Abstract
We used functional connectivity magnetic resonance imaging (fcMRI) to investigate changes in interhemispheric brain connectivity in 11 patients with mild Alzheimer’s disease (AD) following eight weeks of treatment with the cholinesterase inhibitor donepezil. We examined functional connectivity between four homologous temporal, frontal, and occipital regions. These regions were selected to represent sites of AD neuropathology, sites of donepezil-related brain activation change in prior studies, and sites that are minimally affected by the pathologic changes of AD. Based on previous findings of selective, localized frontal responses to donepezil, we predicted that frontal connectivity would be most strongly impacted by treatment. Of the areas we examined, we found that treatment had a significant effect only on functional connectivity between right and left dorsolateral prefrontal cortices. Implications for understanding the impact of donepezil treatment on brain functioning and behavior in patients with AD are discussed. This preliminary report suggests that fcMRI may provide a useful index of treatment outcome in diseases affecting brain connectivity. Future research should investigate these treatment-related changes in larger samples of patients and age-matched controls.
Keywords: Alzheimer’s disease, donepezil, functional connectivity, dorsolateral prefrontal cortex, hippocampus
Introduction
Neurofibrillary tangles (NFTs) and neuritic plaques (NPs) are the neuropathological hallmarks of Alzheimer’s disease (AD). These pathologic changes compromise the integrity of neural connections and disrupt communication among brain regions [1]. Diffusion tensor imaging, electroencephalographic, and positron emission tomography findings of interhemispheric connectivity abnormalities in AD [2] likely reflect the detrimental effects of such pathology on brain connectivity.
Functional connectivity MRI (fcMRI) is another technique for examining in vivo human brain connectivity. It is based on the observation that functionally related brain regions show correlated low-frequency fluctuations in the blood oxygenation level-dependent (BOLD) MRI signal [3]. When applied to patients, fcMRI appears to be sensitive to functional disconnections. For instance, previous studies have found a breakdown in the synchronous activity of neural circuits involving the hippocampus in subjects with mild AD [e.g., 4–6].
Functional connectivity methods may also be useful in evaluating the effectiveness of treatment methods for improving brain function, and thus changing brain connectivity. A number of previous studies have utilized task-based functional magnetic resonance imaging (fMRI) to examine the impact of treatment with acetylcholinesterase (AchE) inhibitors on brain functioning in patients with AD and mild cognitive impairment (MCI) [7]. In these studies, increases in prefrontal activation appear to be a particularly consistent finding [e.g., 8, 9]. More recently, AchE inhibitor treatment has been shown to increase functional connectivity between the hippocampus and precentral gyrus, parahippocampus, insula, lentiform nucleus, thalamus, middle frontal gyrus, pons, and posterior cingulate [10].
For the present investigation, we used fcMRI as an index of change in interhemispheric connectivity following pharmacological treatment with the AChE inhibitor donepezil in patients with mild AD. We investigated functional connectivity of four homologous region of interest (ROI) pairs. ROIs were in a primary site of AD neuropathology (medial temporal lobes) and a primary site of activation response to donepezil (frontal lobes). In addition, due to the lack of control participants, a control brain region (occipital lobes) was studied as well, the rationale being that we would not expect placebo effects to be brain region-specific. We hypothesized that frontal connectivity would be most affected by donepezil treatment.
Materials and Methods
Study participants
Participants were 11 patients with mild AD (4 males, 7 females; mean age=75.5±7.4, range=62–84; mean education=14.3±2.5 years, range=12–18). Patients were recruited from the Alzheimer’s Disease Center and the Mildred Wyatt and Ivor P. Wold Center for Geriatric Care at the University of Texas Southwestern Medical Center at Dallas. Alzheimer’s disease (AD) was diagnosed by a neurologist or geriatrician using NINCDS/ADRDA criteria [11]. Inclusion criteria included a diagnosis of probable AD, a Mini-Mental State Examination score greater than or equal to 15, and adequate sight, hearing, and comprehension to participate. Subjects were excluded if they were already taking any medication that might alter brain function, including donepezil or antipsychotic drugs. They were also screened to rule out neurological diseases other than AD, any condition that could chronically affect cognitive functioning, or major psychopathology such as severe depression. The UT Southwestern Institutional Review Board approved the experimental protocol. Before participation, written informed consent was obtained from each participant and a family member or legal representative.
Cholinesterase inhibition therapy
Donepezil (Aricept™) was initially administered at 5 mg daily for 28 days, and then 10 mg daily for 28 days. Caregivers monitored daily usage, and compliance was verified by pill count. MRI acquisitions were conducted before treatment and following eight weeks of therapy. Pfizer U.S. Pharmaceuticals provided Aricept™ as a gift to the investigators, but did not participate in or provide support for study conception, design, analysis or conduct.
MRI data acquisition
MR images were acquired on a General Electric Horizon LX NV/i 1.5 Tesla scanner (GE Medical Systems, Milwaukee, WI) using the standard GE quadrature birdcage RF head coil. To minimize head motion, each subject’s head was immobilized with tightly fitting foam padding and a forehead strap. For the collection of fcMRI data, a time series of 100 echo-planar image (EPI) volumes was acquired while subjects were at rest in a darkened MRI scanner magnet bore. Sensory stimulation was limited to scanner noise, which was dampened by earplugs and headphones. Whole-brain EPI data were acquired in the axial plane with a single-shot gradient-recalled pulse sequence (sequential slice acquisition; TR=2000 ms; TE=45 ms; flip angle=90°; matrix=64×64; FOV=24 cm; slice thickness=7 mm; gap=0.5 mm). High-resolution coronal images (SPGR sequence: TR=30 ms; TE=5 ms; flip angle=45°; matrix=256×256; FOV=24 cm; slice thickness=2 mm) were acquired during the same scan session for each subject.
MRI data analysis
AFNI software [12] was used for all analyses. Pre-processing steps included de-trending, motion correction, and low-pass filtering to remove frequencies greater than 0.08 Hz. For each subject, frontal, medial temporal, and occipital ROIs were identified on the high-resolution anatomical images. ROIs were traced in both the right and left hemispheres with the assistance of brain atlases [13–16], published tracing protocols and neuroanatomical articles [17–23], and a Brodmann area (BA) probability map overlaid on lateral, medial and dorsal surfaces of the brain (Aboitiz, unpublished data). Frontal ROIs were placed in the dorsolateral prefrontal cortex (DLPFC; BA 46, 46/9, and 9) and the inferior frontal gyrus (IFG; BA 44 and 45). The medial temporal ROI was placed in the hippocampus (HIPP), and the occipital ROI was placed in primary visual cortex (PVC; BA17). These regions were traced on the high-resolution structural images (Figure 1) and then resampled to the lower resolution of the EPI data.
Figure 1.
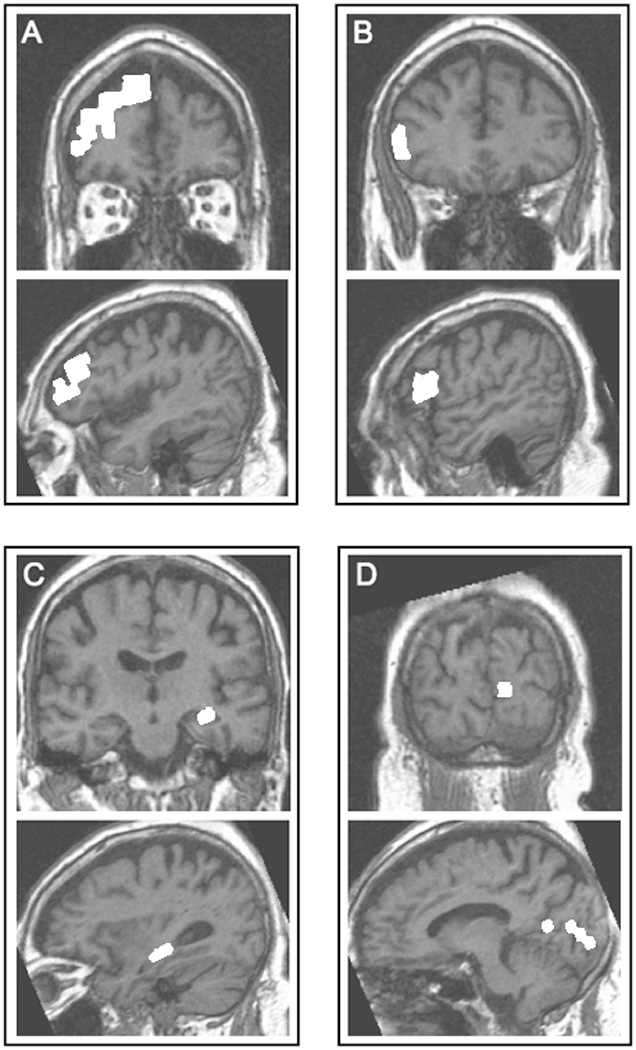
Seed/target ROIs (shown in white) from a representative subject overlaid on that subject’s high-resolution anatomical images. (A) right dorsolateral prefrontal cortex, (B) right inferior frontal gyrus, (C) left hippocampus, and (D) left primary visual cortex. For each ROI, coronal (top of panel) and sagittal (bottom of panel) views are shown.
Left hemisphere ROIs were designated as "seeds" and the homologous right hemisphere ROIs as "targets." For each seed-target pair, pre-processed time series MR signal data from the seed were averaged, and the mean signal time series was used as a reference function for cross-correlation with the time series data from all voxels in the target. From this calculation, the least squares fit coefficient was used as the index of functional connectivity at each voxel. For each ROI pair, the average fit coefficient within the target was the index of interhemispheric functional connectivity.
Four separate planned comparisons examined the effect of donepezil on interhemispheric connectivity within each of the seed-target ROI pairs. Tests for non-normality were all non-significant, confirming the appropriateness of paired t-tests for our analyses. Analyses were conducted using SPSS [24]. Given the small number of a priori comparisons, the criterion for significance was set at a per comparison error rate (α) of .05.
Results
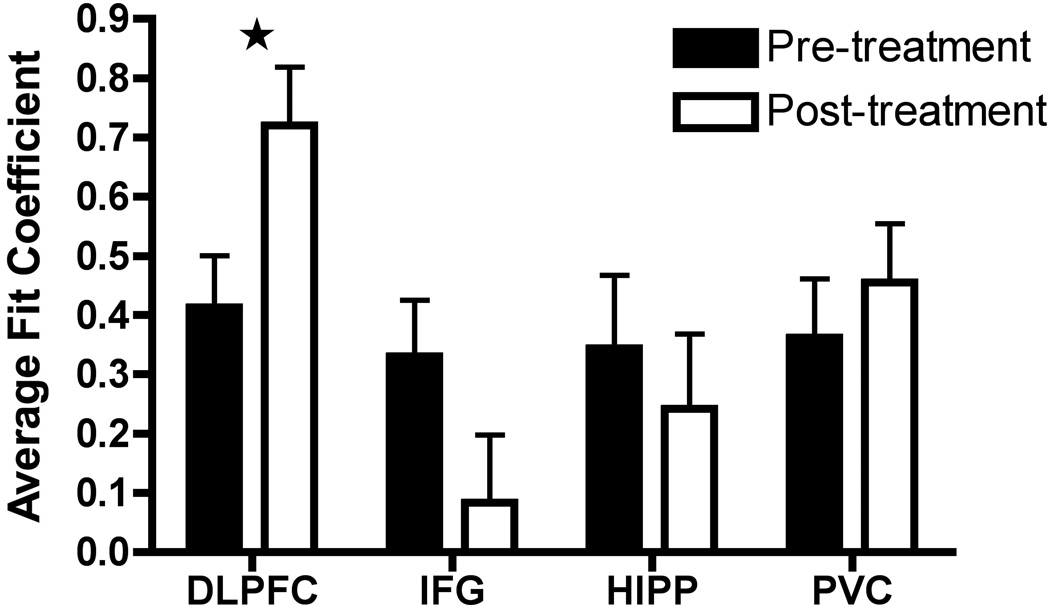
Interhemispheric functional connectivity change following treatment in each of the four ROI pairs is displayed in Figure 2. As shown in the figure, these different brain regions varied in their response to treatment. The left-right DLPFC connection was the only one to show a significant change, reflecting a large increase in functional connectivity [t(10)=−2.54, p=.029]. In contrast, connectivity between the left and right IFG showed a non-significant decrease [t(10)=1.81, p=.10], as did connectivity between the left and right HIPP [t(10)=.54, p=.60]. Lastly, control connectivity between left and right PVC showed a small non-significant increase [t(10)=−.90, p=.39].
Figure 2.
Functional connectivity values for each region of interest (ROI) before and after donepezil treatment. Bars show the mean fit coefficient for each ROI. Error bars represent standard error. Abbreviations: DLPFC = dorsolateral prefrontal cortex; IFG = inferior frontal gyrus; HIPP = hippocampus; PVC = primary visual cortex. Significant change in DLPFC is indicated with a star.
Discussion
Donepezil treatment had a differential impact on the interhemispheric connectivity of distinct brain regions. Our hypothesis that donepezil would exert its greatest effect on the functional connectivity of frontal regions was supported; DLPFC was the only region to show a significant change in connectivity. This is consistent with findings that the effects of cholinesterase inhibitors on regional AChE activity are greater in frontal cortices, when compared to temporal cortices [25]. Goveas and colleagues [10] suggested that AchE inhibitors facilitate reorganization in the neural structures that support cognition, including the DLPFC. Such reorganization might include increases in interhemispheric connectivity as observed in the present study.
We found that in contrast to the significant increase in DLPFC connectivity, interhemispheric connectivity of the hippocampus, one of the regions most severely affected by the neuropathology of AD, did not change with treatment. This may be a reflection of the fact that prefrontal cortex has a greater amount of intact functional tissue than medial temporal regions during the early stages of AD [1].
The DLPFC is especially critical for cognitive control [26], and AChE inhibitors may in fact improve cognition via their impact on attention and executive function [27]. This is supported by the observation that the patients studied here showed improvements in attention and executive control following donepezil treatment, while performance in other domains (e.g., memory) was unaffected [28]. For example, while mean pre-treatment performance on the Controlled Oral Word Association Test (COWAT), a measure of verbal fluency, was in the impaired range, mean post-treatment performance was within normal limits. To explore the association between increases in DLPFC connectivity and verbal fluency, a median split was used to divide the sample into “low” versus “high” levels of DLPFC connectivity change. Using this approach, the “low connectivity change” sub-group showed a mean increase of less than 1 raw score point on the COWAT, while the “high connectivity change” sub-group showed a mean increase of nearly 7 points. This supports a relation between increased DLPFC connectivity and enhanced aspects of executive control.
Limitations and future directions
Although the sample in the present study was small, limiting statistical power, the findings are promising. Future research should investigate treatment-related change in larger samples and more extensive brain networks, and treatment should be contrasted with placebo. In addition, to better understand the mechanisms underlying change in connectivity, future studies might examine the link between connectivity change and change in other indices of neural integrity, such as MRS measurement of oxidative stress and pH [29, 30]. The neurophysiological indices that can be drawn from fcMRI data may provide biomarkers that link neurochemical and neuroanatomical change to cognitive change in this population. Furthermore, and more broadly, our results highlight the potential use of fcMRI as a novel index of treatment outcome in various diseases that involve changes in brain connectivity.
Acknowledgements
This study was supported by a grant from the Margaret and Trammell Crow family. Dr. Weiner received research funding unrelated to the present study from Pfizer/Eisai and Forest Laboratories. Pfizer U.S. Pharmaceuticals provided Aricept™ as a gift to the investigators, but did not participate in or provide support for study conception, design, analysis or conduct. The authors gratefully acknowledge the Alzheimer’s Disease Center at UT Southwestern (NIA grant AG12300) for referral of participants; Dr. Javier Lopez-Calderon, Jennifer Hervey, and Dr. Julie Fields for technical assistance; and the participants and their families, who made this research possible.
Footnotes
Drs. Zaidel, Allen, Cullum, Briggs, Hynan, McColl, Gopinath, and Rubin and Ms. McDonald reported no conflicts of interest.
References
- 1.De Lacoste MC, White CL., III The role of cortical connectivity in Alzheimer's disease pathogenesis: a review and model system. Neurobiol Aging. 1993;14:1–16. doi: 10.1016/0197-4580(93)90015-4. [DOI] [PubMed] [Google Scholar]
- 2.He Y, Chen Z, Gong G, Evans A. Neuronal networks in Alzheimer’s disease. Neuroscientist. 2009;15:333–350. doi: 10.1177/1073858409334423. [DOI] [PubMed] [Google Scholar]
- 3.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 4.Allen G, Barnard H, McColl R, Hester AL, Fields JA, Weiner MF, Ringe WK, Lipton AM, Brooker M, McDonald E, Rubin CD, Cullum CM. Reduced hippocampal functional connectivity in Alzheimer disease. Arch Neurol. 2007;64:1482–1487. doi: 10.1001/archneur.64.10.1482. [DOI] [PubMed] [Google Scholar]
- 5.Li SJ, Li Z, Wu G, Zhang MJ, Franczak M, Antuono PG. Alzheimer Disease: evaluation of a functional MR imaging index as a marker. Radiology. 2002;225:253–259. doi: 10.1148/radiol.2251011301. [DOI] [PubMed] [Google Scholar]
- 6.Wang L, Zang Y, He Y, Liang M, Zhang X, Tian L, Wu T, Jiang T, Li K. Changes in hippocampal connectivity in the early stages of Alzheimer's disease: evidence from resting state fMRI. Neuroimage. 2006;31:496–504. doi: 10.1016/j.neuroimage.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 7.Venneri A. Imaging treatment effects in Alzheimer’s disease. Magn Reson Imaging. 2007;25:953–968. doi: 10.1016/j.mri.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Rombouts SA, Barkhof F, Van Meel CS, Scheltens P. Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002;73:665–671. doi: 10.1136/jnnp.73.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saykin AJ, Wishart HA, Rabin LA, Flashman LA, McHugh TL, Mamourian AC, Santulli RB. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127:1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- 10.Goveas JS, Xie CX, Ward BD, Wu Z, Li W, Franczak M, Jones JL, Antuono PG, Li S. Recovery of hippocampal network connectivity correlates with cognitive improvement in mild Alzheimer’s disease patients treated with donepezil by resting-state fMRI. J Magn Reson Imaging. 2011;34:764–773. doi: 10.1002/jmri.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ARDRA Work Group under the auspices of the Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 12.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 13.Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy with MRI, and Blood Supply. New York: Springer; 1999. [Google Scholar]
- 14.Duvernoy HM. The Human Hippocampus: Functional Anatomy, Vascularization, and Serial Sections with MRI. New York: Springer; 2005. [Google Scholar]
- 15.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. Amsterdam: Elsevier; 2003. [Google Scholar]
- 16.Ono M, Kubik S, Abernathey CD. Atlas of the Cerebral Sulci. New York: Thieme; 1990. [Google Scholar]
- 17.Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 18.Cook MJ, Fish DR, Shorvon SD, Straughan K, Stevens JM. Hippocampal volumetric and morphometric studies in frontal and temporal lobe epilepsy. Brain. 1992;115:1001–1015. doi: 10.1093/brain/115.4.1001. [DOI] [PubMed] [Google Scholar]
- 19.Foundas AL, Weisberg A, Browning CA, Weinberger DR. Morphology of the frontal operculum: a volumetric magnetic resonance imaging study of the pars triangularis. J Neuroimaging. 2001;11:153–159. doi: 10.1111/j.1552-6569.2001.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 20.Mega MS, Small GW, Xu ML, Felix J, Manese M, Tran NP, Dailey JI, Ercoli LM, Bookheimer SY, Toga AW. Hippocampal atrophy in persons with age-associated memory impairment: volumetry within a common space. Psychosom Med. 2002;64:487–492. doi: 10.1097/00006842-200205000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur J Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- 22.Rademacher J, Caviness VS, Jr, Steinmetz H, Galaburda AM. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cereb Cortex. 1993;3:313–329. doi: 10.1093/cercor/3.4.313. [DOI] [PubMed] [Google Scholar]
- 23.Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, Caviness VS., Jr Human cerebral cortex: Localization, parcellation, and morphometry with magnetic resonance imaging. J Cog Neurosci. 1992;4:352–374. doi: 10.1162/jocn.1992.4.4.352. [DOI] [PubMed] [Google Scholar]
- 24.SPSS Inc. SPSS Base 10.0 for Windows User's Guide. Chicago: SPSS Inc.; 1999. [Google Scholar]
- 25.Kaasinen V, Någren K, Järvenpää T, Roivainen A, Yu M, Oikonen V, Kurki T, Rinne JO. Regional effects of donepezil and rivastigmine on cortical acetylcholinesterase activity in Alzheimer's disease. J Clin Psychopharmacol. 2002;22:615–620. doi: 10.1097/00004714-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Baddeley A, Logie R, Bressi S, Della Sala S, Spinnler H. Dementia and working memory. Q J Exp Psychol A. 1986;38:603–618. doi: 10.1080/14640748608401616. [DOI] [PubMed] [Google Scholar]
- 27.Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Koeppe RA, Meltzer CC, Constantine G, Davis JG, Mathis CA, Dekosky ST, Moore RY. Degree of inhibition of cortical acetylcholinesterase activity and cognitive effects by donepezil treatment in Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:315–319. doi: 10.1136/jnnp.2004.038729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hester AL, Cullum CM, Ringe WK, Brooker M, Weiner MF, McColl R, Lipton AM, McDonald E, Rubin CD, Allen G. Improved attention and verbal fluency following short-term donepezil treatment. Arch Clin Neuropsychol. 2006;21:587. [Google Scholar]
- 29.Mandal PK, Tripathi M, Sugunan S. Brain oxidative stress: detection and mapping of anti-oxidant marker 'Glutathione' in different brain regions of healthy male/female, MCI and Alzheimer patients using non-invasive magnetic resonance spectroscopy. Biochem Biophys Res Commun. 2012;417:43–48. doi: 10.1016/j.bbrc.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 30.Mandal PK, Akolkar H, Tripathi M. Mapping of hippocampal pH and neurochemicals from in vivo multi-voxel 31P experiments in healthy normal young male/female, mild cognitive impairment, and Alzheimer's patients. J Alzheimers Dis. doi: 10.3233/JAD-2012-120166. (in press) [DOI] [PubMed] [Google Scholar]