Abstract
Background
Although a general consensus holds that emotional reactivity in youth with conduct disorder (CD) symptoms arises as one of the main causes of successive aggression, it remains to be determined whether automatic emotion processing is altered in this population.
Methods
We measured auditory event-related potentials (ERP) in twenty young offenders and twenty controls, screened for DSM-IV criteria of CD and evaluated using the youth version of Hare Psychopathy Checklist (PCL:YV), State-Trait Anxiety Inventory (STAI) and Barrett Impulsive Scale (BIS-11). In an oddball design, sadly or fearfully spoken “deviant” syllables were randomly presented within a train of emotionally neutral “standard” syllables.
Results
In young offenders meeting with CD criteria, the ERP component mismatch negativity (MMN), presumed to reflect pre-attentive auditory change detection, was significantly stronger for fearful than sad syllables. No MMN differences for fearful vs. sad syllables were observed in controls. Analyses of non-vocal deviants, matched spectrally with the fearful and sad sounds, supported our interpretation that the MMN abnormalities in juvenile offenders were related to the emotional content of sounds, instead of purely acoustic factors. Further, in the young offenders with CD symptoms, strong MMN amplitudes to fearful syllables were associated with high impulsive tendencies (PCL:YV, Factor 2). Higher trait and state anxiety, assessed by STAI, were positively correlated with P3a amplitudes to fearful and sad syllables, respectively. The differences in group-interaction MMN/P3a patterns to emotional syllables and non-vocal sounds could be speculated to suggest that there is a distinct processing route for pre-attentive processing of species-specific emotional information in human auditory cortices.
Conclusions
Our results suggest that youths with CD symptoms may process distressful voices in an atypical fashion already at the pre-attentive level. This auditory processing abnormality correlated with increased impulsivity and anxiety. Our results may help shed light on the neural mechanisms of aggression.
Keywords: Conduct disorder (CD), Distressful voices, Impulsivity, Juvenile delinquents, Mismatch negativity (MMN)
Introduction
Much attention has been directed toward identifying developmental pathways to serious delinquency in order to treat children who are at high risk for the costly outcome of violence. Early-onset conduct disorder (CD), the major childhood precursor to antisocial personality disorder in adulthood, tends to exhibit high levels of aggression and increased risk for incarceration throughout development (Lahey, Loeber, Burke, & Applegate, 2005; Moffitt, Caspi, Harrington, & Milne, 2002). Several lines of evidence suggest that antisocial behavior is closely related to abnormal emotional reactivity (Loney, Frick, Clements, Ellis, & Kerlin, 2003; Sharp, van Goozen, & Goodyer, 2006; Viding, Fontaine, & McCrory, 2012). Youths who frequently experience intense emotions of fear or anger have an increased risk of exhibiting impulsive and violent behavior (Herpertz et al., 2008). Meanwhile, antisocial behavior is also known to be associated with emotional callousness (Frick et al., 2003). However, the neuronal bases of atypical emotional reactivity in adolescents with antisocial behavior problems, and the way how these abnormalities are related to the neurobiological mechanisms that give rise to CD, are still poorly known.
It is not yet fully clear how emotional reactivity is associated with aggression in children and youths with, or without, behavioral problems. The disposition for aggressive behavior at an early age has been hypothesized to arise as a consequence of a deficiency in responding to the distress of others (Blair, 2005; Raine, Venables, & Mednick, 1997). Low autonomic arousal and impaired recognition of emotional faces or words along with reduced amygdala responses to negative emotional stimuli in children and adolescents with conduct problems and callous-unemotional traits lend support to this notion (Jones, Laurens, Herba, Barker, & Viding, 2009; Marsh et al., 2008; Sterzer, Stadler, Krebs, Kleinschmidt, & Poustka, 2005). At the same time, amygdala responses may be enhanced to emotionally provocative stimuli in adolescents with CD (Decety, Michalska, Akitsuki, & Lahey, 2009; Herpertz et al., 2008). It has also been proposed that negative affect in conjunction with poor emotional regulation produce stress that instigates aggression (Campbell, 1990; Gill & Calkins, 2003). When considering these findings, it is important to note that distinct subgroups of youths with aggressive behavior, for instance, might show different patterns of emotional reactivity (Loney et al., 2003; Viding et al., 2012), and that the responsiveness of youths with aggressive behaviors as a whole might be heterogeneous in tasks measuring different aspects of processing of emotions. Moreover, aberrant attention (Dadds, El Masry, Wimalaweera, & Guastella, 2008; Newman, Curtin, Bertsch, & Baskin-Sommers, 2010) and impoverished verbal skills (Savitsky & Czyzewski, 1978) may also moderate emotional reactivity ascribed to aggression.
Neuronal mechanisms of emotional reactivity in delinquents with CD symptoms could be studied with mismatch negativity (MMN), an auditory event-related potential (ERP) component that is presumed to reflect pre-attentive discrimination of sound changes in human auditory cortex (Näätänen et al., 2011). The fact that MMN is generated without the subject paying active attention to sound stimulation may be a benefit in studies on populations with potential comorbid attention deficits. Recent studies suggest that, in addition to many basic features of sounds such as frequency, duration, or phonetic content, MMN can also be utilized as an index of the salience of emotional voice processing (Schirmer & Kotz, 2006). For example, it has been employed to measure perception of emotional voices in infants (Cheng, Lee, Chen, Wang, & Decey, 2012).
In addition to superior temporal auditory-cortex areas, MMN has also been suggested involve higher-order cortex regions linked to regulation of emotions and social cognition, such as dorsolateral prefrontal cortices (Alain, Woods, & Knight, 1998; Alho, Woods, Algazi, Knight, & Näätänen, 1994; Knight, 1984; Woods & Knight, 1986) and the anterior cingulate (Crottaz-Herbette & Menon, 2006). Consistent with these findings, MMN abnormalities have been documented in a great variety of psychiatric disorders involving emotional and mood dysfunctions (Näätänen et al., 2011), and in individuals with moderate intermittent explosive disorder showing strong stress responses to sensory stimuli (Koelsch, 2009). MMN may also correlate with psychological measures of impulsivity and anxiety in healthy subjects with no history of psychiatric illnesses. Specifically, there is evidence that self-reported impulsivity correlates with enhanced MMN amplitude, with high impulsive individuals showing larger MMN amplitudes than low impulsive individuals (Franken, Nijs, & Van Strien, 2005). Interestingly, it has been also shown that MMN to threat is further enhanced if an individual feels anxious (Schirmer & Escoffier, 2010).
When the stimulus change is robust enough, MMN is followed by a frontocentrally positive P3a component (Keage et al., 2006; Polich, 2007). In contrast to the active target-detection response P3b, the P3a component is presumed to reflect involuntary attention switching (Escera, Alho, Schroger, & Winkler, 2000) and associated control process (SanMiguel, Morgan, Klein, Linden, & Escera, 2010). In combination with MMN, P3a might therefore provide a sensitive measure of involuntary attention systems (Light, Swerdlow, & Braff, 2007) and their dysfunctions in psychiatric populations (Hermens et al., 2010). Importantly, it has been found the effects of social relevance on P300 latency and amplitude are most salient over frontal regions and hence most likely associated with the P3a component (Schirmer, Simpson, & Escoffier, 2007).
To provide a better understanding on pre-attentive processing of distressful emotions involved in aggression, we recorded MMN and P3a in response to neutrally, sadly, and fearfully spoken meaningless syllables “dada”, in addition to acoustically matched non-vocal sounds in a passive oddball paradigm. We hypothesized that, if the blunted emotional response hypothesis is true, then young offenders with CD symptoms would show weaker MMN/P3a to emotional syllables than their matched controls. Alternatively, if this hypothesis were not favored, juvenile delinquents would exhibit stronger emotional MMN/P3a than controls. If such group differences were specific to voice processing per se, MMN to correspondingly non-vocal sounds would not differ between young offenders and control counterparts. Given that impulsivity and anxiety are the associated factors of aggression (Fritz, Wiklund, Koposov, af Klinteberg, & Ruchkin, 2008), we also analyzed to which extent emotional MMN correlates with impulsivity and anxiety traits.
Methods
Participants
All participants were Chinese. Twenty male juvenile delinquents (aged 13–19 years) from a high-security juvenile detention center underwent screening for participation in the study. All young inmates were recidivists and met the DSM-IV criteria for childhood-onset CD. Each inmate had onset of at least one criterion prior to age 10 and the symptoms were ascertained. They had a history of aggression toward people or animals, destruction of property, deceitfulness or theft, and serious violations of rules. Seven of them had been accused of murder or attempted murder; the remaining thirteen had been accused of assault, extortion, abrupt taking or larceny. Individuals with mental deficiencies, alcohol or other drug abuse, and sexual offenders were not included. In addition, twenty age-matched typically developing male adolescents, who had no official criminal record, no versatile violent history, and no neurological or psychiatric illness, were recruited as a control group through bulletin board announcements in the community. All subjects were right-handed without hearing or visual impairments. The study was approved by the local Ethics Committee (Yang-Ming University Hospital): According to the Declaration of Helsinki, all participants gave a voluntary informed consent before the procedures, with the approval of their legal representative. They received verbal and written instructions about all details of the experiment, as well as the possibilities to withdraw at any moment.
Stimuli and Tasks
The stimuli consisted of two categories: emotional syllables and acoustically matched non-vocal sounds. A female speaker, recruited from a professional performing arts school, produced the meaningless syllables “dada” with three sets of emotional (neutral, sad, fearful) syllables. Within each kind of emotional syllables, the speaker produced the syllables “dada” for more than ten times for further validation with the use of Cool Edit Pro 2.0 and Sound Forge 9.0. Each set was rated for emotionality by a total of forty listeners (twenty males). For the sad set, listeners classified each stimulus on a 5-point scale from “extremely sad” to “not sad at all”. For the fearful set, listeners classified each stimulus on a 5-point scale from “extremely fearful” to “not fearful at all”. These listeners did not overlap with the participants recruited in the ERP experiment. Two emotional syllables, which consistently were identified as “extremely sad” and “extremely fearful”, were selected as experimental stimuli and the most emotionless stimulus were selected as neutral syllables. The Likert-scale (mean±SD) of neutral, sad and fearful syllables were 2.47±0.87, 4.12±0.87, and 3.92±0.97, respectively.
In order to create a set of stimuli that retained acoustical correspondence, we synthesized non-vocal sounds using Praat (Boersma, 2001) and Matlab software (The MathWorks, Inc., USA). The fundamental frequencies (f0) of the original (neutral, sad, fearful) syllables were extracted to produce the non-vocal sounds using a sine waveform and then multiplied by the original syllable envelope. In this way, non-vocal sounds retained the temporal envelope and f0 of emotional syllables in order to control for temporal and spectral features. All of the stimuli were similar with respect to their duration (550 ms) and loudness (max: 62 dB; mean: 59 dB).
General Procedures
Before EEG recording, juvenile delinquents were assessed by the Barratt Impulsiveness Scale (BIS-11) (Patton, Stanford, & Barratt, 1995), the youth version of the Hare Psychopathy Checklist (PCL:YV) (Forth, Kosson, & Hare, 2003), and the State-Trait Anxiety Inventory (STAI) (Spielberger, Gorssuch, Lushene, & Jacobs, 1983). The BIS-11 is an internally consistent measure of impulsiveness (Cronbach’s alpha=0.82 in healthy subjects, d=0.83 in psychiatric patients) and has potential clinical utility for measuring impulsiveness among inmate populations. The PCL:YV evaluation, including interview, institutional data, psychiatric profiles and behavior reports, measures interpersonal, affective, and behavioral features related to psychopathy in adolescents. Factor 1 involves interpersonal or affective (emotion) personality traits, and higher values are associated with low empathy as well as social dominance and less fear or depression; Factor 2 involves either impulsive-irresponsible behaviors or antisocial behaviors, and it is associated with a maladaptive lifestyle including criminality. The STAI is the leading measure of personal anxiety worldwide. The STAI consists of two twenty-item scales with a range of four possible responses to each. One scale determines anxiety in specific situations; another scale determines anxiety as a general trait. All these scales were self-reported assessments except PCL:YV. The controls were screened by a more limited version including the most critical questions to exclude subjects representing psychopathic characteristics, instead of a complete semi-structured interview (133 questions in total). PCL:YV, especially factor 2, was assessed in juvenile delinquents in order to provide extra information on the correlation between emotional MMN and impulsivity.
During an ERP oddball paradigm, participants were instructed to watch a silent movie with subtitles, and to ignore sound stimulation consisting of neutral-syllable standards (P=80%) and occasional task-irrelevant sad (P=10%) or fearful (P=10%) deviants, presented at a 1.2-s stimulus-onset asynchrony. In a separate block, non-vocal standards, sad-derived deviants, and fearful-derived deviants were presented analogously. The order of emotional vs. non-vocal blocks (600 sound trials in each) was counter-balanced and randomized across participants. The total experimental time, including pauses between blocks, was approximately fifty minutes.
Apparatus and Recordings
EEG was continuously recorded at 600 Hz (band-pass 0.1–100 Hz) from 32 scalp electrodes mounted on an elastic cap in accordance to a modified 10–20 system, with the addition of two mastoid electrodes used as the reference, and ground electrode placed in the forehead. Eye blinks and vertical eye movements were monitored with two electro-oculogram (EOG) electrode pairs located vertically above vs. below the left eye and horizontaly at the outer canthi of both eyes. Electrode/skin impedance was kept<5 kΩ. EEG was epoched to 600-ms trials, including a 100 ms pre-stimulus baseline. Trials containing changes exceeding 70μV at recording electrodes and exceeding 100 μV at the EOG channels were excluded by an automatic rejection system. Furthermore, the quality of ERP traces was ensured by careful visual inspection in every subject and trial and by applying appropriate digital, zero-phase shift band-pass filtered (0.1–50 Hz, 24 dB/octave). ERP traces confirmed that muscle artifacts did not significantly contaminate even the most lateral electrodes. The first ten epochs of each sequence were omitted from averaging in order to exclude unexpected large responses elicited by the initiation of the sequences. The number of accepted standard and deviant trial numbers did not differ significantly across the groups in the emotional (Controls: Neutral: 743±107, Sad: 87±13, Fearful: 82±14; Juvenile delinquents: Neutral: 748±152, Sad: 83±15, Fearful: 81±12) or non-vocal sessions (Controls: N: 788±172, S: 76±12, F: 79±11; Juvenile delinquents: N: 741±191, S: 75±18, F: 75±17). The paradigm was edited by Matlab software (MathWorks, Natick, MA). ERPs were processed and analyzed by Neuroscan 4.3 (Compumedics Ltd., Australia).
MMN source distributions were qualitatively explored using current source density (CSD) mapping ( CSD Tool box , http://psychophysiology.cpmc.columbia.edu/software/CSDtoolbox/index.html), which is a measure of the strength of extracellular current generators underlying the recorded EEG potentials (Nicholson, 1973). The CSD method, which computes the surface Laplacian over the surface potentials implying the dipole sources oriented normal to local skull, has been used in several previous MMN studies (Giard, Perrin, Pernier, & Bouchet, 1990). The smoothing constant λ was set as 10−5. Since voltage distribution was only observed at the electrodes, the procedure of surface interpolation was used to calculate the total voltage distribution. The CSD toolbox employed Biharmonic Spline Interpolation (Sandwell, 1987), which is implemented by the embedded MATLAB function ‘griddata’ with the method option of ‘v4’.
Statistical Analysis
The amplitudes of MMN and P3a were analyzed as an average within a 100-ms window surrounding the peak latency at the electrode sites Fz, Cz, and Pz. The MMN peak was defined as the largest negativity in the subtraction between the deviant and standard sound ERPs 200–300 ms after sound onset. The P3a peak was defined as the largest positivity at 300–450 ms. Statistical analyses were conducted, separately for each experiment (emotional or non-vocal), using a three-way mixed ANOVA with deviant type (sad, fearful) and (2) electrode (Fz, Cz, Pz) as the within-subject factors, and (3) group (delinquents vs. controls) as the between-subject factor with additional a priori group by deviant type ANOVA contrasts calculated within each electrode site (Winer, Brown, & Michels, 1991). The dependent variable was the ERP component amplitude (MMN or P3a). Peak latencies were subjected to separate ANOVAs with deviant type and electrode as repeated-measure factors and group as a between-subject factor. Cohen’s d was calculated to estimate the effect size (i.e., the standardized difference between means). Degrees of freedom were corrected using the Greenhouse-Geisser method. Bonferroni-corrected t-tests were conducted only when preceded by significant main effects. Finally, Pearson correlations were calculated between emotional MMN amplitude measures and questionnaire data.
Results
Demographics and dispositional measures
Analyses of demographic and dispositional data indicated no significant group differences in age and education (Table 1). Scores on the BIS-11 and non-planning sub-trait revealed that the delinquent group was more impulsive, more reactive and less considerate than the controls (p=0.032, Cohen’s d=0.72; p=0.008, Cohen’s d=0.92). A significant difference on the scores of STAI-T (p=0.031, Cohen’s d=0.73) also suggested that the overall anxiety level was higher in the delinquents relative to the controls.
Table 1.
Demographic and dispositional measures.
| Controls (N = 20) |
Juvenile Delinquents (N =
20) |
p | |||
|---|---|---|---|---|---|
| mean | SD | mean | SD | ||
| Age | 17.3 | 1.6 | 16.3 | 1.7 | .067 |
| Education (yrs) | 16.3 | 1.5 | 15.6 | 0.9 | .108 |
| BIS-11 | 64.6 | 7.0 | 70.2 | 8.9 | .032* |
| Attentional | 17.5 | 3.4 | 17.4 | 2.4 | .957 |
| Motor | 22.0 | 3.4 | 24.1 | 4.1 | .087 |
| Non-planning | 25.2 | 2.9 | 28.8 | 4.9 | .008* |
| PCL: YV | -- | -- | 25.0 | 6.7 | |
| Factor 1 | -- | -- | 8.4 | 3.4 | |
| Factor 2 | -- | -- | 13.8 | 3.3 | |
| STAI-T (Trait) | 43.1 | 7.0 | 50.2 | 12.3 | .031* |
| STAI-S (State) | 38.3 | 9.1 | 43.4 | 9.5 | .092 |
Abbreviations: PCL:YV, Psychopathy Checklist: Youth Version; Factor 1, emotional; Factor 2, impulsive; BIS-11, Barratt Impulsiveness Scale Version 11; STAI, State-Trait Anxiety Inventory.
Neurophysiological measures of pre-attentive emotional voice discrimination
Pre-attentive discrimination of emotional voices was studied using MMN, determined by subtracting the neutral ERP from fearful and sad ERPs. The three-way mixed ANOVA analysis showed significant main effects of electrode [F(2, 76)=5.90, p=0.004, Cohen’s d=0.79] and deviant type (fearful MMN vs. sad MMN) [F(1, 38)=4.70, p=0.036, Cohen’s d=0.70] and interaction between deviant type and group (controls vs. delinquents) [F(1, 38)=4.13, p=0.049, Cohen’s d=0.65]. There were no significant effects of electrode x deviant type x group [F(2, 76)=1.10, p=0.337, Cohen’s d=0.33], electrode x group [F(2, 76)=0.86, p=0.426, Cohen’s d=0.30], or electrode x deviant type [F(2, 76)=1.57, p=0.213, Cohen’s d=0.40]. According to our a priori contrasts (Winer et al., 1991) derived from the main ANOVA on emotional MMN amplitudes, there was a significant interaction between the deviant type and group at the electrode site Fz [F(1, 38)=4.92, p=0.033, Cohen’s d=0.72]. A statistically non-significant trend toward a similar deviant type by group interaction was found at the electrode site Cz [F(1, 38)=3.65, p=0.064, Cohen’s d=0.62]. A further planned comparison of means indicated that MMN to fearful deviants was significantly stronger in amplitude than MMN to sad deviants in delinquents [Fz: t(19)=3.13, p=0.005, Cohen’s d=0.71; Cz: t(19)=2.61, p=0.017, Cohen’s d=0.63], whereas no difference between these conditions emerged in controls (Figure 1).
Figure 1. MMN amplitudes to emotional syllables at the electrode cite Fz.
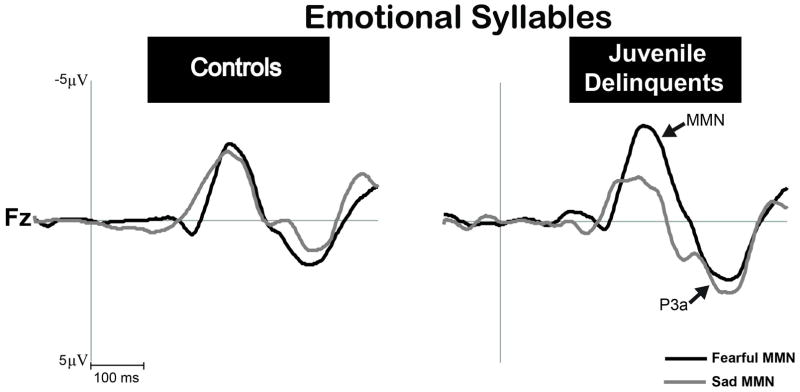
MMN to fearful deviants (black line) was significantly stronger in amplitude than MMN to sad deviants (gray line) in juvenile delinquents with CD symptoms (p=0.005), whereas no differences between these conditions emerged in controls (p=0.846).
To control whether the MMN amplitude effects for fearful vs. sad deviants in young delinquents were stemming from acoustical feature differences, instead of the emotional sound content, an additional MMN analysis was conducted by subtracting the neutral-derived ERP from the fearful-derived and sad-derived ERPs. The ANOVA model showed significant main effects of deviant type (fearful-derived MMN vs. sad-derived MMN) [F(1, 38)=10.99, p=0.002, Cohen’s d=1.07] and electrode [F(1, 38)=10.02, p=0.001, Cohen’s d=1.03]. There were no significant effects of electrode x group [F(2, 76)=1.00, p=0.371, Cohen’s d=0.32], deviant type x group [F(1, 38)=0.00, p=0.958, Cohen’s d=0.00], electrode x deviant type [F(2, 76)=0.86, p=0.426, Cohen’s d=0.30] or electrode x deviant type x group [F(2, 76)=0.43, p=0.647, Cohen’s d=0.21]. Unlike with emotional syllables, for MMNs to the non-vocal sounds the interaction between the deviant type and group was not significant [F(1, 38)<0.01, p=0.958, d<0.01] (Figure 2).
Figure 2. MMN amplitudes to non-vocal sounds matched spectrally with emotional syllables at the electrode cite Fz.
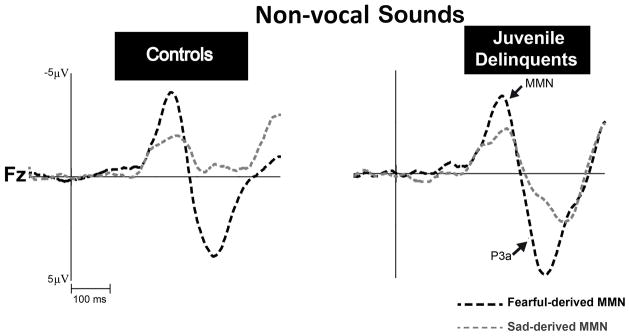
Non-vocal deviants that retained the acoustical features of emotional syllables were derived from sad (“sad-derived”) and fearful (“fearful-derived”) deviants. MMN amplitudes to fearful-derived deviants (black line) and sad-derived deviants (gray line) were comparable in juvenile delinquents with CD symptoms and controls (p=0.958).
The ANOVA model of P3a amplitudes to emotional syllables revealed a significant main effect of group [F(1, 38)=5.83, p=0.021, Cohen’s d=0.78]. A follow-up analysis showed that P3a amplitudes to sad deviants were significantly larger in delinquents than in controls at Fz and Cz [t(38)=2.55, p=0.015, Cohen’s d=0.83; t(38)=2.32, p=0.026, Cohen’s d=0.75, respectively]. The group differences for P3a amplitude to fearful deviants were non-significant. The ANOVA model of P3a peak latencies to emotional deviants revealed no effects involving the group factor.
The ANOVA model of P3a amplitudes to non-vocal sounds showed a significant main effect of group [F(1, 38)=8.58, p=0.006, Cohen’s d=0.95], as well as a significant electrode × deviant type x group interaction [F(2, 76)=4.21, p=0.018, Cohen’s d=0.67]. A follow-up analysis also showed an interaction effect of deviant type x group at Fz [F(1,38)=4.48, p=0.041, Cohen’s d=0.69]. At this location, the amplitude of sad-derived P3a was significantly larger in delinquents than in controls [t(38)=3.90, p<0.001, Cohen’s d=1.27]. As for P3a peak latencies to non-vocal deviants, a significant main effect of deviant type [F(1, 38)=9.61, p=0.004, Cohen’s d=3.51] was observed, suggesting that responses to fearful-derived deviants peaked earlier than those for sad-derived deviants. There was also a significant interaction of electrode x group [F(2, 76)=3.82, p=0.026, Cohen’s d=0.63]. P3a was delayed in delinquents at the frontal region in comparison to controls (p=0.049, Cohen’s d=0.46) but not the central or the parietal region (p=0.903, Cohen’s d=0.03; p=0.879, Cohen’s d=0.04). The grand-mean±SD latency of non-vocal P3a was 352±41 ms in controls and 370±35 ms in delinquents at Fz, 358±40 ms in controls and 359±27 ms in delinquents at Cz, and 367±40 ms in controls and 366±28 ms in delinquents at Pz.
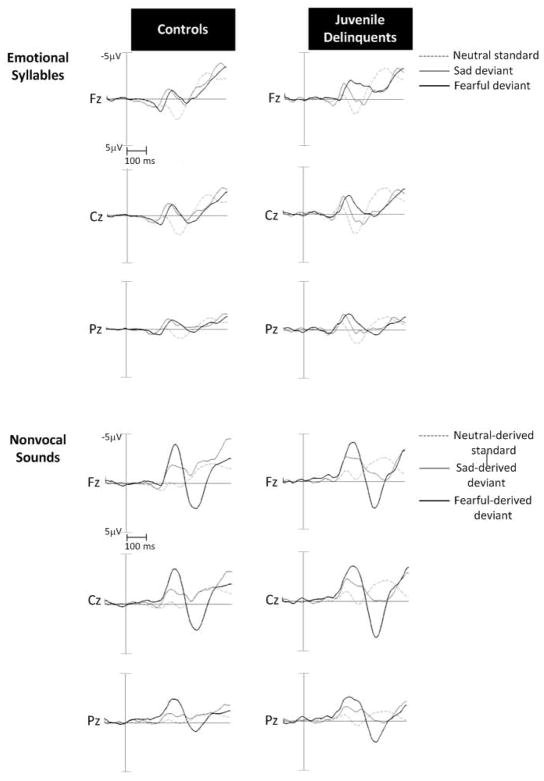
Table 2 shows amplitudes and peak latencies of MMN and P3a subcomponents in the controls and juvenile delinquents, in all stimulus conditions. The ERP waveforms for standard and deviant responses have been shown in Figure 3.
Table 2.
Mean± SE amplitudes and latencies of MMN and P3a.
| MMN | ||||||
|---|---|---|---|---|---|---|
| Controls |
Juvenile Delinquents |
|||||
| Amplitude (μV) | Latency (ms) | Amplitude(μV) | Latency (ms) | |||
| Emotional Syllables | Fearful | Fz | −1.84 ± 0.35 | 249.7 ± 4.9 | −2.39 ± 0.35 | 248.7 ± 4.9 |
| Cz | −1.79 ± 0.37 | 249.4 ± 4.9 | −2.26 ± 0.37 | 239.8 ± 4.9 | ||
| Pz | −1.21 ± 0.35 | 245.8 ± 5.4 | −1.83 ± 0.35 | 241.8 ± 5.4 | ||
|
| ||||||
| Sad | Fz | −1.77 ± 0.37 | 238.9 ± 6.2 | −1.10 ± 0.37 | 226.5 ± 6.2 | |
| Cz | −1.77 ± 0.39 | 240.0 ± 6.5 | −1.11 ± 0.39 | 230.0 ± 6.5 | ||
| Pz | −1.20 ± 0.35 | 247.5 ± 7.8 | −1.07 ± 0.35 | 236.4 ± 7.8 | ||
|
| ||||||
| Non-vocal Sounds | Fearful | Fz | −2.17 ± 0.44 | 237.3 ± 5.6 | −2.39 ± 0.44 | 248.6 ± 5.6 |
| Cz | −2.07 ± 0.38 | 240.4 ± 5.4 | −2.35 ± 0.38 | 246.1 ± 5.4 | ||
| Pz | −1.63 ± 0.33 | 231.9 ± 6.4 | −2.01 ± 0.33 | 239.2 ± 6.4 | ||
|
| ||||||
| Sad | Fz | −1.64 ± 0.29 | 243.9 ± 7.2 | −1.63 ± 0.29 | 238.1 ± 7.2 | |
| Cz | −1.16 ± 0.24 | 233.2 ± 8.4 | −1.41 ± 0.24 | 239.7 ± 8.4 | ||
| Pz | −0.66 ± 0.22 | 236.3 ± 8.5 | −1.22 ± 0.22 | 250.5 ± 8.5 | ||
| P3a | ||||||
|---|---|---|---|---|---|---|
| Controls |
Juvenile Delinquents |
|||||
| Amplitude (μV) | Latency (ms) | Amplitude(μV) | Latency (ms) | |||
| Emotional Syllables | Fearful | Fz | 2.04 ± 0.39 | 382.9 ± 7.2 | 3.10 ± 0.39 | 379.4 ± 7.2 |
| Cz | 2.11 ± 0.46 | 369.4 ± 7.5 | 3.25 ± 0.46 | 382.1 ± 7.5 | ||
| Pz | 1.26 ± 0.47 | 375.0 ± 8.4 | 2.29 ± 0.47 | 375.7 ± 8.4 | ||
|
| ||||||
| Sad | Fz | 1.79 ± 0.46 | 370.9 ± 7.9 | 3.46 ± 0.46 | 373.4 ± 7.9 | |
| Cz | 1.82 ± 0.43 | 382.3 ± 7.1 | 3.24 ± 0.43 | 367.9 ± 7.1 | ||
| Pz | 1.32 ± 0.31 | 393.5 ± 8.5 | 1.91 ± 0.31 | 375.9 ± 8.5 | ||
|
| ||||||
| Non-vocal Sounds | Fearful | Fz | 4.63 ± 0.62 | 349.1 ± 6.5 | 5.60 ± 0.62 | 358.9 ± 6.5 |
| Cz | 4.54 ± 0.62 | 349.2 ± 5.7 | 5.90 ± 0.62 | 353.6 ± 5.7 | ||
| Pz | 2.39 ± 0.48 | 357.7 ± 6.0 | 3.50 ± 0.48 | 356.9 ± 6.0 | ||
|
| ||||||
| Sad | Fz | 0.97 ± 0.49 | 356.6 ± 10.0 | 3.70 ± 0.49 | 381.3 ± 10.0 | |
| Cz | 1.32 ± 0.40 | 367.7 ± 9.1 | 3.33 ± 0.40 | 365.2 ± 9.1 | ||
| Pz | 0.87 ± 0.31 | 376.7 ± 9.0 | 2.12 ± 0.31 | 375.1 ± 9.0 | ||
Figure 3.
Grand average standard and deviant ERP waveforms for emotional syllables and non-vocal sounds in juvenile delinquents and controls.
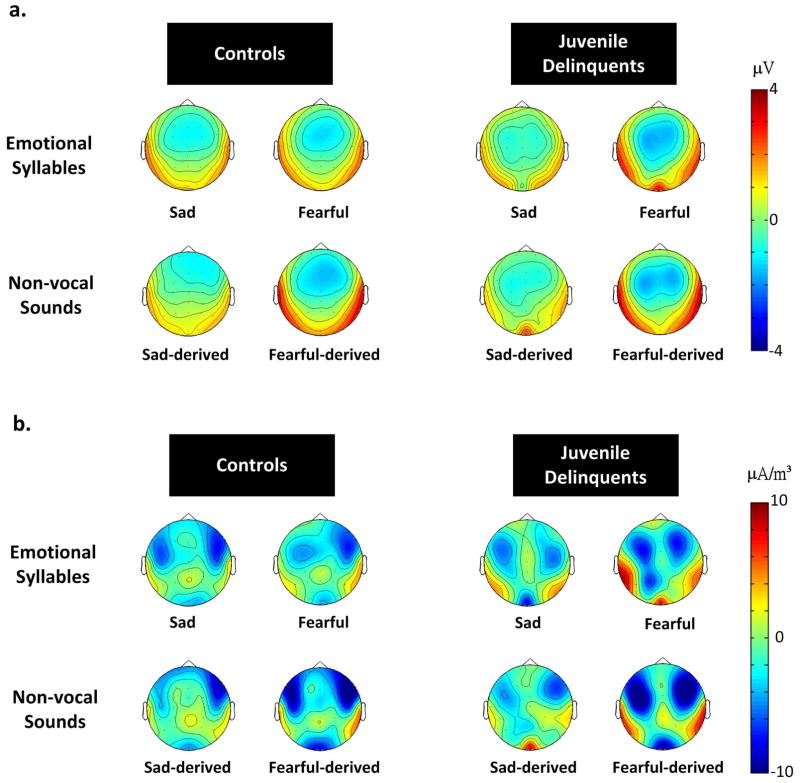
Current source density analyses
The scalp topographies for absolute voltages of MMN for emotional and non-vocal conditions in both groups were consistent with the MMN amplitudes results (Figure 4a). Our exploratory source distribution analyses based on CSDs suggested that MMN received a major contribution from the bilateral auditory cortices (Figure 4b). Additionally, in juvenile delinquents, there was a trend toward an additional posterior temporal / temporoparietal source (Figure 4b).
Figure 4. (a) The MMN scalp potential distributions.
There was a selective increase of MMN to fearful deviants in the juvenile delinquents. However, the MMN scalp potential distributions were relatively similar across the groups and conditions, showing a typical frontocentral minimum (or peak negativity). (b) The respective current source density (CSD) maps. The exploratory source distribution analyses based on CSDs suggested that MMN received a major contribution from the bilateral auditory cortices. Additionally, for MMN to fearful deviants, there was a trend toward an additional posterior temporal / temporoparietal source in juvenile delinquents.
Correlations with Neurophysiological indices and Psychometric variables
Correlation analyses were conducted to test the relationship between emotional MMN, BIS-11, STAI, and PCL:YV. Positive correlations were found between the score on STAI trait and fearful P3a at Cz [r (40)=0.35, p=0.025, Cohen’s d=0.76], and between the score on STAI state and sad P3a at Cz [r (40)=0.32, p=0.042, Cohen’s d=0.68].
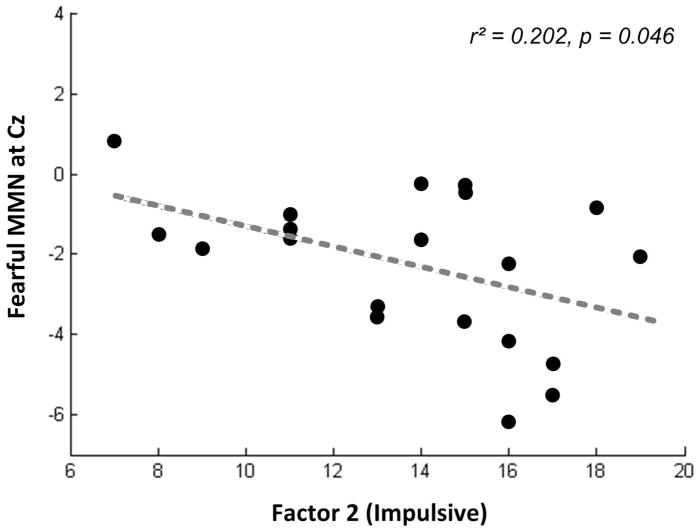
Within the juvenile delinquents, fearful MMN amplitudes were significantly negatively correlated with impulsive tendencies (factor 2, PCL:YV) at Cz [r (20)= −0.45, p=0.046, Cohen’s d= −1.01] (Figure 5). Delinquents with CD with more impulsive tendencies were more likely to display stronger negativities (amplitudes) of fearful MMN. No correlation was found between MMN amplitudes and impulsive trait, psychopathic trait, or unemotional callousness (factor 1) while delinquents and controls were collapsed together [Fz: r (40)=0.02, p=0.91; r (40)= −0.29, p=0.22; r (40)= −0.37, p=0.11; r (40)= −0.39, p=0.09; Cz: r (40)=0.04, p=0.81; r (40)= −0.26, p=0.27; r (40)= −0.38, p=0.10; r (40)= −0.31, p=0.18].
Figure 5. Correlation between MMN amplitudes to fearful deviants and impulsive tendencies.
Within the juvenile delinquents with CD symptoms, MMN amplitudes to fearful deviants were significantly negatively correlated with impulsive tendencies at Cz (p=0.046). Delinquents with CD who showed more impulsive tendencies were more likely to display stronger negativities (amplitudes) of MMN to fearful deviants.
While the differences between fearful and sad MMN were used as the dependent variable, BIS-11 total scores were negatively correlated [Fz: r (40)= −0.31, p=0.048, Cohen’s d= −0.66; Cz: r (40)= −0.38, p=0.015, Cohen’s d= −0.82]. In other words, larger MMN difference values (i.e., increased negativity) predicted high BIS-11 total scores. Specifically, negative correlations were found for the motor subscore [Fz: r (40)= −0.36, p=0.024, Cohen’s d= −0.76; Cz: r (40)= −0.36, p=0.023, Cohen’s d= −0.76] as well as for the non-planning subscore [Cz: r (40)= −0.33, p=0.041, Cohen’s d= −0.68]. No correlation was found for the differences between fearful-derived and sad-derived non-vocal MMN at either Fz [r (40)=0.16, p=0318, Cohen’s d=0.32] or Cz [r (40)=0.25, p=0.128, Cohen’s d=0.52]. Further, within the delinquents, a positive correlation was found between BIS-11 and PCL:YV total score [r (40)=0.48, p=0.031, Cohen’s d=1.11], particularly between BIS-11 and factor 2 [r (40)=0.49, p=0.028, Cohen’s d=1.12].
Discussion
We examined the emotional reactivity to distressful voices in delinquents with CD symptoms using an emotional MMN paradigm. Fearful MMN as compared with sad MMN was significantly larger in amplitude in juvenile delinquents, whereas no such effect was observed in controls. Notably, our control analyses suggested no significant MMN differences between the non-vocal acoustic equivalents of sad and fearful syllables, suggesting that the atypical affective discrimination in young offenders was driven by emotional voice processing, per se, rather than low-level acoustical features. Finally, our correlation analyses suggested that young offenders with strongest impulsive tendencies were likely to display largest amplitudes of fearful MMN.
Enhanced MMN difference to fearful vs. sad voices in juvenile delinquents with CD symptoms might reflect their stronger automatic sensitivity of emotional voices. Higher levels of emotional arousal have been proposed to render antisocial youth to strongly react to perceived provocation, which, in turn, results in aggressive behaviors without forethought and planning (Frick, 2009). A previous study reported that, of the distinct subtraits of impulsiveness, the non-planning impulsiveness was correlated with situations involving hostile aggression, and specifically emotional agitation (Ramirez & Andreu, 2006). Consistent with these findings, we found that the differences between fearful and sad MMN were negatively associated with the BIS-11 total scores as well as the non-planning impulsiveness subscores. That is, a large MMN difference (a negative value) predicted high impulsiveness scores in the young offender group. One might thus speculate that delinquents with CD symptoms could be sensitized to discriminate sadness from fear through voices, indicating stronger emotional reactivity, which, in turn, might be associated with impulsivity.
However, the findings on emotional MMN seem to contradict previous reports that show reduced affective arousal to other’s distress in adolescents with CD and psychopathic tendencies (Blair, 2005; Jones et al., 2009; Marsh et al., 2008; Sterzer et al., 2005). Note however that, in contrast to the present passive-listening design, in these previous studies, decreased responses to the emotional stimuli were observed while participants were required to watch or listen to the stimuli attentively. Active attention, in turn, is a factor that may cause essential modulations on emotional reactivity (Jones et al., 2009; Marsh et al., 2008; Newman et al., 2010). We, thus, speculate that certain differences between the present and the aforementioned active-attention studies may result from aberrantly imbalanced emotional reactivity between conscious and subconscious emotion processing in CD (Smith, 2009).
In line with previous report (Snowden & Gray, 2011), Factor 2 of PCL:YV was positively correlated with BIS-11. At the same time, delinquents with CD symptoms showing higher impulsive tendencies (Factor 2) were most prone to display strong amplitudes of fearful MMN. This association between enlarged MMN and impulsiveness is, more generally, in line of several previous studies. One of such studies suggested that healthy individuals scoring high at scales of impulsiveness elicit larger MMN amplitudes to pure tones than less impulsive persons (Franken et al., 2005). It has been also shown (Kähkönen et al., 2005) that MMN to unattended duration and frequency changes increases after acute trypthophan depletion that reduces brain synthesis of serotonin, a neurotransmitter that is well known to be associated with impulse control and mood regulation. Therefore, noting that MMN has been considered as a clinical marker for genetic predispositions in a variety of disorders (Näätänen et al., 2011), one might speculate that emotional MMN, particularly fearful MMN, could potentially provide a neural correlate of genetic risk for impulsive tendency
Higher scores on STAI trait anxiety in delinquents suggested that trait anxiety might contribute to the symptomatology of CD. In parallel, anxiety was reported to have a positive correlation with reactive aggression in boys with early-onset CD and co-morbid attention-deficit hyperactivity disorder (Cosi, Hernandez-Martinez, Canals, & Vigil-Colet, 2011; Polier, Herpertz-Dahlmann, Matthias, Konrad, & Vloet, 2010). Our findings indicated that higher STAI trait anxiety was associated with stronger P3a amplitudes to fearful syllables and higher STAI state anxiety was associated with larger P3a to sad syllables. P3a amplitudes would increase while mixed anxious-depressed individuals perceived emotional faces (Rossignol, Philippot, Crommelinck, & Campanella, 2008). That is, emotional MMN might reflect impulsivity whereas emotional P3a could represent anxiety traits.
MMN and P3a amplitudes were significantly larger in fearful derived non-vocal sounds than in sad derived non-vocal sounds in both groups. It is thus possible that without considering the stimulus as an emotion-associated sound object, the spectrotemporal properties of fearful-derived (and possibly also the fearful vocal) sounds differed more from the standards than sad-derived sounds, consequently resulting in a larger MMN and P3a to fearful-derived than sad-derived deviants in comparison to standards. However, the fact that this effect was very similar across the two groups also suggests that any group differences in processing of emotion-associated sound objects cannot be explained by more fundamental deficits in auditory cortex feature extraction properties. At the same time, the differences in group-interaction MMN/P3a patterns to emotional syllables (emotional sound objects) and non-vocal sounds (non-meaningful spectrotemporal “basic objects”) could be speculated to suggest that there is a distinct processing route for pre-attentive processing of species-specific emotional information in human auditory cortices. That is, when the sound is biologically relevant and emotion containing, its emotional significance may dominate the change-detection in auditory cortex, instead of the spectrotemporal physical dimension. From an evolutional perspective, hypothesis of a distinct sensory route for emotion-related information would be consistent with the fact that the processing of emotions is independent of attention (Belin & Grosbras, 2010; Vuilleumier, 2005). It has been also shown that there are specific areas of auditory cortex that respond stronger to human vocalization than to other non-vocal sounds (Belin, Zatorre, Lafaille, Ahad, & Pike, 2000; Rauschecker & Scott, 2009), and that emotional modulation effects occur within these region (Ethofer et al., 2012; Ethofer, Van De Ville, Scherer, & Vuilleumier, 2009).
Interestingly, our explorative CSD analyses suggested the major contribution to deviance-standard difference responses is coming from the bilateral auditory cortices. Furthermore, a slight trend toward the left posterior enhancement observed in juvenile delinquents for fearful MMN could possibly reflect an additional left posterior temporal / temporoparietal source. Notably, by combining structural and functional neuroimaing approaches, Ethofer and colleagues (2012) recently suggested that emotion-sensitive areas in posterior lateral non-primary auditory cortices, which is tentatively consistent with the finding of potentially additional MMN source in the left posterior temporal regions (Figure 4b). However, given the known inaccuracies with EEG source localization, these CSD results will need to be confirmed with more accurate source analysis approaches.
A significant group by electrode interaction was observed for the latencies of P3a to non-vocal deviants. P3a was delayed in delinquents at the frontal region in comparison to controls but not the central or the parietal region. The grand-mean latency of non-vocal P3a showed the controls’ response peaked around 352 ms and the delinquents’ peaked around 370 ms. The result suggests that, in addition to abnormalities in automatic reactivity to specific emotional cues, the youths with CD might also have more fundamental differences in their auditory attention processes or working memory performance (Light et al., 2007). However, this finding does not necessarily undermine the interpretations of our main result, as the effect did not seem to be specific to spectral cues (i.e., the interaction involving non-vocal deviant type was not significant).
Potential limitations of our study are the relatively small sample size, and the fact that all subjects were ethnically Chinese, which may somewhat limit the generalizability of results. Due to the experiment design, we cannot disentangle N1-effects and MMN in our study, and the present MMN measures may have “N1 refractoriness” contribution (Kujala, Tervaniemi, & Schroger, 2007; May & Tiitinen, 2010). However, the present interpretation that emotional sound cues may be processed in different ways in CD vs. healthy controls would not change even if an alternative model of neuronal mechanism considering MMN generation, per se, was accepted (e.g., short-term plasticity of populations also generating the N1 response (Jääskeläinen et al., 2011; Jääskeläinen, Ahveninen, Belliveau, Raij, & Sams, 2007; Jääskeläinen et al., 2004)). Further, the heterogeneity of antisocial and aggressive behaviors is likely to give rise to qualitatively different interpretations of the results. Juvenile aggression is usually diagnosed as CD but is often associated with other co-morbid conditions, such as ADHD and mood disorders. The anxiety or negative mood should be taken into consideration because the higher sensitivity to distressful stimuli could be due merely to a deficit in impulse control, but could also be related to the emotional state. A number of maturational changes may still be going for the young participants. Longitudinal researches in clinical populations or large community-based samples will be indispensable for a more thorough pathological understanding of the development and prognosis of impulsivity and aggression during adolescence.
Conclusion
In summary, increased difference in MMN between fearful and sad deviants, and correlation of this phenomenon with high impulsive tendencies, suggest that involuntary pre-attentive processing of distressful voices may be abnormal in youths afflicted with CD symptoms and a history of delinquent behaviors. These results may help understand the neurodevelopment of aggression and impulsivity from childhood to adulthood.
Key points.
Youths with CD process distressful voices in an atypical fashion already at the pre-attentive level.
Enhanced MMN difference between fearful and sad deviants in juvenile delinquents with CD symptoms suggests their stronger emotional sensitivity to distressful voices.
The MMN abnormalities in juvenile delinquents were related to the emotional content of sounds, instead of purely acoustic factors.
Acknowledgments
The study was funded by National Science Council (NSC 99-2314-B-010-037-MY3; NSC 100-2628-H-010-001-MY3) and National Yang-Ming University Hospital (RD2011-005), and was also supported by a grant from the Ministry of Education (A grant from Ministry of Education, Aim for the Top University Plan). Author J.A. was supported by National Institutes of Health Awards R01MH083744, R21DC010060, R01HD040712, and R01NS037462. The authors would like to thank Dr. Sharon Furtak for proofreading the whole article and Dr. Wei-Tang Chang for the MMN scalp potential analyses.
Footnotes
Conflict of interest statement: No conflict declared
References
- Alain C, Woods DL, Knight RT. A distributed cortical network for auditory sensory memory in humans. Brain Res. 1998;812(1–2):23–37. doi: 10.1016/s0006-8993(98)00851-8. [DOI] [PubMed] [Google Scholar]
- Alho K, Woods DL, Algazi A, Knight RT, Näätänen R. Lesions of frontal cortex diminish the auditory mismatch negativity. Electroencephalography & Clinical Neurophysiology. 1994;91(5):353–362. doi: 10.1016/0013-4694(94)00173-1. [DOI] [PubMed] [Google Scholar]
- Belin P, Grosbras MH. Before speech: cerebral voice processing in infants. Neuron. 2010;65(6):733–735. doi: 10.1016/j.neuron.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403(6767):309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn. 2005;14(4):698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Boersma P. Praat, a system for doing phonetics by computer. Glot International. 2001;5(9–10):341–345. [Google Scholar]
- Campbell SB. Behavior problems in preschool childhen: Clinical and developmental issues. New York: Guilford Press; 1990. [Google Scholar]
- Cheng Y, Lee SY, Chen HY, Wang PY, Decey J. Voice and emotion processing in the human neonatal brain. Journal of Cognitive Neuroscience. 2012;24(6):1411–1419. doi: 10.1162/jocn_a_00214. [DOI] [PubMed] [Google Scholar]
- Cosi S, Hernandez-Martinez C, Canals J, Vigil-Colet A. Impulsivity and internalizing disorders in childhood. Psychiatry Res. 2011;190(2–3):342–347. doi: 10.1016/j.psychres.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Crottaz-Herbette S, Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. J Cogn Neurosci. 2006;18(5):766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- Dadds MR, El Masry Y, Wimalaweera S, Guastella AJ. Reduced eye gaze explains “fear blindness” in childhood psychopathic traits. J Am Acad Child Adolesc Psychiatry. 2008;47(4):455–463. doi: 10.1097/CHI.0b013e31816407f1. [DOI] [PubMed] [Google Scholar]
- Decety J, Michalska KJ, Akitsuki Y, Lahey BB. Atypical empathic responses in adolescents with aggressive conduct disorder: a functional MRI investigation. Biol Psychol. 2009;80(2):203–211. doi: 10.1016/j.biopsycho.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escera C, Alho K, Schroger E, Winkler I. Involuntary attention and distractibility as evaluated with event-related brain potentials. Audiol Neurootol. 2000;5(3–4):151–166. doi: 10.1159/000013877. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Bretscher J, Gschwind M, Kreifelts B, Wildgruber D, Vuilleumier P. Emotional voice areas: anatomic location, functional properties, and structural connections revealed by combined fMRI/DTI. Cereb Cortex. 2012;22(1):191–200. doi: 10.1093/cercor/bhr113. [DOI] [PubMed] [Google Scholar]
- Ethofer T, Van De Ville D, Scherer K, Vuilleumier P. Decoding of emotional information in voice-sensitive cortices. Curr Biol. 2009;19(12):1028–1033. doi: 10.1016/j.cub.2009.04.054. [DOI] [PubMed] [Google Scholar]
- Forth AE, Kosson DS, Hare RD. Technical manual, Multi-Healthy Systems. Toronto, Canada: Multi-Health Systems; 2003. The Hare Psychopathy Checklist: Youth Version. [Google Scholar]
- Franken IH, Nijs I, Van Strien JW. Impulsivity affects mismatch negativity (MMN) measures of preattentive auditory processing. Biol Psychol. 2005;70(3):161–167. doi: 10.1016/j.biopsycho.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Frick PJ. Extending the construct of psychopathy to youth: implications for understanding, diagnosing, and treating antisocial children and adolescents. Can J Psychiatry. 2009;54(12):803–812. doi: 10.1177/070674370905401203. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Cornell AH, Bodin SD, Dane HE, Barry CT, Loney BR. Callous-unemotional traits and developmental pathways to severe conduct problems. Dev Psychol. 2003;39(2):246–260. doi: 10.1037//0012-1649.39.2.246. [DOI] [PubMed] [Google Scholar]
- Fritz MV, Wiklund G, Koposov RA, af Klinteberg B, Ruchkin VV. Psychopathy and violence in juvenile delinquents: what are the associated factors? Int J Law Psychiatry. 2008;31(3):272–279. doi: 10.1016/j.ijlp.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Giard MH, Perrin F, Pernier J, Bouchet P. Brain generators implicated in the processing of auditory stimulus deviance: a topographic event-related potential study. Psychophysiology. 1990;27(6):627–640. doi: 10.1111/j.1469-8986.1990.tb03184.x. [DOI] [PubMed] [Google Scholar]
- Gill KL, Calkins SD. Do aggressive/destructive toddlers lack concern for others? Behavioral and physiological indicators of empathic responding in 2-year-old children. Dev Psychopathol. 2003;15(1):55–71. doi: 10.1017/s095457940300004x. [DOI] [PubMed] [Google Scholar]
- Hermens DF, Ward PB, Hodge MA, Kaur M, Naismith SL, Hickie IB. Impaired MMN/P3a complex in first-episode psychosis: cognitive and psychosocial associations. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(6):822–829. doi: 10.1016/j.pnpbp.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, Stoecker T, et al. Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry. 2008;49(7):781–791. doi: 10.1111/j.1469-7610.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen IP, Ahveninen J, Andermann ML, Belliveau JW, Raij T, Sams M. Short-term plasticity as a neural mechanism supporting memory and attentional functions. Brain Res. 2011;1422:66–81. doi: 10.1016/j.brainres.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen IP, Ahveninen J, Belliveau JW, Raij T, Sams M. Short-term plasticity in auditory cognition. Trends Neurosci. 2007;30(12):653–661. doi: 10.1016/j.tins.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen IP, Ahveninen J, Bonmassar G, Dale AM, Ilmoniemi RJ, Levänen S, et al. Human posterior auditory cortex gates novel sounds to consciousness. Proc Natl Acad Sci U S A. 2004;101(17):6809–6814. doi: 10.1073/pnas.0303760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. Am J Psychiatry. 2009;166(1):95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Kähkönen S, Makinen V, Jääskeläinen IP, Pennanen S, Liesivuori J, Ahveninen J. Serotonergic modulation of mismatch negativity. Psychiatry Res. 2005;138(1):61–74. doi: 10.1016/j.pscychresns.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Keage HA, Clark CR, Hermens DF, Kohn MR, Clarke S, Williams LM, et al. Distractibility in AD/HD predominantly inattentive and combined subtypes: the P3a ERP component, heart rate and performance. J Integr Neurosci. 2006;5(1):139–158. doi: 10.1142/s0219635206001070. [DOI] [PubMed] [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalogr Clin Neurophysiol. 1984;59(1):9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Koelsch S. P3a and mismatch negativity in individuals with moderate Intermittent Explosive Disorder. Neurosci Lett. 2009;460(1):21–26. doi: 10.1016/j.neulet.2009.05.047. [DOI] [PubMed] [Google Scholar]
- Kujala T, Tervaniemi M, Schroger E. The mismatch negativity in cognitive and clinical neuroscience: theoretical and methodological considerations. Biol Psychol. 2007;74(1):1–19. doi: 10.1016/j.biopsycho.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Loeber R, Burke JD, Applegate B. Predicting future antisocial personality disorder in males from a clinical assessment in childhood. J Consult Clin Psychol. 2005;73(3):389–399. doi: 10.1037/0022-006X.73.3.389. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Braff DL. Preattentive sensory processing as indexed by the MMN and P3a brain responses is associated with cognitive and psychosocial functioning in healthy adults. J Cogn Neurosci. 2007;19(10):1624–1632. doi: 10.1162/jocn.2007.19.10.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loney BR, Frick PJ, Clements CB, Ellis ML, Kerlin K. Callous-unemotional traits, impulsivity, and emotional processing in adolescents with antisocial behavior problems. J Clin Child Adolesc Psychol. 2003;32(1):66–80. doi: 10.1207/S15374424JCCP3201_07. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 2008;165(6):712–720. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- May PJ, Tiitinen H. Mismatch negativity (MMN), the deviance-elicited auditory deflection, explained. Psychophysiology. 2010;47(1):66–122. doi: 10.1111/j.1469-8986.2009.00856.x. [DOI] [PubMed] [Google Scholar]
- Moffitt TE, Caspi A, Harrington H, Milne BJ. Males on the life-course-persistent and adolescence-limited antisocial pathways: follow-up at age 26 years. Dev Psychopathol. 2002;14(1):179–207. doi: 10.1017/s0954579402001104. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Kujala T, Kreegipuu K, Carlson S, Escera C, Baldeweg T, et al. The mismatch negativity: an index of cognitive decline in neuropsychiatric and neurological diseases and in ageing. Brain. 2011;134(Pt 12):3435–3453. doi: 10.1093/brain/awr064. [DOI] [PubMed] [Google Scholar]
- Newman JP, Curtin JJ, Bertsch JD, Baskin-Sommers AR. Attention moderates the fearlessness of psychopathic offenders. Biol Psychiatry. 2010;67(1):66–70. doi: 10.1016/j.biopsych.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C. Theoretical analysis of field potentials in anisotropic ensembles of neuronal elements. IEEE Trans Biomed Eng. 1973;20(4):278–288. doi: 10.1109/TBME.1973.324192. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51(6):768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polier GG, Herpertz-Dahlmann B, Matthias K, Konrad K, Vloet TD. Associations between trait anxiety and psychopathological characteristics of children at high risk for severe antisocial development. Atten Defic Hyperact Disord. 2010;2(4):185–193. doi: 10.1007/s12402-010-0048-5. [DOI] [PubMed] [Google Scholar]
- Raine A, Venables PH, Mednick SA. Low resting heart rate at age 3 years predisposes to aggression at age 11 years: evidence from the Mauritius Child Health Project. J Am Acad Child Adolesc Psychiatry. 1997;36(10):1457–1464. doi: 10.1097/00004583-199710000-00029. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Andreu JM. Aggression, and some related psychological constructs (anger, hostility, and impulsivity); some comments from a research project. Neurosci Biobehav Rev. 2006;30(3):276–291. doi: 10.1016/j.neubiorev.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP, Scott SK. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nat Neurosci. 2009;12(6):718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol M, Philippot P, Crommelinck M, Campanella S. Visual processing of emotional expressions in mixed anxious-depressed subclinical state: an event-related potential study on a female sample. Neurophysiol Clin. 2008;38(5):267–275. doi: 10.1016/j.neucli.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Sandwell DT. Biharmonic Spline Interpolation of GEOS-3 and SEASAT Altimeter Data. Geophysical Research Letters. 1987;2:139–142. [Google Scholar]
- SanMiguel I, Morgan HM, Klein C, Linden D, Escera C. On the functional significance of Novelty-P3: facilitation by unexpected novel sounds. Biol Psychol. 2010;83(2):143–152. doi: 10.1016/j.biopsycho.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Savitsky JC, Czyzewski D. The reaction of adolescent offenders and nonoffenders to nonverbal emotion displays. J Abnorm Child Psychol. 1978;6(1):89–96. doi: 10.1007/BF00915784. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Escoffier N. Emotional MMN: Anxiety and heart rate correlate with the ERP signature for auditory change detection. Clin Neurophysiol. 2010;121(1):53–59. doi: 10.1016/j.clinph.2009.09.029. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Kotz SA. Beyond the right hemisphere: brain mechanisms mediating vocal emotional processing. Trends Cogn Sci. 2006;10(1):24–30. doi: 10.1016/j.tics.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Simpson E, Escoffier N. Listen up! Processing of intensity change differs for vocal and nonvocal sounds. Brain Res. 2007;1176:103–112. doi: 10.1016/j.brainres.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Sharp C, van Goozen S, Goodyer I. Children’s subjective emotional reactivity to affective pictures: gender differences and their antisocial correlates in an unselected sample of 7-11-year-olds. J Child Psychol Psychiatry. 2006;47(2):143–150. doi: 10.1111/j.1469-7610.2005.01464.x. [DOI] [PubMed] [Google Scholar]
- Smith A. The empathy imbalance hypothesis of autism: A theoretical approach to cognitive and emotional empathy in autistic development. The Psychological Record. 2009;59:489–510. [Google Scholar]
- Snowden RJ, Gray NS. Impulsivity and psychopathy: associations between the barrett impulsivity scale and the psychopathy checklist revised. Psychiatry Res. 2011;187(3):414–417. doi: 10.1016/j.psychres.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorssuch RL, Lushene PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, Inc; 1983. [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biol Psychiatry. 2005;57(1):7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Viding E, Fontaine NM, McCrory EJ. Antisocial behaviour in children with and without callous-unemotional traits. J R Soc Med. 2012;105(5):195–200. doi: 10.1258/jrsm.2011.110223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Winer B, Brown D, Michels K. Statistical Principles in Experimental Design. New York: McGrawhill; 1991. [Google Scholar]
- Woods DL, Knight RT. Electrophysiologic evidence of increased distractibility after dorsolateral prefrontal lesions. Neurology. 1986;36(2):212–216. doi: 10.1212/wnl.36.2.212. [DOI] [PubMed] [Google Scholar]