Lipid rafts are distinct microdomains in the plasma membrane with highly ordered structure due to the presence of cholesterol and sphingolipids. Following receptor engagement, certain adaptor and signaling proteins translocate in and out of lipid rafts to facilitate signaling [1]. Lipid rafts play a crucial role in platelet function by hosting a number of agonist receptors and signaling molecules [2-6]. Despite the appreciation that signaling events in rafts contribute to platelet function, whether serine/threonine (Ser/Thr) phosphatases reside in platelet rafts remains unexplored. Here, we present evidence for a partial translocation of the catalytic subunits of protein phosphatase 1 (PP1c; ppp1c) and protein phosphatase 2A (PP2Ac; ppp2Ac) into the lipid rafts in thrombin and collagen-related peptide (CRP) stimulated platelets, an event that participates in phosphatase activation and platelet aggregation.
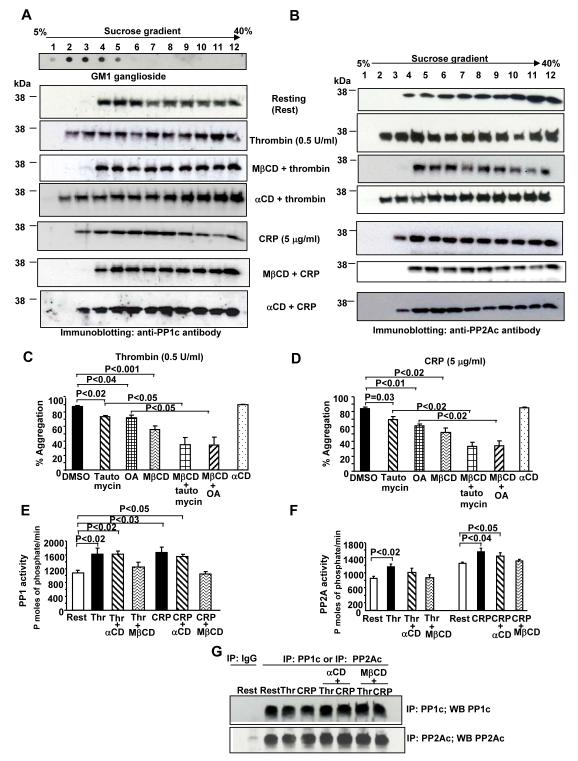
Isolation of rafts was achieved by sucrose density gradient centrifugation of 1% Triton X-100 platelet lysate followed by twelve equal fractionations from the top of the gradient [4]. Dot blot staining of all the fractions with cholera toxin B-subunit, which specifically binds to lipid raft marker ganglioside GM1, revealed that lipid rafts predominantly localized to fractions 2-4 with a minor presence in fraction 5 (Figure 1A, first panel). Immunoblotting of the fractions from resting platelets with anti-PP1c or anti-PP2Ac antibodies revealed the presence of PP1c (Figure 1A, second panel) and PP2Ac (Figure 1B, first panel) in the late raft fractions 4 and 5. However, the majority of PP1c/PP2Ac was localized to the non-lipid raft fractions (6 through 12). Following platelet activation with either thrombin or CRP, additional PP1c (Figure 1A, third and sixth panel) and PP2Ac (Figure 1B, second and fifth panel) were prominently detected in the early raft fractions 2 and 3 (thrombin) and fraction 3 (CRP). These observations indicate that the engagement of protease activated receptor 1 (PAR1) and the GPVI-FcRγ receptor in platelets is associated with the partial translocation of Ser/Thr phosphatases to the lipid rafts. Consistent with these observations, the receptors for thrombin (PAR1) [7] and CRP (GPVI) [5] also partition to platelet lipid rafts. Ser/Thr phosphatases facilitated PAR1 and GPVI mediated platelet aggregation because compared to the control DMSO, low dose inhibitors of PP1/PP2A (tautomycin) or PP2A (okadaic acid) revealed a modest but significant reduction in thrombin (Figures 1C) and CRP (Figures 1D) induced aggregation. These observations are consistent with the previous findings that platelet functions are impaired by pharmacological inhibitors that block the activity of Ser/Thr phosphatases [8-10].
Figure 1.
Washed platelets (resting or stimulated with 0.5 U/ml thrombin, 5 μg/ml CRP for 2 minutes) were lysed using ice cold 1% Triton X-100 lysis buffer. In some experiments, platelets were incubated with 10 mM MβCD or 10 mM αCD for 30 minutes prior to stimulation and lysis. Lysate was subjected to sucrose gradient centrifugation at 4 °C for 18 hours and 25 μl from twelve fractions were separated by SDS-PAGE and immunoblotted with anti-PP1c (recognizes all isoforms) (A) or anti-PP2Ac (B) antibodies. Blots are representative from 3-4 experiments. Aggregation (C and D) was studied using platelets treated with 0.01% DMSO, 2.5 μM tautomycin (Tocris Bioscience; Ellisville, MO) 10 nM okadaic acid (Tocris) for 30 minutes and then challenged with thrombin (Haematologic Technologic Inc; Essex Junction, VT) or CRP (synthesized at the Protein Core Facility of Baylor College of Medicine and cross linked by glutaraldehyde). In some assays, platelets were pretreated with MβCD or αCD (10 mM) for 30 minutes prior to treatment with phosphatase inhibitors and agonist. Data is expressed as mean ± SE from 3-4 experiments. For phosphatase assays (E and F), platelets were suspended in phosphate free buffer prior to raft disruption and 2 minutes challenge with agonist. Platelet lysate obtained after sonication was immunoprecipitated with control mouse IgG, anti-PP1c and anti-PP2Ac antibodies. The immunoprecipitates were incubated with 250-500 μM of either PP1c specific substrate phospho-Rb peptide HIPR(pS)PYKFPS(pS)PLR [14] (Peptide 2.0;Chantilly, VA), or the PP2Ac specific substrate phosphopeptide K-R-pT-I-R-R [Ser/Thr phosphatase assay kit (Millipore)] for 4 hours. The only exception was that the PP2Ac immunoprecipitate in the (F, left group) comprising the resting, thrombin, thrombin with MβCD or αCD treated platelets was incubated for 1 hour with the phosphopeptide. Phosphate released following the dephosphorylation of phosphopeptides was detected by malachite green, and the concentration (expressed as pmoles of phosphatase/minute) determined by comparing the absorbance value to the standard curve generated using the phosphate standard solution from the phosphatase assay kit. Variation in the incubation time of the substrate with the PP2Ac immunoprecipitate accounted for the two resting samples with different PP2Ac activity. Data is expressed as mean ± SE from 3-4 experiments. Mouse IgG, PP1c and PP2Ac immunoprecipitates were immunoblotted with anti-PP1c or anti-PP2Ac antibodies (G). Paired Student’s t test was used and P=0.05 was considered significant.
Disruption of platelet rafts by methyl-β-cyclodextrin (MβCD), which depletes membrane cholesterol prior to thrombin or CRP treatment, resulted in the loss of PP1c (Figure 1A, fourth and seventh panel) and PP2Ac (Figure 1B, third and sixth panel) from the early raft fractions 2 and 3. Since MβCD may exhibit raft dependent and independent effects [11], α cyclodextrin (αCD), an inactive cyclodextrin analogue was utilized as a control to demonstrate raft specificity [12]. PP1c (Figure 1A, fifth and eighth panel) and PP2Ac (Figures 1B, fourth and seventh panel) were retained in the lipid rafts when agonist-stimulated platelets were pretreated with αCD. How PP1c/PP2Ac localizes to the lipid rafts is unclear. PP1c and PP2Ac exhibit several cytosine residues in close proximity and may undergo palmitoylation, a modification that facilitates raft localization. In 3T3 cells, PP2A was localized to lipid rafts via its association with the cholesterol-regulated scaffolding protein OSBP [13].
To evaluate if the localization of phosphatases to rafts following agonist stimulation affected its activity, we disrupted rafts and evaluated phosphatase activity. Compared to the resting platelets, treatment with thrombin and CRP resulted in a moderate but significant increase in PP1c (Figure 1E) and PP2Ac (Figure 1F) activity. PP1c and PP2Ac enzymatic activities were specific because the mouse IgG immunoprecipitates detected only the base line phosphate levels (~100-150 pmoles of phosphate/minute) (not shown). Raft disruption by MβCD, but not by αCD decreased agonist-induced activation of PP1c and PP2Ac (Figures 1E and 1F). The amount of phosphatases available for the activity assays in the immune precipitates was comparable across various treatments (Figure 1G). Furthermore, agonist-induced platelet aggregation was also impaired in MβCD but not in αCD treated platelets (Figures 1C and 1D). Thus, disrupting lipid rafts reduced agonist-induced phosphatase activation with a concomitant impairment in platelet aggregation. To investigate if raft localization of phosphatases influenced platelet function, we assessed the impact of PP1c/PP2Ac inhibitors on platelet aggregation in the presence of a raft disruptor. Compared to platelets treated with only Ser/Thr phosphatase inhibitors, a combination of Ser/Thr phosphatase inhibitors and MβCD treatment significantly decreased agonist-induced platelet aggregation (Figures 1C and 1D). This suggests that the association of Ser/Thr phosphatases with rafts contribute to platelet aggregation. MβCD treatment does not alter integrin αIIbβ3 surface expression [4] and cannot account for the decreased aggregation. To summarize, previous studies have identified kinases (Syk, palmitolyated Fyn and Lyn) but not phosphatases in rafts. We show that Ser/Thr phosphatases can localize to lipid rafts following platelet activation with thrombin and CRP. Translocation of Ser/Thr phosphatases to lipid rafts facilitates complete agonist-induced phosphatase activation and platelet aggregation.
Acknowledgements
Supported by a grant from the NIH HL081613. K.V.V. was supported by the Mary R. Gibson Foundation and the Alkek Foundation.
Footnotes
Authorship details: S.P. designed study, generated and analyzed data. K.V.V. designed study, analyzed and interpreted data and wrote the paper.
Disclosure of Conflicts of Interest: The authors state that they have no conflict of interest.
References
- 1.Galbiati F, Razani B, Lisanti MP. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–11. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- 2.Lee FA, van LM, Relou IA, Foley L, Akkerman JW, Heijnen HF, Farndale RW. Lipid rafts facilitate the interaction of PECAM-1 with the glycoprotein VI-FcR gamma-chain complex in human platelets. J. Biol. Chem. 2006;281:39330–38. doi: 10.1074/jbc.M607930200. [DOI] [PubMed] [Google Scholar]
- 3.Pollitt AY, Grygielska B, Leblond B, Desire L, Eble JA, Watson SP. Phosphorylation of CLEC-2 is dependent on lipid rafts, actin polymerization, secondary mediators, and Rac. Blood. 2010;115:2938–46. doi: 10.1182/blood-2009-12-257212. [DOI] [PubMed] [Google Scholar]
- 4.Shrimpton CN, Borthakur G, Larrucea S, Cruz MA, Dong JF, Lopez JA. Localization of the adhesion receptor glycoprotein Ib-IX-V complex to lipid rafts is required for platelet adhesion and activation. J. Exp. Med. 2002;196:1057–66. doi: 10.1084/jem.20020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke D, Chen H, Liu Y, Liu C, Kahn ML. Lipid rafts orchestrate signaling by the platelet receptor glycoprotein VI. J. Biol. Chem. 2002;277:18801–09. doi: 10.1074/jbc.M111520200. [DOI] [PubMed] [Google Scholar]
- 6.Quinton TM, Kim S, Jin J, Kunapuli SP. Lipid rafts are required in Gα(i) signaling downstream of the P2Y12 receptor during ADP-mediated platelet activation. J. Thromb. Haemost. 2005;3:1036–41. doi: 10.1111/j.1538-7836.2005.01325.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Wang Y, Xiang Y, Lee W, Zhang Y. Prohibitins are involved in protease-activated receptor 1-mediated platelet aggregation. J. Thromb. Haemost. 2012;10:411–418. doi: 10.1111/j.1538-7836.2011.04607.x. [DOI] [PubMed] [Google Scholar]
- 8.Nishikawa M, Toyoda H, Saito M, Morita K, Tawara I, Deguchi K, Kuno T, Shima H, Nagao M, Shirakawa S. Calyculin A and okadiac acid inhibit human platelet aggregation by blocking protein phosphatases types 1 and 2A. Cell Signal. 1994;6:59–71. doi: 10.1016/0898-6568(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 9.Hoyt CH, Lerea KM. Aggregation-dependent signaling in human platelets is sensitive to protein serine/threonine phosphatase inhibitors. Biochemistry. 1995;34:9565–9570. doi: 10.1021/bi00029a033. [DOI] [PubMed] [Google Scholar]
- 10.Lerea KM, Cordero KP, Sakariassen KS, Kirk RI, Fried VA. Phosphorylation sites in the integrin β3 cytoplasmic domain in intact platelets. J. Biol. Chem. 1999;274:1914–1919. doi: 10.1074/jbc.274.4.1914. [DOI] [PubMed] [Google Scholar]
- 11.Zidovetzki R, Levitan I. Use of cyclodextrins to manipulate plasma membrane cholesterol contents: evidence, misconceptions and control strategies. Biochim. Biophys. Acta. 2007;1768:1311–1324. doi: 10.1016/j.bbamem.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassme H, Jekle A, Riehle A, Schwarz H, Berger J, Sandhoff K, Kolesnick R, Gulbins E. CD95 signaling via ceramide-rich membrane rafts. J. Biol. Chem. 2001;276:20589–20596. doi: 10.1074/jbc.M101207200. [DOI] [PubMed] [Google Scholar]
- 13.Wang PY, Liu P, Weng J, Sontag E, Anderson RG. A cholesterol-regulated PP2A/HePTP complex with dual specificity ERK1/2 phosphatase activity. EMBO J. 2003;22:2658–67. doi: 10.1093/emboj/cdg255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ammosova T, Obukhov Y, Kotelkin A, Breuer D, Beullens M, Gordeuk VR, Bollen M, Nekhai S. Protein phosphatase-1 activates CDK9 by dephosphorylating Ser175. PLoS. One. 2011;6:e18985. doi: 10.1371/journal.pone.0018985. [DOI] [PMC free article] [PubMed] [Google Scholar]