Abstract
Long-term breast cancer trends in incidence in the United States (US) show rising ER positive rates and falling ER negative rates. We hypothesized that these divergent trends reflect etiologic heterogeneity and that comparable trends should be observed in other countries with similar risk factor profiles. We, therefore, analyzed invasive female breast cancers in Denmark, a country with similar risk factors as the US. We summarized the overall trend in age-standardized rates with the estimated annual percentage change (EAPC) statistic (1993–2010) and used age-period-cohort models to estimate age-specific EAPCs, cohort rate ratios (CRRs), and projections for future time periods (2011–2018). In Denmark, the overall rate of ER positive cancers rose between 1993 and 2010 by 3·0%/year (95% CI: 2·8 to 3·3%/yr) while the overall rate of ER negative cancers fell by 2·1%/year (95% CI: −2·5 to −1·6%/yr). The ER positive rate increased fastest among postmenopausal women and the ER negative rate decreased fastest among premenopausal women, reflecting that cohorts born after 1944 were at relatively higher risk of ER positive tumors and lower risk of ER negative tumors. If current trends continue, ER positive cancers will increase at least 13% by 2018 in Denmark, ER negative cancers will fall 15% by 2018, and breast cancer overall will increase at least 7% by 2018. Divergent ER-specific trends are consistent with distinct etiologic pathways. If trends in known risk factors are responsible, the Danish and US experience may foreshadow a common pattern worldwide.
Keywords: Breast cancer, Estrogen receptor, Epidemiology, Age-period-cohort models
INTRODUCTION
Following decades of rising breast cancer incidence in the United States (US), there were abrupt declines circa 2000 that stabilized during 2003–2004.1 The rapid fall in incidence occurred mostly among older women with estrogen receptor (ER) positive cancers. Much less attention was given to the steady decline in ER negative cancers, which was first reported in the Pacific Northwest region of the US.2 However, subsequent studies using the National Cancer Institute’s Surveillance Epidemiology and End Results database showed that the rate of ER negative breast cancers was declining nationwide, while the rate of ER positive breast cancers was rising over the long-term.1, 3
We hypothesized that the distinct ER trends in the US reflected etiologic heterogeneity due to changes in the prevalence of risk factor exposures with opposite effects for ER positive and negative cancers. However, alternative explanations included statistical anomaly, changes in assay or classification with lower threshold for ER positive designation,4 and/or implementation of organized screening mammography with increased sensitivity for ER positive cancers.5 If genuine etiologic heterogeneity were responsible for the US trends, then analogous patterns should be observed among other populations with similar risk factor exposures. Denmark is an excellent test case because reproductive and other risk factors have changed like the US,6–9 characteristics of the ER test are known,10 and the introduction of screening mammography has been well-documented.11
In this report, we describe ER positive and negative trends in Denmark for the time period 1993 through 2010. We used a previously developed and validated approach to account for cases with missing ER status3 and fitted age-period-cohort (APC) models to elucidate underlying trends by ER expression.
MATERIAL AND METHODS
Data
We obtained breast cancer case data from the Danish Breast Cancer Group (DBCG)12 and population data from Statistics Denmark. The DBCG registered nearly 95% of primary breast cancers in Denmark during the time period 1993–2010,13 and is the premier professional organization for breast cancer treatment in Denmark.14 Our project was approved by the DBCG Institutional Review Board. The research was exempt from review by the NIH Office of Human Subject Research because it did not involve interaction with human subjects or use personal identifying information.
We assembled a comprehensive and anonymized dataset of invasive female breast cancers by single year of age at diagnosis (30–84 years), calendar year of diagnosis (1993 through 2010), and ER expression. ER analysis was introduced in Denmark in 1977.10 During the early 1990s, biochemical assays (BCAs) for ER evaluation were replaced with immunohistochemistry (IHC) nuclear staining.15 DBCG specified 10–100% IHC staining for ER positive and 0–9% for ER negative expression throughout our study period.10, 16 ER status was unknown for a small fraction of cases. We allocated the ER unknown cases to ER positive and negative categories according to the observed proportions of ER positive and negative breast cancer cases by age and year of diagnosis, as previous described.3 Prior to 2007, organized screening mammography was sporadic in Denmark and only offered to about 20% of the Danish population;17 after which, nationwide biennial screening mammography was mandated for all women aged 50–69 years.11 Estimates suggest that 50% to 80% of eligible women participated in the Denmark screening programs.18
Statistical Analysis
The age-standardized incidence rate (ASR) for each calendar year from 1993 through 2010 was calculated by the direct method, using the 2000 US standard population. The overall secular trend in the ASR was summarized with the estimated annual percentage change (EAPC) statistic, calculated by weighted log-linear regression assuming an underlying Poisson distribution. A bootstrap procedure was used to estimate the total uncertainty of the EAPC from imputation and random variation.3 Age-period-cohort (APC) models were fitted to characterize variations of the incidence rates accounting for age, period, and birth cohort effects.19 To obtain stable APC estimates (supplemental figure), we grouped single-year data (excluding age 30 years) into twenty-seven 2-year age groups (31–32, 33–34, …, 83–84) and nine 2-year time periods (1993–1994, 1995–1996, …, 2009–2010), spanning thirty-five partially overlapping 4-year birth cohorts referred to by mid-year of birth (1910, 1912, …, 1978). All APC models were fitted with Poisson regression and included an overdispersion parameter to account for potential extra Poisson variation.19
We used age 50 years as a population-based menopausal surrogate and the central 1944 birth cohort as our reference cohort. During our study period (1993–2010, see supplemental figure), earlier cohorts born before 1944 (i.e., 1910–1942) were observed during post-menopausal ages 52–84 years, middle cohorts born 1946–1958 were observed through peri-menopausal and menopausal ages 36–64 years, and more recent cohorts born 1960–1978 were observed prior and up to menopausal ages 32–50 years.
APC parameters and functions included the net drift,20 local drifts,21 longitudinal fitted age at onset curve,22 and the recently described cohort rate ratios (CRRs).23 Net drift measures the overall log-linear trend by calendar period and birth cohort and is analogous to the EAPC of the ASR. Local drifts provide efficient estimates of the corresponding EAPCs for individual age groups. The fitted age at onset curve summarizes the longitudinal age-specific incidence rates for birth cohorts; and by construction, it is adjusted for period and cohort effects.22 CRR values describe the incidence rates for each cohort relative to an arbitrary reference cohort (herein the central 1944 birth cohort), adjusted for period effects such as changing diagnostic practice. Finally, we used the longitudinal fitted age at onset curve, CRR values, and net drift to project breast cancer incidence rates into future time periods.3 In brief, incidence rates for observed birth cohorts were extrapolated based on the longitudinal fitted age at onset curve and CRR values, and rates for future cohorts were extrapolated using the longitudinal age at onset curve and net drift.
To assess the impact of nationwide screening, projections were made with and without data for 2007–2010 among women ages 50–69 years; ages 49 and 70 were also excluded because the APC models used 2-year age groups. Even without the excluded data, the APC model for the restricted data set included parameter estimates for all of the age groups, time periods, and birth cohorts necessary to estimate expected values for 2007–2010 as well as to project future trends for 2011–2018. Bootstrapped 95% confidence intervals were constructed that incorporated uncertainty of parameters estimated from the observed rates as well as the expected variability of unobserved future cohort and period deviations. All statistical tests were two-sided and p values less than 0.05 were considered statistically significant.
RESULTS
The DBCG registered 62549 invasive female breast cancer patients ages 30–84 in 30066960 woman-years at risk from 1993 through 2010 (Table 1). There were 45571 (72·9%) ER positive, 12016 (19·2%) ER negative, and 4962 (7·9%) ER unknown cancers. The overall age-standardized incidence rate was 188·7 per 100000 woman-years, reflecting the sum of the rates for ER positive (136·5), negative (37·4), and unknown cancers (14·8). ER positive compared to ER negative cancers occurred at older ages at diagnosis, were smaller in size, more likely lymph node negative and low tumor grade (p < 0·001 for heterogeneity by ER). Overall, 78% of ER unknowns were imputed to ER positive and the remainder to ER negative categories. All subsequent results pertain to the corrected ER positive and negative data. Age-period-cohort models were successfully fitted, residual plots did not reveal any systematic lack of fit, and fitted rates consistently tracked close to observed rates.
Table 1.
Descriptive statistics for invasive female breast cancer among women aged 30–84 years in the Danish Breast Cancer Cooperative Group (DBCG: 1993 through 2010)
| Total cases | ER positive | ER negative | ER unknown | p value for heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|---|
| Sample size | 62,549 | 45,571 | 12,016 | 4,962 | |||||
| % Total cases | 100% | 72·9% | 19·2% | 7·9% | |||||
| Mean age (SE) | 60.5 (0.05) | 60·9 (0·05) | 57·9 (0·11) | 62·7 (0·19) | p < 0·001 | ||||
| Rates per 100,000 | 188.7 | 136·5 | 37·4 | 14·8 | |||||
| Variable | Count | Percent | Count | Percent | Count | Percent | Count | Percent | p value |
| Menopausal surrogate | |||||||||
| <50 years | 12,291 | 19.7% | 8,221 | 18·0% | 3,158 | 26·3% | 912 | 18·4% | |
| 50+ years | 50,258 | 80.3% | 37,350 | 82·0% | 8,858 | 73·7% | 4,050 | 81·6% | p < 0·001 |
| Tumor Size | |||||||||
| ≤ 2.0 cm | 33,906 | 54.2% | 27,257 | 59·8% | 5,316 | 44·2% | 1,333 | 26·9% | |
| > 2.0 cm | 24,514 | 39.2% | 17,379 | 38·1% | 6,189 | 51·5% | 946 | 19·1% | |
| Other or unknown | 4,129 | 6.6% | 935 | 2·1% | 511 | 4·3% | 2,683 | 54·1% | p < 0·001 |
| Lymph nodes | |||||||||
| negative | 30,142 | 48.2% | 23,032 | 50·5% | 5,806 | 48·3% | 1,304 | 26·3% | |
| positive | 27,055 | 43.3% | 20,549 | 45·1% | 5,590 | 46·5% | 916 | 18·5% | |
| Other or unknown | 5,352 | 8.6% | 1,990 | 4·4% | 620 | 5·2% | 2,742 | 55·3% | p < 0·001 |
| Tumor Grade | |||||||||
| I (low) | 15,966 | 25.5% | 14,621 | 32·1% | 743 | 6·2% | 602 | 12·1% | |
| II–III (high) | 33,158 | 53.0% | 23,287 | 51·1% | 8,886 | 74·0% | 985 | 19·9% | |
| Other or unknown | 13,425 | 21.5% | 7,663 | 16·8% | 2,387 | 19·9% | 3,375 | 68·0% | p < 0·001 |
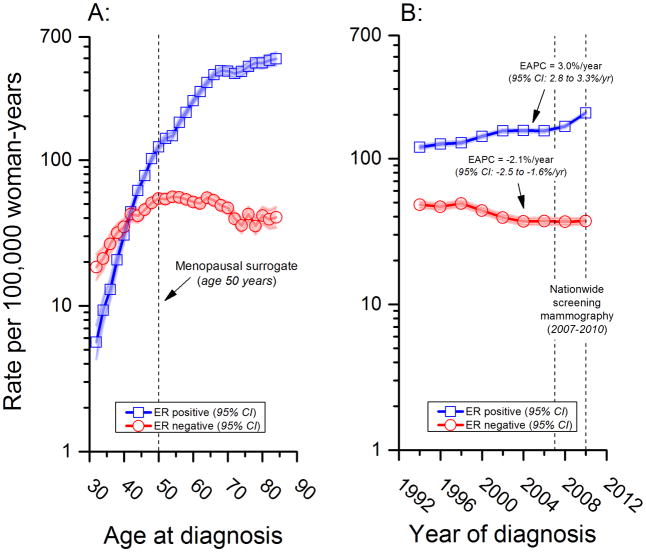
As in the US, the longitudinal fitted age at onset curve in Denmark revealed a qualitative (or reversing) age interaction,24 Figure 1A. The ER negative to positive incidence rate ratio (IRR ERN:ERP) was >1·0 prior to age 40 years after which it was <1·0. Specifically, the IRR ERN:ERP was 3.3 for the age group 31–32 years and 0.1 for the age group 71–72 years.
Figure 1.
Also similar to the US experience,3 ER positive and negative secular trends in Denmark diverged over time with statistically significant rising and falling log-linear trends, respectively (Figure 1B). The EAPC was 3·0% per year (95% CI: 2·8 to 3·3% per year) for ER positive and negative 2·1% per year (95% CI: −2·5 to −1·6% per year) for ER negative cancers. ER positive incidence rates rose abruptly in 2007 from 155 to 206 per 100000, likely due to the introduction of nationwide screening mammography that year.
In contrast, ER negative rates fell from 48 to 37 per 100000 woman-years over the entire study period (1993–2010). Age-specific incidence rate trends for ER positive tumors (Figure 2A) and corresponding local drifts (Figure 2C) revealed significantly greater increases among postmenopausal women ages 50+ years (peak increasing trend ~4%/year near ages 60–65) and significantly greater decreases in ER negative tumors (Figures 2B and 2D) among premenopausal women ages 30–49 years (peak decreasing trend ~−5%/year at age 45), compared to the respective global net drifts (or EAPCs) for all age groups combined.
Figure 2.
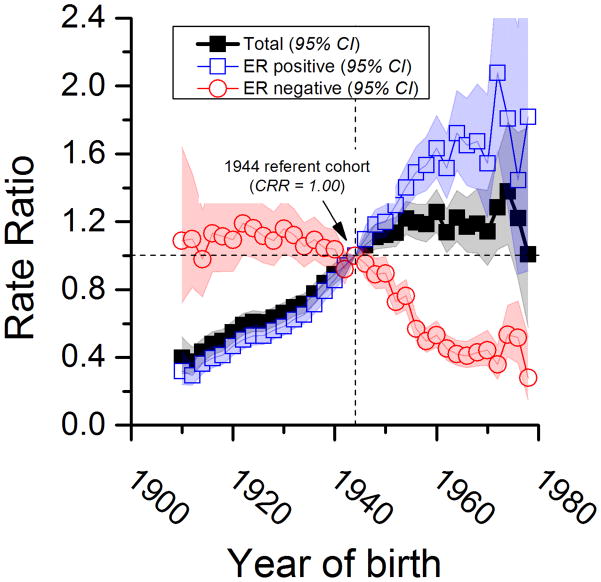
Compared to women born circa 1944, more recent cohorts had around a 20% higher rate for breast cancer overall (Figure 3). Furthermore, between the 1910 and 1978 birth cohorts, breast cancer overall increased more than 3 fold, rising from a CRR of approximately 0·3 to 1·2. The overall pattern reflects the confluence of ER positive CRRs that rose about 5-fold (from ~0.3 to ~1.6) and ER negative CRRs that fell about 3-fold (from ~1.1 to ~0.4).
Figure 3.
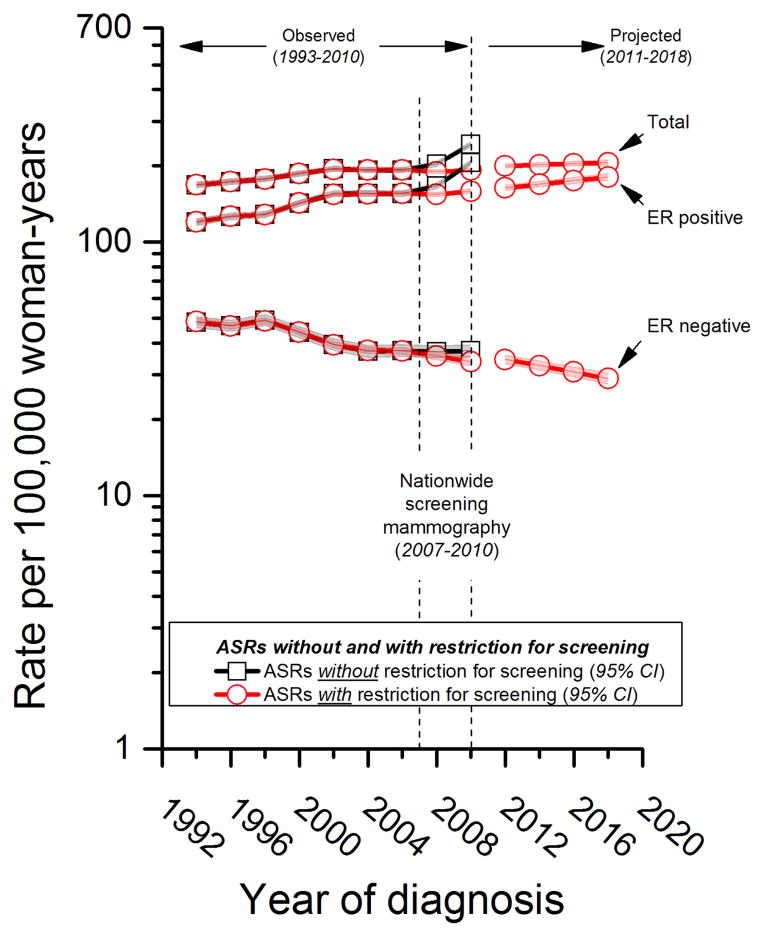
To obtain conservative estimates of future breast cancer trends (Figure 4), we excluded follow-up most likely to be impacted by organized screening in Denmark that included the 11562 breast cancers diagnosed in the age groups 49–50 through 69–70 during 2007–2010. Under the counterfactual assumption of no organized screening mammography, the expected ASR for ER positive cancers during 2009–2010 is 159 per 100000 women-years (95% CI: 156 to 162) with restriction for screening versus 206 per 100000 woman-years (95% CI: 201 to 210) actually observed without restriction for screening. Also during 2009–2010, the expected ASR for ER negative cancers is 34 per 100000 women-years (95%CI: 32 to 35) with restriction for screening versus 37 per 100000 (95% CI: 35 to 39) actually observed without restriction for screening. If the patterns driving the expected rates in the absence of screening were to persist, ER positive cancers would continue to rise, reaching 180 per 100000 by 2018 (13% increase from 159 to 180 per 100,000). On the other hand, ER negative cancers would continue to decline to 29 per 100000 (15% decrease from 34 to 29 per 100000). In absolute terms, the expected decline in ER negative cancers of 5 per 100000 is about ¼ the expected increase in ER positive cancers of 21 per 100000; breast cancer overall is expected to rise by at least 7% from 192 to 206 per 100000.
Figure 4.
DISCUSSION
Measurement of ER expression over the last 18 years has opened an important window on the dynamics of breast cancer trends in Denmark. An earlier report noted significant declines in ER negative cancers in both premenopausal and postmenopausal Danish women from 1996 to 2007.25 After correction for missing ER data, our analysis confirms these observations and also shows that the ER negative trends began earlier and continued beyond the time period of the previous report. Furthermore, our analysis reveals that the age-specific incidence and birth cohort patterns were also strikingly different for ER positive and negative cancers, consistent with the hypothesis of etiological heterogeneity by ER expression in the general population of Danish women.
ER positive cancers were increasing among both earlier and middle aged cohorts. These observations imply that a confluence of risk factor exposures is resulting in more ER positive tumors from the peri-menopausal through the post-menopausal periods. At the same time, it appears that risk factor changes have suppressed ER negative tumors among more recent pre-menopausal cohorts during early reproductive life.
Compared to the 1944 reference cohort, in the more recent cohorts (1946 to 1978, figure 3), the relative risk reduction for ER negative tumors of ~70% (from 1.0 to 0.3) is similar to the relative risk increase for ER positive tumors of ~80% (from 1.0 to 1.8). However, the conservative projected absolute rise in ER positive tumors from 2010–2018 in the absence of screening (21 per 100000) will greatly exceed the absolute decline in ER negative tumors (5 per 100000). Therefore, for every one fewer ER negative tumor, we expect four additional ER positive tumors. Given that screening differentially detects ER positive over ER negative tumors, the actual excess may be larger.
The underlying breast cancer patterns in Denmark are also remarkably similar to the US3 and possibly Scotland.26 Notably, the data for Denmark were less likely impacted by screening mammography than in the US, and we could control for the introduction of nationwide screening mammography since we had pre- and post-screening data. Furthermore, there is little likelihood that our results for Denmark are artifacts of trends in ER measurement since ER has been measured consistently and nearly completely over time.
The major limitation of our study (besides the usual caveats regarding interpretation of purely observational data) is that the precise quantitative conclusions depend on the accuracy of our weighting method to correct the small fraction of cases with ER unknown expression. Our conceptually and computationally simple method estimated the total ER positive and negative breast cancers for each year of age and year of diagnosis. In previous work in the US, a more complex scheme that accounted for race, tumor grade, and stage in addition to age and year of diagnosis produced similar results to age and year alone.3 Our weighting method also requires less intensive modeling than multiple imputation methods,1 by not requiring a statistical imputation model to impute each woman’s ER test result.
The striking divergence between ER positive and negative breast cancer rates in Denmark and the US could be explained by trends in environmental and lifestyle risk factors with dual effects; two such possible risk factors are pregnancy (or parity)27, 28 and obesity,29, 30 which are decreasing and increasing, respectively, in both the US6, 7 and Denmark.8, 9 In fact, new breast cancer cases appear to be rising worldwide due to the consequences of changing reproductive, hormonal, and dietary risk factors.31 For this reason, it is plausible that the same risk factors, or at the least risk factors acting through common mechanisms, are operative in Denmark and the US as well as many other countries. If so, then, the worldwide rising trends in breast cancer overall will also reflect a decreasing ratio of ER negative to ER positive breast cancers. This is somewhat good news since ER negative breast cancers include the more aggressive subtypes such as triple negative tumors. Nonetheless, as molecularly-targeted therapeutics continue to advance the treatment for hormone sensitive and insensitive breast cancers, the concurrent development of ER-specific intervention strategies may be needed to prevent ER positive and negative cancers worldwide. Though the concept of ER-specific breast cancer therapy is well-established, the notion of targeted ER positive and negative breast cancer prevention is a novel idea.
Supplementary Material
Novelty and Impact.
Estrogen receptor (ER) negative breast cancer incidence rates are declining nationwide in Denmark and the United States, whereas rates for ER positive breast cancers are rising. Divergent ER trends are consistent with distinct etiologic pathways due to the prevalence of risk factor exposures with opposite effects for ER positive and ER negative breast cancers. The Danish and United States experience may foreshadow a common pattern for many countries worldwide.
Acknowledgments
The study was funded in part by the intramural program of the National Institutes of Health, National Cancer Institute.
Footnotes
Conflicts of Interest: None of the authors have any financial and/or personal relationships that could bias this work.
References
- 1.Desantis C, Howlader N, Cronin KA, Jemal A. Breast cancer incidence rates in US women are no longer declining. Cancer Epidemiol Biomarkers Prev. 2011;20:733–9. doi: 10.1158/1055-9965.EPI-11-0061. [DOI] [PubMed] [Google Scholar]
- 2.Glass AG, Lacey JV, Jr, Carreon JD, Hoover RN. Breast Cancer Incidence, 1980 2006: Combined Roles of Menopausal Hormone Therapy, Screening Mammography, and Estrogen Receptor Status. J Natl Cancer Inst. 2007;99:1152–61. doi: 10.1093/jnci/djm059. [DOI] [PubMed] [Google Scholar]
- 3.Anderson WF, Katki HA, Rosenberg PS. Breast cancer incidence in the United States: Current and Future Trends. J Natl Cancer Inst. 2011;103:1397–402. doi: 10.1093/jnci/djr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porter PL, El-Bastawissi AY, Mandelson MT, Lin MG, Khalid N, Watney EA, Cousens L, White D, Taplin S, White E. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 1999;91:2020–8. doi: 10.1093/jnci/91.23.2020. [DOI] [PubMed] [Google Scholar]
- 6.Tarone RE. Breast cancer trends among young women in the United States. Epidemiology. 2006;17:588–90. doi: 10.1097/01.ede.0000229195.98786.ee. [DOI] [PubMed] [Google Scholar]
- 7.F as in Fat: How obesity threatens America’s future 2012. Robert Wood Johnson Foundation; 2012. [Google Scholar]
- 8.Lidegarrd O, Kroman N. The epidemiology of breast cancer. Eur Clinics Obstet Gynaecol. 2005;1:24–8. [Google Scholar]
- 9.Lassen TH, Sobotka T, Jensen TK, Jacobsen R, Erb K, Skakkebaek NE. Trends in rates of natural conceptions among Danish women born during 1960–1984. Hum Reprod. 2012;27:2815–22. doi: 10.1093/humrep/des207. [DOI] [PubMed] [Google Scholar]
- 10.Talman ML, Rasmussen BB, Andersen J, Christensen IJ. Estrogen Receptor analyses in the Danish Breast Cancer Cooperative Group. History, methods, prognosis and clinical implications. Acta Oncol. 2008;47:789–94. doi: 10.1080/02841860801982741. [DOI] [PubMed] [Google Scholar]
- 11.Vejborg I, Mikkelsen E, Garne JP, Bak M, Lernevall A, Mogensen NB, Schwartz W, Lynge E. Mammography screening in Denmark. Dan Med Bull. 2011;58:C4287. [PubMed] [Google Scholar]
- 12.Blichert-Toft M, Christiansen P, Mouridsen HT. Danish Breast Cancer Cooperative Group--DBCG: History, organization, and status of scientific achievements at 30-year anniversary. Acta Oncol. 2008;47:497–505. doi: 10.1080/02841860802068615. [DOI] [PubMed] [Google Scholar]
- 13.Association of the Nordic Cancer Registries (NORDCAN) 2013 Feb 25; 2013, http://www-dep.iarc.fr/NORDCAN/english/table2.asp?cancer=180&period=2010&period=2009&period=2008&period=2007&period=2006&period=2005&period=2004&period=2003&period=2002&period=2001&period=2000&period=1999&period=1998&period=1997&period=1996&period=1995&period=1994&period=1993&sex=2&type=0&age_from=7&age_to=17&sort=1®istry=1&submit=Execute.
- 14.Rostgaard K, Holst H, Mouridsen HT, Lynge E. Do clinical databases render population-based cancer registers obsolete? The example of breast cancer in Denmark. Cancer Causes Control. 2000;11:669–74. doi: 10.1023/a:1008928204121. [DOI] [PubMed] [Google Scholar]
- 15.Laenkholm AV, Knoop A, Ejlertsen B, Rudbeck T, Jensen MB, Muller S, Lykkesfeldt AE, Rasmussen BB, Nielsen KV. ESR1 gene status correlates with estrogen receptor protein levels measured by ligand binding assay and immunohistochemistry. Mol Oncol. 2012;6:428–36. doi: 10.1016/j.molonc.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danish Breast Cancer Cooperative Group. 2012 Sep 1; 2012, http://www.dbcg.dk/
- 17.Olsen AH, Jensen A, Njor SH, Villadsen E, Schwartz W, Vejborg I, Lynge E. Breast cancer incidence after the start of mammography screening in Denmark. Br J Cancer. 2003;88:362–5. doi: 10.1038/sj.bjc.6600712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Euler-Chelpin M, Olsen AH, Njor S, Vejborg I, Schwartz W, Lynge E. Socio-demographic determinants of participation in mammography screening. Int J Cancer. 2008;122:418–23. doi: 10.1002/ijc.23089. [DOI] [PubMed] [Google Scholar]
- 19.Holford TR. Understanding the effects of age, period, and cohort on incidence and mortality rates. Annu Rev Public Health. 1991;12:425–57. doi: 10.1146/annurev.pu.12.050191.002233. [DOI] [PubMed] [Google Scholar]
- 20.Clayton D, Schifflers E. Models for temporal variation in cancer rates. II: Age-period-cohort models. Stat Med. 1987;6:469–81. doi: 10.1002/sim.4780060406. [DOI] [PubMed] [Google Scholar]
- 21.Mbulaiteye SM, Anderson WF, Ferlay J, Bhatia K, Chang C, Rosenberg PS, Devesa SS, Parkin DM. Pediatric, elderly, and emerging adult-onset peaks in Burkitt’s lymphoma incidence diagnosed in four continents, excluding Africa. Am J Hematol. 2012;87:573–8. doi: 10.1002/ajh.23187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson WF, Rosenberg PS, Menashe I, Mitani A, Pfeiffer RM. Age-related crossover in breast cancer incidence rates between black and white ethnic groups. J Natl Cancer Inst. 2008;100:1804–14. doi: 10.1093/jnci/djn411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jemal A, Ma J, Rosenber PS, Siegel R, Anderson WF. Increasing lung cancer death rates among young women in southern and midwestern states. J Clin Oncol. 2012;30:2739–44. doi: 10.1200/JCO.2012.42.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson WF, Jatoi I, Sherman ME. Qualitative age interactions in breast cancer studies: mind the gap. J Clin Oncol. 2009;27:5308–11. doi: 10.1200/JCO.2009.22.9450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bigaard J, Stahlberg C, Jensen M-B, Ewertz M, Kroman N. Breast cancer incidence by estrogen receptor status in Denmark from 1996 to 2007. Breast Cancer Res Treat. 2012 doi: 10.1007/s10549-012-2269-0. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 26.Sharpe KH, McClements P, Clark DI, Collins J, Springbett A, Brewster DH. Reduced risk of oestrogen receptor positive breast cancer among peri- and post-menopausal women in Scotland following a striking decrease in use of hormone replacement therapy. Eur J Cancer. 2010;46:937–43. doi: 10.1016/j.ejca.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 27.Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331:5–9. doi: 10.1056/NEJM199407073310102. [DOI] [PubMed] [Google Scholar]
- 28.Colditz GA, Rosner BA, Chen WY, Holmes MD, Hankinson SE. Risk factors for breast cancer according to estrogen and progesterone receptor status. J Natl Cancer Inst. 2004;96:218–28. doi: 10.1093/jnci/djh025. [DOI] [PubMed] [Google Scholar]
- 29.Yang XR, Chang-Claude J, Goode EL, Couch FJ, Nevanlinna H, Milne RL, Gaudet M, Schmidt MK, Broeks A, Cox A, Fasching PA, Hein R, et al. Associations of Breast Cancer Risk Factors With Tumor Subtypes: A Pooled Analysis From the Breast Cancer Association Consortium Studies. J Natl Cancer Inst. 2011;103:250–63. doi: 10.1093/jnci/djq526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Gapstur SM, Giles GG, Giovannucci E, Goodman G, Hankinson SE, et al. Carotenoid intakes and risk of breast cancer defined by estrogen receptor and progesterone receptor status: a pooled analysis of 18 prospective cohort studies. Am J Clin Nutr. 2012;95:713–25. doi: 10.3945/ajcn.111.014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): a population-based study. The lancet oncology. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.