Abstract
Primary Aim
Examine the effectiveness of transdermal selegiline for producing cigarette smoking abstinence
Design
Randomized double blind placebo controlled trial. Eight weeks of treatment, follow-ups conducted at 25 and 52 weeks
Setting
Community smoking cessation clinic
Participants
243 adult smokers (≥18 years of age; ≥ 10 cigarettes/day)
Intervention
Selegiline transdermal system (STS) or placebo given for 8 weeks. All participants received CBT
Primary Outcome Measurement
Expired-air carbon monoxide confirmed 7-day point prevalence abstinence
Results
STS was not superior to placebo. More women than men were abstinent at 52 week follow-up (28% vs 16%, p<.05). Behavioral activation (BAS) moderated treatment response (p=.01). The survival rate through week 52 for those with high “drive” scores on the BAS was 47% if assigned to selegiline and 34% if assigned to placebo. The survival rate for those with low “drive scores” on the BAS was 35% if assigned to selegiline compared to 53% if assigned to placebo.
Conclusion
The provision of CBT to all participants may have masked potential short-term effects of selegiline. The superior response of women is additional evidence that the alleged gender difference in response to smoking cessation treatment is more apparent than real. Because we did not stratify randomization on BAS score, the result will require replication in future research that employs a larger sample size and appropriate randomization methodology.
Keywords: smoking cessation, nicotine dependence, selegiline
Cigarette smoking cessation is typically accompanied by urges and cravings that can weaken a smoker’s resolve to maintain abstinence [1–3]. The development of cravings and other withdrawal symptoms is common to a variety of drugs of abuse, including nicotine, and may result from neuroadaptations that occur in response to chronic drug use [4,5]. Koob and co-workers have elaborated a model of the development of withdrawal symptoms built upon a conceptual framework provided by opponent process theory [6,7]. In Koob’s model, withdrawal phenomena are triggered by the depletion of dopamine, serotonin and other neurotransmitter systems that occur under conditions of drug deprivation. The emergence of withdrawal phenomena reflects the operation of a homeostatic opponent process in which initial drug effects (“A processes”) are countered by homeostatic mechanisms (“B processes”) that attempt to restore system parameters [5,8]. Under conditions of drug deprivation, “B processes”, which are long lasting in comparison to “A processes”, are unopposed leading to neurotransmitter depletion and the emergence of craving, negative affect and various other subjective abstinence effects. In accord with this model, there is evidence that nicotine increases central DA [9–11] and that central DA depletion is associated with nicotine deprivation [12].
Selegiline is a selective inhibitor of MAO B and is used clinically in combination with levodopa to treat late-stage Parkinsonism and, in transdermal form (EMSAM), to treat depression. Selegiline permits the stabilization of DA levels in the brain by preventing the rapid degradation of DA via MAO B. It is used as an adjunct to levodopa therapy causing a dose-sparing effect and enhancing DA transmission [13]. Because tobacco smoke inhibits CNS MAO B [14], we reasoned that treatment with selegiline might produce better smoking cessation outcomes by more effectively preventing or alleviating craving and depression symptoms through its inhibiting effects on MAO B and resulting potentiation of central DA.
Although no previous smoking cessation trials have been conducted with the selegiline transdermal system (“STS”), the oral form of the medication has been examined in two short-term studies and one randomized clinical trial. Houtsmiller and colleagues [15] reported that selegiline reduced craving and cigarette consumption relative to placebo in a two-week study of 15 smokers given selegiline and placebo in counter-balanced order. George and colleagues [16] randomized 40 smokers to an 8-week course of selegiline or placebo. At the end of treatment, 7-day point prevalence abstinence rates were 45% for selegiline and 15% for placebo (p≤.05). Finally, Biberman and colleagues [17] randomized 109 smokers to 26 weeks of selegiline or placebo. In addition, all received nicotine patch for 8 weeks. At 52 week follow-up, continuous abstinence rates were 25% for selegiline and 11% for placebo (p≤.08).
An analysis of moderators of treatment response was also included in this trial. A “moderator” of treatment is a pre-randomization factor (hence uncorrelated with treatment in a randomized clinical trial (RCT) that has an interactive effect with treatment on outcome [18]. Subgroups of the population sampled in an RCT, identified by strata of a moderator variable, may have different treatment effect sizes. Moderators may help clinicians to develop better treatment targeting algorithms, can be used by researchers to identify appropriate study inclusion/exclusion criteria and serve as factors on which a study may be stratified to amplify power.
Method
Study Design
The study was an 8-week double blind, randomized, placebo-controlled trial of the selegiline transdermal system (STS) with follow-up assessments conducted at 25 and 52 weeks. Treatment assignments were generated prior to study entry. Between May 2, 2006 and July 30, 2008 we recruited and randomized 243 cigarette smokers 18–65 years of age and smoking ≥10 cigarettes per day. The Stanford University Panel for the Protection of Human Subjects in Medical Research approved the study protocol. A data safety and monitoring board provided additional safety monitoring. The trial was registered with clinicaltrials.gov (NCT00218647).
Randomization was conducted using a permuted block method (block size =2) to obtain balance between groups, separately for each gender. When a participant was assigned to the next available ID number within the corresponding gender, they received the associated treatment assignment. This associated treatment assignment was achieved by using the random number generator function RANUNI available in SAS 9.2. The drug (active or placebo) associated with each ID was pre-packaged and labeled by ID only at an off site location by an individual who had no association with the participants. Treatment assignment was concealed from staff and both research staff and participants were blind through week 52.
Primary hypothesis
Treatment with STS would produce higher expired-air carbon monoxide (CO)-confirmed 7-day point prevalence abstinence rates at 25 and 52-week follow-ups. With 120 participants per treatment group, the trial had, in general, 85% power at a two-tailed alpha of .05 to detect a difference in abstinence rates of at least 20% over a range of success probabilities [19]. All analyses are based on intention-to-treat principles.
Study population
Participants were recruited through advertisements on the radio, in local newspapers, on a community website, and by notices distributed through various local organizations. Eligible smokers were scheduled for an initial clinic visit which included signing consent forms, responding to a structured clinical interview designed to detect current and past depression, venipuncture for liver and kidney function tests, and receiving instructions to complete a physical examination at one of two local medical clinics.
Screening
Individuals were excluded for pregnancy, lactation, intent to become pregnant within 6 months, bipolar disorder, schizophrenia, current liver or kidney disease, uncontrolled diabetes, Parkinson’s disease, Alzheimer’s disease, unstable thyroid disorder, active treatment for or reporting current depression or substance abuse, history of heart problems in the previous 6 months, uncontrolled hypertension, orthostatic hypotension, current use of medications intended to assist in smoking cessation, or use of medications contraindicated for use with selegiline. All participants were required to complete a medical history and physical examination. Participation was contingent on physician approval after review of the history, physical examination, and blood test results.
Treatments
Selegiline transdermal system (STS)
The STS is a monoamine oxidase inhibitor (MAOI) developed as a transdermal treatment for depression. The recommended starting dose and target dose for the STS is 6mg/24hrs. The STS has been shown to be effective for treating depression in a dose range of 6mg/24hrs to 12mg/24hrs. However, the trials were not designed to assess if higher doses are more effective than the lowest effective dose of 6mg/24hrs.
The most common side effect associated with the use of the STS is reddening of the skin at the site of patch application. As a class, MAOI medicines have been associated with hypertensive crises caused by the ingestion of foods containing high amounts of tyramine. Data from safety studies conducted with STS 6mg/24hrs indicate that a modified diet is not required at this dose. Due to the more limited data available for STS 9mg/24hrs and 12mg/24hrs, patients receiving these doses are instructed to avoid ingestion of foods rich in tyramine. Given these restrictions and the lack of data suggesting increased efficacy at higher doses, STS 6mg/24hrs was chosen for this study.
Half of the participants received an 8-week supply of STS and half received placebo, identical in appearance. Participants were instructed to begin using the patch on their designated quit day, and to continue to use the patch for the full eight weeks. Each patch was worn for a full 24 hours, unless a participant experienced severe and persistent insomnia, in which case they were instructed to remove the patch at night.
CBT treatment
At each clinic session, trained staff met with participants individually to develop cognitive and behavioral skills to resist urges to smoke. Staff used self-efficacy questionnaires to assess participants’ confidence in their abilities to resist urges to smoke in specific situations and completed behavioral worksheets to help articulate treatment plans to be used in managing their behavior in specific situations without smoking.
Measures
Primary study end point
Expired-air carbon monoxide (CO)-confirmed 7-day point prevalence abstinence evaluated at both 25 and 52 weeks. This end point measure is defined as a report of non-smoking for seven consecutive days prior to contact plus a CO level below 10 PPM. CO was measured at all clinic visits with the Bedfont EC50 Smokerlyzer (www.Bedfont.com).
Secondary outcome measure
Time-to-relapse was defined as at least seven consecutive days of smoking [20] and was used to explore moderators of treatment response. The relapse date was treated as the first day on which smoking occurred for seven consecutive days.
Potential moderators of treatment response
Gender was used to stratify randomization in this trial and examined as a hypothesized moderator of treatment response. Some post-hoc analyses indicate that cessation therapy may work better for men [21–24] although the effect, if present, would appear to be small [25]. However, men and women may respond differently to different types of cessation interventions [26].
The five-question modified Fagerström Tolerance Questionnaire (mFTQ), an instrument designed to assess tobacco dependence, was administered at baseline [27,28].
Past and current major depressive disorder (MDD) were assessed with the mood disorders portion of the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (SCID), fourth edition (DSM-IV) [29]. Those with current depression were excluded from participation, encouraged to seek treatment and, as needed, given help in obtaining treatment.
Craving was measured at baseline and at all clinic and phone appointments. Craving was measured with the following two questions: “Have you felt cravings for a cigarette?” and “Have you felt strong urges to smoke?” Participants rated how upsetting cravings and urges had been in the past 24 hours (0=None; 6 = Extremely Upsetting). A craving score was obtained by averaging the two items. [1].
Depression symptoms were measured at all clinic visits during treatment with the 20-item Center for Epidemiological Studies depression instrument (CES-D) [30]. Gray argued that two separable neural systems regulate human motivation [31]. Aversive motivation is regulated by a behavioral inhibition system (BIS) and appetitive motivation is regulated by a behavioral activation system (BAS). Because drugs of abuse are hypothesized to produce their effects by acting upon human motivational systems we elected to measure BIS and BAS at baseline with the scales developed by Carver and White [32]. The scales have good discriminant and convergent validity [32].
Height was measured on a wall-mounted stadiometer. Weight was recorded on a Scale-Tronix 5600 electronic scale (www.scale-tronix.com/).
Safety Measures
Occurrence, duration, and severity of adverse events were assessed at all clinic visits.
Heart rate and blood pressure were measured with an automated blood pressure device (DINAMAP Procare 120, www.gehealthcare.com). At baseline, participants with blood pressure exceeding 160 (systolic) or 100 (diastolic) were asked to see their physicians for treatment before they could enter the trial.
Medication compliance
During clinic visits, participants were asked if they “applied [their] patch this morning”, and if they had “applied [their] patch every day in the past week”.
Treatment group guess
At the end of treatment and again at 25-week follow up, participants guessed their treatment assignment.
Statistical Analysis
Logistic regression analysis was used to test the primary hypothesis. Because gender was used to stratify the randomization, gender and the gender X treatment interaction were examined along with treatment in the model [33].
Moderator effects were examined in secondary analyses. A statistically significant interaction between treatment and a pre-randomization factor is indication of a moderator effect [34, 35].
Results
Screening
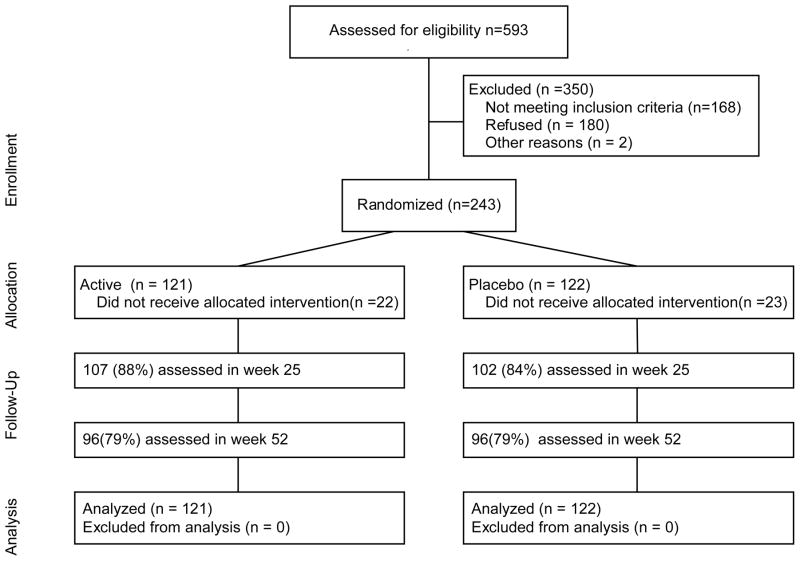
A total of 593 smokers were screened to yield the analysis sample of 243 (169 men and 74 women). The ethnic distribution of the sample was: White: 77%; Hispanic: 9%; Asian: 4%; Black: 1%; multiple ethnicity: 16%; other: 1%.
Of those not randomized, 168 were not eligible, 180 declined to participate after screening and 2 did not participate for other reasons. Smokers failed the eligibility screen for the following principal reasons: too few cigarettes (34); taking exclusionary medication (61); skin problems (10); residence outside target sample geographic area (9); failed depression screen (20); current alcohol/drug abuse (15); age >65 (1); exclusionary medical conditions (8), pregnant, breastfeeding or no use of birth control (2); not approved by study MD (3); other medical problems (4); another household member already a participant (1).
Comparability of treatment groups
As is evident from Table 1, differences between treatment groups on various baseline measures were not statistically significant.
Table 1.
Participant Characteristics
| Selegiline | Placebo | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Male | Female | Male | Female | Tx | Gender | |||||
|
| ||||||||||
| Variable | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | p |
| Cigarettes smoked/day | 20.51 | 6.84 | 16.76 | 5.79 | 20.55 | 8.02 | 19.24 | 6.13 | .20 | .01 |
| mFagerström (5–25) | 15.43 | 3.11 | 14.89 | 2.89 | 15.58 | 2.88 | 15.76 | 2.68 | .22 | .66 |
| CES-D (0–60) | 7.52 | 8.13 | 7.27 | 6.59 | 8.51 | 7.86 | 6.76 | 7.83 | .83 | .36 |
| BIS | 17.21 | 4.04 | 17.32 | 4.31 | 17.04 | 3.55 | 17.59 | 3.82 | .99 | .49 |
| BAS Reward | 16.14 | 3.01 | 17.16 | 2.20 | 16.20 | 2.68 | 16.54 | 1.82 | .44 | .06 |
| BAS Drive | 10.99 | 2.96 | 11.65 | 2.90 | 10.78 | 2.43 | 10.54 | 2.58 | .09 | .60 |
| BAS Fun | 11.43 | 3.07 | 12.08 | 2.66 | 11.56 | 2.57 | 10.76 | 1.99 | .11 | .81 |
| BMI (kg/m2) | 28.10 | 5.64 | 27.05 | 4.54 | 29.20 | 5.39 | 28.65 | 6.35 | .08 | .30 |
| Age (years) | 41.73 | 10.88 | 47.49 | 10.39 | 44.46 | 10.54 | 45.30 | 10.10 | .85 | .03 |
| Education (years) | 13.85 | 1.92 | 14.32 | 2.04 | 13.94 | 2.16 | 14.14 | 1.87 | .87 | .23 |
| BMI | 28.10 | 5.64 | 25.05 | 4.54 | 29.20 | 5.39 | 28.65 | 6.35 | .08 | .30 |
| History of MDD (%) | 9.5% | 10.8% | 8.2% | 18.9% | .57 | .21 | ||||
| Married (%) | 36.9% | 37.8% | 44.7% | 32.4% | .88 | .41 | ||||
| Minority (%) | 21.7% | 19.4% | 22.4% | 24.3% | .63 | .97 | ||||
| Sample size | 84 | 37 | 85 | 37 | ||||||
Reclassification
Of self-reported non-smokers, 92%, 71%, and 75% percent provided biochemical confirmation of abstinence at 8, 25 and 52 weeks. Those reporting abstinence but failing to provide breath samples for CO verification were reclassified as smokers unless an individual was no longer living in the area (two participants at week 8; three at week 25 and four at week 52).
Test of primary hypothesis
Separate models were fitted for each follow-up point (8, 25 and 52 weeks). Abstinence rates are presented in Table 2. At 8 and 25 weeks, main effects tests and the interaction did not meet criteria for statistical significance. At week 52, there was a main effect for gender (χ2 (1)=4.2, p<.05, OR = .50, 95% CI: .26 – .97) with women achieving a higher confirmed abstinence rate than men (28% vs 16%).
Table 2.
Expired-Air CO confirmed 7-day point prevalence Abstinence Rates
| N Abstinent | % Abstinent | |
|---|---|---|
| End of Treatment | ||
| Selegiline | 32 | 26.4% |
| Placebo | 36 | 29.5% |
| 25 week Follow-up | ||
| Selegiline | 21 | 17.4% |
| Placebo | 23 | 18.8% |
| 52 week Follow-up | ||
| Selegiline | 24 | 19.8% |
| Placebo | 25 | 20.5% |
Did STS Reduce Craving?
There was no effect of STS on craving monitored during the course of treatment (selegiline: mean change: −.63 (1.8); placebo mean change: −.68 (2.1).
Moderator analysis
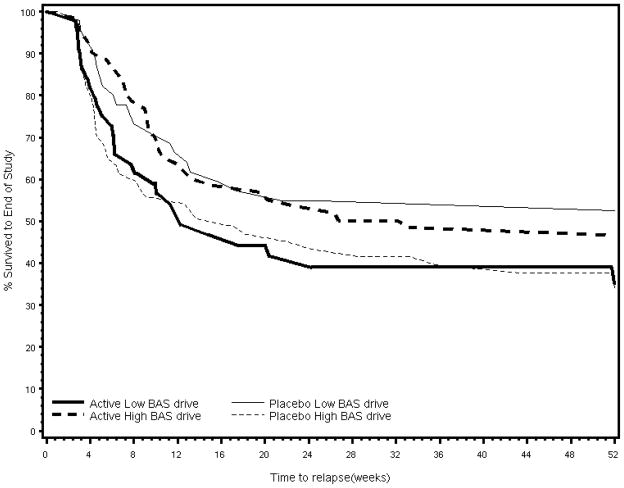
Putative moderators explored in the analysis were gender, depression (history of MDD, CESD score), behavioral inhibition/activation, nicotine dependence (cigarettes/day, expired-air CO, modified Fagerström, immediate post-cessation craving), Body Mass Index (weight (kg)/height(m2)) and age. For each putative moderator, a Cox proportional hazard model was formed with treatment, the putative moderator and the treatment X putative moderator interaction as independent factors and time-to-relapse through week 52 as the dependent variable. The “drive” subscale of the BAS was a moderator of treatment response [χ2(1)=7.5, p=.01; OR = .82, 95% CI=.71, .94]. The survival rate through week 52 for those with high “drive” scores on the BAS was 47% if assigned to STS (n= 72) and 34% if assigned to placebo (n=72). The survival rate for those with low “drive scores” on the BAS was 35% if assigned to STS (n=49) compared to 53% if assigned to placebo (n=50).
Medication compliance
Medication compliance was assessed on 8 occasions during treatment; 15% of participants responded “Yes” on all 8 occasions when asked if they were currently wearing their patch; 45% of participants answered “Yes” at all visits attended” 19% responded “No” on all occasions. There were no treatment or gender differences in compliance rates; participants with high compliance in both treatment groups were more likely to be abstinent (40%) at end of treatment.
Treatment session attendance
The average number of treatment sessions attended was 5 (out of 9) (sd=3.5) with no treatment or gender differences.
Safety and tolerability
Mean (sd) blood pressure and heart rate at baseline were: STS: systolic: 124.3 (14.7), diastolic: 73.4 (10.6), HR: 77.9 (11.1); placebo: systolic: 124.2 (13.6), diastolic: 72.9 (8.3), HR: 78.4 (10.9); Mean (sd) blood pressure and heart rate at the end of treatment were: STS: systolic: 122.39 (13.59), diastolic: 72.22 (8.66), HR: 71.16 (11.17); placebo: systolic: 123.05 (12.65), diastolic: 74.06 (9.17), HR: 71.78 (10.36).
Adverse events reported by five percent or more of the participants during treatment are listed in Table 3.
Table 3.
Adverse Events Reported By At Least 5% of Participants During Treatment
| Adverse Event | Selegiline | Placebo |
|---|---|---|
| Insomnia | 47.1% | 42.6% |
| Headache | 33.9% | 35.2% |
| Skin rash | 24.8% | 20.5% |
| Racing thoughts | 20.7% | 23.0% |
| Lightheaded | 16.5% | 16.4% |
| Vivid Dreams | 10.7% | 9.8% |
| Other | 42% | 35% |
No effects of treatment: all p-values > .05
Adverse event rates were similar in both STS and placebo groups. However, 19 participants (16%) discontinued STS and 9 participants (7%) discontinued placebo because of adverse events experienced during treatment (χ2 (1) = 4.3, p<.05; OR = 2.6, 95% CI= 1.05, 6.20). Two participants died during the course of the trial. Causes of death were pulmonary emphysema and metastatic lung cancer. Neither death was considered related to trial participation.
Treatment guess
At end of treatment, 50% of those receiving STS correctly guessed their treatment assignment and 47% of those assigned to placebo correctly guessed their assignment. The corresponding percent correct guess at week 25 was 38% and 40%.
At end of treatment, treatment staff correctly guessed 53% of those randomized to STS and 48% of those randomized to placebo.
Discussion
Transdermal selegiline was not superior to placebo in promoting smoking abstinence. We hypothesized that STS would increase abstinence rates by preventing or alleviating the post-cessation cravings that can induce relapse [1]. However, STS was no more effective than placebo in reducing craving. Our prediction was based on the results of pilot studies conducted with the oral form of selegiline and our own pilot work with STS [15–17]. Indeed, the STS appeared to offer several important advantages over the oral form for the treatment of nicotine dependence. The transdermal system bypasses first pass effects. This in turn allows a once-daily dosing regimen, higher and sustained levels of the parent drug at sites of action and reduced toxicity through reduction of active metabolites (amphetamine and methamphetamine) [13].
Although compliance data from smoking cessation trials are seldom reported, in general, compliance to medication and behavioral treatment regimens is poor [36,37]. In this study, almost 20% of participants never reported wearing a patch when queried. On the other hand, almost half reported wearing a patch at all scheduled clinic visits that were completed. There was no difference in adherence between active and placebo conditions. Ultimately, the fact that higher compliance produced similar confirmed abstinence rates in both groups (i.e. 40% abstinence at end of treatment) argues against poor compliance as an explanation for the null result.
This was a single dose study and the limitations of single dose examinations are well known. A better result might have been achieved at higher doses. However, at higher doses, there is evidence that selectivity for brain MAO-B is lost leading to the need for strict dietary restrictions. Thus, the costs and benefits would have to be weighed carefully before examining outcomes associated with higher dosing.
CBT may have masked a short-term effect of pharmacotherapy. We think this “masking effect” is a plausible account for two reasons. First, almost 30% of smokers assigned to placebo were abstinent at end of treatment. By comparison, in meta-analyses comparing nicotine patch and placebo response, on average, 27% of those assigned to active medication and only 13% assigned to placebo were abstinent at end of treatment [38]. Second, we have conducted two trials comparing the effects of bupropion and CBT in promoting longer-term smoking abstinence. In the most recent trial, CBT was more effective than supportive therapy (42% vs 29%) [39]. In the first trial, bupropion was no more effective than placebo although at 6 months the overall abstinence rate of 40% represented a higher-than-average treatment response [40]. Significantly, in addition to bupropion or placebo, all participants received CBT. The provision of CBT during treatment may have been a key factor contributing to the comparatively high abstinence rates achieved in the earlier studies.
Although the primary hypothesis was not supported, two findings merit attention. First, women did better than men. At 52-week follow-up, 28% of women but only 16% of men were abstinent. The gender difference in baseline cigarette consumption levels does not account for this result and, indeed, as indexed by the mFTQ, men and women in this trial were equally dependent on nicotine.
A number of analyses have compared the performance of men and women. Some reported no differences in abstinence rates [41–43] while others reported results favoring men [21–24]. A problem with most, if not all, of these analyses is that they have been post-hoc in nature. Yet it is well known that post-hoc subgroup analyses of clinical trial results can be very misleading [44,45]. Thus, post-hoc comparisons of smoking cessation trial results in men and women should be interpreted with caution. If researchers wish to examine the hypothesized gender effect in smoking cessation in a methodologically sound manner, they must power studies accordingly and stratify randomization on gender.
Second, BAS score moderated treatment response. Smokers with high BAS reactivity on the drive scale were more likely to avoid relapse through week 52 if given STS whereas low scorers performed best if given a placebo. This result is consistent with some hypotheses about the nature of neural regulation of appetitive motivation. Gray proposed that a hard-wired behavioral approach or activation system (BAS) responds to stimuli associated with reward and that the biological basis of this system is at least partly dopaminergic [46]. He speculated that variation in individual BAS reactivity could stem from variation in number/functioning of central DA receptors and/or more intense DA cell firing in response to primary or secondary positive reinforcers.
Several researchers have proposed that individuals with highly reactive BAS systems are more prone to seek out and experience more positive effects in situations associated with rewarding stimuli because they are particularly sensitive to DA neurotransmission that occurs in response to cues signaling reward or to the reward itself [32,47]. Following from this line of reasoning, STS may have helped smokers with high BAS scores by facilitating dopaminergic transmission in the absence of smoking.
This result is interesting inasmuch as there are very few firmly established moderators of smoking cessation treatment response. However, because we did not stratify randomization on BAS score, this result will require replication in future research that employs a larger sample size and appropriate randomization methodology.
Figure 1.
Flow Chart Showing Attrition of Smokers Over 52 Weeks
Figure 2.
Survival Curve for BAS Drive by Treatment Group
Acknowledgments
Support was provided solely by a grant from the National Institute on Drug Abuse (R01 DA017457) awarded to the first author. Medication and matching placebo were provided by Somerset Pharmaceuticals, Inc. Dr. Schatzberg served as a consultant to the manufacturer.
Footnotes
TRIAL REGISTRATION: clinicaltrials.gov
Dr. Schatzberg served as a consultant for Somerset Pharmaceuticals
References
- 1.Killen JD, Fortmann SP. Craving is associated with smoking relapse: Findings from three prospective studies. Exp Clin Psychopharm. 1997;5:137–142. doi: 10.1037//1064-1297.5.2.137. [DOI] [PubMed] [Google Scholar]
- 2.Shiffman S. The tobacco withdrawal syndrome. In: Krasnegor NA, editor. Cigarette smoking as a dependence process. NIDA Res Mon. Vol. 23. Washington, DC: DHEW; 1979. pp. 158–184. [Google Scholar]
- 3.West RJ, Schneider N. Craving for cigarettes. Brit J Addict. 1987;82:407–415. doi: 10.1111/j.1360-0443.1987.tb01496.x. [DOI] [PubMed] [Google Scholar]
- 4.Hafeley W. Biological basis of drug-induced tolerance, rebound, and dependence. Contribution of recent research on benzodiazepines. Pharmacopsychiat. 1986;19:353–361. doi: 10.1055/s-2007-1025061. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF. Drug addiction: The Yin and Yang of hedonic homeostasis. Neuron. 1996;16:893–896. doi: 10.1016/s0896-6273(00)80109-9. [DOI] [PubMed] [Google Scholar]
- 6.Solomon RL. Drug addiction: An opponent process theory. In: Maser JD, Seligman MEP, editors. Psychopathology: Experimental Models. WH Freeman; San Francisco: 1977. pp. 124–145. [Google Scholar]
- 7.Wikler A. Dynamics of drug dependence. Arch Gen Psychiat. 1973;28:611–619. doi: 10.1001/archpsyc.1973.01750350005001. [DOI] [PubMed] [Google Scholar]
- 8.Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropsychopharm. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 9.Grenhoff J, Aston-Jones G, Svensson TH. Nicotinic effects on the firing pattern of mid brain dopamine neurons. Act Physiol Scan. 1986;128:351–358. doi: 10.1111/j.1748-1716.1986.tb07988.x. [DOI] [PubMed] [Google Scholar]
- 10.Imperato A, Mulas A, Di Chiara G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur J Pharmacol. 1986;132:337–338. doi: 10.1016/0014-2999(86)90629-1. [DOI] [PubMed] [Google Scholar]
- 11.Mereu G, Yoon K-WP, Boi V, Gesesa GI, Naes L, Westfall TC. Preferential stimulation of ventral tegmental area dopaminergic neurons by nicotine. Eur J Pharmacol. 1987;141:395–399. doi: 10.1016/0014-2999(87)90556-5. [DOI] [PubMed] [Google Scholar]
- 12.Epping-Jordan MP, Watkins SS, Koob GF, Markou A. Dramatic decreases in brain reward function during nicotine withdrawal. Nature. 1998;393:76–79. doi: 10.1038/30001. [DOI] [PubMed] [Google Scholar]
- 13.Barrett JS, Hochadel TJ, Morales RJ, Rohatagi S, DeWitt KE, Watson SK, DiSanto AR. Pharmacokinetics and safety of a selegiline transdermal system relative to single-dose oral administration in the elderly. Am J Therapeutics. 1996;3:688–698. doi: 10.1097/00045391-199610000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Fowler JS, Volkow ND, Wang G-J, Pappas N, Logan J, MacGregor R, Alexoff D, Shea C, Schlyer D, Wolf AP, Warner D, Zezulkova I, Cilento R. Inhibition of monoamine oxidase B in the brains of smokers. Nature. 1996;379:733–736. doi: 10.1038/379733a0. [DOI] [PubMed] [Google Scholar]
- 15.Houtsmiller EJ, Thornton JA, Stitzer ML. Effects of selegiline (l-deprenyl) during smoking and short-term abstinence. Psychopharm. 2002;163:213–220. doi: 10.1007/s00213-002-1152-9. [DOI] [PubMed] [Google Scholar]
- 16.George TP, Vessoccjo JC, Termine A, Jatlow PI, Kosten TR, O’Malley SS. A preliminary placebo-controlled trial of selegiline hydrochloride for smoking cessation. Biol Psychiat. 2003;53:136–143. doi: 10.1016/s0006-3223(02)01454-3. [DOI] [PubMed] [Google Scholar]
- 17.Biberman R, Neumann R, Katzir I, Gerber Y. A randomized controlled trial of oral selegiline plus nicotine skin patch compared with placebo nicotine skin patch for smoking cessation. Addict. 2003;98:1403–1407. doi: 10.1046/j.1360-0443.2003.00524.x. [DOI] [PubMed] [Google Scholar]
- 18.Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: clinical, research, and policy importance. JAMA. 2006;296:1286–9. doi: 10.1001/jama.296.10.1286. [DOI] [PubMed] [Google Scholar]
- 19.Fleiss JL. Statistical methods for rates and proportions. John Wiley; New York: 1981. [Google Scholar]
- 20.Ossip-Klein DJ, Bigelow G, Curry S, Hall S, Kirkland S. Task Force 1: Classification and Assessment of Smoking Behavior, Proceedings of the National Working Conference on Smoking Relapse. Health Psychol. 1986;5 (Suppl):3–11. [PubMed] [Google Scholar]
- 21.Bjornsen W, Rand C, Connett JE, Lindgren P, Nides M, Pope F, et al. Gender differences in smoking cessation after 3 years in the lung health study. Am J Pub Health. 1995;85:223–230. doi: 10.2105/ajph.85.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.COMMIT Research Group. Community intervention trial for smoking cessation (COMMIT): I. Cohort results from a four-year community intervention. Am J Pub Health. 1995;85:183–192. doi: 10.2105/ajph.85.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gourlay SG, Forbes A, Marriner T, Pethica D, McNeil JJ. Prospective study of factors predicting outcome of transdermal nicotine treatment in smoking cessation. BMJ. 1994;309:842–846. doi: 10.1136/bmj.309.6958.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wetter DW, Kenford SL, Smith SS, Fiore MC, Jorenby DE, Baker TB. Gender differences in smoking cessation. J Consult Clin Psychol. 1999;67:555–562. doi: 10.1037//0022-006x.67.4.555. [DOI] [PubMed] [Google Scholar]
- 25.Killen JD, Fortmann SP, Kraemer HC, Varady A. Do men outperform women in smoking cessation studies? Maybe – But Not By Much. Exp Clin Psychopharm. 2002;10:295–301. doi: 10.1037//1064-1297.10.3.295. [DOI] [PubMed] [Google Scholar]
- 26.Perkins KA. Sex differences in nicotine vs. nonnicotine reinforcement as determinants of tobacco smoking. Exp Clin Psychopharm. 1996;4:166–177. [Google Scholar]
- 27.Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- 28.Killen JD, Fortmann SP, Telch MJ, Newman B. Are heavy smokers different from light smokers? A comparison after 48 hours without cigarettes. JAMA. 1988;260:1581–1585. [PubMed] [Google Scholar]
- 29.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) Washington, D.C: American Psychiatric Press, Inc; 1996. [Google Scholar]
- 30.Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. App Psychol Meas. 1977;1:385–401. [Google Scholar]
- 31.Gray JA. Framework for a taxonomy of psychiatric disorder. In: van Grozen S, van de Poll N, Sergeant JA, editors. Emotions: Essays on emotion theory. New Jersey: Lawrence Erlbaum Associates, Inc; 1993. pp. 29–59. [Google Scholar]
- 32.Carver CS, White TL. Behavioral Inhibition, Behavioral Activation, and Affective Responses to Impending Reward and Punishment: The BIS/BAS Scales. J Person Soc Psychol. 1994;2:319–333. [Google Scholar]
- 33.Kraemer HC, Blasely CM. Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Meth Psychiat Res. 2004;13:141–51. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraemer HC, Frank E, Kupfer DJ. Moderators of treatment outcomes: clinical, research, and policy importance. JAMA. 2006;296:1286–9. doi: 10.1001/jama.296.10.1286. [DOI] [PubMed] [Google Scholar]
- 35.Kraemer HC, Wilson GT, Fariburn CG, Agras WS. Mediators and Moderators of treatment effects in randomized clinical trials. Archiv Gen Psychiat. 2002;50:877–78. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 36.Kozak J, Fagerström KO, Sawe U. High-dose treatment with the nicotine patch. Int J Smoking Cessation. 1995;4:26–28. [Google Scholar]
- 37.O’Brien CP, McLellan AT. Myths about the treatment of addiction. Lancet. 1996;347:237–240. doi: 10.1016/s0140-6736(96)90409-2. [DOI] [PubMed] [Google Scholar]
- 38.Fiore MC, Smith SS, Jorenby DE, Baker TB. The effectiveness of the nicotine patch for smoking cessation. JAMA. 1994;271:1940–1947. [PubMed] [Google Scholar]
- 39.Killen JD, Fortmann SP, Schatzberg AF, Arredondo CJ, Murphy G, Hayward C, Celio M, Cromp D, Fong D, Pandurangi M. Extended cognitive behavior therapy for cigarette smoking cessation. Addiction. 2008;103:1381–1390. 94. doi: 10.1111/j.1360-0443.2008.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Killen JD, Fortmann SP, Murphy G, Hayward C, Arredondo CJ, Cromp D, Abe L, Schatzberg AF. Efficacy of extended treatment with bupropion for cigarette smoking cessation. J Consult Clin Psychol. 2004;74:286–94. doi: 10.1037/0022-006X.74.2.286. [DOI] [PubMed] [Google Scholar]
- 41.Blondal T. Controlled trial of nicotine polacrilex gum with supportive measures. Arch Intern Med. 1989;149:1818–1821. doi: 10.1001/archinte.1989.00390080080018. [DOI] [PubMed] [Google Scholar]
- 42.Hughes JR, Gust SW, Keenan RM, Fenwick JW, Healey ML. Nicotine vs placebo gum in general medical practice. JAMA. 1989;261:1300–1305. [PubMed] [Google Scholar]
- 43.Sachs DPL, Sawe U, Leischow SJ. Effectiveness of a 16hr transdermal nicotine patch in a medical practice setting, without intensive group counseling. Archiv Int Med. 1993;153:1881–1890. [PubMed] [Google Scholar]
- 44.Moye LA. Random Research. Circulation. 2001;1003:3150–3153. doi: 10.1161/hc2501.090955. [DOI] [PubMed] [Google Scholar]
- 45.Yusuf S, Wittes J, Probstfield J. Analysis and interpretation of treatment effects in subgoups of patients in randomized clinical trials. JAMA. 1991;266:93–98. [PubMed] [Google Scholar]
- 46.Pickering AD, Gray JA. Dopamine, appetitive reinforcement, and the neuropsychology of human learning: An individual differences approach. In: Eliasz A, Angleitner A, editors. Advances in Research on Temperament. Pabst Science Publishers; Lengerich, Germany: 2001. pp. 113–149. [Google Scholar]
- 47.Franken HA, Muris P, Georgieva I. Gray’s model of personality and addiction. Addict Behav. 2006;31:399–403. doi: 10.1016/j.addbeh.2005.05.022. [DOI] [PubMed] [Google Scholar]