Abstract
Background
Available research has suggested that affiliation with prosocial peers reduces child and adolescent antisocial behavior. However, the etiologic mechanisms driving this association remain unclear. The current study sought to evaluate whether this association takes the form of a gene-environment interaction (GxE) in which prosocial peer affiliation acts to reduce the consequences of genetic risk for non-aggressive antisocial behavior during childhood.
Methods
Our sample consisted of 500 twin pairs aged 6 to 10 years from the Michigan State University Twin Registry (MSUTR).
Results
Results robustly supported moderation by prosocial peer affiliation: genetic influences on non-aggressive antisocial behavior were observed to be several-fold larger in those with lower levels of prosocial peer affiliation as compared to those with higher levels of prosocial peer affiliation. This pattern of results persisted even after controlling for gene-environment correlations and deviant peer affiliation, and when restricting our analyses to those twins who share all or nearly all of their friends.
Conclusions
Such findings not only suggest that prosocial peer affiliation moderates genetic influences on non-aggressive antisocial behaviors during childhood, but also provide support for the theoretical notion that protective environmental experiences may exert their influence by promoting resilience to genetic risk.
Keywords: Non-aggressive antisocial behavior, Rule-breaking, Prosocial Peers, GxE, Resilience
Affiliation with prosocial peers is thought to provide a critical buffer against the development of youth antisocial behavior (Deater-Deckard, 2001, Hektner et al., 2000, Kendler et al., 2008, Simonoff et al., 2004). It is thus not surprising to learn that a number of antisocial behavior interventions target peer affiliations (Feldman et al., 1983, Huey et al., 2000, Kazdin, 1987, Tremblay et al., 1995). Multisystemic Therapy, for example, is a well-regarded and highly disseminated treatment (Curtis et al., 2004) that, among other things, seeks to increase association with prosocial peers and decrease association with antisocial peers, thereby removing sources of reinforcement for antisocial behaviors and replacing them with reinforcement for prosocial activities.
Given this relationship, it would be important to identify the processes underlying their association. The above treatment studies clearly point to an effect of socialization, such that prosocial peers can positively influence children’s behavior. In day-to-day life, however, children both select, and are selected by, each other as friends. Indeed, there is ample empirical evidence that children with antisocial behavior seek out and/or attract peers who are similarly inclined to engage in antisocial behaviors (Granic and Patterson, 2006, Kendler et al., 2008, Quinton et al., 1993). Although partially a function of their shared interests, there is also evidence that the affiliation of antisocial children with delinquent peers is the result of their rejection by prosocial peers because of their disruptive behaviors (Deater-Deckard, 2001, Hektner et al., 2000).
Put together, the above results indicate that both socialization and selection (or more specifically, rejection) contribute to the negative association between prosocial peer affiliation and child antisocial behavior. Critically, however, we still know virtually nothing about the etiologic mechanisms through which these processes influence child antisocial behavior. One interesting possibility is that prosocial peer affiliation may exert its influence via a gene-environment interaction (GxE) process, whereby prosocial peer affiliation suppresses or deactivates the expression of genetic influences on antisocial behavior. Although researchers have discussed the notion that protective experiences might promote resilience to genetic risk (Lahey and Waldman, 2003, Rutter et al., 2006, Shanahan and Hofer, 2005), these discussions have been largely theoretical to date (for an exception, see Feinberg et al., 2007). We thus have little empirical insight into the pervasiveness of ‘GxE protection’ or the moderators that are most crucial.
Importantly, the ability to identify GxE, including ‘GxE protection’, with any certainty hinges in part on a meaningful consideration of the gene-environment correlation (rGE), or genetically-influenced exposure to particular “environmental” experiences (Plomin et al., 1977, Scarr and McCartney, 1983). It may well be the case, for example, that what appears to be the suppression of genetic risk for antisocial behavior in the presence of prosocial peer affiliation may in fact reflect the rejection of children at genetic risk for antisocial behavior by their prosocial peers. In other words, what appears to be the moderation of genetic risk by environmental experience in fact reflects rGE processes. Researchers studying these processes should thus employ analytic techniques that circumvent possible rGE confounds.
The current study sought to clarify whether prosocial peer affiliation reduces genetic influences on antisocial behavior, while controlling for the effects of selection (i.e., rGE). We specifically examined whether prosocial peer affiliation moderated the etiology of childhood antisocial behavior in a sample of 500 twin pairs, and conducted a series of analytic checks of those results to ensure that any positive GxE findings were indeed reflective of moderation by prosocial peer affiliation per se.
METHODS
PARTICIPANTS
The Michigan State University Twin Registry (MSUTR) includes several independent twin projects (Burt and Klump, 2013). The 500 twin pairs examined here were assessed as part of the Twin Study of Behavioral and Emotional Development in Children (TBED-C) within the MSUTR. Recruitment procedures are outlined in detail in Burt & Klump (2013). To be eligible for participation, neither twin could have a cognitive or physical condition (e.g., significant developmental delays) that would preclude completion of the roughly 4-hour assessment (as assessed via parental report during the initial phone screen).
Our final sample was broadly representative of the area population and of recruited families (see Burt & Klump, 2013, for detailed information). In brief, participating families endorsed ethnic group memberships at rates comparable to area inhabitants (e.g., Caucasian: 86.4% and 85.5%, African-American: 5.4% and 6.3% for the participating families and the local census, respectively). Moreover, participating twins did not differ from non-participating twins in their average levels of conduct problems, emotional symptoms, or hyperactivity (Cohen’s d = −.047, .010, and −.076, respectively; all p ≥ .29).
The twins were 47.0% female and ranged from in age 6 to 10 years, although a small handful (n=14 pairs) had turned 11 by the time the family participated (mean age (SD) = 8.2 years (1.46)). Zygosity was established using physical similarity questionnaires administered to the twins’ primary caregiver (Peeters et al., 1998). On average, the physical similarity questionnaires used by the MSUTR have accuracy rates of 95% or better. Approximately half of the twin pairs (N=251) were monozygotic. Of the DZ pairs, nearly all (N=227) were same-sex. Our conclusions were identical with and without the 22 opposite sex pairs, and thus they were retained for analysis.
MEASURES
Child Antisocial Behavior
Mothers and fathers completed the Child Behavior Checklist (CBCL; Achenbach and Rescorla, 2001) separately for each twin. Parents rated the extent to which a series of statements described each twins’ behavior over the past six months using a three point scale (0=never to 2=often/mostly true). We utilized the well-known rule-breaking scale (RB; 17 items; e.g., breaks rules, cheats or lies, steals), as prior research has linked peer influences specifically to non-aggressive antisocial behaviors (as opposed to physically aggressive behaviors, which may or may not be committed in the company of others; Burt, 2009b, Burt and Klump, in press, Moffitt, 1993, 2003). Consistent with manual recommendations (Achenbach & Rescorla, 2001), analyses were conducted on the raw RB scores.
Maternal-reported RB data was available for 996 twins; paternal-reported RB data was available on 862 twins. Consistent with the cross-informant correlations of .2 to .3 found in meta-analyses of informant effects (Achenbach et al., 1987), maternal and paternal RB were moderately correlated (r = .34, p<.01). When only one informant report was available, that report was used for analyses. When both informant reports were available, data were averaged to create an RB composite. The use of this combined informant approach is thought to allow for a more complete assessment of twin symptomatology than would the use of either informant alone (Achenbach et al., 1987). RB data were available for all twins following the creation of the composite. RB was log-transformed prior to analysis (skews before and after transformation were 2.38 and 0.42, respectively).
Prosocial Peer Affiliation
Parents reported on each of their twins’ peer group affiliations using the Friends Inventory (Walden et al., 2004). Parents were instructed to provide ratings for each of their twins’ entire peer groups, with items scored using a 4-choice response format (ranging from 1 = none of my child’s friends are like that to 4 = all of my child’s friends are like that). Item ratings were summed to yield a prosocial peer affiliation score (5 items; “My child’s friends get good grades”; α = .92 for maternal and paternal informant reports). Maternal reports were available for 98.9% of the twins; paternal reports were available for 84.9% of the twins. As done for RB, maternal and paternal informant-reports were combined to create a composite score. Following the creation of the composite, peer data were available for 998 twins.
Teacher reports of prosocial peer affiliation were also available on 64% of the sample (teacher data collection is still on-going)1, thereby allowing us to preliminarily evaluate the validity of our parental reports. When examining participants with Friends data from all three informants, teacher reports of prosocial peer affiliation were correlated .21 (p<.05) with parental reports of prosocial peer affiliation, an association that is statistically equivalent to that between maternal and paternal reports (r = .26, p<.05). Similarly, mother and twin reports of prosocial peer affiliation were correlated 0.38 (p<.05) in an independent sample of 222 twins in late childhood/early adolescence. In short, extant data indicate that parents are able to provide a reasonable assessment of twin prosocial peer affiliation.
One additional item, also administered to parents as part of the Friends scale, was used to determine the extent to which the twins’ peer groups overlapped (ranging from 1 = all or nearly all of the twins’ friends overlap to 4 = none of the twins’ friends overlap). Consistent with the observation that twins tend to share friends, 54% of twins shared “all or nearly all” of their friends. Of those twins that did not share all of their friends, most (83%) shared “many but not all” of their friends, 15% shared “a few” friends, and 2% did not share any friends. As one would expect, the extent to which twins shared their friends moderated the similarity in their prosocial peer affiliation. Those twins who shared all or nearly all of their friends were experiencing very similar levels of prosocial peer affiliation (r = .82), while those who shared many or only a few friends were less similar in their prosocial peer affiliation (r = .56 and .39, respectively).
ANALYSES
Twin studies leverage the difference in the proportion of genes shared between monozygotic or MZ twins (who share 100% of their segregating genes) and dizygotic or DZ twins (who share roughly 50% of their segregating genes) to estimate additive genetic (A), shared environmental (i.e., environmental factors that make twins similar to each other; C), and non-shared environmental (i.e., factors that make twins different from each other, including measurement error; E) contributions to a given phenotype. More information on twin studies is provided elsewhere (Neale and Cardon, 1992).
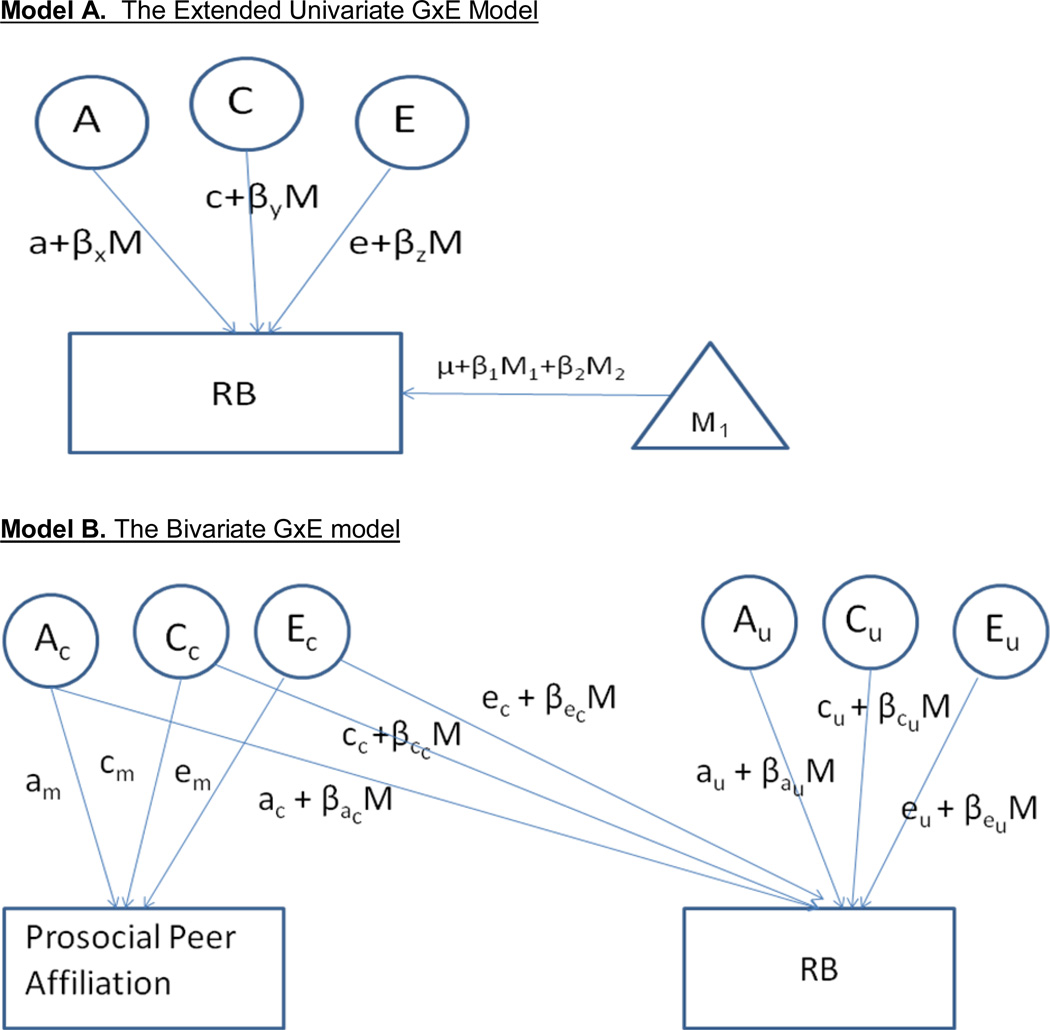
For our primary analyses, we evaluated how prosocial peer affiliation might moderate the etiology of RB using the ‘extended univariate GxE’ model (van der Sluis et al., 2012), an extension of the univariate GxE model (Purcell, 2002). Using the extended univariate GxE model (see Figure 1a), the variance decomposition of RB was modeled as a function of prosocial peer affiliation. To circumvent possible rGE confounds, the moderator values of both twins were entered in a means model of each twin’s RB. Moderation was then modeled on the residual RB variance (i.e., that which does not overlap with prosocial peer affiliation). The first and least restrictive of these models allows for both linear and non-linear moderation of A, C, and E contributions to RB. We then fitted a series of more restrictive moderator models, constraining the linear and non-linear moderators to be zero and evaluating the reduction in model fit.
Figure 1.
Note. A, C, and E represent genetic, shared environmental, and non-shared environmental influences, respectively. For ease of presentation, the co-twin variables and paths are omitted here, though they are estimated in the models. In the extended univariate twin model (van der Sluis et al., 2012), interactions with the linear moderator are added to the genetic and environmental paths, and are estimated separately for each component of variance (i.e., βxM, βYM, and βZM for a, c, and e paths, respectively). The non-linear moderators are not shown. In the bivariate GxE model (Purcell, 2002), AC and AU respectively represent genetic influences on rule-breaking (RB) held in common with the moderator (prosocial peer affiliation; labeled M above) and those unique to RB. Interactions with the moderator are added to these common and unique genetic influences. Only the latter are thought to index “true” GxE.
The extended univariate GxE model is quite flexible. Twins are not required to be concordant on the value of the moderator (although they can be), and the moderator can be either continuous or categorical, but should include zero. A continuous moderator was examined here, although it was floored at zero prior to analysis (and thus ranged from 0 to 10). Purcell (2002) also recommends that unstandardized estimates be presented for all GxE models, as standardized or proportional estimates can obscure absolute changes with the moderator. We thus standardized our log-transformed RB score to have a mean of zero and a standard deviation of one to facilitate interpretation of the unstandardized values.
Mx, a structural-equation modeling program (Neale et al., 2003), was used to fit models to the transformed raw data using Full-Information Maximum-Likelihood raw data techniques. When fitting models to raw data, variances, covariances, and means are first freely estimated to get a baseline index of fit (minus twice the log-likelihood; −2lnL). Model fit for the more restrictive biometric GxE models was then evaluated using four information theoretic indices that balance overall fit (via −2lnL) with model parsimony: the Akaike’s Information Criterion (AIC; Akaike, 1987), the Bayesian Information Criteria (BIC; Raftery, 1995), the sample-size adjusted Bayesian Information Criterion (SABIC; Sclove, 1987), and the Deviance Information Criterion (DIC; Spiegelhalter et al., 2002). The lowest or most negative AIC, BIC, SABIC, and DIC among a series of nested models is considered best. Because fit indices do not always agree (they place different values on parsimony, among other things), we reasoned that the best fitting model should yield lower or more negative values for at least 3 of the 4 fit indices (as done in Hicks et al., 2009).
Confirmatory Analyses
To evaluate the robustness of our primary GxE results, we conducted four sets of confirmatory analyses.
Analysis 1
van der Sluis et al. (2012) recommended that researchers confirm positive findings of etiological moderation using the bivariate GxE model (see Figure 1b; Purcell, 2002), since the extended univariate GxE model is unable to disambiguate moderation of the covariance between the moderator and the outcome from moderation that is unique to the moderator (only the latter of which represents “true” GxE). The bivariate GxE model overcomes this limitation because the moderator is entered twice: once as a variable that is allowed to correlate with the outcome and once as the moderator. Although useful for ensuring that positive univariate GxE results index true etiological moderation, the bivariate GxE model suffers from issues of identifiability (Rathouz et al., 2008). Given these problems, we restricted our core GxE analyses to the extended univariate model, and made use of the bivariate model to confirm those results.
Analysis 2
We also sought to confirm that our results persisted to individual informant-reports of RB and prosocial peer affiliation. We thus re-ran our primary GxE analyses separately by informant, examining whether evidence of moderation persisted to maternal and paternal informant-reports, respectively.
Analysis 3
We sought to evaluate whether the effects of prosocial peer affiliation on RB were in fact a function of (reverse-scored) delinquent peer affiliation. In other words, prosocial peer affiliation may influence RB by limiting (or, in its absence, promoting) opportunities to affiliate with delinquent peers. We empirically examined this possibility by allowing prosocial and delinquent peer affiliation (as assessed via 5 items on the Friends Inventory; e.g., “My child’s friends steal things”; α = .95) to simultaneously moderate the etiology of RB via a two moderator model (Purcell, 2002).
Analysis 4
As a final check, we sought to address the well-known observation that MZ twins share friends more often than do DZ twins. In these data, for example, 67.9% of MZ twins shared all or nearly all of their friends versus 40.7% of DZ twins. To evaluate whether this differential sharing of friends influenced our results, we repeated our primary analyses on those twin pairs who shared all or nearly all of their friends (267 pairs, of which 168 were MZ).
RESULTS
Mean levels of RB and prosocial peer affiliation varied significantly across sex (see Table 1), such that boys evidenced higher rates of RB and lower rates of prosocial peer affiliation as compared to girls (both p<.05). Although prosocial peer affiliation was not significantly associated with twin age (r = −.05, ns), RB demonstrated a small negative associated with age (r = −.08, p<.05). As such, sex, age, and their interaction were regressed out of the data prior to analysis (McGue and Bouchard, 1984). RB was negatively associated with prosocial peer affiliation (r = −.22, p<.001).
Table 1.
Descriptive Statistics.
| Females | Males | Cohen’s d effect size |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) |
n | % above -off |
Min | Max | Mean (SD) |
n | % above cut-off |
Min | Max | ||
| RB | 1.22 (1.42) |
470 | 7.0 | 0 | 14 | 1.75 (1.85) |
530 | 7.2 | 0 | 14 | −.32** |
|
Prosocial Peer Affiliation |
15.58 (1.62) |
470 | --- | 10 | 20 | 14.90 (1.63) |
528 | --- | 10 | 20 | .42** |
Note. % above cut-off refers to the proportion of participants who scored in the marginal- or clinically-significant range on the Rule-Breaking (RB) scale by either mother or father report (as defined in the CBCL manual; Achenbach and Rescorla, 2001). RB could conceivably range from 0 to 34. Prosocial peer affiliation could conceivably range from 5 to 20. Means were compared across boys and girls using independent samples t-tests,
p<.01.
PRIMARY ANALYSES
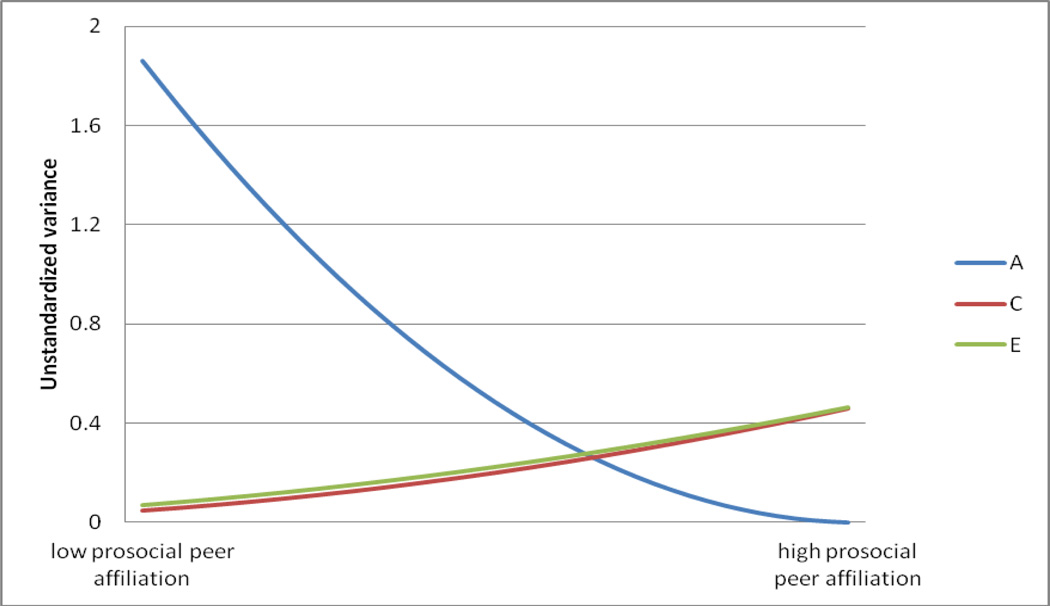
There was clear evidence of linear moderation of RB by prosocial peer affiliation (see Table 2)2. Such results imply that the etiology of RB varies with prosocial peer affiliation, and does so independently of any rGE processes3. We made use of the estimated paths and moderators from the linear moderation models (see Table 3) to calculate and plot (see Figure 2) the unstandardized genetic and environmental variance components at each level of prosocial peer affiliation. Non-shared environmental effects were observed to increase slightly, if significantly, with increasing prosocial peer affiliation. Genetic variation, by contrast, was observed to decrease with increasing levels of prosocial peer affiliation, such that genetic influences on RB at high levels of prosocial peer affiliation were several-fold smaller than those at low levels of prosocial peer affiliation.
Table 2.
Fit Indices
| Model | −2lnL | df | AIC | BIC | SABIC | DIC |
|---|---|---|---|---|---|---|
| Primary Analyses | ||||||
| 1a) Linear and non-linear ACE moderation | 2489.80 | 981 | 527.80 | −1801.40 | −244.53 | −899.92 |
| 1b) Linear ACE moderation | 2489.98 | 984 | 521.98 | −1810.63 | −249.00 | −906.39 |
| 1c) No moderation | 2513.49 | 987 | 539.49 | −1808.19 | −241.79 | −901.19 |
| Confirmatory Analyses | ||||||
| Bivariate GxE model | ||||||
| 2a) Linear ACE moderation | 5963.47 | 1975 | 2013.47 | −3151.23 | −16.86 | −1336.33 |
| 2b) No moderation | 5997.29 | 1981 | 2035.29 | −3152.96 | −9.06 | −1332.54 |
| Father reports of prosocial peer affiliation and child RB | ||||||
| 3a) Linear ACE moderation | 2167.48 | 824 | 519.48 | −1402.88 | −95.49 | −645.67 |
| 3b) No moderation | 2178.45 | 827 | 524.45 | −1406.45 | −94.29 | −646.48 |
| Mother reports of prosocial peer affiliation and child RB | ||||||
| 4a) Linear ACE moderation | 2449.37 | 974 | 501.37 | −1794.96 | −249.22 | −899.92 |
| 4b) No moderation | 2457.46 | 977 | 503.46 | −1800.22 | −249.72 | −902.42 |
| Only those twin pairs who share all or nearly all their friends | ||||||
| 5a) Linear ACE moderation | 1263.10 | 526 | 211.10 | −839.86 | −5.98 | −356.50 |
| 5b) No moderation | 1286.63 | 529 | 228.63 | −836.49 | 2.15 | −350.37 |
Note. The best fitting model for a given set of analyses is highlighted in bond font, and is indicated by the lowest AIC (Akaike’s Information Criterion), BIC (Bayesian Information Criterion), SABIC (sample size adjusted Bayesian Information Criterion), and DIC (Deviance Information Criterion) values for at least 3 of the 4 fit indices.
Table 3.
Unstandardized path and moderator estimates for full linear ACE moderation models.
| PATHS | LINEAR MODERATORS | ||||
|---|---|---|---|---|---|
| a | c | e | A1 | C1 | E1 |
| Primary Analyses | |||||
| 1.36* | 0.22 | 0.27* | −0.13* | 0.05 | 0.04* |
| (1.03, 1.65) | (−0.43, 0.68) | (0.15, 0.41) | (−0.19, −0.07) | (−0.03, 0.12) | (0.02, 0.07) |
| Confirmatory Analyses | |||||
| Bivariate GxE model | |||||
| 1.22* | 0.53 | 0.29* | −0.10* | −0.02 | 0.04* |
| (0.82, 1.57) | (−1.09, 1.09) | (0.17, 0.43) | (−0.17, −0.03) | (−0.13, 0.08) | (0.01, 0.06) |
| Paternal reports of prosocial peer affiliation and child RB | |||||
| 0.93* | −0.66* | 0.44* | −0.08* | 0.02 | 0.03* |
| (0.53, 1.26) | (−1.01, −0.23) | (0.31, 0.60) | (−0.14, −0.01) | (−0.04, 0.09) | (0.002, 0.05) |
| Maternal reports of prosocial peer affiliation and child RB | |||||
| 1.14* | 0.12 | 0.35* | −0.08* | 0.06 | 0.02 |
| (0.85, 1.37) | (−0.49, 0.53) | (0.23, 0.49) | (−0.13, −0.03) | (−0.01, 0.12) | (−0.003, 0.04) |
| Only those twin pairs who share all or nearly all their friends | |||||
| 1.49* | 0.08 | 0.18* | −0.16* | 0.07 | 0.05* |
| (1.10, 1.91) | (−0.81, 0.65) | (0.05, 0.33) | (−0.25, −0.08) | (−0.01, 0.17) | (0.02, 0.08) |
Note. A, C, and E (upper and lower case) respectively represent genetic, shared, and non-shared environmental parameters on rule-breaking (RB).
Bold font and an asterisk indicate that the estimate is significant at p<.05. Primary analytic results are presented in the top half of the table. Because low prosocial peer affiliation was dummy coded as 0, the genetic and environmental contributions to RB at this level can be obtained by squaring the path estimates (i.e., a, c, and e). At each subsequent level, linear moderators (i.e., A1, C1, E1) were added to the paths using the following equation: Unstandardized VarianceTotal = (a + A1(prosocial peer))2 + (c + C1(prosocial peer))2 + (e + E1(prosocial peer))2. The variance component estimates calculated this way are presented in Figure 2. Confirmatory analytic results are presented in the bottom half of the table. Note that, for the bivariate GxE model, only the moderators of the unique (i.e., RB-specific) genetic and environmental influences are presented above (the moderators of the common genetic, shared and non-shared environmental influences were uniformly non-significant).
Figure 2.
Etiological moderation of rule-breaking (RB) by prosocial peer affiliation
Note. A, C, and E represent genetic, shared, and non-shared environmental influences, respectively. These estimates index the absolute (unstandardized) changes in genetic and environmental variance in RB by prosocial peer affiliation in the best-fitting model (Model 1b in Table 2). The specific path estimates are presented in Table 3.
CONFIRMATORY ANALYSES
1) Do our findings of moderation persist to the bivariate GxE model?
We sought to further confirm the above results using the bivariate GxE model (Purcell, 2002), as recommended by van der Sluis et al. (2012). Results (presented in Tables 2 and 3) indicated that the above results index actual genetic moderation of RB by prosocial peer affiliation, rather than moderation of the covariance between RB and prosocial peer affiliation, bolstering confidence in our primary results.
2) Do our results persist to individual informant-reports?
To confirm that our results were not unduly influenced by our use of RB and prosocial peer affiliation composites, we re-ran our primary GxE analyses separately by informant. Although the linear moderation model did not provide a particularly good fit to the data relative to the no moderation model (see Table 2), inspection of the path and moderator estimates (see Table 3) suggest that this is due to the small and largely non-significant C and E moderators. Indeed, the A moderators were statistically significant and similar in magnitude to those reported above. We thus conclude that our results are largely robust to informant considerations.
3) Do the above results stem from an absence of affiliation with delinquent peers?
To confirm that our primary GxE results were a function of prosocial peer affiliation itself, rather than a reflection of low delinquent peer affiliation, we fitted a two-moderator model to the data, in which we allowed prosocial and delinquent peer affiliation to simultaneously moderate the etiology of RB. The best fitting model (results not shown) was one in which A and E contributions to RB were uniquely moderated by prosocial peer affiliation (the moderators were estimated at −.11 and .06, respectively; both p<.05), while shared environmental contributions to RB were uniquely moderated by delinquent peer affiliation (the C moderator was estimated to be .11; p<.05; see Burt & Klump (in press) for a more detailed exploration of the delinquent peer affiliation results). There is thus little empirical support for the proposition that the moderation of genetic influences by prosocial peer affiliation is in fact a function of reverse-scored delinquent peer affiliation.
4) Does the differential sharing of peers by MZ and DZ twins influence our results?
As a final check on our results, we sought to evaluate whether our finding of genetic moderation was influenced in some way by the fact that MZ twins share peers more often than DZ twins. To evaluate this question, we repeated analyses on those pairs who shared all or nearly all of their friends. Results were very much in line with those reported above (see Tables 2 and 3). We thus conclude that the higher level of peer similarity seen for MZ as compared to DZ twins does not appear to be substantively influencing our results.
Discussion
The goal of the current study was to evaluate whether prosocial peer affiliation served to suppress genetic influences on non-aggressive antisocial behavior. Results robustly supported this possibility: genetic influences on RB were observed to be several-fold larger in those with low levels of prosocial peer affiliation as compared to those with high levels of prosocial peer affiliation. In other words, RB appears to be primarily genetic in origin at low levels of prosocial peer affiliation, but primarily environmental in origin at high levels of prosocial peer affiliation. Confirmatory analyses further revealed that these results were independent of delinquent peer affiliation, and persisted when restricting our analyses to those twins who shared all or nearly all of their friends. Moreover, the moderation of genetic influences could not be explained by rGE. Such findings collectively suggest that prosocial peer affiliation acts as a potent moderator of genetic influences on non-aggressive antisocial behaviors.
These results are notably consistent with those of the only similar study conducted to date. Hicks and colleagues (2009) examined whether several different environmental risk factors, including reverse-scored prosocial peer affiliation, moderated the etiology of a broad substance abuse/externalizing composite in a large sample of late adolescent twin pairs. Results revealed that genetic influences on adolescent externalizing were significantly more pronounced in those with low levels of prosocial peer affiliation. The current study replicated and extended these results to the developmental period of childhood, an important advance given that childhood-onset antisocial behavior is thought to represent a more severe and persistent form of the disorder (Moffitt, 1993).
Despite this consistency with prior work, there are limitations to the above study. First, the current sample consists largely of healthy families from middle-class backgrounds. Clinically meaningful levels of RB were thus relatively low in our data (roughly 8–10%). Future research should seek to extend the current findings to higher risk samples. Second, although our sample is only moderately-sized by current twin study samples, previous power analyses (Purcell, 2002) suggest that it is more than adequate for the GxE models used here. Nevertheless, analyses incorporating sex would likely be unwieldy and underpowered in this sample. It thus remains unclear whether the GxE identified here vary across sex (although it is worth noting that CP heritability estimates in general do not vary significantly across sex (Burt, 2009a, c)).
Conclusions
The findings of the current study have several important implications. First, delinquent and prosocial peer affiliation do not appear to function as mirror images of one another at the etiologic level, at least during childhood. Prosocial peer affiliation was found to moderate genetic influences on RB, whereas delinquent peer affiliation moderated only the shared environmental component of variance. Although it is unclear what may account for these differences, they may stem from the fact that affiliation with delinquent peers during childhood appears to stem primarily from social rejection/limited social opportunities (Deater-Deckard, 2001, Hektner et al., 2000). By adolescence, however, genetic influences largely account for the link between delinquent peer affiliation and RB (Beaver et al., 2009, Button et al., 2007, Cleveland et al., 2005, Harden et al., 2008, Hicks et al., 2009, Rowe and Osgood, 1984). As an example, Kendler and colleagues (2008) examined retrospectively-reported Conduct Disorder and delinquent peer affiliation at ages 8–11, 12–14, and 15–17 years. Shared environmental contributions to peer deviance influenced Conduct Disorder, but did so only during late childhood and mid-adolescence (rC = .92, .51, and .00 at ages 8–11 years, 12–14 years, and 15–18 years; Kendler et al., 2008). Such findings have collectively been interpreted to suggest that while socialization may underlie the association between peer deviance and antisocial behavior during childhood, their association in adolescence stems more from selection processes (Kendler et al., 2008). The current results support this possibility, while also suggesting that it does not extend to prosocial peer affiliation.
Our findings of latent GxE also have key implications for molecular genetic research (Kendler, 2005). The suppression of genetic influences by prosocial peer affiliation implies that efforts to identify genetic main effects may be hampered by genetic suppression in particular environmental contexts. It further implies that efforts to identify the genes underlying GxE should be extended beyond specific candidate genes. In particular, molecular GxE research to date has focused all but exclusively on the moderation of a single polymorphism within a single gene. Their results are thus so specific that they are likely to represent only a very small part of the overall causal pathway in complex biobehavioral phenomena (such as RB). Future molecular genetic research should seek to examine GxE for multiple genes in concert, perhaps via GWAS data.
Next, empirical studies of GxE have focused almost exclusively on the activation of genetic vulnerabilities by environmental risk factors. This conceptualization of GxE is based primarily on the diathesis-stress model, in which a biological vulnerability (the diathesis) interacts with environmental events (stressors) in the onset of a particular disorder. Although this particular manifestation of GxE has received extensive empirical support (see Hicks et al., 2009, for one example), other manifestations are also possible. Indeed, from a biological standpoint, it seems unlikely that genotypic expression would be altered only in response to deleterious experiences. Positive or protective experiences may also modulate the expression of genetic risk (note that protective factors are specifically conceived of as positive or prosocial aspects of the environment rather than just the absence of risk; e.g., the absence of parental criticism does not equate to the presence of parental praise). We would specifically expect protective experiences to promote resilience to genetic risk (sometimes referred to as ‘social context as compensation’; Shanahan & Hofer, 2005). In other words, ‘GxE protection’ could serve to suppress genetic influences on a given disorder by reducing the consequences of inherited genetic risk (Lahey and Waldman, 2003, Rutter et al., 2006, Shanahan and Hofer, 2005). Although a provocative idea, very little empirical research to date had examined this proposition. The current study did just this, and found compelling evidence that (at least one) protective aspect of the social environment promotes resilience to genetic risk. Future research should continue to explore the role of protective experiences in modulating genetic risk for psychopathology.
Finally, the results of the current study also help us understand how socialization with prosocial peers protects against the development of antisocial behavior. Rather than solely reflecting a main effect of the environment, prosocial peer affiliation appears to suppress genetic influences on antisocial behavior. And because prosocial peer affiliation is thought to reduce antisocial behavior via the reinforcement of prosocial inclinations and activities (Huey, et al., 2000), such results could imply that behavioral reinforcement may act to shape the biology underlying those behaviors. Consistent with this possibility, prior work has linked behavioral reinforcement conditioning to dopaminergic neurons in the midbrain and prefrontal cortex (Dayan and Balleine, 2002, Schultz, 2002, Schultz et al., 1997). Future work should thus explore the possibility that prosocial peer affiliation may (de)activate genes in the dopaminergic system, and moreover, may accomplish this moderation via simple reward and reinforcement learning.
Acknowledgements
This project was supported by R01-MH081813 from the National Institute of Mental Health, awarded to Drs. Burt and Klump. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors thank all participating twins and their families for making this work possible.
Footnotes
The limited number of teacher reports, combined with the fact that data collection is on-going, means that the teacher report data were less suited to the GxE analyses conducted here. It is nevertheless worth noting that our primary conclusions are identical when teacher reports are included in the prosocial peer affiliation composite.
Note that the findings of etiologic moderation by prosocial peer affiliation fully persisted to categorical operationalizations of the moderator (in which prosocial peer affiliation was trichotomized into low, average, and high groups), indicating that results are robust to the measurement of our moderator variable.
Confounding by rGE was expressly avoided by our choice of models. Nevertheless, it is worth noting that the genetic correlation between RB and prosocial peer affiliation was estimated to be quite small in a simple bivariate ACE model (rA = -.16, ns). The above GxE are thus not a function of rGE in disguise.
References
- Achenbach TM, McConaughy SH, Howell CT. Child/adolescent behavioral and emotional problems: Implications of cross-informant correlations for situational specificity. Psychological Bulletin. 1987;101:213–232. [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Beaver KM, DeLisi M, Wright JP, Vaughn MG. Gene-environment interplay and delinquent involvement: Evidence of direct, indirect, and interactive effects. Journal of Adolescent Research. 2009;24:147–168. [Google Scholar]
- Burt SA. Are there meaningful etiological differences within antisocial behavior? Results of a meta-analysis. Clinical Psychology Review. 2009a;29:163–178. doi: 10.1016/j.cpr.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Burt SA. A mechanistic explanation of popularity: Genes, rule-breaking, and evocative gene-environment correlations. Journal of Personality and Social Psychology. 2009b;96:783–794. doi: 10.1037/a0013702. [DOI] [PubMed] [Google Scholar]
- Burt SA. Rethinking environmental contributions to child and adolescent psychopathology: A meta-analysis of shared environmental influences. Psychological Bulletin. 2009c;135:608–637. doi: 10.1037/a0015702. [DOI] [PubMed] [Google Scholar]
- Burt SA, Klump KL. The Michigan State University Twin Registry (MSUTR): An update. Twin Research and Human Genetics. 2013;16:344–350. doi: 10.1017/thg.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt SA, Klump KL. Delinquent peer affiliation as an etiological moderator of childhood delinquency. Psychological Medicine. doi: 10.1017/S0033291712000013. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button TMM, Corley RP, Rhee SH, Hewitt JK, Young SE, Stallings MC. Delinquent peer affiliation and conduct problems: A twin study. Journal of Abnormal Psychology. 2007;116:554–564. doi: 10.1037/0021-843X.116.3.554. [DOI] [PubMed] [Google Scholar]
- Cleveland HH, Wiebe RP, Rowe DC. Sources of exposure to smoking and drinking friends among adolescents: A behavioral-genetic evaluation. The Journal of Genetic Psychology. 2005;166:153–169. [PubMed] [Google Scholar]
- Curtis NM, Ronan KR, Borduin CM. Mutisystemic Treatment: A meta-analysis of outcome studies. Journal of Family Psychology. 2004;18:411–419. doi: 10.1037/0893-3200.18.3.411. [DOI] [PubMed] [Google Scholar]
- Dayan P, Balleine BW. Reward, movtivation, and reinforcement learning. Neuron. 2002;36:285–298. doi: 10.1016/s0896-6273(02)00963-7. [DOI] [PubMed] [Google Scholar]
- Deater-Deckard K. Annotation: Recent research examining the role of peer relationships in the development of psychopathology. Journal of Child Psychology and Psychiatry. 2001;42:565–579. [PubMed] [Google Scholar]
- Feinberg ME, Button TMM, Neiderhiser JM, Hetherington EM, Reiss D. Parenting and adolescent antisocial behavior and depression: Evidence for genotype by parenting interaction. Archives of General Psychiatry. 2007;64:457–465. doi: 10.1001/archpsyc.64.4.457. [DOI] [PubMed] [Google Scholar]
- Feldman RA, Caplinger TE, Wodarski JS. The St. Louis conundrum: The effective treatment of antisocial youth. Englewood Cliffs, N.J.: Prentice-Hall; 1983. [Google Scholar]
- Granic I, Patterson GR. Towards a comprehensive model of antisocial development: A dynamic systems approach. Psychological Bulletin. 2006;113:101–131. doi: 10.1037/0033-295X.113.1.101. [DOI] [PubMed] [Google Scholar]
- Harden PW, Hill JE, Turkheimer E, Emery RE. Gene-envrionment correlation and interaction on peer effects on adolescent alcohol and tobacco use. Behavior Genetics. 2008;38:339–347. doi: 10.1007/s10519-008-9202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hektner JM, August GJ, Realmuta GM. Patterns and temporal changes in peer affiliation among aggressive and non-aggressive children particpating in a summer school program. Journal of Clinical Child Psychology. 2000;29:603–614. doi: 10.1207/S15374424JCCP2904_12. [DOI] [PubMed] [Google Scholar]
- Hicks BM, South SC, DiRago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Archives of General Psychiatry. 2009;66:640–648. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey SJ, Henggeler SW, Brondino MJ, Pickrel SG. Mechanisms of change in Multisystemic Therapy: Reducing delinquent behavior through therapist adherence and improved family and peer functioning. Journal of Consulting and Clinical Psychology. 2000;68:451–467. [PubMed] [Google Scholar]
- Kazdin AE. Treatment of antisocial behavior in children: Current status and future directions. Psychological Bulletin. 1987;102:187–203. [PubMed] [Google Scholar]
- Kendler KS. Psychiatric genetics: A methodological critique. American Journal of Psychiatry. 2005;162:3–11. doi: 10.1176/appi.ajp.162.1.3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Myers JM, Eaves LJ. A genetically informative developmental study of the relationship between Conduct Disorder and peer deviance in males. Psychological Medicine. 2008;38:1001–1011. doi: 10.1017/S0033291707001821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahey BB, Waldman ED. Causes of Conduct Disorder and Juvenile Delinquency. New York: The Guilford Press; 2003. A developmental propensity model of the origins of conduct problems during childhood and adolescence; pp. 76–117. [Google Scholar]
- McGue M, Bouchard TJJ. Adjustment of twin data for the effects of age and sex. Behavior Genetics. 1984;14:325–343. doi: 10.1007/BF01080045. [DOI] [PubMed] [Google Scholar]
- Moffitt TE. Adolescence-limited and life-course-persistent antisocial behavior: A developmental taxonomy. Psychological Review. 1993;100:674–701. [PubMed] [Google Scholar]
- Moffitt TE. Life-course persistent and adolescence-limited antisocial behavior: A research review and a research agenda. In. In: Lahey B, Moffitt TE, Caspi A, editors. The causes of conduct disorder and serious juvenile delinquency. New York: Guilford; 2003. pp. 49–75. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6th Edition. VCU Box 900126, Richmond, VA 23298: Department of Psychiatry; 2003. [Google Scholar]
- Neale MC, Cardon LR. Methodology for Genetic Studies of Twins and Families. Boston, MA: Kluwer Academic Publishers; 1992. [Google Scholar]
- Peeters H, Van Gestel S, Vlietinck R, Derom C, Derom R. Validation of a telephone zygosity questionnaire in twins of known zygosity. Behavior Genetics. 1998;28:159–161. doi: 10.1023/a:1021416112215. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Loehlin JC. Genotype-environment interaction and correlation in the analysis of human behavior. Psychological Bulletin. 1977;84:309–322. [PubMed] [Google Scholar]
- Purcell S. Variance components model for gene-environment interaction in twin analysis. Twin Research. 2002;5:554–571. doi: 10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Quinton D, Pickles A, Maughan B, Rutter M. Partners, peers, and pathways: Assortative pairing and continuities in conduct disorder. Development and Psychopathology. 1993;5:763–783. [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Sociological Methodology. 1995;25:111–163. [Google Scholar]
- Rathouz PJ, Van Hulle CA, Rodgers JL, Waldman ID, Lahey BB. Specification, testing, and interpretation of gene-by-measured-environment interaction models in the presence of gene-environment correlation. Behavior Genetics. 2008;38:301–315. doi: 10.1007/s10519-008-9193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe DC, Osgood DW. Heredity and sociological theories of delinquency: A reconsideration. American Sociological Review. 1984;49:526–540. [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Gene-environment interplay and psychopathology: Multiple varieties but real effects. Journal of Child Psychology and Psychiatry. 2006;47:226–261. doi: 10.1111/j.1469-7610.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- Scarr S, McCartney K. How people make their own environments: A theory of genotype-environment effects. Child Development. 1983;54:424–435. doi: 10.1111/j.1467-8624.1983.tb03884.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sclove LS. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika. 1987;53:333–343. [Google Scholar]
- Shanahan MJ, Hofer SM. Social context and gene-environment interactions: Retrospect and prospect. Journals of Gerontology. 2005;60B:65–76. doi: 10.1093/geronb/60.special_issue_1.65. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Elander J, Holmshow J, Pickles A, Murray R, Rutter M. Predictor of antisocail personality: Continuities from childhood to adult life. British Journal of Psychiatry. 2004;184:118–127. doi: 10.1192/bjp.184.2.118. [DOI] [PubMed] [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. Journal of the Royal Statistical Society: Series B. 2002;64:583–639. [Google Scholar]
- Tremblay RE, Pagani-Kurtz L, Masse LC, Vitaro F, Pihl RO. A bimodal preventive intervention for disruptive Kindergarten boys: Its impact through mid-adolescence. Journal of Consulting and Clinical Psychology. 1995;63:560–568. doi: 10.1037//0022-006x.63.4.560. [DOI] [PubMed] [Google Scholar]
- van der Sluis S, Posthuma D, Dolan CV. A note on false positives and power in GxE modeling of twin data. Behavior Genetics. 2012;42:170–186. doi: 10.1007/s10519-011-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden SB, McGue M, Iacono WG, Burt SA, Elkins I. Identifying shared environmental contributions to early substance use: The importance of peers and parents. Journal of Abnormal Psychology. 2004;113:440–450. doi: 10.1037/0021-843X.113.3.440. [DOI] [PubMed] [Google Scholar]