Abstract
This study explored the knowledge, attitude, practice and barriers to prescribe human papillomavirus (HPV) vaccines among private primary care physicians in Hong Kong. A self-administered questionnaire survey was conducted by sending letters to doctors who had joined a vaccination program for school girls. From 720 surveys sent, 444 (61.7%) completed questionnaires were returned and analyzed. For knowledge, few responded to questions accurately on the prevalence of cervical HPV (27.9%) and genital wart infection (13.1%) among sexually active young women in Hong Kong, and only 44.4% correctly answered the percentage of cervical cancers caused by HPV. For attitude, most agreed that HPV vaccination should be fully paid by the Government (68.3%) as an important public health strategy. Vaccination against HPV was perceived as more important than those for genital herpes (52.2%) and Chlamydia (50.1%) for adolescent health, and the majority selected adolescents aged 12–14 years as the ideal group for vaccination. Gardasil® (30.9%) and Cervarix® (28.0%) were almost equally preferred. For practice, the factors influencing the choice of vaccine included strength of vaccine protection (61.1%), long-lasting immunity (56.8%) and good antibody response (55.6%). The most significant barriers to prescribe HPV vaccines consisted of parental refusal due to safety concerns (48.2%), and their practice of advising vaccination was mostly affected by local Governmental recommendations (78.7%). A substantial proportion of physicians had recommended HPV vaccines for their female clients/patients aged 18–26 years for protection of cervical cancer (83.8%) or both cervical cancer and genital warts (85.5%). The knowledge on HPV infection was low among physicians in Hong Kong. Prescription of HPV vaccine was hindered by the perceived parental concerns and was mostly relied on Governmental recommendations. Educational initiatives should be targeted towards both physicians and parents, and the Government should consider full subsidy to enhance vaccine uptake rate.
Introduction
Human papillomavirus (HPV) infection is the most common sexually transmitted disease worldwide. It has been estimated that at least 50% of sexually active people had acquired genital HPV infection during their lifetime [1], and in a recent report the prevalence of genital HPV infection was 14% in females aged 26–30 years [2]. Virtually all cases of cervical cancer are attributed to HPV, with HPV 16 and 18 accounting for 58% to 75% of all cervical cancers in Asia Pacific countries which was similar to Western countries [3]–[8]. Two vaccines (Gardasil®, Merck & Co. and Cervarix®, GlaxoSmithKline) have demonstrated high efficacy against cervical intraepithelial neoplasia and invasive cancers associated with vaccine-types (HPV16 and 18) [9]–[13], and their administration have been anticipated to be a crucial measure to reduce the incidence of cervical cancer worldwide [14], [15].
However, in order for the vaccination programmes to be successful, a high uptake rate is needed. Recommendation of HPV immunization by primary care physicians (PCPs) has been recognized as one the most influential factors in the individual’s willingness to receive the vaccine. Updated knowledge and positive attitude towards the vaccine among PCPs, as well as the removal of any barriers to prescription are the main determinant factors [16], [17]. PCPs represent a taskforce in the healthcare system which offered first-contact, coordinative preventive care to the general public and are in a privileged position to prescribe HPV immunizations. Many studies showed that physicians’ experience and attitudes towards HPV vaccine were major motivators affecting women and adolescent girls to receive the immunization [18]–[20].
Nevertheless, most of the existing studies were conducted among physicians in Western countries [21]–[29]. Half of the cervical cancer cases are in Asia [30], but there is a scarcity of original research conducted in Asia [31]. The differences in cultural environment and clinical practice among physicians practicing in different cultural and clinical environment remain to be explored.
The objectives of this study were to evaluate the knowledge on HPV infection, and the attitude towards and perceived barriers of HPV vaccination among PCPs in Hong Kong. Their practice of prescribing HPV vaccine according to recommendations and patient characteristics was also explored.
Methods
Sampling Frame and Recruitment Strategies
As HPV is not under universal coverage in Hong Kong, a Family-School-Doctor Model was developed by university academics in 2011 to improve the initiation of HPV vaccine among adolescent girls by tackling the socio-cultural barriers and also the barriers related to cost and convenience [32]. In Hong Kong, HPV vaccination is not provided in the public sector and patients have to pay out-of-pocket to receive the immunization in the private sector. Online education platform was built to provide free education videos for students and parents (http://www.youitv.com/partner/hps/cervicalcancer/). The contents of the online platform include the prevalence of cervical cancer, causes, symptoms, preventive measure and information of the vaccine. Schools were invited to join a special seminar conducted by the Chinese University of Hong Kong for knowledge enrichment. Schools were given an option to join the voluntary HPV vaccination programme, and participated students had the first dose (Cervarix®) free of charge on site at their schools. The acceptance of the 1st free-of-charge dose of Cervarix at school was over 80%, and there has been no local recommendations regarding interchangeable use of two HPV vaccines. Physicians were not recommended to prescribe Gardasil after an initial dose of Cervarix. The second and third doses were then administered in the clinics of PCPs at market price determined by individual physicians. The PCPs in Hong Kong consist of general practitioners, family physicians, and specialists in the private sector who practice in the community setting including medical internists, gynecologists, pediatricians and oncologists. All 720 PCPs who participated in the school HPV vaccination programme received an invitation letter to complete an anonymous questionnaire designed for the current study.
Since part of this survey assessed the knowledge of the doctors on the research topic, the surveys were kept anonymous and did not require participants’ signatures. The completion of the survey by the study participants implied their consent. In terms of the measures that were taken to document the process, the reseachers invited the participants to return their completed surveys in an enclosed envelope after which they could sign their name in an attendance sheet to obtain Continuous Medical Education (CME) points. The return of their surveys was recorded to evaluate the response rate. The Survey and Behavioral Research Ethics Committee of The Chinese University of Hong Kong has approved this study and the consent procedure. The study did not involve minors/children participants, thus the concerns related to minors/children participants were not applicable in this study.
Survey Instrument
The survey used in this study was designed by an academic physician, and validated by an expert panel consisting of epidemiologists, public health specialists, family physicians, microbiologists and academic professionals. The survey was pilot-tested by 20 physicians for face-validity and comprehensibility before study commencement. In addition, the information provided in the online platform was not related to the questions in the survey to ensure accurate assessment of the PCPs’ knowledge. We used a five-point Likert scale for survey items which assessed attitude towards the HPV vaccine. These questions are close-ended with possible responses including “extremely important”, “very important”, “important”, “somewhat important”, and “not at all”. The same applied to questions on their perceived barriers and likelihood to follow recommendations on administration of HPV vaccines. The questionnaire consists of 10 pages with 33 questions, and English was used in the survey as all PCPs in Hong Kong know English. For assessment of PCPs’ knowledge on HPV vaccine, the correctness of an answer was established from a thorough literature search and validation by the expert panel who designed the survey. For comparison of importance among different vaccines, one question item was phrased as “Compared to the following other vaccines that are in use or development, how would you rank the HPV vaccine in terms of importance for adolescent health?” According to our pilot study, the PCPs will need an average of 10 minutes to complete the questionnaire.
Statistical Analysis
The Statistical Package for Social Sciences (SPSS, Chicago, IL) version 16.0 was used for all data entry and analyses. All descriptive statistics were presented as proportions. Chi square tests of heterogeneity were used to compare proportions. As an exploratory analysis, the proportions of physicians choosing Cervarix® vs. Gardasil® in their routine practice were compared according to the reasons of vaccine choice. P values <0.05 were regarded as statistically significant.
Results
Participant Characteristics
Of the 720 questionnaires distributed, 444 completed ones were collected (Table 1). The average age was 48.5 years (SD 12.1). 77.3% were male, and half (52.8%) had post-registration experience of more than 20 years (Table 1). The proportions of general practitioners or specialists in family medicine, pediatricians, and gynecologists were 76.4%, 12.2% and 4.3%, respectively. 44.8% of them reported seeing 26–99 patients aged between 10 to 17 years per week, and 42.1% saw 26–99 parents with teenage children per week. 82.2% indicated that they did not see any patients with cervical cancer in a typical month. In a typical week, 63.0% did not perform any Pap smears, and 34.1% reported performing 1–25 Pap smears. The majority of these physicians prescribed 1–25 HPV vaccines in the past 3 months for girls aged 10–17 years (77.8%), girls aged 18–26 years (84.3%), and women aged 27–45 years (74.0%). HPV vaccinations were rarely prescribed for older women, boys and men. Nearly 15% of PCPs never advised their adolescent patients on sex related matters and about two thirds advised in less than 25% of their consultation with adolescents (Table 1).
Table 1. Participant Characteristics (N = 444).
| N | % | |||||||
| Age (mean +/− S.D.) | 48.5 | 12.1 | ||||||
| Gender | ||||||||
| Female | 98 | 22.1 | ||||||
| Male | 343 | 77.3 | ||||||
| Years after post registration | ||||||||
| Over 30 years | 117 | 26.4 | ||||||
| 21 to 30 years | 117 | 26.4 | ||||||
| 11–20 years | 147 | 33.1 | ||||||
| 10 years or below | 58 | 13.1 | ||||||
| Clinical specialty | ||||||||
| Pediatrician | 54 | 12.2 | ||||||
| Gynecologist | 19 | 4.3 | ||||||
| Oncologist | 1 | 0.2 | ||||||
| Internal Medicine specialist | 12 | 2.7 | ||||||
| General Practice/Family Medicine | 339 | 76.4 | ||||||
| Others | 14 | 3.2 | ||||||
| Number of patients seen in a typical week | ||||||||
| a). Aged 10–17 years | ||||||||
| None | 14 | 3.2 | ||||||
| Jan-25 | 150 | 33.8 | ||||||
| 26–99 | 199 | 44.8 | ||||||
| ≥100 | 79 | 17.8 | ||||||
| b). Parents with teenage children | ||||||||
| None | 16 | 3.6 | ||||||
| Jan-25 | 135 | 30.4 | ||||||
| 26–99 | 187 | 42.1 | ||||||
| ≥100 | 100 | 22.5 | ||||||
| Number of patients with cervical cancer seen in a typical month | ||||||||
| None | 365 | 82.2 | ||||||
| Jan-25 | 66 | 14.9 | ||||||
| 26–99 | 7 | 1.6 | ||||||
| ≥100 | 1 | 0.2 | ||||||
| Number of Pap smear performed in a typical week | ||||||||
| None | 277 | 63 | ||||||
| Jan-25 | 150 | 34.1 | ||||||
| 26–99 | 11 | 2.5 | ||||||
| ≥100 | 2 | 0.5 | ||||||
| Number of HPV vaccines prescribed in the past 3 months | None | 1–25 | 26–99 | ≥100 | ||||
| n | % | n | % | n | % | n | % | |
| Girls 10–17 years | 86 | 21.0 | 318 | 77.8 | 4 | 1.0 | 1 | 0.2 |
| Girls 18–26 years | 56 | 13.3 | 354 | 84.3 | 7 | 1.7 | 3 | 0.7 |
| Women 27–45 years | 98 | 23.4 | 310 | 74.0 | 10 | 2.4 | 1 | 0.2 |
| Women >45 years | 327 | 87.9 | 43 | 11.6 | 1 | 0.3 | 1 | 0.3 |
| Boys 10–15 years | 356 | 95.4 | 15 | 4.0 | 1 | 0.3 | 1 | 0.3 |
| Boys/men >15 years | 357 | 94.9 | 17 | 4.5 | 0 | 0.0 | 2 | 0.5 |
| Frequency of discussion of sexual activity with adolescent patients during a usual visit | n | % | ||||||
| Never | 61 | 13.9 | ||||||
| ≤25% of visits | 291 | 66.4 | ||||||
| 25–50% of visits | 45 | 10.3 | ||||||
| 50–90% of visits | 21 | 4.8 | ||||||
| >90% of visits | 10 | 2.3 | ||||||
| Not applicable | 10 | 2.3 | ||||||
HPV: Human Papillomavirus.
Knowledge of HPV Vaccination
Only 27.9% and 13.1% of physicians correctly responded to questions on the prevalence of cervical HPV infection and genital wart infection, respectively, among sexually active young women in Hong Kong (Table 2). Only 44.4% correctly answered the percentage of cervical cancers caused by HPV. However, high proportions of physicians could accurately identify the disease entities associated with HPV 6 and 11 (84.7%) and HPV 16 and 18 (88.1%).
Table 2. Knowledge on human papillomavirus (HPV) infection (N = 444).
| n | % | N (correct answers given) | % (correct answers given) | |
| Prevalence of HPV infection in sexually active young female in HK | ||||
| 5–10% | 106 | 23.9 | 124 | 27.9 |
| 11–30% | 124 | 27.9 | ||
| 31–50% | 71 | 16.0 | ||
| 51–75% | 54 | 12.2 | ||
| 76–100% | 18 | 4.1 | ||
| Prevalence of genital warts infection in sexually active young female in HK | ||||
| ≤1% | 58 | 13.1 | 58 | 13.1 |
| 2–5% | 129 | 29.1 | ||
| 6–10% | 95 | 21.4 | ||
| 11–20% | 57 | 12.8 | ||
| >20% | 31 | 7.0 | ||
| Percentage of cervical cancer caused by infection with HPV | ||||
| 0–25% | 37 | 8.3 | 197 | 44.4 |
| 26–50% | 32 | 7.2 | ||
| 51–75% | 108 | 24.3 | ||
| 76–100% | 197 | 44.4 | ||
| HPV subtypes 6 & 11 commonly associated with what disease | ||||
| Genital warts | 376 | 84.7 | 376 | 84.7 |
| Plantar warts | 4 | 0.9 | ||
| Cervical carcinoma | 30 | 6.8 | ||
| Unsure | 20 | 4.5 | ||
| HPV subtypes 16 & 18 commonly associated with what disease | ||||
| Genital warts | 15 | 3.4 | 391 | 88.1 |
| Plantar warts | 4 | 0.9 | ||
| Cervical carcinoma | 391 | 88.1 | ||
| Unsure | 18 | 4.1 | ||
Attitudes towards Payment, Importance and Ideal Age Range of HPV Vaccination
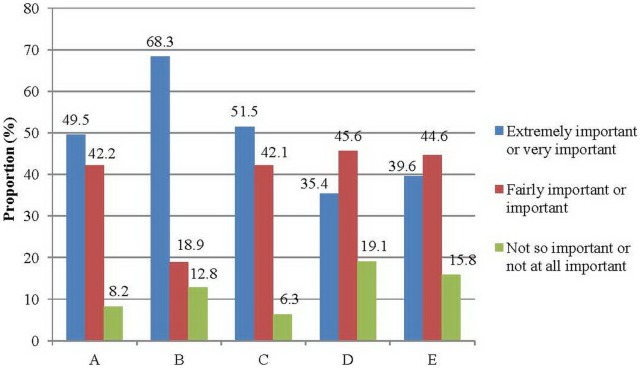
The participants were asked about the importance of different public health strategies for promoting HPV vaccination (Fig. 1). They were of the view that HPV vaccination should be fully paid by the Government as an “extremely important” or “very important” public health strategy (68.3%). This was followed by “prices offered at a 50% discount” (51.5%) and “partial subsidy by the Government at a 30% discount” (49.5%). Immunization program jointly organized by school and community doctors with market price (39.6%), and school immunization programmes at market price (33.4%) were not regarded as important strategies. When the participants were asked whether HPV vaccination was more important than other vaccines for adolescent health, most agreed that HPV immunization was more important than those for genital herpes (52.2%) and chlamydia (50.1%). Many regarded HPV vaccination as equally important when compared with hepatitis B (61.7%), tuberculosis (57.4%), influenza (45.4%) and chickenpox (47.8%).
Figure 1. Attitude towards the importance of public health strategies to promote human papillomavirus vaccination.
(A: Partial (∼30%) subsidy by the Government; B: Fully paid by the Government; C: Price offered at 50% discount; D: School immunization program with market price; E: Immunization program jointly organized by school and community doctors with market price).
The attitudes of participating PCPs towards the ideal age range for HPV vaccine administration were explored. Most physicians indicated that 12–14 years (45.6%), 10–11 years (31.6%), and 15–17 years (20.3%) were the ideal age groups. Very few regarded ages 18 years or older as ideal for HPV vaccination.
Preference and Reasons to Choose HPV Vaccines for Adolescent Girls Aged 10–17 Years
41.1% of participants did not express any preference to the two available vaccines (Table 3). Among those indicated a preference, Gardasil® (30.9%) and Cervarix® (28.0%) were approximately equally preferred. The major reason for choosing a vaccine regarded by the participants as “extremely important” or “very important” included its strength of protection (61.1%), immunity being long-lasting (56.8%), good antibody response (55.6%), more HPV types covered (53.5%), cross-protection for other cancer-associated HPV types (53.4%) and vaccine safety (51.2%). Factors which were less considered included vaccine price (27.9%), credibility of manufacturers (28%) and comprehensive service of manufacturers (29.2%) (Table 3). Among physicians who preferred Cervarix®, greater proportions regarded stronger protection, better antibody response and long-lasting immunity as extremely or very important when compared with those who preferred Gardasil® (all p<0.05) (Fig. 2). Alternatively, physicians choosing Gardasil® had significantly higher proportions regarding more HPV types to be covered, its protection against genital warts, better patient perception, and Government selection (though our Government did not express any preference) as important reasons for the choice (all p<0.05) (Fig. 2).
Table 3. Preference and reasons to choose human papillomavirus (HPV) vaccines (bivalent vs. quadrivalent) in practice for adolescent girls aged 10–17 years.
| n | %a | ||||
| Bivalent - Cervarix® | 116 | 28.0 | |||
| Quadrivalent - Gardasil® | 128 | 30.9 | |||
| No preference | 170 | 41.1 | |||
| Extremely or very important | Important | Fairly important | Somewhat important | Not at all important | |
| Stronger protection | 138 (61.1) | 49 (21.7) | 14 (6.2) | 8 (3.5) | 17 (7.5) |
| Safety | 134 (51.2) | 79 (30.2) | 23 (8.8) | 8 (3.1) | 18 (6.9) |
| Lower price | 67 (27.9) | 73 (30.4) | 39 (16.3) | 18 (7.5) | 43 (17.9) |
| More HPV types | 138 (53.5) | 53 (20.5) | 19 (7.4) | 17 (6.6) | 31 (12.0) |
| Protect genital warts | 108 (44.6) | 51 (21.1) | 23 (9.5) | 21 (8.7) | 39 (16.1) |
| Better antibody response | 145 (55.6) | 63 (24.1) | 23 (8.8) | 12 (4.6) | 18 (6.9) |
| Long-lasting immunity | 141 (56.8) | 63 (25.4) | 19 (7.7) | 8 (3.2) | 17 (6.9) |
| Cross-protection for other cancer-associated HPV types | 139 (53.4) | 78 (30.0) | 18 (6.9) | 7 (2.7) | 18 (6.9) |
| Better adjuvant | 86 (34.4) | 80 (32.0) | 36 (14.4) | 12 (4.8) | 36 (14.4) |
| Patients think it’s better | 86 (33.4) | 73 (28.4) | 36 (14.0) | 20 (7.8) | 42 (16.3) |
| Practice management | 66 (26.8) | 78 (31.7) | 27 (11.0) | 15 (6.1) | 60 (24.4) |
| Selected by Government | 79 (30.6) | 88 (34.1) | 29 (11.2) | 19 (7.4) | 43 (16.7) |
| Credibility of manufacturer | 68 (28) | 85 (35.0) | 29 (11.9) | 22 (9.1) | 39 (16.0) |
| Comprehensive service of manufacturer | 68 (29.2) | 74 (31.8) | 27 (11.6) | 24 (10.3) | 40 (17.2) |
Percentages in brackets were valid % which excluded missing variables.
Figure 2. The assoication between preferred vaccine (Cervarix® vs. Gardasil® vs. no preference) and the reasons to choose vaccines (A to N).
(A: Stronger protection; B: Safety; C: Lower price; D: More HPV types; E: Protect against genital warts; F: Better antibody response; G: Long-lasting immunity; H: Cross-protection for other cancer-associated HPV types; I: Better adjuvant; J: Patients think it’s better; K: Practice management; L: Selected by the Government; M: Credibility of manufacturer; N: Comprehensive service of manufacturer). All comparisons among vaccine preference groups across reasons to choose vaccine categories (A to N) were statistically significant (p<0.05).
Perceived Barriers to Advise Adolescents Aged 10–17 Years for HPV Vaccination
The most frequent factors perceived as “extremely likely” or “somewhat likely” barriers to advise HPV vaccination among adolescents included parental refusal due to safety concerns (78.6%), parental reluctance to discuss sexuality and sexually transmitted diseases (70.4%), and physicians being regarded as hard selling an expensive vaccine (65.0%) (Table 4). Most perceived that parents were less attentive to vaccines once the child was older than 5 years (62.6%). The participating physicians also concerned that parents would refuse based on too many vaccines for their children (56.1%), or parental disbelief in vaccination (56.8%). Physicians also perceived the difficulty to get adolescents showing up for well visits (58.0%), too low parental awareness or being time-consuming (51.8%), as well as parental perception of labeling their children at risk for sexually transmitted diseases (44.9%), were extremely or somewhat important barriers (Table 4).
Table 4. Perceived barriers to advising adolescents aged 10–17 years for human papillomavirus (HPV) vaccination.
| Extremely Likely or Somewhat Likely | Extremely Unlikely, Somewhat unlikely, or Neutral | |
| Parental refusal | ||
| Child too many vaccine | 198 (56.1) | 155 (43.9) |
| Safety concern | 282 (78.6) | 77 (21.4) |
| Not believe in vaccine | 201 (56.8) | 153 (43.2) |
| Less attentive to vaccine once child is >5 years | 229 (62.6) | 137 (37.4) |
| Parental reluctance to discuss sexuality/STD | 254 (70.4) | 107 (29.6) |
| Parental perception of singling out their child as at risk for STD | 164 (44.9) | 201 (55.1) |
| Parental concern of increasing risky behavior | 149 (41.0) | 214 (59.0) |
| Frequent changes of recommendation for immunization | 155 (42.9) | 206 (57.1) |
| Doctors’ reluctance to offer multiple vaccines | 107 (30.1) | 249 (69.9) |
| Doctors’ reluctance to discuss sexuality/STD | 118 (32.3) | 247 (67.7) |
| Difficult to get adolescents to show up for well visits/immunizations | 207 (58.0) | 150 (42.0) |
| Doctors’ reluctance since regular pap smear is still needed after vaccine | 96 (26.4) | 268 (73.6) |
| Doctors’ reluctance as vaccine’s protection is <100% | 102 (28.2) | 260 (71.8) |
| Too low parental awareness, time-consuming | 187 (51.8) | 174 (48.2) |
| Hard selling an expensive vaccine | 234 (65.0) | 126 (35.0) |
| Vaccination not related to the reasons of consultation | 201 (55.7) | 160 (44.3) |
| Not the doctors’ responsibility | 65 (18.5) | 286 (81.5) |
| Difficulty to initiate the conversation | 125 (34.4) | 238 (65.6) |
| Most adolescent patients not at risk for HPV infection | 86 (24.2) | 269 (75.8) |
| Most adolescent patients not at risk for cervical cancer | 85 (23.5) | 277 (76.5) |
STD: Sexually Transmitted Disease.
Practice of HPV Vaccination According to Recommendations, Age Groups and Sex
A majority of the physicians reported that the recommendations to prescribe HPV vaccine to adolescents were “extremely likely” or “somewhat likely” influenced by the local Government (the Department of Health) (95.8%), the respective colleges of their specialties (93.8%), local University academics (90.7%) and overseas authorities or colleges (86.8%) (Table 5). Very few physicians recommended HPV vaccine among males irrespective of patients’ age and the nature of vaccine protection (Table 5). A substantial proportion recommended HPV vaccines among females aged 18–26 years for prevention of cervical cancer alone (95.7%), and genital warts and cervical cancer combined (96.9%). The proportions were similarly high among females aged 10–17 years and 27–36 years, whilst the proportion recommending HPV vaccine was fewer among women aged 37–45 years (Table 5).
Table 5. Practice of human papillomavirus (HPV) vaccination according to recommendations, age groups and sex.
| Extremely Likely or Somewhat Likely to follow recommendation | Extremely Unlikely, Somewhat unlikely, or neither likely or unlikely to follow recommendation | |
| Your colleagues | 346 (83.6) | 68 (16.4) |
| The Hong Kong College of your specialty | 393 (93.8) | 26 (6.2) |
| The Department of Health | 415 (95.8) | 18 (4.2) |
| The oversea authorities or colleges | 363 (86.8) | 55 (13.2) |
| Local university academics | 380 (90.7) | 39 (9.3) |
| A pharmaceutical representative | 285 (68.0) | 134 (32.0) |
| Cervical cancer only | ||
| 10–17 year old girl | 403 (94.8) | 22 (5.2) |
| 10–17 year old boy | 141 (34.0) | 274 (66.0) |
| 18–26 year old girl | 402 (95.7) | 18 (4.3) |
| 18–26 year old boy | 132 (32.4) | 275 (67.6) |
| 27–36 year old female | 387 (92.8) | 30 (7.2) |
| 27–36 year old male | 113 (27.6) | 296 (72.4) |
| 37–45 year old female | 352 (84.4) | 65 (15.6) |
| 37–45 year old male | 105 (25.4) | 308 (74.6) |
| Genital warts and cervical cancer | ||
| 10–17 year old girl | 408 (96.9) | 13 (3.1) |
| 10–17 year old boy | 281 (68.2) | 131 (31.8) |
| 18–26 year old girl | 405 (96.9) | 13 (3.1) |
| 18–26 year old boy | 279 (68.4) | 129 (31.6) |
| 27–36 year old female | 394 (94.9) | 21 (5.1) |
| 27–36 year old male | 242 (59.5) | 165 (40.5) |
| 37–45 year old female | 361 (87.0) | 54 (13.0) |
| 37–45 year old male | 219 (53.2) | 193 (46.8) |
Discussion
Major Findings
This study found that the knowledge on HPV prevalence was low yet the knowledge on the role of different HPV types in cervical cancer and anogenital warts was high among PCPs in Hong Kong. They regarded HPV vaccine relatively important for adolescent health, and should preferably be administered at the age range of 12–14 years. Cervarix® and Gardasil® were almost equally preferred, with the major factors for vaccine choice being their strength of protection, duration of immunity, cross-protection for other cancer-related HPV types, types of HPV covered and safety. Parental refusal based on vaccine safety and reluctance to discuss sexually transmitted diseases as perceived by physicians represented the most frequently encountered barriers. Most physicians recommended HPV vaccination among females aged 18–26 years, and followed guidelines from the Government and their respective colleges.
Compared with Other Studies - knowledge
A survey among 214 Vaccine For Children (VFC) providers in Georgia reported high levels of knowledge about the epidemiology of HPV infection, the causative role of HPV for cervical cancer, and the status of FDA approval for quadrivalent HPV vaccines for use in adolescents aged 9–26 years (range 76.7% to 97.2%) [23]. In addition, one multi-centre survey involving 1,282 Canadian physicians also showed that their knowledge about causation of HPV to cervical cancer and anogenital cancer was high (56.2% to 96.1%) [29]. On the contrary, a large-scale cross-sectional study among a nationally representative sample of primary care pediatricians in US showed that almost one-third were unaware that HPV has a causative role in cervical cancer [21]. A recent survey in West Yorkshire among 222 general practitioners, pediatricians and gynecologists showed that 38% felt inadequately informed about the HPV vaccine, and up to 55% had lack of knowledge about the aetiology of cervical cancer [28]. Hence, current literature pointed towards heterogeneity of knowledge levels among physicians depending on the location of survey and their specialties. Our study pointed towards lower levels of knowledge on the epidemiology of HPV infection but higher levels of knowledge on etiology when compared with these surveys.
Compared with Other Studies - Attitude
Turning to physicians’ attitude towards HPV vaccine, a high degree of overall acceptance for HPV vaccine use directed against cervical cancer and genital warts was consistently reported by several large-scale studies, including primary care providers like family physicians, gynecologists and pediatricians [21], [22], [24]–[29]. A recent systematic review [33] including 25 studies showed strong support for HPV vaccination in their practice although gynecologists did not perceive themselves as traditional vaccinators [22]. These were compatible with our findings that PCPs perceived HPV vaccine was an important strategy to promote adolescent health. Of interest is the discrepancy between the ideal age range (12–14 years) for HPV vaccination perceived by PCPs and the age group (18–26 years) most commonly recommended for vaccination. This may reflect some underlying barriers preventing PCPs to recommend vaccination to young teenagers despite their wish to do so.
Compared with Other Studies – Practice and Barriers
For the ideal age of adolescent girls to receive HPV vaccine from the PCPs’ perspective, the present study reported 12–14 years as the range most commonly selected by our study participants. One study reported that 89% of pediatricians in the US recommended HPV vaccines to girls aged 16–18 years, whilst only 46% recommended vaccines for adolescent girls aged 10–12 years [21]. Similarly, another study in US showed that fewer family physicians and pediatricians recommended HPV vaccines for children aged 11–12 years than for older female subjects [25]. The current US Food and Drug Administration approved quadrivalent HPV vaccine to be used for females aged 9 to 26 years in 2006 [34], and the Advisory Committee on Immunization Practices (ACIP) voted to administer HPV vaccines among adolescents aged 11–12 years [35]. Our study found that a large proportion of PCPs perceived 10–11 years (31.6%) and 12–14 years (45.6%) as the ideal age ranges. This is compatible with recommendations from international authorities. Of note is the relatively high proportion of PCPs who were influenced by recommendations by pharmaceutical representatives. One possible reason is the high level of drug marketing activities in the primary care sector of Hong Kong, and that most PCPs develop a good relationship with drug salespersons who become more influential on prescription practices.
From a recent systematic review [33], cost of vaccine and/or problems with its reimbursement, providers’ and parental concern about vaccine safety and efficacy, perception of clients being too young for HPV vaccination, the potential disadvantages of HPV vaccine as promoting sexual activities, organizational influence, communication related to sexuality, and lack of education were identified as major barriers of HPV vaccine prescriptions. Our study findings on barriers were in general similar with current literature.
Implications to Clinical Practice and Future Research
This study has several important implications. Firstly, more educational initiatives should be organized for PCPs working in the private sector so as to enhance their perceived importance of prescribing HPV vaccine to their clients and patients. The focus should be on the epidemiology of HPV infection and genital wart, as well as proportion of cervical cancer attributed to HPV types covered by current vaccines. This should be well received as PCPs strongly agreed that HPV vaccine has a higher priority in improving adolescent health as compared with other vaccines. Several aspects in these educational initiatives should be emphasized, including the ability of HPV vaccines to achieve strong protection with long-lasting immunity, some degrees of cross-protection against the important oncogenic types of HPV, and their good safety profiles. Secondly, since parental refusal was mainly on the vaccine’s safety, parents should be particularly reassured about its low rates of adverse effects in lieu of its many health benefits. The parents’ concerns on discussing sexuality and sexually transmitted diseases signify the crucial role played by PCPs in adopting a holistic approach in their consultations, lending higher levels of attention to discuss sensitive issues. Consultations with prospective clients and patients eligible to receive HPV vaccine should be cautiously conducted with good communication skills taking possible embarrassment to girls and their parents into account. Also, since the Governmental recommendations and the PCPs’ respective academic colleges were perceived as the most influential in affecting their practice of prescribing HPV vaccine, they should be the primary agents to deliver educational messages. That a high proportion of PCPs suggesting full subsidy of the vaccine by the Government also implied that the vaccine might raise an affordability issue. At the early stage to enhance vaccine uptake rate among adolescent girls, the Government should consider at least partial subsidy to eligible clients to overcome vaccine cost which is one of the important barriers.
In light of the present findings, future research should evaluate interventions which could effectively enhance PCPs’ knowledge of HPV and its vaccination in a sustainable manner; remove the barriers of offering immunization, and optimize proper practice of HPV vaccines in suitable subjects.
Strengths and Limitations
To our knowledge this study is the first-ever evaluation among Chinese PCPs on their prescriptions of HPV vaccination. The validity of survey instrument, the high response rate and the sampling methodology enhanced the generalizability of the findings. However, several limitations should be addressed here. Firstly, this is a cross-sectional study and one could not establish causal relationships between PCPs’ knowledge or attitude and their HPV prescriptions. In addition, we had invited PCPs working in the private sector only, and the attitudes and barriers of HPV vaccine prescription might differ to a certain extent with physicians working in the public sector and also doctors in the academic field; the PCPs were heterogeneous with respect to their specialties and we have adopted an aggregate analysis. Also, since the demographic and practice characteristics of all PCPs in Hong Kong are not known, the generalizability of the present findings could not be ascertained. Furthermore, this is a descriptive analysis and there could be potential confounders affecting clinical practice, like the choice between Gardasil and Cervarix. Lastly, this cross-sectional study was conducted in 2011–2012, five years after the licensure of the HPV vaccines in Hong Kong, and it is anticipated that the perceptual variables pertinent to the vaccine will change with time as more promotional materials and media communications emerge. A time series of cross-sectional surveys is needed to reflect the trends of changing knowledge, attitude and perceived barriers.
Conclusions
There is still room to equip PCPs the knowledge on HPV epidemiology infection and skills in motivating behavioural change in discussing sex related issues not directly related common presentations by adolescents. Public health education on safety and efficacy of the HPV vaccination is still needed. Recommendation by Government including subsidy would be an important factor to enhance vaccination rates.
What Is Already Known on This Topic
Human papillomavirus (HPV) infection could be effectively prevented by vaccination, and physician recommendation has been known to be the most influential factor for patients’ uptake of the vaccine. However, few studies explored the knowledge, attitude, barriers and practice of HPV vaccination among primary care physicians.
What This Study Adds
The knowledge of 444 physicians on HPV infection was low. Vaccination against HPV was perceived as more important than other vaccines, and Government subsidy was regarded as an important public health strategy. The most significant barriers to prescribe HPV vaccines consisted of parental refusal due to safety concerns. Public health education on safety and efficacy of vaccination is needed, and support by Governmental funding would be an important factor to enhance vaccination rates.
Acknowledgments
We thank all the doctors who participated in this study.
Funding Statement
A Lee has received support from GlaxoSmithKline to conduct educational activities. PKS Chan has received support for attending academic conferences from GlaxoSmithKline. This study was partly supported by an unrestricted educational grant from GlaxoSmithKline. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Centers for Disease Control and Prevention. Available: http://www.cdc.gov/hpv/. Accessed: 2010 May.
- 2. Chan PKS, Ho WCS, Wong MC, Chang AR, Chor JSY, et al. (2009) Epidemiologic risk profile of infection with different groups of human papillomaviruses. J MedVirol 81: 1635–44. [DOI] [PubMed] [Google Scholar]
- 3. Schiffman M, Castle PE (2003) Human papillomavirus: epidemiology and public health. Arch Pathol Lab Med 127: 930–4. [DOI] [PubMed] [Google Scholar]
- 4. Trottier H, Franco EL (2005) The epidemiology of genital human papillomavirus infection. Vaccine 24: S1–15. [DOI] [PubMed] [Google Scholar]
- 5. Franco E, Harper DM (2005) Vaccination against human papillomavirus infection: a new paradigm in cervical cancer control. Vaccine 23: 2388–94. [DOI] [PubMed] [Google Scholar]
- 6. Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, et al. (2003) Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Eng J Med 348: 518–27. [DOI] [PubMed] [Google Scholar]
- 7. Garland S, Park S, Ngan H, Frazer I, Tay EH, et al. (2008) The need for public education on HPV and cervical cancer prevention in Asia: Opinions of experts at the AOGIN Conference. Vaccine 26: 5435–40. [DOI] [PubMed] [Google Scholar]
- 8.Lee A, Chan PKS (2012) Interim result for school based cervical cancer education and prevention programme. 50th Anniversary Multidisciplinary Conference, The Hong Kong Paediatric Society. Hong Kong, 17–19 Aug 2012.
- 9. Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, et al. (2005) Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomized double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 6: 271–8. [DOI] [PubMed] [Google Scholar]
- 10. Villa LL, Costa RL, Petta CA, Andrade RP, Paavonen J, et al. (2006) High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 95: 1459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, et al. (2004) Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet 364: 1757–65. [DOI] [PubMed] [Google Scholar]
- 12. Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, et al. (2006) Sustained efficacy up to 4–5 years of a bivalent L1 virus-like particle against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 367: 1247–55. [DOI] [PubMed] [Google Scholar]
- 13. Saslow D, Boetes C, Burke W, Harms S, Leach MO, et al. (2007) American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin 57: 75–89. Erratum in: CA Cancer J Clin 2007 57(3): 185. [DOI] [PubMed] [Google Scholar]
- 14. Goldie SJ, Kohli M, Grima D, Weinstein MC, Wright TC, et al. (2004) Projected clinical benefits and cost-effectiveness of a human papillomavirus 16/18 vaccine. J Natl Cancer Inst 96: 604–15. [DOI] [PubMed] [Google Scholar]
- 15. Adams M, Jasani B, Fiander A (2007) Human papillomavirus (HPV) prophylactic vaccination: challenges for public health and implications for screening. Vaccine 25: 3007–13. [DOI] [PubMed] [Google Scholar]
- 16. Zimet GD, Mays RM, Fortenberry JD (2000) Vaccines against sexually transmitted infections: promise and problems of the magic bullets for prevention and control. Sex Transm Dis 27: 49–52. [DOI] [PubMed] [Google Scholar]
- 17. Riedesel JM, Rosenthal SL, Zimet GD, Bernstein DI, Huang B, et al. (2005) Attitudes about human papillomavirus vaccine among family physicians. J Pediatr Adolesc Gynecol 18: 391–8. [DOI] [PubMed] [Google Scholar]
- 18. Dinh TA, Rosenthal SL, Doan ED, Trang T, Pham VH, et al. (2007) Attitudes of mothers in Da Nang, Vietnam toward a human papillomavirus vaccine. J Adolesc Health 40: 559–63. [DOI] [PubMed] [Google Scholar]
- 19. Gerend MA, Lee SC, Shepherd JE (2007) Predictors of human papillomavirus vaccination acceptability among underserved women. Sex Transm Dis 34: 468–71. [DOI] [PubMed] [Google Scholar]
- 20. Ogilvie GS, Remple VP, Marra F, McNeil SA, Naus M, et al. (2007) Parental intention to have daughters receive the human papillomavirus vaccine. CMAJ 177(12): 1506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daley MF, Liddon N, Crane LA, Beaty BL, Barrow J, et al. (2006) A national survey of pediatrician knowledge and attitudes regarding human papillomavirus vaccination. Pediatrics 118: 2280–9. [DOI] [PubMed] [Google Scholar]
- 22.Jaspan DM, Dunton CJ, Cook TL (2008) Acceptance of human papillomavirus vaccine by Gynecologists in an urban setting. J Low Genital Tract Dis 12: 11 8–21. [DOI] [PubMed]
- 23.Dixon E (2011) An assessment of HPV vaccination by Georgia physicians: knowledge, barriers, supports, practices, and adherence to ACIP guidelines and recommendations. A Dissertation Submitted to the Graduate Faculty of Georgia Southern University in Partial Fulfillment of the Requirements for the Degree Doctor of Public Health, Statesboro, Georgia, 2011.
- 24. Raley JC, Followwill KA, Zimet GD, Ault KA (2004) Gynecologists’ attitudes regarding human papilloma virus vaccination: a survey of fellows of the American College of Obstetricians and Gynecologists. Infect Dis Obstet Gynecol 12: 127–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daley MF, Crane LA, Markowitz LE, Black SR, Beaty BL, et al. (2010) Human Papillomavirus vaccination practices: a survey of US physicians 18 months after licensure. Pediatrics 126: 425–33. [DOI] [PubMed] [Google Scholar]
- 26. Huey NL, Clark AD, Kluhsman BC, Lengerich EJ (2009) the ACTION Health Cancer Task Force (2009) HPV vaccine attitudes and practices among primary care providers in Appalachian Pennsylvania. Prev Chronic Dis 6: A49. [PMC free article] [PubMed] [Google Scholar]
- 27. Tan J, Farrell L, Allen DG (2010) The attitudes of Australian gynaecologists to HPV vaccination: An ASCCP survey. Aust N Z J Obstet Gynaecol 50: 472–7. [DOI] [PubMed] [Google Scholar]
- 28. Hopkins TG, Wood NJ, West RM, Darling JC (2009) UK health professionals’ attitudes and knowledge regarding Human Papillomavirus (HPV) vaccination: A West Yorkshire Study. J Paediatr Child Health 45: 652–5. [DOI] [PubMed] [Google Scholar]
- 29. Duval B, Gilca V, McNeil S, Dobson S, Money D, et al. (2007) Vaccination against human papillomavirus: A baseline survey of Canadian clinicians’ knowledge, attitudes and beliefs. Vaccine 25: 7841–7. [DOI] [PubMed] [Google Scholar]
- 30. Chan PKS, Picconi MA, Cheung TH, Giovannelli L, Park JS (2012) Laboratory and clinical aspects of human papillomavirus testing. Crit Rev Clin Lab Sci 49: 117–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan ZC, Chan TS, Lam YM, Lau LM, Li KK, et al. (2011) HPV vaccination in Hong Kong: implications for medical education. Asian Pac J Cancer Prev 12: 1095–9. [PubMed] [Google Scholar]
- 32. Chan PKS, Cheung TH, Li WH, Yu MY, Chan MYM, et al. (2012) Attribution of human papillomavirus types to cervical intraepithelial neoplasia and invasive cancers in southern China. Int J Cancer 131: 692–705. [DOI] [PubMed] [Google Scholar]
- 33.Franco MFE (2011) Physicians’ attitudes towards human papillomavirus vaccination programme: a systematic review. Dissertation submitted to the University of Chester for the Degree of Master of Science (Public Health) in partial fulfilment of the Modular Programme in Public Health, 2011.
- 34.US Food and Drug Administration (2006) FDA licenses new vaccine for prevention of cervical cancer and other diseases in females caused by human papillomavirus. Available: www.fda.gov/bbs/topics/NEWS/2006/NEW01385.html. Accessed 2012 Sep.
- 35.Centers for Disease Control and Prevention (2012) CDC’s Advisory Committee recommends human papillomavirus virus vaccination. Available: www.cdc.gov/od/oc/media/pressrel/r060629.htm. Accessed 2012 Sep.