A, Walsh CA. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297:365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- Dahlhoff M, Siegmund A, Golub Y, Wolf E, Holsboer F, Wotjak CT. AKT/GSK-3beta/beta-catenin signalling within hippocampus and amygdala reflects genetically determined differences in posttraumatic stress disorder like symptoms. Neuroscience. 2010;169:1216–1226. doi: 10.1016/j.neuroscience.2010.05.066. [DOI] [PubMed] [Google Scholar]
- Dash PK, Johnson D, Clark J, Orsi SA, Zhang M, Zhao J, Grill RJ, Moore AN, Pati S. Involvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcome. PLoS One. 2011;6:e24648. doi: 10.1371/journal.pone.0024648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GV, Chacon MA, Barria MI, Garrido JL, Godoy JA, Olivares G, Reyes AE, Alvarez A, Bronfman M, Inestrosa NC. Activation of Wnt signaling rescues neurodegeneration and behavioral impairments induced by beta-amyloid fibrils. Mol Psychiatry. 2003;8:195–208. doi: 10.1038/sj.mp.4001208. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer’s disease. Brain Res Brain Res Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- Ding S, Wu TY, Brinker A, Peters EC, Hur W, Gray NS, Schultz PG. Synthetic small molecules that control stem cell fate. Proc Natl Acad Sci U S A. 2003;100:7632–7637. doi: 10.1073/pnas.0732087100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einat H. Different behaviors and different strains: potential new ways to model bipolar disorder. Neurosci Biobehav Rev. 2007;31:850–857. doi: 10.1016/j.neubiorev.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Elias MF, Beiser A, Wolf PA, Au R, White RF, D’Agostino RB. The preclinical phase of alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch Neurol. 2000;57:808–813. doi: 10.1001/archneur.57.6.808. [DOI] [PubMed] [Google Scholar]
- Farias GG, Alfaro IE, Cerpa W, Grabowski CP, Godoy JA, Bonansco C, Inestrosa NC. Wnt-5a/JNK signaling promotes the clustering of PSD-95 in hippocampal neurons. J Biol Chem. 2009;284:15857–15866. doi: 10.1074/jbc.M808986200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farías GG, Godoy JA, Cerpa W, Varela-Nallar L, Inestrosa NC. Wnt signaling modulates pre- and postsynaptic maturation: Therapeutic considerations. Developmental Dynamics. 2010 doi: 10.1002/dvdy.22065. [DOI] [PubMed] [Google Scholar]
- Faure A, Conde F, Cheruel F, el Massioui N. Learning-dependent activation of Fra-1: involvement of ventral hippocampus and SNc/VTA complex in learning and habit formation. Brain Res Bull. 2006;68:233–248. doi: 10.1016/j.brainresbull.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Fujio J, Hosono H, Ishiguro K, Ikegami S, Fujita SC. Tau phosphorylation in the mouse brain during aversive conditioning. Neurochem Int. 2007;51:200–208. doi: 10.1016/j.neuint.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Gao X, Arlotta P, Macklis JD, Chen J. Conditional knock-out of beta-catenin in postnatal-born dentate gyrus granule neurons results in dendritic malformation. J Neurosci. 2007;27:14317–25. doi: 10.1523/JNEUROSCI.3206-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, Goebbels S, Eisch AJ, Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon MD, Nusse R. Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J Biol Chem. 2006;281:22429–22433. doi: 10.1074/jbc.R600015200. [DOI] [PubMed] [Google Scholar]
- Gottardi CJ, Gumbiner BM. Distinct molecular forms of beta-catenin are targeted to adhesive or transcriptional complexes. J Cell Biol. 2004;167:339–349. doi: 10.1083/jcb.200402153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TD, Chen G, Manji HK. In vivo evidence in the brain for lithium inhibition of glycogen synthase kinase-3. Neuropsychopharmacology. 2004a;29:32–38. doi: 10.1038/sj.npp.1300283. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004b;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- Gould TD, Dow ER, O’Donnell KC, Chen G, Manji HK. Targeting signal transduction pathways in the treatment of mood disorders: recent insights into the relevance of the Wnt pathway. CNS Neurol Disord Drug Targets. 2007a;6(3):193–204. doi: 10.2174/187152707780619308. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, O’Donnell KC, Picchini AM, Schloesser RJ, Manji HK. Beta-catenin overexpression in the mouse brain phenocopies lithium-sensitive behaviors. Neuropsychopharmacology. 2007b;32:2173–2183. doi: 10.1038/sj.npp.1301338. [DOI] [PubMed] [Google Scholar]
- Gould TD, O’Donnell KC, Picchini AM, Dow ER, Chen G, Manji HK. Generation and behavioral characterization of beta-catenin forebrain-specific conditional knock-out mice. Behav Brain Res. 2008;189:117–125. doi: 10.1016/j.bbr.2007.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Hannah-Poquette C, Anderson GW, Flaisher-Grinberg S, Wang J, Meinerding TM, Einat H. Modeling mania: further validation for Black Swiss mice as model animals. Behav Brain Res. 2011;223:222–226. doi: 10.1016/j.bbr.2011.04.047. [DOI] [PubMed] [Google Scholar]
- Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Borrell J, Guaza C, Avila J, Lucas JJ. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem. 2002;83:1529–1533. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Alvarez A, Godoy J, Reyes A, De Ferrari GV. Acetylcholinesterase-amyloid-beta-peptide interaction and Wnt signaling involvement in Abeta neurotoxicity. Acta Neurol Scand Suppl. 2000;176:53–59. doi: 10.1034/j.1600-0404.2000.00308.x. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Kang DE, Soriano S, Xia X, Eberhart CG, De Strooper B, Zheng H, Koo EH. Presenilin couples the paired phosphorylation of beta-catenin independent of axin: implications for beta-catenin activation in tumorigenesis. Cell. 2002;110:751–762. doi: 10.1016/s0092-8674(02)00970-4. [DOI] [PubMed] [Google Scholar]
- Karasawa T, Yokokura H, Kitajewski J, Lombroso PJ. Frizzled-9 is activated by Wnt-2 and functions in Wnt/beta-catenin signaling. J Biol Chem. 2002;277:37479–37486. doi: 10.1074/jbc.M205658200. [DOI] [PubMed] [Google Scholar]
- Kim AS, Lowenstein DH, Pleasure SJ. Wnt receptors and Wnt inhibitors are expressed in gradients in the developing telencephalon. Mech Dev. 2001;103:167–172. doi: 10.1016/s0925-4773(01)00342-2. [DOI] [PubMed] [Google Scholar]
- Kuwabara T, Hsieh J, Muotri A, Yeo G, Warashina M, Lie DC, Moore L, Nakashima K, Asashima M, Gage FH. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Peng IF, Ng YG, Yanagisawa M, Bamji SX, Elia LP, Balsamo J, Lilien J, Anastasiadis PZ, Ullian EM, et al. Synapses are regulated by the cytoplasmic tyrosine kinase Fer in a pathway mediated by p120catenin, Fer, SHP-2, and beta-catenin. J Cell Biol. 2008;183:893–908. doi: 10.1083/jcb.200807188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy K, Ando K, Heraud C, Yilmaz Z, Authelet M, Boeynaems JM, Buee L, De Decker R, Brion JP. Lithium treatment arrests the development of neurofibrillary tangles in mutant tau transgenic mice with advanced neurofibrillary pathology. J Alzheimers Dis. 2010;19:705–719. doi: 10.3233/JAD-2010-1276. [DOI] [PubMed] [Google Scholar]
- Li X, Jope RS. Is glycogen synthase kinase-3 a central modulator in mood regulation? Neuropsychopharmacology. 2010;35:2143–54. doi: 10.1038/npp.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lie DC, Colamarino SA, Song HJ, Desire L, Mira H, Consiglio A, Lein ES, Jessberger S, Lansford H, Dearie AR, et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437:1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Zhang AH, Li HL, Wang Q, Deng HM, Netzer WJ, Xu H, Wang JZ. Overactivation of glycogen synthase kinase-3 by inhibition of phosphoinositol-3 kinase and protein kinase C leads to hyperphosphorylation of tau and impairment of spatial memory. J Neurochem. 2003;87:1333–1344. doi: 10.1046/j.1471-4159.2003.02070.x. [DOI] [PubMed] [Google Scholar]
- Liu XS, Xue QS, Zeng QW, Li Q, Liu J, Feng XM, Yu BW. Sevoflurane impairs memory consolidation in rats, possibly through inhibiting phosphorylation of glycogen synthase kinase-3beta in the hippocampus. Neurobiol Learn Mem. 2010;94:461–467. doi: 10.1016/j.nlm.2010.08.011. [DOI] [PubMed] [Google Scholar]
- Lochhead PA, Kinstrie R, Sibbet G, Rawjee T, Morrice N, Cleghon V. A chaperone-dependent GSK3beta transitional intermediate mediates activation-loop autophosphorylation. Mol Cell. 2006;24:627–633. doi: 10.1016/j.molcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol. 1997;192:31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- Ma L, Liu Y, Ky B, Shughrue PJ, Austin CP, Morris JA. Cloning and characterization of Disc1, the mouse ortholog of DISC1 (Disrupted-in-Schizophrenia 1) Genomics. 2002;80:662–672. doi: 10.1006/geno.2002.7012. [DOI] [PubMed] [Google Scholar]
- Machado-Vieira R, Kapczinski F, Soares JC. Perspectives for the development of animal models of bipolar disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:209–224. doi: 10.1016/j.pnpbp.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Newton SS, Eaton ME, Russell DS, Duman RS. Chronic electroconvulsive seizure up-regulates beta-catenin expression in rat hippocampus: role in adult neurogenesis. Biol Psychiatry. 2003;54:1006–1014. doi: 10.1016/s0006-3223(03)00700-5. [DOI] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. Beta-catenin is required for memory consolidation. Nat Neurosci. 2008;11:1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. Wnt signaling in amygdala-dependent learning and memory. J Neurosci. 2011;31:13057–13067. doi: 10.1523/JNEUROSCI.3248-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Y, Ge X, Frank CL, Madison JM, Koehler AN, Doud MK, Tassa C, Berry EM, Soda T, Singh KK, et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136:1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelidis TM, Lie DC. Wnt signaling and neural stem cells: caught in the Wnt web. Cell Tissue Res. 2008;331:193–210. doi: 10.1007/s00441-007-0476-5. [DOI] [PubMed] [Google Scholar]
- Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- Murase S, Mosser E, Schuman EM. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H, Voleti B, Banasr M, Sarhan M, Duric V, Girgenti MJ, Dileone RJ, Newton SS, Duman RS. Wnt2 expression and signaling is increased by different classes of antidepressant treatments. Biol Psychiatry. 2010;68:521–527. doi: 10.1016/j.biopsych.2010.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Yu LM, Cingolani LA, Kemler R, Goda Y. beta-Catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proc Natl Acad Sci U S A. 2007;104:13479–13484. doi: 10.1073/pnas.0702334104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng YR, He S, Marie H, Zeng SY, Ma J, Tan ZJ, Lee SY, Malenka RC, Yu X. Coordinated changes in dendritic arborization and synaptic strength during neural circuit development. Neuron. 2009;61:71–84. doi: 10.1016/j.neuron.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedra J, Martinez D, Castano J, Miravet S, Dunach M, de Herreros AG. Regulation of beta-catenin structure and activity by tyrosine phosphorylation. J Biol Chem. 2001;276:20436–20443. doi: 10.1074/jbc.M100194200. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Moechars D, Cryns K, Lenaerts I, van Craenendonck H, Goris I, Daneels G, Bouwknecht JA, Steckler T. Trans-genic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. J Neurosci. 2006;26:9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purro SA, Ciani L, Hoyos-Flight M, Stamatakou E, Siomou E, Salinas PC. Wnt regulates axon behavior through changes in microtubule growth directionality: a new role for adenomatous polyposis coli. J Neurosci. 2008;28:8644–8654. doi: 10.1523/JNEUROSCI.2320-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, Saftig P. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roura S, Miravet S, Piedra J, Garcia de Herreros A, Dunach M. Regulation of E-cadherin/Catenin association by tyrosine phosphorylation. J Biol Chem. 1999;274:36734–36740. doi: 10.1074/jbc.274.51.36734. [DOI] [PubMed] [Google Scholar]
- Salinas PC, Price SR. Cadherins and catenins in synapse development. Curr Opin Neurobiol. 2005;15:73–80. doi: 10.1016/j.conb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Sawamura N, Sawa A. Disrupted-in-schizophrenia-1 (DISC1): a key susceptibility factor for major mental illnesses. Ann N YAcad Sci. 2006;1086:126–133. doi: 10.1196/annals.1377.018. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Wolfe MS. Presenilin: running with scissors in the membrane. Cell. 2007;131:215–221. doi: 10.1016/j.cell.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Shimogori T, VanSant J, Paik E, Grove EA. Members of the Wnt, Fz, and Frp gene families expressed in postnatal mouse cerebral cortex. J Comp Neurol. 2004;473:496–510. doi: 10.1002/cne.20135. [DOI] [PubMed] [Google Scholar]
- Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leao P, Almeida OF, Sousa N. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience. 2008;152:656–669. doi: 10.1016/j.neuroscience.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Steiner H, Fluhrer R, Haass C. Intramembrane proteolysis by gamma-secretase. J Biol Chem. 2008;283:29627–29631. doi: 10.1074/jbc.R800010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Aiga M, Yoshida E, Humbert PO, Bamji SX. Scribble interacts with beta-catenin to localize synaptic vesicles to synapses. Mol Biol Cell. 2009;20:3390–3400. doi: 10.1091/mbc.E08-12-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M, Abe K. Synaptic contact dynamics controlled by cadherin and catenins. Trends Cell Biol. 2005;15:216–221. doi: 10.1016/j.tcb.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Thotala DK, Hallahan DE, Yazlovitskaya EM. Inhibition of glycogen synthase kinase 3 beta attenuates neurocognitive dysfunction resulting from cranial irradiation. Cancer Res. 2008;68:5859–5868. doi: 10.1158/0008-5472.CAN-07-6327. [DOI] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol Psychiatry. 2010;15(272–285):228. doi: 10.1038/mp.2009.72. [DOI] [PubMed] [Google Scholar]
- Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, Dietz D, Covington H, 3rd, Russo S, Neve R, et al. A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, 3rd, Birchmeier W, Birchmeier C. beta-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hartmann H, Do VM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Sommer B, van de Wetering M, Clevers H, Saftig P, et al. Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395:698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]
- Zhao C, Pleasure SJ. Frizzled-9 promoter drives expression of transgenes in the medial wall of the cortex and its chief derivative the hippocampus. Genesis. 2004;40:32–39. doi: 10.1002/gene.20058. [DOI] [PubMed] [Google Scholar]
- Zhao C, Aviles C, Abel RA, Almli CR, McQuillen P, Pleasure SJ. Hippocampal and visuospatial learning defects in mice with a deletion of frizzled 9, a gene in the Williams syndrome deletion interval. Development. 2005a;132:2917–2927. doi: 10.1242/dev.01871. [DOI] [PubMed] [Google Scholar]
- Zhao X, Zhuang S, Chen Y, Boss GR, Pilz RB. Cyclic GMP-dependent protein kinase regulates CCAAT enhancer-binding protein beta functions through inhibition of glycogen synthase kinase-3. J Biol Chem. 2005b;280(38):32683–92. doi: 10.1074/jbc.M505486200. [DOI] [PubMed] [Google Scholar]
- Zhou J, Liyanage U, Medina M, Ho C, Simmons AD, Lovett M, Kosik KS. Presenilin 1 interaction in the brain with a novel member of the Armadillo family. Neuroreport. 1997;8:2085–2090. doi: 10.1097/00001756-199705260-00054. [DOI] [PubMed] [Google Scholar]