Abstract
Inflammatory bowel disease is associated with industrialization, and its incidence has increased markedly over time. The prospect of reversing these trends motivates the search for the agent(s) involved. Modernity entails several physical and behavioral modifications that compromise both the photosynthesis of cholecalciferol in the skin, and of its bioavailability. Although deficiency in this “vitamin” has therefore emerged as a leading candidate, and despite the publication of a randomized control trial that showed a trend towards statistically significant benefit in Crohn’s disease, its causal agency has yet to be demonstrated by an adequately powered study. We discuss the strengths and weaknesses of the case being made by epidemiologists, geneticists, clinicians and basic researchers, and consolidate their findings into a model that provides mechanistic plausibility to the claim. Specifically, converging data sets suggest that local activation of vitamin D coordinates the activity of the innate and adaptive arms of immunity, and of the intestinal epithelium, in a manner that promotes barrier integrity, facilitates the clearance of translocated flora and diverts CD4 T cell development away from inflammatory phenotypes. Since smoking is an important risk-altering exposure, we also discuss its newly established melanizing effect, as well as other emerging evidence linking tobacco use to immune function through vitamin D pathways.
Keywords: Vitamin D, Inflammatory Bowel Disease, Crohn’s Disease, Ulcerative Colitis
Introduction
Vitamin D deficiency is not an “answer in search of a question”. It is one of a limited set of variables credibly proposed to mediate the observed association between environmental exposures and the inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC). The rising incidence rates of these diseases over time, as well as their association with industrialization, limits plausible explanations to those that invoke variables that have changed over that time and with economic development1. The evidence concerning a role for vitamin D deficiency in promoting IBD should, then, be viewed with an appreciation for the burden shared by the IBD field collectively to explain these trends, and researchers must be prepared to address the question “if not vitamin D deficiency, then what?” Although not explored here, several environmental risk factors have been proposed to link IBD to its increasing incidence and to industrialization. These include antibiotics, oral contraceptives, dietary changes (including increased reliance on infant formula over breastfeeding), and improved hygiene1-5. Here, we discuss the evidence and rationale that implicate vitamin D deficiency in the pathogenesis of IBD.
The perceived credibility of vitamin D deficiency as a common contributor to IBD rests on the understanding that the molecule in question was poorly named. The word “vitamin” was originally meant to denote a dietary micronutrient, the absence of which results in disease. The notion that countries populated by affluent and obese citizens could experience widespread deficiency in any vitamin would, then, seem counterintuitive. However, vitamin D is something of a misnomer6, insofar as exposure of the skin to ultraviolet B (UVB) light leads to the production of vitamin D at levels that notably exceed what can be obtained from most foods. For perspective, one minimal erythemal dose of sunshine can generate as many as 20,000 international units (IU) of vitamin D, which is, for example, 200-fold more than the amount in 8 ounces of milk that has been deliberately fortified with vitamin D7. The functional consequence of the cutaneous production of vitamin D is implied by one of the most overtly variable of phenotypic human traits: pigmentation. Positive selective pressure appears to have favored depigmentation in early Eurasians8, 9, and the driving force for this is most commonly attributed to the enhanced rate of vitamin D synthesis afforded by pale skin in the face of reduced exposure to UVB10-12.
Our use of the term “deficiency” likewise warrants clarification. Although the definition of “deficiency” and “insufficiency”, in the context of bone health, is a matter of some debate13, 14, in the context of immune-mediated disease such threshold values can only be guessed at, and attempts to do so might risk erroneously implying a discontinuous relationship between serum levels of vitamin D (specifically 25(OH)D, described in the next section) and disease. Here we use the word deficiency to denote a presumed, and as-yet undefined suboptimal range of serum 25(OH)D values that may place an individual, or her offspring, at an incrementally greater risk for a given disease as that individual’s vitamin D status falls. By this definition, the relationship between serum 25(OH)D concentrations and immune-mediated disease is not known. Furthermore, we do not frame this discussion in terms of a putative ability of vitamin D to ameliorate IBD, referring instead to vitamin D “deficiency” and “repletion” in order to highlight the basic premise that vitamin D deficiency may describe an average state of the population, but does not describe the state of each individual within that population. If deficiency influences the onset or course of IBD, then it does so in a subset of patients. This is in contrast to what is implied by the various randomized controlled trials (RCTs) that do not employ relatively low vitamin D status as an inclusion criterion.
The Basic Biology of Vitamin D
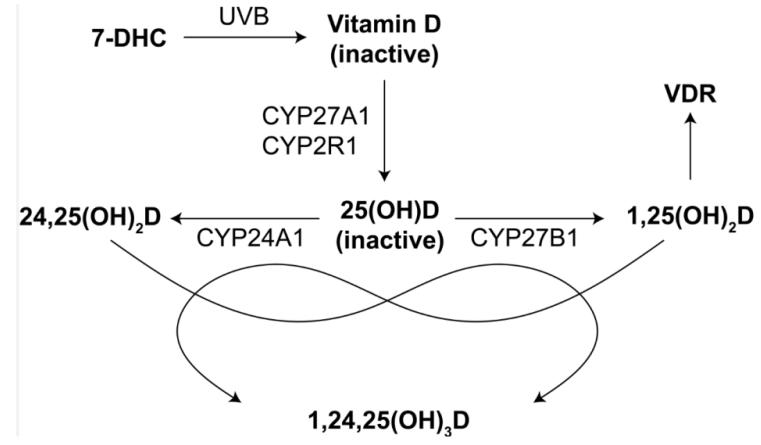
The evidence regarding vitamin D and IBD cannot be appreciated without an understanding of the basic aspects of vitamin D biology, comprehensively reviewed elsewhere7, 15-18. In this section, we provide an abbreviated, but conventional view of vitamin D metabolism and signaling. As depicted in figure 1, UVB light converts cutaneous 7-dehydrocholesterol (7-DHC) to cholecalciferol (i.e., vitamin D). Vitamin D is an inactive precursor that is converted to yet another inactive precursor, 25-hydroxyvitamin D [25(OH)D]. The most commonly cited genes that encode the 25-hydroxylase(s) that catalyze this reaction are CYP27A1 and CYP2R1, though other genes have been implicated. 25(OH)D circulates in the blood with a half-life of, roughly, one month, and serves as the reservoir of substrate from which cells that activate vitamin D can draw. To do so, these cells express 1α-hydroxylase from CYP27B1, thereby converting 25(OH)D to the active form of vitamin D, 1α,25-dihydroxyvitamin D [1,25(OH)2D]. In practice, the term vitamin D is sometimes used to refer, depending on the context, to 25(OH)D or 1,25(OH)2D, but formally denotes cholecalciferol. Thus, for example, one’s vitamin D status, which can be measured in the clinic, refers to the serum concentration of 25(OH)D, even though 25(OH)D is not, formally, vitamin D. In target cells, 1,25(OH)2D binds and activates a transcription factor, the vitamin D receptor (VDR). Ligand-activated VDR heterodimerizes with the retinoid X receptor (RXR), and this heterodimer subsequently binds DNA at vitamin D response elements (VDREs) that are distributed across a target gene’s extended locus (i.e., not exclusively at the proximal promoter). Binding of the heterodimer to VDREs leads to the recruitment of cofactors that subsequently induce or repress gene expression. CYP24A1 encodes 24-hydroxylase, which converts 25(OH)D and 1,25(OH)2D to 24,25-dihydroxyvitamin D [24,25(OH)2D] and 1,24,25-trihydroxyvitamin D [1,24,25(OH)3D], respectively. 1,24,25(OH)3D is also formed from 24,25(OH)2D by the action of CYP27B1. These latter molecules retain activity19-21, but the functional consequences of these metabolites are less well characterized than for 1,25(OH)2D.
Figure 1. Vitamin D metabolism.
As discussed in more detail in the text, the conversion of vitamin D from 7-DHC by UVB is followed by two hydroxylation reactions to generate the active ligand for VDR, a transcription factor that regulates gene expression. Catabolic pathways are also shown.
Vitamin D is commonly referred to as an endocrine molecule, since that is the first mode of signaling that was identified for it. However, as the concentration of serum 25(OH)D falls, the concentration of serum 1,25(OH)2D changes only modestly, if at all22-24, except when 25(OH)D becomes very low25—lower than is experienced by the large majority of modern humans. The maintenance of serum 1,25(OH)2D concentrations reflects the secondary hyperparathyroidism that increases CYP27B1 activity when 25(OH)D levels fall. If VDR that is expressed in immune cells is liganded by 1,25(OH)2D that is produced by the kidneys, then the activation of VDR in those cells should change only modestly as vitamin D status fluctuates through the range that is typical for the industrialized world, and the incentive for investigating vitamin D “deficiency” as a cause of IBD is diminished. However, subsequent to the discovery of vitamin D activation in the kidneys numerous laboratories have reported the expression of CYP27B1 in extra-renal tissues, including macrophages and dendritic cells, thereby supporting the view that intracrine and paracrine roles for vitamin D signaling may be commonplace26. Various endpoints that were measured in cultured antigen-presenting cells (APCs), and in humans, correlated with 25(OH)D concentrations when those concentrations were manipulated to vary across the normal human range27-31, indicating the biological relevance of the local activation of vitamin D by tissues other than the kidneys.
Observational Studies
Epidemiology
Depending on the study, the vitamin D status of IBD patients is32, 33, or is not34, 35, lower than that of healthy controls, and does36, 37, or does not33, 38, correlate with disease severity. The studies that report a relationship between vitamin D status and IBD entail the possibility that vitamin D deficiency is a cause of the disease, but it is also possible that low vitamin D status marks the influence of causal confounders (see below) or that vitamin D deficiency is a consequence of disease (i.e., reverse causation). At least three forms of reverse causation may be operative here. First, sickness may lead patients to spend less time outdoors photosynthesizing vitamin D. Second, CD may result in the malabsorption of vitamin D39, 40. Finally, the expression of CYP24A1 and CYP27B1 in inflamed tissue (as with the expression of CYP27B1 in colonic tissue of CD patients41) results in the consumption of 25(OH)D thereby placing downward pressure on vitamin D status. Consistent with this, lipopolysaccharide (LPS) induces the expression of CYP27B1 in human monocytes and dendritic cells29, 42, and induced endotoxemia causes vitamin D status to fall in dogs43. For some perspective on the likelihood of vitamin D deficiency as a cause of IBD, we turn first to the field of epidemiology.
Several factors that influence vitamin D status fall within the purview of epidemiological investigations, and would be expected to co-vary with IBD, assuming that vitamin D deficiency is one of its causes. That is, vitamin D status is, or may be, non-randomly allocated, in part, by industrialization, urbanization, pollution, time, ethnicity, body mass, geography and the orbit of our tilted planet around its sun. More specifically, vitamin D status is set largely by the availability of UVB radiation (e.g., by season, latitude and pollution), the time spent outdoors exposed to that radiation (e.g., by industrialization), the rate at which exposure results in the photosynthesis of vitamin D (e.g., by pigmentation) and the effect that the production of vitamin D has on vitamin D status (e.g., by body mass). Since the interpretation of epidemiological data sets is especially vulnerable to confounding, biases and reverse causation44, 45, the presence or absence of an association between IBD and any of the vitamin D status-modifying exposures is only suggestive of a link, or its absence, between vitamin D deficiency and IBD. These studies are, however, available and informative.
The industrial revolution heralded an age of rickets, which led to the discovery of vitamin D and of its production by UVB. The concentration of children into the factories and narrow streets of heavily polluted cities compromised access to sunlight to such an extent that adequate calcium metabolism for some children was lost6. Even today, city dwellers typically have lower vitamin D status than do residents of outlying areas46-53, and this has been attributed either to absorption of UVB by pollution54-58 or to the physical obstruction of sunlight by tall buildings59. Furthermore, the amount of time spent indoors dramatically affects vitamin D status60. If vitamin D deficiency is a cause of IBD, then it might be expected that industrialization, urbanization and pollution are associated with IBD. Industrialization1, 2, 4, 61-63 and urbanicity64 are indeed clear risk factors for IBD, and two of three studies also link air pollution to IBD65-67.
Furthermore, in the United States and globally, vitamin D status is declining, at least in recent years68-70. To estimate the change in vitamin D status over a longer time frame, it was recently established that traditionally living Maasai and Hadzabe have an average serum 25(OH)D concentration of 46 ng/ml (i.e., 115 nM)71, whereas the mean level in the US is roughly half that69. If vitamin D deficiency is a cause of IBD, then it might be expected that IBD has increased over time, which it clearly has72. The incidence of pediatric IBD, for example, doubled between 1991 and 2002 in the US62.
Since fat sequesters73, or body mass dilutes74, vitamin D, the aforementioned obesity that is associated with affluent societies is itself a risk factor for vitamin D deficiency. If deficiency contributes to IBD, then obesity might be expected to correlate with IBD. In fact, IBD is generally associated with reduced weight75, and recent weight loss is a common feature at presentation76, though the relationship between the two may be more complex than conventionally believed77, 78. Attempts to link obesity to IBD are, however, confounded by the anorexia and malabsorption that result from disease76.
Since melanin absorbs UVB, slowing the rate at which the skin photosynthesizes vitamin D, African Americans have a collective vitamin D status that is lower than that of Caucasian Americans79. If vitamin D deficiency is a cause of IBD, then it might be expected that African Americans would be at enhanced risk for this disease, as they clearly are for rickets80. It is commonly believed, however, that their risk for IBD is lower, not higher. However, concerns have been raised that this could reflect under-diagnosis of this population81. Additionally, ethnic groups differ not only with respect to pigmentation, but with respect to non-pigmentation-related genetic variations as well. Ashkenazi Jews, for example, have genetically-determined greater risk for CD than do other Europeans82.
Both the position of people on the earth and the migration of the earth around the sun are additional parameters that affect vitamin D status, and for the same reason. The higher the sun appears off of the horizon (i.e, the lower the solar zenith angle) the less atmosphere the sunlight travels through before striking the earth’s surface. Since the atmosphere absorbs UVB, this results in a latitudinal gradient of UVB radiation, with UVB intensity decreasing as the distance from the equator is increased. Furthermore, the axial tilt of the earth establishes, in a manner dependant on latitude, a seasonal oscillation of the UVB radiation to which people are exposed. Thus, the amount of UVB radiation that is available to catalyze the production of vitamin D varies with season and latitude83, 84.
Despite rare reports to the contrary85, 86, a north-south gradient of IBD is now well established for both CD and UC87-94, with risk positively correlated with latitude. In France, however, the effect of latitude was noted only for CD, not UC91, 92. Vitamin D status is being considered as a candidate variable to explain the latitude effect on IBD2, 93. However, the presumed relationship of vitamin D status to latitude needs further investigation95. For example dietary intake of vitamin D in Europe positively correlates with latitude96. Furthermore, much of the human population has not migrated far from where their ancestors resided, and therefore exhibit a level of pigmentation that is adapted to their environment. This would be expected to reduce or nullify the effect of latitude on vitamin D production, and may be why a latitude gradient of vitamin D status was reported only for Caucasians97. Although vitamin D status negatively correlated with latitude in the adult urban population of France98, and in postmenopausal women worldwide during winter99, when Europe was analyzed separately, vitamin D status of these women positively correlated with latitude, and this was accounted for almost entirely by per capita Gross Domestic Product (GDP)99. Indeed, per capita GDP increases with latitude99, and this correlation was documented as a potential confounder of the IBD latitude gradient89.
Of all the commonly cited risk factors for vitamin D deficiency that are discussed here, only season seems to vary independently of the others. Although the seasonal fluctuation of vitamin D status100, 101 makes season an especially attractive variable to follow, the studies that have investigated IBD as a function of season are so numerous, and their conclusions so conflicting, that we leave it to the reader to evaluate them in detail102-121. Instead we segue directly into a discussion of their weaknesses in order to aid in the interpretation and planning of existing and future studies, respectively. Firstly, where studies showed seasonal variations in IBD, causal agency was usually suggested of pathogens, not vitamin D. For example, periodicity of IBD tracks with bacterial infections120, 121. Secondly, where studies failed to find a correlation between season and diagnosis, the null results may be explained away by the variable and sometimes long time lag between onset and diagnosis, which would decouple the time of diagnosis from a seasonal effect on disease onset109, 122. Thirdly, if vitamin D deficiency provokes IBD, but a relatively long and variable time lag separates vitamin D deficiency from the onset or exacerbation of disease, then seasonal fluctuations in vitamin D status will not impact the seasonality of IBD. Fourthly, the magnitude with which vitamin D status varies across the seasons is influenced by latitude and race123. Furthermore, in locations where heat and humidity become extreme, summer may lead people to take shelter, away from UVB, resulting in a counterintuitive relationship between season and vitamin D status124.
Although epidemiologists typically make efforts to correct for the influence of confounders44, the extent to which the exposures discussed above confound each other bears emphasizing. Obesity is becoming more prevalent125, and is associated with industrialization125 and ethnicity126. Pollution, urbanization and industrialization are likewise related, and, as noted above, latitude correlates with both vitamin D status of Caucasians and per capita GDP89, 97, 99. Thus, on the one hand, the ability to rationalize many of these risk exposures in terms of mechanistically independent causes of vitamin D deficiency seems to lend some credence to the notion that vitamin D deficiency promotes IBD. On the other hand, these risk exposures appear highly interdependent, making the value of rationalizing their influence over vitamin D status by independent mechanisms unclear. Modernity may embody various environmental exposures that conspire to enhance the risk for IBD through the agency of vitamin D deficiency, but possibly also presents a myriad other candidate exposures that may promote IBD independent of this deficiency.
Since vitamin D is not only generated by exposure to UVB, but is a marker of that exposure as well, vitamin D-independent effects of that exposure may be the most difficult group of confounders for which to control127. Cutaneous urocanic acid (UCA), for example, is converted, by exposure to UVB, from its inactive trans conformer to the active, systemically-immunosuppressive cis conformer. Importantly, subcutaneous injections of cis-UCA ameliorated disease in the dextran sodium sulfate (DSS) mouse model of colitis128.
Genetic Association Studies
Although Mendelian randomization studies are correlative in nature, the random assignment of parental alleles to offspring at the moment of conception minimizes confounding, precludes reverse causation, and may justify causal inferences45. If we equate variations in vitamin D status with variations in vitamin D signaling, then genetic association studies may qualify as Mendelian randomization studies, where genetic variants that influence vitamin D signaling serve as instrumental variables that proxy for vitamin D status. In this regard, VDR is localized to a region of chromosome 12 that has been linked to IBD susceptibility, and, given the biological plausibility of relatively weak VDR activation in the etiology of IBD, its associated polymorphisms have been selected for analysis. Until recently, it was mainly the restriction fragment length polymorphisms (RFLPs) ApaI, BsmI, FokI & TaqI that were analyzed, and, for the most part, study results are highly discrepant129-134. Two groups have, however, reported that the TaqI variant (consisting of a synonymous change in codon 352 of exon 8) is more frequent in male CD patients than in female CD patients or in healthy controls135, 136. It has also been reported that variants of the open reading frame for the vitamin D binding protein may influence IBD137. As a cautionary note, early reports linking VDR RFLPs to bone mineral density and fracture risk appear not to have survived close scrutiny138. More recently, a very large study that combined genome-wide association scans and network-based analyses implicated VDR in the pathogenesis of both CD and UC139.
Intervention Studies
Animal Experimentation
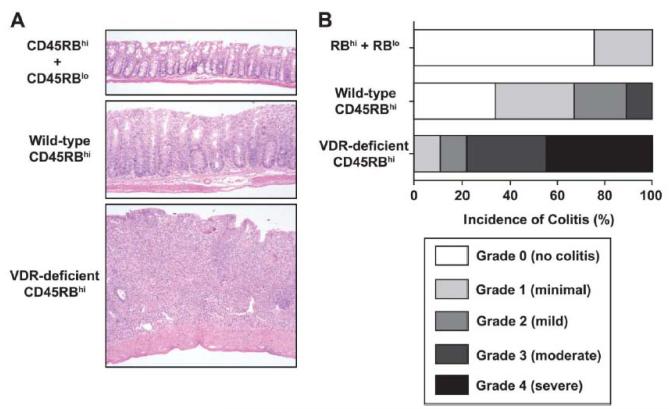
To formally test a causal relationship between vitamin D status and IBD, serum 25(OH)D must be experimentally manipulated and the consequences of this on IBD measured. To the extent that vitamin D status influences vitamin D signaling, the manipulation of vitamin D pathways in animal models also informs the causal relationship between 25(OH)D and colitis. In the context of IL-10 deficiency, mice rendered vitamin D deficient developed diarrhea and began dying by 9 weeks of age, in contrast to vitamin D sufficient mice140. Oral administration of 1,25(OH)2D to the IL-10/vitamin D double-deficient mice was therapeutic140, 141. Deficiency in VDR aggravates IBD in the CD45RBhi transfer model142, an effect we confirm here (Fig. 2), as well as in IL-10 KO mice142, 143. VDR and 1,25(OH)2D had similar effects on colitis induced by DSS144. DSS-induced colitis is likewise exacerbated by deficiency in either Cyp27b1 or vitamin D145, 146, and, when administered intraperitoneally, 1,25(OH)2D or a low calcemic analog of 1,25(OH)2D reduces the severity of colitis induced by trinitrobenzene sulfonic acid147, 148.
Figure 2. T cell deficiency of VDR aggravates CD45RBhi CD4 T cell-mediated colitis.
CD4+CD45RBhi and CD4+CD45RBlo T cells were from isolated from secondary lymphoid tissues of WT or VDR-deficient donors by FACS sorting and 3 × 105 of the indicated cells were injected i.p. into congenic, C57BL/6 RAG-deficient recipients. (A) Five weeks post-transfer, mice were sacrificed and intestinal tissues collected for histological processing and analysis as previously described195. Shown are representative hematoxylin and eosin (H&E)-stained sections of colon from the indicated experimental groups of one of two experiments; all magnifications = 40×. (B) Incidence and severity of colitis five weeks post-transfer of the indicated T cell populations. Samples were coded and scored by a pathologist in a blinded fashion, as previously described195. Data are pooled from two independent experiments. The total number of recipients in each group were: CD45RBhi +CD45RBlo, 4; WT, 9; and VDR-deficient, 9.
Granting that, when examined in isolation, several of the experimental designs that were used to collect the data reported above may be suspected to suffer demonstrated (i.e, calcemic effects) or potential (e.g., ligand-independent effects of VDR15) confounders, vitamin D deficiency, which was shown in studies referenced above to worsen disease in two colitis models, cannot be its own confounder. By whatever pathway(s), then, vitamin D deficiency aggravates IBD in mouse models. Instead, the main weakness of these studies is that the results obtained from mouse models do not always generalize to humans. We know, for example, that 1,25(OH)2D induces the expression of CAMP (an antimicrobial peptide; AMP) in human, but not mouse, cells and that this difference mirrors a VDRE within a retroelement that is present in the promoter for human, but not mouse, CAMP149.
Clinical Trials
Although we are not aware of any clinical trials that have evaluated the effects of vitamin D on UC, two trials published the effects on CD150, 151. One of them150 compared vitamin D to an active analog, not a placebo, and will not be discussed here. In the second study—a double-blind, placebo-controlled trial—94 remitted CD patients had been randomized to receive either 1,200 IU vitamin D or placebo once daily for one year151. Both groups also received 1,200 mg calcium daily. Treatment insignificantly reduced (P = 0.06) the rate of clinical relapse, which was defined in terms of a Crohn’s Disease Activity Index. The decision to set alpha at 0.05 as the criterion of significance151 does not follow from mathematics, but reflects the tolerance of the investigators for committing a type I error. That is, one can conclude from this study that 1,200 IU vitamin D (with 1,200 mg calcium) daily is therapeutic for CD, with a 6% chance of doing so in error.
A primary weakness of this study is that, by convention152, it was underpowered (i.e, at 70%)151, and the authors recommended larger sample sizes for future studies. We also note that this study’s design is not consistent with the premise that the increasing incidence of CD over time, and its association with the Western lifestyle, reflects restricted exposure to sunshine with an attendant average decline in serum 25(OH)D within the population. Relative to placebo, treatment of the CD patients with 1,200 IU vitamin D daily increased serum 25(OH)D concentration by 40% (i.e, 38.4 ng/ml versus 27.6 ng/ml)151. The selection criteria for this study did not exclude patients with relatively high vitamin D status, and the study did not dose for what may now be considered an ancestral level of 25(OH)D71. If, for example, selection of study subjects had resulted in an average initial serum 25(OH)D concentration of 15 ng/ml and subjects in the treatment arm had received enough vitamin D to increase 25(OH)D to 45 ng/ml, then 25(OH)D would have increased 300%, not 40%, and this difference may have increased the study’s statistical power. Finally, it is possible that vitamin D deficiency may promote disease onset without influencing the course of disease, such that vitamin D repletion will not reverse pathology that was initiated by deficiency. Vitamin D repletion will not, for example, reverse limb deformities in adults who suffered childhood rickets, and adaptive immunity is well known for its memory.
Mechanistic Plausibility
Vitamin D is locally activated in disease-affected tissue of CD patients41, and data from several areas of enquiry converge to suggest that this signaling coordinates the activity of multiple cell types, intervening at several stages, to promote homeostatic coexistence between the host and its intestinal microbiota. We present a preliminary model that incorporates what are most likely to be core elements involved in the putative failure to maintain this tolerance during the vitamin D deficient state in otherwise predisposed individuals.
The intestinal epithelium and its associated mucus constitute a barrier that physically separates the host from its gastrointestinal commensal microorganisms. Intestinal permeability is a hallmark of IBD153, and a role for mucus is indicated by the identification of MUC1, which encodes a constituent of mucus, as a candidate gene whose locus harbors a CD risk-conferring variant154. In this regard, vitamin D deficient mice have 50-fold more bacteria in colonic tissue than do non-deficient controls and this was attributed in part to reduced expression of the Paneth cell-produced AMP angiogenin 4146. This bacterial translocation may also reflect the loss of VDR-dependent intercellular tight junctions that aid gut epithelial barrier integrity155.
Dysregulated innate immunity also contributes to the pathophysiology of CD. Genome-wide association studies (GWASs) have, for example, implicated the genes NOD2 and ATG16L1, whose products interact within DCs and macrophages to facilitate autophagy, antigen presentation and bacterial clearance156. NOD2 is a receptor for peptidoglycans from Gram-positive bacteria, and it has been reported that, in the absence of a NOD2 ligand, 1,25(OH)2D induces expression of NOD2 in human monocytes (and other cells) by directing VDR to distal VDREs along the NOD2 locus30. Furthermore, in normal human macrophages, 1,25(OH)2D and liganded NOD2 synergistically induce the expression of CAMP and β-defensin 2 (i.e, DEFB4A; formerly DEFB2 and HBD-2), but not in macrophages obtained from CD patients homozygous for loss-of-function variants of NOD230. LPS, a toll-like receptor (TLR) 4 ligand, induces CYP27B129 and IL-631 in human monocytes, and addition of 25(OH)D reduces IL-631. TLR2/1 activation induces CYP27B1, VDR and IL-1β, along with its receptor, in human monocytes, with VDR inducing CAMP independent of IL-1β signaling, but inducing DEFB4A, which may be important in IBD157, in concert with IL-1β-activated NF-kB158. 1,25(OH)2D induces the expression of CAMP in human monocytes, which in turn promotes autophagy through transcriptional activation of autophagy-related genes, including ATG5159. Similar effects have been recorded in human macrophages160. ATG5 interacts with ATG16L1 in this process159, 160. CAMP not only facilitates the formation of autophagosomes that sequester Mycobacterium tuberculosis (Mtb), but also promotes fusion of these structures with lysosomes, and subsequently enters the lumen of the resulting autophagolysosomes to effect direct anti-Mtb activity as well159. Induction of CYP27B1 and VDR similarly links IFN-γ signaling in human monocytes/macrophages to AMP expression and autophagy, with IL-15 serving as an intermediary161.
A role for adaptive immunity is also well established. Flagellin-derived antigens expressed by the host microbiota are immunodominant in patients, CD4 T cells that recognize these antigens are pathogenic in an animal model of colitis162, and CD4 T cell depletion during AIDS limits relapse of CD163. GWASs and animal data suggest that Th1 and Th17 cells are especially relevant CD4 T cell subsets, but their relative contribution, and their relationship to each other, has not been fully elucidated169. The development and maintenance of Th1 cells is directed by the cytokine IL-12, and by the transcription factors T-bet, STAT1 and STAT4, whereas the development and maintenance of Th17 cells is directed by the cytokines TGF-β, IL-1β, IL-6 and IL-23, and by transcription factors, the most important of which are STAT3 and RORγt (encoded by RORC). Th1 cells produce IFN-γ, whereas Th17 cells produce IL-17A, IL-17F and IL-22. On the one hand, a variant of IL-23R, a receptor whose activation by IL-23 promotes Th17 cell development, is protective for CD164, RORC and STAT3 are associated with IBD139, and the loss of Rorc function in CD4 T cells strongly limits colitis in a mouse model169. On the other hand, STAT1, STAT4 and IFNG are associated with IBD139, and T cell-deficiency of either T-bet or STAT4 reduces disease severity in transfer models of colitis169. The gene that encodes p40, the subunit that is common to both IL-12 and IL-23, is implicated in CD by GWAS154, and the neutralization of p40 is therapeutic for CD165, 166. These and other data suggest that elements of Th1 and Th17 cells are jointly protagonistic, which may reflect the transition of Th17 cells to a Th1 phenotype, the co-expression of T-bet in some Th17 cells, or both169. Importantly, GWASs also implicate IL-10 in CD154 and UC167, and mice deficient for IL-10 in CD4 T cells spontaneously develop colitis comparably to mice globally deficient in IL-10168.
As discussed above, T cell expression of VDR limits colitis, and we have shown that 1,25(OH)2D partially suppresses in vitro Th17 cell developmental programming (including suppression of mRNA that encodes RORγt and IL-23R), while increasing the expression of IL-10. Importantly, this suppression occurred even when IL-1β and IL-23 were used for polarization. In contrast to previous reports, 1,25(OH)2D has negligible effects on Th1 cell development in our hands and we further reported that VDR mRNA was ~30 fold lower in Th1 cells than in Th17 cells170. The failure to inhibit Th1 cell differentiation from naïve murine precursors was corroborated by another recent study171. In dendritic cells, however, 1,25(OH)2D suppresses the expression of p40172, which is expected to reduce the polarization of both Th1 and Th17 cells.
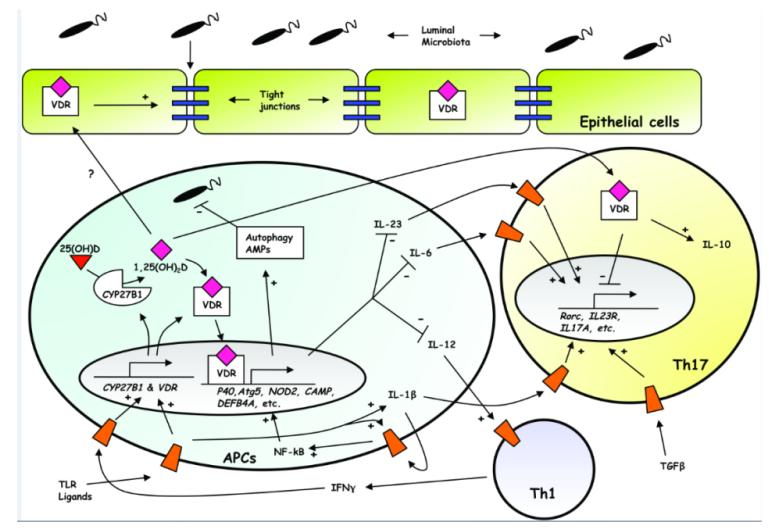
Collectively, these data suggest that vitamin D sufficiency assists epithelial barrier integrity, and that, when the barrier is breached by luminal microbiota, activation of TLRs on APCs solicits intracrine vitamin D signaling to further contain these microbes through autophagy and the expression of AMPs, while also limiting the development and maintenance of Th1 and Th17 effector CD4 T cells through paracrine signaling (Fig. 3), and enhancing IL-10 production. Conversely, in the vitamin D deficient state, flux through CYP27B1 is likely reduced and the formation of tight junctions, as well as the responsiveness of APCs to bacteria, is thereby diminished. Ligands to TLRs and NOD2 subsequently accumulate, and the production of IFN-γ is increased. Consequently, expression of CYP27B1 and VDR, and engagement of NOD2, increases still further, until enough autophagy and AMPs are recruited by VDR to place an upper limit on the microbial excess. In this scenario, inordinate inflammation occurs in the deficient state as Th1 and Th17 cell effector functions are favored over innate mechanisms of homeostatic control.
Figure 3. Model for vitamin D-mediated intestinal homeostasis.
Induction of CYP27B1 and VDR by TLR and IFN-γ signaling leads to intracrine and paracrine signaling involving the several cell types depicted. Activated VDR alters the expression of downstream target genes which promote the formation of tight junctions (blue rectangles), as well as the expression of AMPs and proteins involved in autophagy, and diverts development of CD4 T cells away from the Th1 and Th17 lineages with enhanced expression of IL-10. Receptors for cognate ligands are shown as orange trapezoids. See text for more details.
A model for vitamin D sufficiency-mediated protection against UC is more tentative. Many of the same loci are implicated in both CD and UC, and this includes roles for autophagy, Th1/Th17 pathways, IL-10 and VDR139. Thus, some of the mechanisms proposed in figure 3 may apply to UC as well. Importantly, however, a few polymorphisms have opposing effects on CD and UC139, and this includes risk-altering polymorphisms that implicate NOD2, a gene whose expression is induced by 1,25(OH)2D.
Vitamin D and Smoking
New links between smoking, vitamin D signaling, multiple sclerosis (MS) and IBD have become apparent recently, and are worth highlighting separately. Immune-mediated diseases are related to each other173, and we note here overlapping areas of interest with MS. As with IBD, the distribution of MS exhibits a latitude gradient174, and recent GWASs identify risk-conferring polymorphisms that implicate CYP24A1 and CYP27B1 in MS175, 176, thereby giving more weight to the idea that the latitude effect reflects vitamin D status.
Moreover, smoking is one of the clearest risk factors for both MS174 and CD177. Very recently, and very surprisingly, the lungs have been reported to be an immunological staging ground in a rodent model of MS178, and it has therefore been speculated that smoking may activate auto-reactive, pulmonary T cells that subsequently traffic to the central nervous system (CNS)179. Importantly, a recent GWAS of lung cancer provides evidence that links smoking to vitamin D signaling, and suggests a mechanism that, we speculate, might apply to MS and IBD as well. Dong et al. report that the single nucleotide polymorphisms (SNPs) rs48009957 and rs1663689 confer risk for lung cancer180. The former occurs in the 3′ UTR of CYP24A1 and interacts with smoking to contribute to lung cancer risk. Although the latter SNP is almost one megabase downstream of the nearest gene, that gene is GATA3, which encodes an essential Th2-programming transcription factor. Furthermore, a third SNP (i.e., rs247008) was found to interact with smoking to contribute to lung cancer risk, and occurs just downstream from IL3 and CSF2, and near the Th2 cytokine cluster. Consistent with a relationship between Th2 cells and CYP24A1, the Th2 cytokine IL-4 has been shown to enhance the monocyte-mediated catabolism of vitamin D in a manner dependent on CYP24A1181. Collectively, these data suggest interactions not only between vitamin D signaling and the immune system, which was already well established, but also between these variables and smoking. Dong et al. noted180 that benzo[a]pyrene (BaP), a component of tobacco smoke and a ligand for the aryl hydrocarbon receptor (AhR), enhances, within a human monocyte/macrophage-derived cell line, the induction of CYP24A1 by 1,25(OH)2D in an AhR-dependent manner182. Perhaps, then, smoking promotes MS by inducing the CYP24A1-mediated catabolism of 25(OH)D and 1,25(OH)2D, with loss of VDR-mediated regulation of auto-reactive T cells that subsequently migrate to the CNS. It will be interesting to see if the lungs likewise harbor colitogenic T cells, and if this could link smoking and vitamin D to CD. Alternatively, BaP may distribute systemically183, directly affecting immune cells residing in the gastrointestinal tract. We also wonder how this may be related to the observation that smoking cessation actually increases risk for UC177. Liganding of the nicotinic receptor on macrophages may underpin this latter effect184.
To this we would add a simpler putative mechanism relating smoking to vitamin D-mediated effects on IBD and MS. Nicotine accumulates in melanin-expressing tissues185 and activates amphibian dermal melanocytes in vitro185, while cigarette smoking promotes pigmentation in humans186, 187. That surplus melanin should slow the rate of UVB-mediated vitamin D production, thereby lowering vitamin D status. Smoking is indeed associated with lower vitamin D status188-193. This suggests a causal relationship between tobacco use and reduced vitamin D status, but further research is needed to formally demonstrate this link and to assess the extent to which the risk of IBD and MS that is conferred by smoking is mediated by effects on the photosynthesis of vitamin D.
Directions for Future Research
In conclusion, we remain agnostic regarding any causal relationship between vitamin D status and human IBD, and emphasize, instead, the need to accelerate the research efforts that can generate the answers that physicians and patients await. Repeating the aforementioned RCT, but with a design that increases the study’s power, should be a priority. To expand on the recent analyses that implicate VDR in IBD139, while minimizing the multiple testing burden innate to GWASs, it may be useful to target polymorphisms known to affect vitamin D status194, as well as those that may link vitamin D signaling to MS175, 176 and lung cancer180, for future study. A better understanding of vitamin D signaling during mouse models of colitis will inform our efforts to understand the context in which vitamin D status affects human IBD, if that is what it does. The prospect of reversing pathology that arises from the Western lifestyle with something as simple as vitamin D repletion gives this research some urgency.
Acknowledgments
Supported by the US National Institutes of Health (C.T.W. and M.T.P.) and the Crohn’s and Colitis Foundation of America (C.T.W. and M.T.P.). The authors thank Daniel D. Bikle for helpful comments, and Yun Kyung Lee for technical assistance.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Molodecky NA, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. Challenges associated with identifying the environmental determinants of the inflammatory bowel diseases. Inflamm Bowel Dis. 2011 Aug;17(8):1792–1799. doi: 10.1002/ibd.21511. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008 Sep;57(9):1185–1191. doi: 10.1136/gut.2007.122143. [DOI] [PubMed] [Google Scholar]
- 3.Bernstein CN. Assessing environmental risk factors affecting the inflammatory bowel diseases: a joint workshop of the Crohn’s & Colitis Foundations of Canada and the USA. Inflamm Bowel Dis. 2008 Aug;14(8):1139–1146. doi: 10.1002/ibd.20494. [DOI] [PubMed] [Google Scholar]
- 4.Molodecky NA, Kaplan GG. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2010 May;6(5):339–346. [PMC free article] [PubMed] [Google Scholar]
- 5.Shores DR, Binion DG, Freeman BA, Baker PR. New insights into the role of fatty acids in the pathogenesis and resolution of inflammatory bowel disease. Inflamm Bowel Dis. 2011 Oct;17(10):2192–2204. doi: 10.1002/ibd.21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hochberg Z. Rickets--past and present. Introduction. Endocr Dev. 2003;6:1–13. doi: 10.1159/000072763. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF. Vitamin D deficiency. N Engl J Med. 2007 Jul 19;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 8.Norton HL, Kittles RA, Parra E, et al. Genetic evidence for the convergent evolution of light skin in Europeans and East Asians. Mol Biol Evol. 2007 Mar;24(3):710–722. doi: 10.1093/molbev/msl203. [DOI] [PubMed] [Google Scholar]
- 9.Lamason RL, Mohideen MA, Mest JR, et al. SLC24A5, a putative cation exchanger, affects pigmentation in zebrafish and humans. Science. 2005 Dec 16;310(5755):1782–1786. doi: 10.1126/science.1116238. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons A. American Association of Physical Anthropologists meeting. European skin turned pale only recently, gene suggests. Science. 2007 Apr 20;316(5823):364. doi: 10.1126/science.316.5823.364a. [DOI] [PubMed] [Google Scholar]
- 11.Diamond J. Evolutionary biology: geography and skin colour. Nature. 2005 May 19;435(7040):283–284. doi: 10.1038/435283a. [DOI] [PubMed] [Google Scholar]
- 12.Jablonski NG, Chaplin G. Colloquium paper: human skin pigmentation as an adaptation to UV radiation. Proc Natl Acad Sci U S A. 2010 May 11;107(Suppl 2):8962–8968. doi: 10.1073/pnas.0914628107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011 Jul;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 14.Rosen CJ, Abrams SA, Aloia JF, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012 Apr;97(4):1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008 Oct;29(6):726–776. doi: 10.1210/er.2008-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25-dihydroxyvitamin D(3) Endocrinol Metab Clin North Am. 2010 Jun;39(2):255–269. doi: 10.1016/j.ecl.2010.02.007. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adams JS, Chen H, Chun R, et al. Substrate and enzyme trafficking as a means of regulating 1,25-dihydroxyvitamin D synthesis and action: the human innate immune response. J Bone Miner Res. 2007 Dec;22(Suppl 2):V20–24. doi: 10.1359/jbmr.07s214. [DOI] [PubMed] [Google Scholar]
- 18.Bikle DD. Vitamin D and the skin. J Bone Miner Metab. 2010 Mar;28(2):117–130. doi: 10.1007/s00774-009-0153-8. [DOI] [PubMed] [Google Scholar]
- 19.Henry HL, Norman AW. Vitamin D: two dihydroxylated metabolites are required for normal chicken egg hatchability. Science. 1978 Sep 1;201(4358):835–837. doi: 10.1126/science.684411. [DOI] [PubMed] [Google Scholar]
- 20.Ornoy A, Goodwin D, Noff D, Edelstein S. 24, 25-dihydroxyvitamin D is a metabolite of vitamin D essential for bone formation. Nature. 1978 Nov 30;276(5687):517–519. doi: 10.1038/276517a0. [DOI] [PubMed] [Google Scholar]
- 21.Harant H, Spinner D, Reddy GS, Lindley IJ. Natural metabolites of 1alpha,25-dihydroxyvitamin D(3) retain biologic activity mediated through the vitamin D receptor. J Cell Biochem. 2000 Apr;78(1):112–120. [PubMed] [Google Scholar]
- 22.Need AG, Horowitz M, Morris HA, Nordin BC. Vitamin D status: effects on parathyroid hormone and 1, 25-dihydroxyvitamin D in postmenopausal women. Am J Clin Nutr. 2000 Jun;71(6):1577–1581. doi: 10.1093/ajcn/71.6.1577. [DOI] [PubMed] [Google Scholar]
- 23.Vieth R, Ladak Y, Walfish PG. Age-related changes in the 25-hydroxyvitamin D versus parathyroid hormone relationship suggest a different reason why older adults require more vitamin D. J Clin Endocrinol Metab. 2003 Jan;88(1):185–191. doi: 10.1210/jc.2002-021064. [DOI] [PubMed] [Google Scholar]
- 24.Rejnmark L, Vestergaard P, Heickendorff L, Mosekilde L. Plasma 1,25(OH)2D levels decrease in postmenopausal women with hypovitaminosis D. Eur J Endocrinol. 2008 Apr;158(4):571–576. doi: 10.1530/EJE-07-0844. [DOI] [PubMed] [Google Scholar]
- 25.Need AG, O’Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res. 2008 Nov;23(11):1859–1863. doi: 10.1359/jbmr.080607. [DOI] [PubMed] [Google Scholar]
- 26.Hewison M, Burke F, Evans KN, et al. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007 Mar;103(3-5):316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 27.Hewison M, Freeman L, Hughes SV, et al. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003 Jun 1;170(11):5382–5390. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 28.Liu PT, Stenger S, Li H, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006 Mar 24;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 29.Adams JS, Ren S, Liu PT, et al. Vitamin d-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009 Apr 1;182(7):4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang TT, Dabbas B, Laperriere D, et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010 Jan 22;285(4):2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012 Mar 1;188(5):2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silvennoinen J. Relationships between vitamin D, parathyroid hormone and bone mineral density in inflammatory bowel disease. J Intern Med. 1996 Feb;239(2):131–137. doi: 10.1046/j.1365-2796.1996.420765000.x. [DOI] [PubMed] [Google Scholar]
- 33.El-Matary W, Sikora S, Spady D. Bone mineral density, vitamin D, and disease activity in children newly diagnosed with inflammatory bowel disease. Dig Dis Sci. 2011 Mar;56(3):825–829. doi: 10.1007/s10620-010-1380-5. [DOI] [PubMed] [Google Scholar]
- 34.Harries AD, Brown R, Heatley RV, Williams LA, Woodhead S, Rhodes J. Vitamin D status in Crohn’s disease: association with nutrition and disease activity. Gut. 1985 Nov;26(11):1197–1203. doi: 10.1136/gut.26.11.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tajika M, Matsuura A, Nakamura T, et al. Risk factors for vitamin D deficiency in patients with Crohn’s disease. J Gastroenterol. 2004 Jun;39(6):527–533. doi: 10.1007/s00535-003-1338-x. [DOI] [PubMed] [Google Scholar]
- 36.Ulitsky A, Ananthakrishnan AN, Naik A, et al. Vitamin D deficiency in patients with inflammatory bowel disease: association with disease activity and quality of life. JPEN J Parenter Enteral Nutr. 2011 May;35(3):308–316. doi: 10.1177/0148607110381267. [DOI] [PubMed] [Google Scholar]
- 37.Fu YT, Chatur N, Cheong-Lee C, Salh B. Hypovitaminosis D in adults with inflammatory bowel disease: potential role of ethnicity. Dig Dis Sci. 2012 Aug;57(8):2144–2148. doi: 10.1007/s10620-012-2130-7. [DOI] [PubMed] [Google Scholar]
- 38.Levin AD, Wadhera V, Leach ST, et al. Vitamin D deficiency in children with inflammatory bowel disease. Dig Dis Sci. 2011 Mar;56(3):830–836. doi: 10.1007/s10620-010-1544-3. [DOI] [PubMed] [Google Scholar]
- 39.Lo CW, Paris PW, Clemens TL, Nolan J, Holick MF. Vitamin D absorption in healthy subjects and in patients with intestinal malabsorption syndromes. Am J Clin Nutr. 1985 Oct;42(4):644–649. doi: 10.1093/ajcn/42.4.644. [DOI] [PubMed] [Google Scholar]
- 40.Farraye FA, Nimitphong H, Stucchi A, et al. Use of a novel vitamin D bioavailability test demonstrates that vitamin D absorption is decreased in patients with quiescent Crohn’s disease. Inflamm Bowel Dis. 2011 Oct;17(10):2116–2121. doi: 10.1002/ibd.21595. [DOI] [PubMed] [Google Scholar]
- 41.Abreu MT, Kantorovich V, Vasiliauskas EA, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004 Aug;53(8):1129–1136. doi: 10.1136/gut.2003.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fritsche J, Mondal K, Ehrnsperger A, Andreesen R, Kreutz M. Regulation of 25-hydroxyvitamin D3-1 alpha-hydroxylase and production of 1 alpha,25-dihydroxyvitamin D3 by human dendritic cells. Blood. 2003 Nov 1;102(9):3314–3316. doi: 10.1182/blood-2002-11-3521. [DOI] [PubMed] [Google Scholar]
- 43.Holowaychuk MK, Birkenheuer AJ, Li J, Marr H, Boll A, Nordone SK. Hypocalcemia and hypovitaminosis D in dogs with induced endotoxemia. J Vet Intern Med. 2012 Mar-Apr;26(2):244–251. doi: 10.1111/j.1939-1676.2012.00886.x. [DOI] [PubMed] [Google Scholar]
- 44.Grimes DA, Schulz KF. Bias and causal associations in observational research. Lancet. 2002 Jan 19;359(9302):248–252. doi: 10.1016/S0140-6736(02)07451-2. [DOI] [PubMed] [Google Scholar]
- 45.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008 Apr 15;27(8):1133–1163. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 46.Gannage-Yared MH, Chemali R, Yaacoub N, Halaby G. Hypovitaminosis D in a sunny country: relation to lifestyle and bone markers. J Bone Miner Res. 2000 Sep;15(9):1856–1862. doi: 10.1359/jbmr.2000.15.9.1856. [DOI] [PubMed] [Google Scholar]
- 47.Maddah M, Sharami SH, Neyestani TR. Vitamin D insufficiency among postmenopausal women in urban and rural areas in Guilan, Northern Iran. J Nutr Elder. 2009 Oct;28(4):386–393. doi: 10.1080/01639360903393523. [DOI] [PubMed] [Google Scholar]
- 48.Heere C, Skeaff CM, Waqatakirewa L, Vatucawaqa P, Khan AN, Green TJ. Serum 25-hydroxyvitamin D concentration of Indigenous-Fijian and Fijian-Indian women. Asia Pac J Clin Nutr. 2010;19(1):43–48. [PubMed] [Google Scholar]
- 49.Chailurkit LO, Aekplakorn W, Ongphiphadhanakul B. Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Health. 2011;11:853. doi: 10.1186/1471-2458-11-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bailey BA, Manning T, Peiris AN. The Impact of Living in Rural and Urban Areas: Vitamin D and Medical Costs in Veterans. J Rural Health. 2012 Apr 4; doi: 10.1111/j.1748-0361.2012.00407.x. [DOI] [PubMed] [Google Scholar]
- 51.Choi EY. 25(OH)D status and demographic and lifestyle determinants of 25(OH)D among Korean adults. Asia Pac J Clin Nutr. 2012;21(4):526–535. [PubMed] [Google Scholar]
- 52.Nguyen HT, von Schoultz B, Nguyen TV, et al. Vitamin D deficiency in northern Vietnam: Prevalence, risk factors and associations with bone mineral density. Bone. 2012 Aug 2; doi: 10.1016/j.bone.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 53.Nichols EK, Khatib IM, Aburto NJ, et al. Vitamin D status and determinants of deficiency among non-pregnant Jordanian women of reproductive age. Eur J Clin Nutr. 2012 Jun;66(6):751–756. doi: 10.1038/ejcn.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mims FM., 3rd Significant reduction of UVB caused by smoke from biomass burning in Brazil. Photochem Photobiol. 1996 Nov;64(5):814–816. doi: 10.1111/j.1751-1097.1996.tb01839.x. [DOI] [PubMed] [Google Scholar]
- 55.Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Arch Dis Child. 2002 Aug;87(2):111–113. doi: 10.1136/adc.87.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Manicourt DH, Devogelaer JP. Urban tropospheric ozone increases the prevalence of vitamin D deficiency among Belgian postmenopausal women with outdoor activities during summer. J Clin Endocrinol Metab. 2008 Oct;93(10):3893–3899. doi: 10.1210/jc.2007-2663. [DOI] [PubMed] [Google Scholar]
- 57.Hosseinpanah F, Pour SH, Heibatollahi M, Moghbel N, Asefzade S, Azizi F. The effects of air pollution on vitamin D status in healthy women: a cross sectional study. BMC Public Health. 2010;10:519. doi: 10.1186/1471-2458-10-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baiz N, Dargent-Molina P, Wark JD, Souberbielle JC, Slama R, Annesi-Maesano I. Gestational Exposure to Urban Air Pollution Related to a Decrease in Cord Blood Vitamin D Levels. J Clin Endocrinol Metab. 2012 Aug 17; doi: 10.1210/jc.2012-1943. [DOI] [PubMed] [Google Scholar]
- 59.McKinley A, Janda M, Auster J, Kimlin M. In vitro model of vitamin D synthesis by UV radiation in an Australian urban environment. Photochem Photobiol. 2011 Mar-Apr;87(2):447–451. doi: 10.1111/j.1751-1097.2010.00865.x. [DOI] [PubMed] [Google Scholar]
- 60.Scragg R, Camargo CA., Jr. Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008 Sep 15;168(6):577–586. doi: 10.1093/aje/kwn163. discussion 587-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Economou M, Pappas G. New global map of Crohn’s disease: Genetic, environmental, and socioeconomic correlations. Inflamm Bowel Dis. 2008 May;14(5):709–720. doi: 10.1002/ibd.20352. [DOI] [PubMed] [Google Scholar]
- 62.Malaty HM, Fan X, Opekun AR, Thibodeaux C, Ferry GD. Rising incidence of inflammatory bowel disease among children: a 12-year study. J Pediatr Gastroenterol Nutr. 2010 Jan;50(1):27–31. doi: 10.1097/MPG.0b013e3181b99baa. [DOI] [PubMed] [Google Scholar]
- 63.Loftus EV., Jr. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology. 2004 May;126(6):1504–1517. doi: 10.1053/j.gastro.2004.01.063. [DOI] [PubMed] [Google Scholar]
- 64.Soon IS, Molodecky NA, Rabi DM, Ghali WA, Barkema HW, Kaplan GG. The relationship between urban environment and the inflammatory bowel diseases: a systematic review and meta-analysis. BMC Gastroenterol. 2012 May 24;12(1):51. doi: 10.1186/1471-230X-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kaplan GG, Hubbard J, Korzenik J, et al. The inflammatory bowel diseases and ambient air pollution: a novel association. Am J Gastroenterol. 2010 Nov;105(11):2412–2419. doi: 10.1038/ajg.2010.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Beamish LA, Osornio-Vargas AR, Wine E. Air pollution: An environmental factor contributing to intestinal disease. J Crohns Colitis. 2011 Aug;5(4):279–286. doi: 10.1016/j.crohns.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 67.Ananthakrishnan AN, McGinley EL, Binion DG, Saeian K. Ambient air pollution correlates with hospitalizations for inflammatory bowel disease: an ecologic analysis. Inflamm Bowel Dis. 2011 May;17(5):1138–1145. doi: 10.1002/ibd.21455. [DOI] [PubMed] [Google Scholar]
- 68.Looker AC, Pfeiffer CM, Lacher DA, Schleicher RL, Picciano MF, Yetley EA. Serum 25-hydroxyvitamin D status of the US population: 1988-1994 compared with 2000-2004. Am J Clin Nutr. 2008 Dec;88(6):1519–1527. doi: 10.3945/ajcn.2008.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ginde AA, Liu MC, Camargo CA., Jr. Demographic differences and trends of vitamin D insufficiency in the US population, 1988-2004. Arch Intern Med. 2009 Mar 23;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mithal A, Wahl DA, Bonjour JP, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009 Nov;20(11):1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 71.Luxwolda MF, Kuipers RS, Kema IP, Janneke Dijck-Brouwer DA, Muskiet FA. Traditionally living populations in East Africa have a mean serum 25-hydroxyvitamin D concentration of 115 nmol/l. Br J Nutr. 2012 Jan 23;:1–5. doi: 10.1017/S0007114511007161. [DOI] [PubMed] [Google Scholar]
- 72.Molodecky NA, Soon IS, Rabi DM, et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012 Jan;142(1):46–54. e42. doi: 10.1053/j.gastro.2011.10.001. quiz e30. [DOI] [PubMed] [Google Scholar]
- 73.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000 Sep;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 74.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin d status of obesity. Obesity (Silver Spring) 2012 Jul;20(7):1444–1448. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 75.Yarur AJ, Deshpande AR, Pechman DM, Tamariz L, Abreu MT, Sussman DA. Inflammatory bowel disease is associated with an increased incidence of cardiovascular events. Am J Gastroenterol. 2011 Apr;106(4):741–747. doi: 10.1038/ajg.2011.63. [DOI] [PubMed] [Google Scholar]
- 76.Gerasimidis K, McGrogan P, Edwards CA. The aetiology and impact of malnutrition in paediatric inflammatory bowel disease. J Hum Nutr Diet. 2011 Aug;24(4):313–326. doi: 10.1111/j.1365-277X.2011.01171.x. [DOI] [PubMed] [Google Scholar]
- 77.Long MD, Crandall WV, Leibowitz IH, et al. Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis. 2011 Oct;17(10):2162–2168. doi: 10.1002/ibd.21585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mendall MA, Gunasekera AV, John BJ, Kumar D. Is obesity a risk factor for Crohn’s disease? Dig Dis Sci. 2011 Mar;56(3):837–844. doi: 10.1007/s10620-010-1541-6. [DOI] [PubMed] [Google Scholar]
- 79.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002 May;30(5):771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 80.Weisberg P, Scanlon KS, Li R, Cogswell ME. Nutritional rickets among children in the United States: review of cases reported between 1986 and 2003. Am J Clin Nutr. 2004 Dec;80(6 Suppl):1697S–1705S. doi: 10.1093/ajcn/80.6.1697S. [DOI] [PubMed] [Google Scholar]
- 81.Veluswamy H, Suryawala K, Sheth A, et al. African-American inflammatory bowel disease in a Southern U.S. health center. BMC Gastroenterol. 2010;10:104. doi: 10.1186/1471-230X-10-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kenny EE, Pe’er I, Karban A, et al. A genome-wide scan of Ashkenazi Jewish Crohn’s disease suggests novel susceptibility loci. PLoS Genet. 2012;8(3):e1002559. doi: 10.1371/journal.pgen.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol. 2000 Jul;39(1):57–106. doi: 10.1006/jhev.2000.0403. [DOI] [PubMed] [Google Scholar]
- 84.Kimlin MG. Geographic location and vitamin D synthesis. Mol Aspects Med. 2008 Dec;29(6):453–461. doi: 10.1016/j.mam.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 85.Abakar-Mahamat A, Filippi J, Pradier C, Dozol A, Hebuterne X. Incidence of inflammatory bowel disease in Corsica from 2002 to 2003. Gastroenterol Clin Biol. 2007 Dec;31(12):1098–1103. doi: 10.1016/s0399-8320(07)78343-4. [DOI] [PubMed] [Google Scholar]
- 86.Ahuja V, Tandon RK. Inflammatory bowel disease in the Asia-Pacific area: a comparison with developed countries and regional differences. J Dig Dis. 2010 Jun;11(3):134–147. doi: 10.1111/j.1751-2980.2010.00429.x. [DOI] [PubMed] [Google Scholar]
- 87.Sonnenberg A, McCarty DJ, Jacobsen SJ. Geographic variation of inflammatory bowel disease within the United States. Gastroenterology. 1991 Jan;100(1):143–149. doi: 10.1016/0016-5085(91)90594-b. [DOI] [PubMed] [Google Scholar]
- 88.Tragnone A, Hanau C, Bazzocchi G, Lanfranchi GA. Epidemiological characteristics of inflammatory bowel disease in Bologna, Italy--incidence and risk factors. Digestion. 1993;54(3):183–188. doi: 10.1159/000201036. [DOI] [PubMed] [Google Scholar]
- 89.Shivananda S, Lennard-Jones J, Logan R, et al. Incidence of inflammatory bowel disease across Europe: is there a difference between north and south? Results of the European Collaborative Study on Inflammatory Bowel Disease (EC-IBD) Gut. 1996 Nov;39(5):690–697. doi: 10.1136/gut.39.5.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Armitage EL, Aldhous MC, Anderson N, et al. Incidence of juvenile-onset Crohn’s disease in Scotland: association with northern latitude and affluence. Gastroenterology. 2004 Oct;127(4):1051–1057. doi: 10.1053/j.gastro.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 91.Nerich V, Monnet E, Etienne A, et al. Geographical variations of inflammatory bowel disease in France: a study based on national health insurance data. Inflamm Bowel Dis. 2006 Mar;12(3):218–226. doi: 10.1097/01.MIB.0000206540.38834.8c. [DOI] [PubMed] [Google Scholar]
- 92.Nerich V, Monnet E, Weill A, et al. Fine-scale geographic variations of inflammatory bowel disease in France: correlation with socioeconomic and house equipment variables. Inflamm Bowel Dis. 2010 May;16(5):813–821. doi: 10.1002/ibd.21122. [DOI] [PubMed] [Google Scholar]
- 93.Khalili H, Huang ES, Ananthakrishnan AN, et al. Geographical variation and incidence of inflammatory bowel disease among US women. Gut. 2012 Jan 11; doi: 10.1136/gutjnl-2011-301574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sonnenberg A, Genta RM. Geographic distributions of microscopic colitis and inflammatory bowel disease in the United States. Inflamm Bowel Dis. 2012 Feb 28; doi: 10.1002/ibd.22932. [DOI] [PubMed] [Google Scholar]
- 95.Kimlin MG, Olds WJ, Moore MR. Location and vitamin D synthesis: is the hypothesis validated by geophysical data? J Photochem Photobiol B. 2007 Mar 1;86(3):234–239. doi: 10.1016/j.jphotobiol.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 96.Freisling H, Fahey MT, Moskal A, et al. Region-specific nutrient intake patterns exhibit a geographical gradient within and between European countries. J Nutr. 2010 Jul;140(7):1280–1286. doi: 10.3945/jn.110.121152. [DOI] [PubMed] [Google Scholar]
- 97.Hagenau T, Vest R, Gissel TN, et al. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: an ecologic meta-regression analysis. Osteoporos Int. 2009 Jan;20(1):133–140. doi: 10.1007/s00198-008-0626-y. [DOI] [PubMed] [Google Scholar]
- 98.Chapuy MC, Preziosi P, Maamer M, et al. Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int. 1997;7(5):439–443. doi: 10.1007/s001980050030. [DOI] [PubMed] [Google Scholar]
- 99.Kuchuk NO, van Schoor NM, Pluijm SM, Chines A, Lips P. Vitamin D status, parathyroid function, bone turnover, and BMD in postmenopausal women with osteoporosis: global perspective. J Bone Miner Res. 2009 Apr;24(4):693–701. doi: 10.1359/jbmr.081209. [DOI] [PubMed] [Google Scholar]
- 100.Hypponen E, Power C. Hypovitaminosis D in British adults at age 45 y: nationwide cohort study of dietary and lifestyle predictors. Am J Clin Nutr. 2007 Mar;85(3):860–868. doi: 10.1093/ajcn/85.3.860. [DOI] [PubMed] [Google Scholar]
- 101.Shoben AB, Kestenbaum B, Levin G, et al. Seasonal variation in 25-hydroxyvitamin D concentrations in the cardiovascular health study. Am J Epidemiol. 2011 Dec 15;174(12):1363–1372. doi: 10.1093/aje/kwr258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Don BA, Goldacre MJ. Absence of seasonality in emergency hospital admissions for inflammatory bowel disease. Lancet. 1984 Nov 17;2(8412):1156–1157. doi: 10.1016/s0140-6736(84)91590-3. [DOI] [PubMed] [Google Scholar]
- 103.Myszor M, Calam J. Seasonality of ulcerative colitis. Lancet. 1984 Sep 1;2(8401):522–523. doi: 10.1016/s0140-6736(84)92600-x. [DOI] [PubMed] [Google Scholar]
- 104.Riley SA, Mani V, Goodman MJ, Lucas S. Why do patients with ulcerative colitis relapse? Gut. 1990 Feb;31(2):179–183. doi: 10.1136/gut.31.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ekbom A, Helmick C, Zack M, Adami HO. The epidemiology of inflammatory bowel disease: a large, population-based study in Sweden. Gastroenterology. 1991 Feb;100(2):350–358. doi: 10.1016/0016-5085(91)90202-v. [DOI] [PubMed] [Google Scholar]
- 106.Tysk C, Jarnerot G. Seasonal variation in exacerbations of ulcerative colitis. Scand J Gastroenterol. 1993 Jan;28(1):95–96. doi: 10.3109/00365529309096052. [DOI] [PubMed] [Google Scholar]
- 107.Sonnenberg A, Jacobsen SJ, Wasserman IH. Periodicity of hospital admissions for inflammatory bowel disease. Am J Gastroenterol. 1994 Jun;89(6):847–851. [PubMed] [Google Scholar]
- 108.Bellaiche G, Beaugerie L, Carbonnel F, et al. [The clinical activity of Crohn’s disease in the Paris area is maximal in the spring] Ann Gastroenterol Hepatol (Paris) 1995 May-Jun;31(3):150–153. [PubMed] [Google Scholar]
- 109.Moum B, Aadland E, Ekbom A, Vatn MH. Seasonal variations in the onset of ulcerative colitis. Gut. 1996 Mar;38(3):376–378. doi: 10.1136/gut.38.3.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zeng L, Anderson FH. Seasonal change in the exacerbations of Crohn’s disease. Scand J Gastroenterol. 1996 Jan;31(1):79–82. doi: 10.3109/00365529609031631. [DOI] [PubMed] [Google Scholar]
- 111.Karamanolis DG, Delis KC, Papatheodoridis GV, Kalafatis E, Paspatis G, Xourgias VC. Seasonal variation in exacerbations of ulcerative colitis. Hepatogastroenterology. 1997 Sep-Oct;44(17):1334–1338. [PubMed] [Google Scholar]
- 112.Vergara M, Fraga X, Casellas F, Bermejo B, Malagelada JR. Seasonal influence in exacerbations of inflammatory bowel disease. Rev Esp Enferm Dig. 1997 May;89(5):357–366. [PubMed] [Google Scholar]
- 113.Lewis JD, Aberra FN, Lichtenstein GR, Bilker WB, Brensinger C, Strom BL. Seasonal variation in flares of inflammatory bowel disease. Gastroenterology. 2004 Mar;126(3):665–673. doi: 10.1053/j.gastro.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 114.Auslander JN, Lieberman DA, Sonnenberg A. Lack of seasonal variation in the endoscopic diagnoses of Crohn’s disease and ulcerative colitis. Am J Gastroenterol. 2005 Oct;100(10):2233–2238. doi: 10.1111/j.1572-0241.2005.50127.x. [DOI] [PubMed] [Google Scholar]
- 115.Aratari A, Papi C, Galletti B, et al. Seasonal variations in onset of symptoms in Crohn’s disease. Dig Liver Dis. 2006 May;38(5):319–323. doi: 10.1016/j.dld.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 116.Soncini M, Triossi O, Leo P, et al. Seasonal patterns of hospital treatment for inflammatory bowel disease in Italy. Digestion. 2006;73(1):1–8. doi: 10.1159/000090036. [DOI] [PubMed] [Google Scholar]
- 117.Bai A, Guo Y, Shen Y, Xie Y, Zhu X, Lu N. Seasonality in flares and months of births of patients with ulcerative colitis in a Chinese population. Dig Dis Sci. 2009 May;54(5):1094–1098. doi: 10.1007/s10620-008-0453-1. [DOI] [PubMed] [Google Scholar]
- 118.Romberg-Camps MJ, Hesselink-van de Kruijs MA, Schouten LJ, et al. Inflammatory Bowel Disease in South Limburg (the Netherlands) 1991-2002: Incidence, diagnostic delay, and seasonal variations in onset of symptoms. J Crohns Colitis. 2009 Jun;3(2):115–124. doi: 10.1016/j.crohns.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 119.Bours PH, Wielders JP, Vermeijden JR, van de Wiel A. Seasonal variation of serum 25-hydroxyvitamin D levels in adult patients with inflammatory bowel disease. Osteoporos Int. 2011 Nov;22(11):2857–2867. doi: 10.1007/s00198-010-1484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sonnenberg A. Seasonal variation of enteric infections and inflammatory bowel disease. Inflamm Bowel Dis. 2008 Jul;14(7):955–959. doi: 10.1002/ibd.20408. [DOI] [PubMed] [Google Scholar]
- 121.Sonnenberg A. Seasonal variation of enteric infections and inflammatory bowel disease. Inflamm Bowel Dis. 2009 Jun;15(6):809. doi: 10.1002/ibd.20770. [DOI] [PubMed] [Google Scholar]
- 122.Basaranoglu M. Symptom date is better than the endoscopic diagnosis date in seasonal variation studies in IBD. Am J Gastroenterol. 2006 Nov;101(11):2668–2669. doi: 10.1111/j.1572-0241.2006.00809_7.x. [DOI] [PubMed] [Google Scholar]
- 123.McCullough ML, Weinstein SJ, Freedman DM, et al. Correlates of circulating 25-hydroxyvitamin D: Cohort Consortium Vitamin D Pooling Project of Rarer Cancers. Am J Epidemiol. 2010 Jul 1;172(1):21–35. doi: 10.1093/aje/kwq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Al-Daghri NM, Al-Attas OS, Alokail MS, et al. Increased vitamin D supplementation recommended during summer season in the gulf region: a counterintuitive seasonal effect in vitamin D levels in adult, overweight and obese Middle Eastern residents. Clin Endocrinol (Oxf) 2012 Mar;76(3):346–350. doi: 10.1111/j.1365-2265.2011.04219.x. [DOI] [PubMed] [Google Scholar]
- 125.James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008 Dec;32(Suppl 7):S120–126. doi: 10.1038/ijo.2008.247. [DOI] [PubMed] [Google Scholar]
- 126.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA. 2010 Jan 20;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 127.Hart PH, Gorman S, Finlay-Jones JJ. Modulation of the immune system by UV radiation: more than just the effects of vitamin D? Nat Rev Immunol. 2011 Sep;11(9):584–596. doi: 10.1038/nri3045. [DOI] [PubMed] [Google Scholar]
- 128.Albert E, Walker J, Thiesen A, Churchill T, Madsen K. cis-Urocanic acid attenuates acute dextran sodium sulphate-induced intestinal inflammation. PLoS One. 2010;5(10):e13676. doi: 10.1371/journal.pone.0013676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn’s disease susceptibility. Gut. 2000 Aug;47(2):211–214. doi: 10.1136/gut.47.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Martin K, Radlmayr M, Borchers R, Heinzlmann M, Folwaczny C. Candidate genes colocalized to linkage regions in inflammatory bowel disease. Digestion. 2002;66(2):121–126. doi: 10.1159/000065592. [DOI] [PubMed] [Google Scholar]
- 131.Dresner-Pollak R, Ackerman Z, Eliakim R, Karban A, Chowers Y, Fidder HH. The BsmI vitamin D receptor gene polymorphism is associated with ulcerative colitis in Jewish Ashkenazi patients. Genet Test. 2004;8(4):417–420. doi: 10.1089/gte.2004.8.417. Winter. [DOI] [PubMed] [Google Scholar]
- 132.Naderi N, Farnood A, Habibi M, et al. Association of vitamin D receptor gene polymorphisms in Iranian patients with inflammatory bowel disease. J Gastroenterol Hepatol. 2008 Dec;23(12):1816–1822. doi: 10.1111/j.1440-1746.2008.05525.x. [DOI] [PubMed] [Google Scholar]
- 133.Hughes DJ, McManus R, Neary P, O’Morain C, O’Sullivan M. Common variation in the vitamin D receptor gene and risk of inflammatory bowel disease in an Irish case-control study. Eur J Gastroenterol Hepatol. 2011 Sep;23(9):807–812. doi: 10.1097/MEG.0b013e328349283e. [DOI] [PubMed] [Google Scholar]
- 134.Pei FH, Wang YJ, Gao SL, et al. Vitamin D receptor gene polymorphism and ulcerative colitis susceptibility in Han Chinese. J Dig Dis. 2011 Apr;12(2):90–98. doi: 10.1111/j.1751-2980.2011.00483.x. [DOI] [PubMed] [Google Scholar]
- 135.Noble CL, McCullough J, Ho W, et al. Low body mass not vitamin D receptor polymorphisms predict osteoporosis in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2008 Apr 1;27(7):588–596. doi: 10.1111/j.1365-2036.2008.03599.x. [DOI] [PubMed] [Google Scholar]
- 136.Bentley RW, Keown D, Merriman TR, et al. Vitamin D receptor gene polymorphism associated with inflammatory bowel disease in New Zealand males. Aliment Pharmacol Ther. 2011 Apr;33(7):855–856. doi: 10.1111/j.1365-2036.2011.04588.x. [DOI] [PubMed] [Google Scholar]
- 137.Eloranta JJ, Wenger C, Mwinyi J, et al. Association of a common vitamin D-binding protein polymorphism with inflammatory bowel disease. Pharmacogenet Genomics. 2011 Sep;21(9):559–564. doi: 10.1097/FPC.0b013e328348f70c. [DOI] [PubMed] [Google Scholar]
- 138.Uitterlinden AG, Ralston SH, Brandi ML, et al. The association between common vitamin D receptor gene variations and osteoporosis: a participant-level meta-analysis. Ann Intern Med. 2006 Aug 15;145(4):255–264. doi: 10.7326/0003-4819-145-4-200608150-00005. [DOI] [PubMed] [Google Scholar]
- 139.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012 Nov 1;491(7422):119–124. doi: 10.1038/nature11582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000 Nov;130(11):2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 141.Zhu Y, Mahon BD, Froicu M, Cantorna MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. 2005 Jan;35(1):217–224. doi: 10.1002/eji.200425491. [DOI] [PubMed] [Google Scholar]
- 142.Froicu M, Weaver V, Wynn TA, McDowell MA, Welsh JE, Cantorna MT. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003 Dec;17(12):2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 143.Froicu M, Zhu Y, Cantorna MT. Vitamin D receptor is required to control gastrointestinal immunity in IL-10 knockout mice. Immunology. 2006 Mar;117(3):310–318. doi: 10.1111/j.1365-2567.2005.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu N, Nguyen L, Chun RF, et al. Altered endocrine and autocrine metabolism of vitamin D in a mouse model of gastrointestinal inflammation. Endocrinology. 2008 Oct;149(10):4799–4808. doi: 10.1210/en.2008-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lagishetty V, Misharin AV, Liu NQ, et al. Vitamin D deficiency in mice impairs colonic antibacterial activity and predisposes to colitis. Endocrinology. 2010 Jun;151(6):2423–2432. doi: 10.1210/en.2010-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Daniel C, Radeke HH, Sartory NA, et al. The new low calcemic vitamin D analog 22-ene-25-oxa-vitamin D prominently ameliorates T helper cell type 1-mediated colitis in mice. J Pharmacol Exp Ther. 2006 Nov;319(2):622–631. doi: 10.1124/jpet.106.107599. [DOI] [PubMed] [Google Scholar]
- 148.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008 Jan;324(1):23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 149.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005 Jul;19(9):1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 150.Miheller P, Muzes G, Hritz I, et al. Comparison of the effects of 1,25 dihydroxyvitamin D and 25 hydroxyvitamin D on bone pathology and disease activity in Crohn’s disease patients. Inflamm Bowel Dis. 2009 Nov;15(11):1656–1662. doi: 10.1002/ibd.20947. [DOI] [PubMed] [Google Scholar]
- 151.Jorgensen SP, Agnholt J, Glerup H, et al. Clinical trial: vitamin D3 treatment in Crohn’s disease - a randomized double-blind placebo-controlled study. Aliment Pharmacol Ther. 2010 Aug;32(3):377–383. doi: 10.1111/j.1365-2036.2010.04355.x. [DOI] [PubMed] [Google Scholar]
- 152.Halpern SD, Karlawish JH, Berlin JA. The continuing unethical conduct of underpowered clinical trials. JAMA. 2002 Jul 17;288(3):358–362. doi: 10.1001/jama.288.3.358. [DOI] [PubMed] [Google Scholar]
- 153.Salim SY, Soderholm JD. Importance of disrupted intestinal barrier in inflammatory bowel diseases. Inflamm Bowel Dis. 2011 Jan;17(1):362–381. doi: 10.1002/ibd.21403. [DOI] [PubMed] [Google Scholar]
- 154.Franke A, McGovern DP, Barrett JC, et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat Genet. 2010 Dec;42(12):1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kong J, Zhang Z, Musch MW, et al. Novel role of the vitamin D receptor in maintaining the integrity of the intestinal mucosal barrier. Am J Physiol Gastrointest Liver Physiol. 2008 Jan;294(1):G208–216. doi: 10.1152/ajpgi.00398.2007. [DOI] [PubMed] [Google Scholar]
- 156.Netea MG, Joosten LA. A NOD for autophagy. Nat Med. 2010 Jan;16(1):28–30. doi: 10.1038/nm0110-28. [DOI] [PubMed] [Google Scholar]
- 157.Zilbauer M, Jenke A, Wenzel G, et al. Expression of human beta-defensins in children with chronic inflammatory bowel disease. PLoS One. 2010;5(10):e15389. doi: 10.1371/journal.pone.0015389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu PT, Schenk M, Walker VP, et al. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4(6):e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yuk JM, Shin DM, Lee HM, et al. Vitamin D3 induces autophagy in human monocytes/macrophages via cathelicidin. Cell Host Microbe. 2009 Sep 17;6(3):231–243. doi: 10.1016/j.chom.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 160.Campbell GR, Spector SA. Hormonally active vitamin D3 (1alpha,25-dihydroxycholecalciferol) triggers autophagy in human macrophages that inhibits HIV-1 infection. J Biol Chem. 2011 May 27;286(21):18890–18902. doi: 10.1074/jbc.M110.206110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fabri M, Stenger S, Shin DM, et al. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011 Oct 12;3(104):104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004 May;113(9):1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Viazis N, Vlachogiannakos J, Georgiou O, et al. Course of inflammatory bowel disease in patients infected with human immunodeficiency virus. Inflamm Bowel Dis. 2010 Mar;16(3):507–511. doi: 10.1002/ibd.21077. [DOI] [PubMed] [Google Scholar]
- 164.Duerr RH, Taylor KD, Brant SR, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006 Dec 1;314(5804):1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mannon PJ, Fuss IJ, Mayer L, et al. Anti-interleukin-12 antibody for active Crohn’s disease. N Engl J Med. 2004 Nov 11;351(20):2069–2079. doi: 10.1056/NEJMoa033402. [DOI] [PubMed] [Google Scholar]
- 166.Sandborn WJ, Feagan BG, Fedorak RN, et al. A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn’s disease. Gastroenterology. 2008 Oct;135(4):1130–1141. doi: 10.1053/j.gastro.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 167.Franke A, Balschun T, Karlsen TH, et al. Sequence variants in IL10, ARPC2 and multiple other loci contribute to ulcerative colitis susceptibility. Nat Genet. 2008 Nov;40(11):1319–1323. doi: 10.1038/ng.221. [DOI] [PubMed] [Google Scholar]
- 168.Roers A, Siewe L, Strittmatter E, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. J Exp Med. 2004 Nov 15;200(10):1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Maynard CL, Weaver CT. Intestinal effector T cells in health and disease. Immunity. 2009 Sep 18;31(3):389–400. doi: 10.1016/j.immuni.2009.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Palmer MT, Lee YK, Maynard CL, et al. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J Biol Chem. 2011 Jan 14;286(2):997–1004. doi: 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chang JH, Cha HR, Lee DS, Seo KY, Kweon MN. 1,25-Dihydroxyvitamin D3 inhibits the differentiation and migration of T(H)17 cells to protect against experimental autoimmune encephalomyelitis. PLoS One. 2010;5:e12925. doi: 10.1371/journal.pone.0012925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A. 2001 Jun 5;98(12):6800–6805. doi: 10.1073/pnas.121172198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cotsapas C, Hafler DA. Immune-mediated disease genetics: the shared basis of pathogenesis. Trends Immunol. 2012 Sep 29; doi: 10.1016/j.it.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 174.Ascherio A, Munger KL, Simon KC. Vitamin D and multiple sclerosis. Lancet Neurol. 2010 Jun;9(6):599–612. doi: 10.1016/S1474-4422(10)70086-7. [DOI] [PubMed] [Google Scholar]
- 175.Genome-wide association study identifies new multiple sclerosis susceptibility loci on chromosomes 12 and 20. Nat Genet. 2009 Jul;41(7):824–828. doi: 10.1038/ng.396. [DOI] [PubMed] [Google Scholar]
- 176.Sawcer S, Hellenthal G, Pirinen M, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011 Aug 11;476(7359):214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Higuchi LM, Khalili H, Chan AT, Richter JM, Bousvaros A, Fuchs CS. A prospective study of cigarette smoking and the risk of inflammatory bowel disease in women. Am J Gastroenterol. 2012 Sep;107(9):1399–1406. doi: 10.1038/ajg.2012.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Odoardi F, Sie C, Streyl K, et al. T cells become licensed in the lung to enter the central nervous system. Nature. 2012 Aug 30;488(7413):675–679. doi: 10.1038/nature11337. [DOI] [PubMed] [Google Scholar]
- 179.Ransohoff RM. Immunology: Licensed in the lungs. Nature. 2012 Aug 30;488(7413):595–596. doi: 10.1038/488595a. [DOI] [PubMed] [Google Scholar]
- 180.Dong J, Hu Z, Wu C, et al. Association analyses identify multiple new lung cancer susceptibility loci and their interactions with smoking in the Chinese population. Nat Genet. 2012 Aug;44(8):895–899. doi: 10.1038/ng.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Edfeldt K, Liu PT, Chun R, et al. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proc Natl Acad Sci U S A. 2010 Dec 28;107(52):22593–22598. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Matsunawa M, Amano Y, Endo K, et al. The aryl hydrocarbon receptor activator benzo[a]pyrene enhances vitamin D3 catabolism in macrophages. Toxicol Sci. 2009 May;109(1):50–58. doi: 10.1093/toxsci/kfp044. [DOI] [PubMed] [Google Scholar]
- 183.Gerde P, Muggenburg BA, Lundborg M, Tesfaigzi Y, Dahl AR. Respiratory epithelial penetration and clearance of particle-borne benzo[a]pyrene. Res Rep Health Eff Inst. 2001 Apr;(101):5–25. discussion 27-32. [PubMed] [Google Scholar]
- 184.Libert C. Inflammation: A nervous connection. Nature. 2003 Jan 23;421(6921):328–329. doi: 10.1038/421328a. [DOI] [PubMed] [Google Scholar]
- 185.Yerger VB, Malone RE. Melanin and nicotine: A review of the literature. Nicotine Tob Res. 2006 Aug;8(4):487–498. doi: 10.1080/14622200600790039. [DOI] [PubMed] [Google Scholar]
- 186.King G, Yerger VB, Whembolua GL, Bendel RB, Kittles R, Moolchan ET. Link between facultative melanin and tobacco use among African Americans. Pharmacol Biochem Behav. 2009 Jun;92(4):589–596. doi: 10.1016/j.pbb.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 187.Cho YH, Jeong DW, Seo SH, et al. Changes in skin color after smoking cessation. Korean J Fam Med. 2012 Mar;33(2):105–109. doi: 10.4082/kjfm.2012.33.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Diaz-Gomez NM, Mendoza C, Gonzalez-Gonzalez NL, et al. Maternal smoking and the vitamin D-parathyroid hormone system during the perinatal period. J Pediatr. 2007 Dec;151(6):618–623. doi: 10.1016/j.jpeds.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 189.Pasco JA, Henry MJ, Nicholson GC, Brennan SL, Kotowicz MA. Behavioural and physical characteristics associated with vitamin D status in women. Bone. 2009 Jun;44(6):1085–1091. doi: 10.1016/j.bone.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 190.Ardawi MS, Sibiany AM, Bakhsh TM, Qari MH, Maimani AA. High prevalence of vitamin D deficiency among healthy Saudi Arabian men: relationship to bone mineral density, parathyroid hormone, bone turnover markers, and lifestyle factors. Osteoporos Int. 2012 Feb;23(2):675–686. doi: 10.1007/s00198-011-1606-1. [DOI] [PubMed] [Google Scholar]
- 191.Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999 Dec;53(12):920–926. doi: 10.1038/sj.ejcn.1600870. [DOI] [PubMed] [Google Scholar]
- 192.Morabia A, Bernstein MS, Antonini S. Smoking, dietary calcium and vitamin D deficiency in women: a population-based study. Eur J Clin Nutr. 2000 Sep;54(9):684–689. doi: 10.1038/sj.ejcn.1601074. [DOI] [PubMed] [Google Scholar]
- 193.Cutillas-Marco E, Fuertes-Prosper A, Grant WB, Morales-Suarez-Varela M. Vitamin D deficiency in South Europe: effect of smoking and aging. Photodermatol Photoimmunol Photomed. 2012 Jun;28(3):159–161. doi: 10.1111/j.1600-0781.2012.00649.x. [DOI] [PubMed] [Google Scholar]
- 194.Wang TJ, Zhang F, Richards JB, et al. Common genetic determinants of vitamin D insufficiency: a genome-wide association study. Lancet. 2010 Jul 17;376(9736):180–188. doi: 10.1016/S0140-6736(10)60588-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lee YK, Turner H, Maynard CL, et al. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009 Jan 16;30(1):92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]