Abstract
Current MEG instruments derive the whole-head coverage by utilizing a helmet-shaped opening at the bottom of the dewar. These helmets, however, are quite a bit larger than most people’s heads so subjects commonly lean against the back wall of the helmet in order to maintain a steady position. In such cases the anterior brain sources may be too distant to be picked up by the sensors reliably. Potential “invisibility” of the frontal and anterior temporal sources may be particularly troublesome for the studies of cognition and language, as they are subserved significantly by these areas. We examined the sensitivity of the distributed anatomically-constrained MEG (aMEG) approach to the head position (“front” vs. “back”) secured within a helmet with custom-tailored bite-bars during a lexical decision task. The anterior head position indeed resulted in much greater sensitivity to language-related activity in frontal and anterior temporal locations. These results emphasize the need to adjust the head position in the helmet in order to maximize the “visibility” of the sources in the anterior brain regions in cognitive and language tasks.
Keywords: MEG, Cognition, Language, Frontal cortex, Head position, Bite-bar, Dewar
INTRODUCTION
The extraordinary capability of MEG to detect very weak magnetic signals relies on the cryogenic methodology. Its sensors are immersed in a fixed array in liquid helium, surrounding the head in the helmet-shaped opening at the bottom of the dewar. In order to accommodate a variety of head shapes and sizes (typically designed to fit 95% of the population), the helmet is quite a bit larger than most people’s heads. When measured with gradiometers, magnitude of the dipolar magnetic field decreases with the cube of the distance [Lewine, 1995]. Hence, the sensors located closer to the sources in the brain are more sensitive to the signal than those further away. Because most recordings are performed without imposing any physical constraints on the position of the head inside the dewar, subjects find it necessary to find a spatial “frame of reference” in an effort to comply with the requirements to sit still. Commonly, they recline and lean their heads against the back of the helmet, as this makes it less effortful to maintain a constant position during the recording. However, this results in uneven distances between the sensors and the cortical sources. In this case, the posterior regions of the brain will be close to the sensors and will contribute more to the overall signal. In contrast, the frontal sources will be at a disadvantage and their contributions may not be picked up by the sensors at a distance. On the other hand, the dSPM solution (see in Methods) is normalized to the noise estimated to arise from each dipole location. Since most MEG noise is biological, the decreased signal arising from sources at an increased distance from the sensors should be partially compensated by decreased noise assigned to that location. If, however, posterior head positions render MEG insensitive to anterior sources, this would create a serious problem for many cognitive studies in general and for language studies in particular, as the frontal and anterior temporal areas crucially contribute to those functions [Halgren, 1994A] [Halgren, 1994B] [Buckner, 2000] [Fiez, 1998]. Therefore, we wished to explore effects of the head position on the “visibility” of the anterior brain sources during a standard language task. The primary aim of our study was to examine the sensitivity of the distributed solutions to the head position (“front” vs. “back”) in a standardized helmet-shaped whole-head dewar.
METHODS
Five healthy subjects (4 men and 1 woman, mean age: 23.8) participated in a lexical decision task that required responding to real words with one hand and to pronounceable (e.g. belez) and nonpronounceable (e.g. rtjkzd) letter strings with the other. The letter-strings (150 per condition) were presented for 278 ms every 2.1 seconds and subtended <50. Response-hands were switched mid-way through each experiment and the order was counterbalanced across subjects. Each subject participated in two successive experiments during the same session whereby the head was positioned towards the front end of the helmet during one run and towards the back of the helmet during the other run. In both cases the subject’s head was touching the top of the helmet, so that the main difference in positioning was along the posterior-to-anterior oriented y-axis. (The coordinate system was defined with the help of anatomical landmarks: the x-axis goes from left to right through pre-auricular points, the y-axis goes through nasion, and the z-axis points up.) The order was counterbalanced across subjects as they performed the same task but with different stimuli during each run. The position of the head in the dewar was determined by digitizing the four magnetic coils that were affixed to the head (behind the ears and just below the hairline on each side of the midline on the top of the scalp) and remained constant throughout both successive runs. In addition, a series of points across the head surface were digitized with 3Space Isotrak II system for subsequent precise co-registration with MRI images. The two respective head positions were secured with the use of custom-tailored bite-bars. A different bite-bar was made for the “front” and the “back” positions for each subject. In order to create a bite-bar mouthpiece, a plaster cast of the upper teeth was first made. A layer of thermo-forming mouthguard material was molded over the cast and then a layer of rigid baseplate material was molded over the mouthguard inner layer. The mouthpiece was attached to a U-shaped bar with a series of connectors that provided flexibility in choosing the head position. Once the head position has been selected, the connector joints were firmly glued resulting in reproducible head position. The U-shaped bar was then firmly affixed to the sides of the dewar (Fig. 1). By fixing the upper jaw, these bite-bars provided head positions for the “front” and “back” runs respectively, while minimizing motion.
Figure. 1.
Upper-jaw bite-bar mouthpieces were made for each subject and for each head position. They are attached to a bar that is firmly affixed to the sides of the dewar.
MEG signals were recorded from 204 planar gradiometers with a whole-head instrument (Elekta-Neuromag) in a magnetically and electrically shielded room. The signals were recorded continuously with 600 Hz sampling rate and minimal filtering (0.1 to 200 Hz). Artifact-free averages for each stimulus type were constructed on-line. Each person’s cortical surface was reconstructed from high-resolution T1-weighted MRI structural images (1.5T Siemens Allegra) [Dale, 1999] [Fischl, 1999] in order to obtain the anatomically-constrained MEG (aMEG) solution estimates. Group average was attained by aligning cortical folding patterns [Fischl, 1999]. Dipole orientation was unconstrained and the forward solution used a boundary element model [Oostendrop, 1991]. Dipole strength power was estimated at each cortical location using a noise-normalized linear minimum-norm approach [Dale, 1993] [Hämäläinen, 1994], resulting in “brain movies”. Group average dynamic statistical parametric maps (dSPMs) of the estimated activity are presented below.
RESULTS
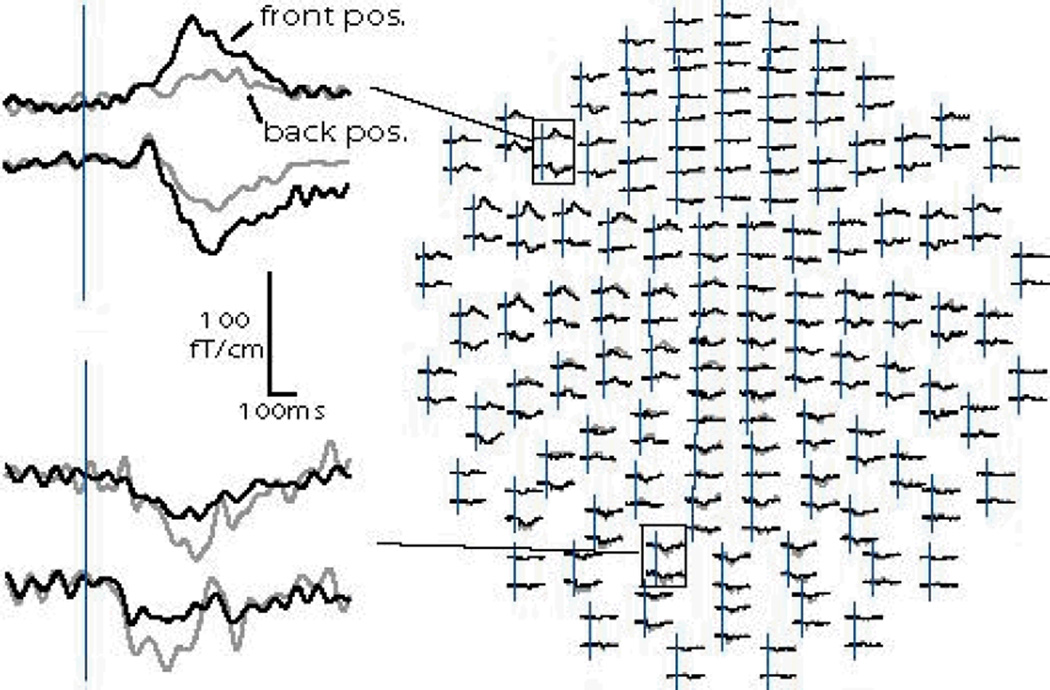
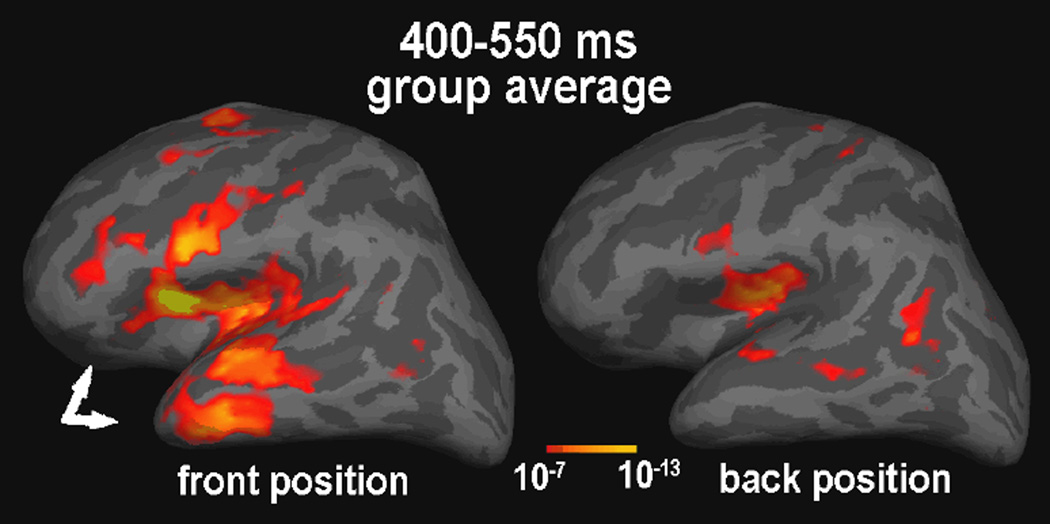
Subjects were indeed able to maintain constant head positions throughout the “front” and “back” runs, as evidenced by an average displacement of 0.25 mm as measured before and after each of the two runs. The coil position displacement between the “front” and the “back” positions was apparent mainly in the y axis (posterior-to-anterior, 16.1 mm on average), whereas the differences in the coil positions along the x (left-to-right) and z (down-to-up) axes were comparably small (1.2 and 2.1 mm respectively). Even in a subject with a large head (59.5 mm circumference), the head origin differed by 14 mm in the y direction, but only 0.4 and 1 mm in the x and z directions respectively, in „front“ to „back“ comparison. Effects of the head position difference on the currents estimated with the aMEG approach are presented below. Figure 2 illustrates the difference detected by the sensors in one subject. Average waveforms to real words recorded in the “front” (black lines) and “back” (gray lines) positions are superimposed. Two gradiometer sensor pairs show larger evoked fields over the left frontal area when the head is positioned towards the front, than when it is in an unrestrained “default” position leaning against the back of the helmet. Figure 3 illustrates the difference in dSPM solutions obtained under two head positions (“front” on the left and “back” on the right) during 400–550 ms latency window. Estimates in the “back” position primarily include the posterior temporal (Wernicke’s area) and the posterior inferior prefrontal regions. In contrast, the “front” position reveals a more extended pattern of the estimated sources at this latency, particularly in the frontal and anterior temporal regions. In an effort to provide a rather conservative test, four out of five of our subjects were males and some of them had quite large heads. Nevertheless, significant differences in sensitivity to anterior sources emerged as a function of the head position in the helmet.
Figure. 2.
Average waveforms to real words recorded in the “front” and “back” positions for one subject are superimposed. The left panel shows two gradiometer sensor pairs. Sensors over the left frontal area show larger evoked fields in the “front” position. Conversely, the evoked fields picked up by the posterior sensors are stronger in the “back” position.
Figure. 3.
Averaged dSPMs to word-specific activity observed during the “front” and the “back” head position runs in the same subjects. The white arrows point to the fronto-temporal activity estimates that are “visible” only in the “front” head position.
DISCUSSION
These results indicate that the head position in the whole-head MEG helmet has a significant effect on the sensitivity to the sources in the brain. Because the strength of the magnetic field decreases with the square of the distance, it is important to position the brain sources of primary interest as close to the sensors as possible. A device (such as a bite-bar) that helps the subject to fix his/her head comfortably in a particular position, to maintain the same position throughout the run and minimize motion artifacts seems to be essential in achieving that goal [Adjamian, 2004]. In cognitive tasks, particularly those involving language, the fronto-temporal regions may be of utmost interest to a researcher as they subserve semantic and mnemonic processing of both spoken and written words as suggested by MEG [Marinkovic, 2004], functional neuroimaging [Buckner, 2000] [Fiez, 1998] [Wagner, 2000], and intracranial studies [Halgren, 1994A] [Halgren, 1994B]. This may be particularly critical for the N400m (magnetic equivalent of the “N400”), which indexes semantic and contextual integration, or access to meaning [Halgren, 2002]. Previous MEG studies of language relying on the distributed aMEG approach [Dhond, 2001] [Marinkovic, 2003] indicated left temporo-prefrontal regions as the main N400m sources, whereas the MEG studies utilizing the ECD approach estimated the main dipole in the superior posterior temporal area [Helenius, 1998] [Sekiguchi, 2001]. The differences could be explained by the respective source modeling techniques [Halgren, 2002], but also potentially by the head position in the helmet. The commonly adopted “back” position may put the anterior sources at a disadvantage because of the increased distance between the brain and the sensors. Comparable conclusions have been suggested by other recent studies [Takeuchi, 2004]. In sum, these results emphasize the need to adjust the head position in the helmet in order to maximize the “visibility” of the sources in the anterior brain regions in cognitive and language tasks.
ACKNOWLEDGEMENTS
Supported by NIH grants AA13402 (K.M.) NS 18741 (E.H.), P41RR14075 (Martinos Center) and the MIND Institute. We thank Anders Dale, Thomas Witzel, Matti Hämäläinen and Bruce Rosen.
REFERENCES
- Adjamian P, Barnes GR, Hillebrand A, et al. Co-registration of magnetoencephalography with magnetic resonance imaging using bite-bar-based fiducials and surface-matching. Clin Neurophysiol. 2004;115(3):691–698. doi: 10.1016/j.clinph.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR. Functional MRI evidence for a role of frontal and inferior temporal cortex in a modal components of priming. Brain. 2000;123(3):620–640. doi: 10.1093/brain/123.3.620. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dhond RP, Buckner RL, Dale AM, Marinkovic K, Halgren E. Sequence of brain activity underlying word-stem completion. Journal of Neuroscience. 2001;21(10):3564–3571. doi: 10.1523/JNEUROSCI.21-10-03564.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proc Natl Acad Sci USA. 1998;95(3):914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K. Spatio-temporal stages in face and word processing. I. Depth-recorded potentials in the human occipital, temporal and parietal lobes. J Physiol Paris. 1994;88(1):1–50. doi: 10.1016/0928-4257(94)90092-2. [corrected] [published erratum appears in J Physiol Paris 1994;88(2):following 151] [DOI] [PubMed] [Google Scholar]
- Halgren E, Baudena P, Heit G, Clarke JM, Marinkovic K, Chauvel P. Spatio-temporal stages in face and word processing. 2. Depth-recorded potentials in the human frontal and Rolandic cortices. J Physiol Paris. 1994;88(1):51–80. doi: 10.1016/0928-4257(94)90093-0. [published erratum appears in J Physiol Paris 1994;88(2):following 151] [DOI] [PubMed] [Google Scholar]
- Halgren E, Dhond RP, Christensen N, et al. N400-like magnetoencephalography responses modulated by semantic context, word frequency, and lexical class in sentences. Neuroimage. 2002;17(3):1101–1116. doi: 10.1006/nimg.2002.1268. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput. 1994;32(1):35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Helenius P, Salmelin R, Service E, Connolly JF. Distinct time courses of word and context comprehension in the left temporal cortex. Brain. 1998;121(6):1133–1142. doi: 10.1093/brain/121.6.1133. [DOI] [PubMed] [Google Scholar]
- Lewine JD, Orrison WW. Magnetoencephalography and magnetic source imaging. In: Orrison WW, Lewine JD, Sanders JA, Hartshorne MF, editors. Functional Brain Imaging. Mosby: St. Louis; 1995. pp. 369–418. [Google Scholar]
- Marinkovic K. Spatiotemporal dynamics of word processing in the human cortex. The Neuroscientist. 2004;10(2):142–152. doi: 10.1177/1073858403261018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovic K, Dhond RP, Dale AM, Glessner M, Carr V, Halgren E. Spatiotemporal dynamics of modality-specific and supramodal word processing. Neuron. 2003;38(3):487–497. doi: 10.1016/s0896-6273(03)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostendorp T, van Oosterom A. The potential distribution generated by surface electrodes in inhomogeneous volume conductors of arbitrary shape. IEEE Trans Biomed Eng. 1991;38(5):409–417. doi: 10.1109/10.81559. [DOI] [PubMed] [Google Scholar]
- Sekiguchi T, Koyama S, Kakigi R. The effect of stimulus repetition on cortical magnetic responses evoked by words and nonwords. Neuroimage. 2001;14(1 Pt 1):118–128. doi: 10.1006/nimg.2001.0774. [DOI] [PubMed] [Google Scholar]
- Takeuchi F, Kamada K, Kuriki S, Kawaguchi H. Paper presented at: Biomag. Boston: 2004. MEG responses from the frontal regions using a whole head SQUID system. [Google Scholar]
- Wagner AD, Koutstaal W, Maril A, Schacter DL, Buckner RL. Task-specific repetition priming in left inferior prefrontal cortex. Cereb Cortex. 2000;10(12):1176–1184. doi: 10.1093/cercor/10.12.1176. [DOI] [PubMed] [Google Scholar]