Abstract
Purpose
In diabetes, endothelial dysfunction and subsequent structural damage to blood vessels can lead to heart attacks, retinopathy and strokes. However, it is unclear whether prediabetic subjects exhibit microvascular dysfunction indicating early stages of arteriosclerosis and vascular risk. The purpose of this study was to examine whether retinal reactivity may be impaired early in the hyperglycemic continuum and may be associated with markers of inflammation.
Methods
Individuals with prediabetes (n = 22), type 2 diabetes (n = 25) and healthy age and body composition matched controls (n = 19) were studied. We used the Dynamic Vessel Analyzer to assess retinal vasoreactivity (percent change in vessel diameter) during a flickering light stimulation. Fasting highly sensitive c-reactive protein (hs-CRP), a marker of inflammation, was measured in blood plasma.
Results
Prediabetic and diabetic individuals had attenuated peak vasodilator and relative amplitude changes in retinal vein diameters to the flickering light stimulus compared to healthy controls (peak dilation: prediabetic subjects 3.3 ± 1.8 %, diabetic subjects 3.3 ± 2.1% controls 5.6 ± 2.6%, p = .001; relative amplitude: prediabetic subjects 4.3 ± 2.2%, diabetic subjects 5.0 ± 2.6% and control subjects 7.2 ± 3.2%, p = .003). Similar findings were observed in retinal arteries. Levels of hs-CRP were not associated with either retinal vessel response parameters.
Conclusion
Retinal reactivity was impaired in prediabetic and type 2 diabetic individuals in parallel with reduced insulin sensitivity but not associated with levels of hs-CRP. Retinal vasoreactivity measurements may be a sensitive tool to assess early vascular risk.
Keywords: prediabetes, type 2 diabetes, retinal reactivity, vasodilation, flickering light stimulation
Introduction
More than 70 million Americans have prediabetes, an early stage in the hyperglycemic continuum associated with an increased risk of developing future diabetes (Haffner 2003; Ford et al. 2010) and vascular complications (Milman & Crandall 2011). The retina is a unique site to study the human microcirculation. Retinal blood flow is controlled by autoregulatory metabolic and pressure mechanisms which are impaired in diabetes and may contribute to retinopathy and vision loss (Pournaras et al. 2008). Understanding the pathophysiologic basis for changed blood vessel responses across the hyperglycemic continuum is important for the discovery of new treatments and preventive strategies during early disease stages.
A flickering light stimulus has been used to assess retinal vascular dysfunction. In healthy individuals, flickering light stimulus increases retinal blood flow and blood vessel diameter (Michelson et al. 2002; Dorner et al. 2003; Nagel & Vilser 2004; Lott et al. 2012), whereas in diabetic individuals retinal vasodilation is attenuated (Garhofer et al. 2004; Mandecka et al. 2007; Bek et al. 2008; Nguyen et al. 2009; Pemp et al. 2009; Lott et al. 2012) but it is unknown whether retinal vascular impairment exists in prediabetes.
Several studies indicate that inflammation plays an important role in the development of atherosclerosis and diabetes (Mazzone et al. 2008; D’Souza et al. 2009). While diabetes has been associated with elevated inflammatory biomarkers (Thomsen et al. 2010) as well as impaired macrovascular reactivity (Toda et al. 2010), whether inflammation is associated with retinal microvascular dysfunction across the hyperglycemic continuum is not clear.
Thus, the purpose of this study was to examine whether retinal reactivity may be impaired early in the hyperglycemic continuum and may be associated with markers of inflammation such as hs-CRP levels. We hypothesized that individuals with prediabetes have an impaired retinal vasoreactivity and that attenuated retinal reactivity would be associated with higher levels of hs-CRP.
Methods
Subjects
The study was approved by the Institutional Review Board at Penn State Hershey Medical Center and followed the Tenets of the Declaration of Helsinki. Middle aged to older non-smoking individuals with prediabetes (n = 22), type 2 diabetes (n = 25) and body mass index (BMI) and age similar matched healthy controls (n = 19) between 21 and 75 years of age participated in this study (Table 1). Diagnosis of diabetes and prediabetes was based on the American Diabetic Association’s new classification standards for prediabetes (HbA1c ≥ 5.7 and < 6.5%) and type 2 diabetes (HbA1c ≥ 6.5%) (2010). Subjects were recruited through physician letters and flyers in the general community. After signing informed consent, all subjects completed a medical history, physical exam, and ocular screening which included a measurement of visual acuity and intraocular pressure. Subjects had a normal eye examination with corrected acuity of 20/30 or better and an intraocular pressure below 21 mmHg. Subjects were free from stroke, coronary, heart, lung and eye diseases (e.g. retinopathy, age related macular degeneration and glaucoma), and were not morbidly obese (BMI > 45 kg/m2) or currently pregnant. All subjects were non-smokers and controls had no history of hypertension. Diabetic subjects’ blood pressures were controlled by medications.
Table 1.
Subject Demographics.
| Healthy Controls | Prediabetes | Type 2 Diabetes | P Value | |
|---|---|---|---|---|
| Number of subjects | 19 | 22 | 25 | |
| Number of women/men | 12 / 7 | 12/ 10 | 16 / 9 | |
| Ethnicity | ||||
| Caucasian | 100% | 95% | 92% | |
| African/American | 0% | 0% | 8% | |
| Asian | 0% | 5% | 0% | |
| Age (yrs.) | 52 ± 9 | 60 ± 10* | 56 ± 9 | .04 |
| Weight (lbs.) | 184 ± 45 | 183 ± 40 | 193 ± 41 | .69 |
| BMI (kg/m2) | 28.4 ± 5.0 | 29.0 ± 5.5 | 30.5 ± 5.2 | .42 |
| Fasting glucose (mg/dl) | 85 ± 9 | 95 ± 11 | 123 ± 53*# | .001 |
| Fasting insulin (IU/ml) | 4 ± 4 | 5 ± 3 | 18 ± 29*# | .02 |
| Insulin Sensitivity (units) | .41 ± .05 | .39 ± .05 | .34 ± .06*# | .001 |
| HbA1c (%) | 5.3 ± 0.3 | 6.0 ± 0.3 | 7.5 ± 1.8*# | .001 |
| Total Cholesterol (mg/dl) | 200 ± 34 | 206 ± 34 | 180 ± 39# | .04 |
| Low density lipoprotein (mg./l) | 125 ± 28 | 128 ± 30 | 105 ± 36 # | .03 |
| High density lipoprotein (md/dl) | 55 ± 19 | 54 ± 16 | 48 ± 11 | .21 |
| High/Low Ratio lipoproteins | 3.8 ± 1.4 | 4.1 ± 1.2 | 4.0 ± 1.2 | .75 |
| Triglycerides (mg/dl) | 91 ± 36 | 129 ± 71 | 137 ± 61* | .03 |
| Medications | ||||
| Oral diabetic (total % of individuals on) | 0% | 0% | 84%*# | .001 |
| Specifics – number of people on: | ||||
| Sulfonylureas | 12 | |||
| Glucophage | 13 | |||
| Thiazolidinediones | 3 | |||
| Dipeptidyl Peptidase IV Inhibitors | 5 | |||
| Insulin (total % of individuals on) | 0% | 0% | 28%*# | .001 |
| Specifics – number of people on: | ||||
| Glucagon-like peptide agonist | 4 | |||
| Rapid acting insulin | 2 | |||
| Long acting insulin | 4 | |||
| Anti-hypertension (total % of individuals on) | 0% | 32%* | 80%2*# | .001 |
| Specifics – number of people on: | ||||
| Hydrochlorothiazide | 2 | 3 | ||
| Ace inhibitors | 4 | 9 | ||
| Beta blockers | 1 | 7 | ||
| Calcium channel blockers | 3 | 7 | ||
| Alpha 2 adrenergic agonists | 1 | 0 | ||
| Angiotensin II receptors blockers | 1 | 3 | ||
| Fish Oil | 26% | 36% | 16% | .28 |
| Statins | 0% | 23%* | 68%*# | .001 |
BMI = body mass index; Hb = Hemoglobin; % = percentage; Mean ± SD
significantly different from controls;
significantly different from prediabetic individuals
Experimental Design
This comparative study examined retinal reactivity (i.e. changes in vessel diameter to flickering light) using the Dynamic Vessel Analyzer (DVA) in three groups of subjects (healthy controls and individuals with prediabetes and type 2 diabetes). Subjects refrained from alcohol, caffeine (Terai et al. 2012), and exercise for 24 hours prior to testing and fasted for approximately 10 hours prior to testing. Measurements were performed in a dimly lit room at room temperature. Individuals with type 2 diabetes held their diabetic medications on the morning of the study. None of the prediabetic subjects were on diabetic medications. Aspirin and non-steroidal anti-inflammatory medications were held for 24 hours prior to the study. After a 15-minute rest period, venous blood samples of hs-CRP, glucose, insulin, lipid panel and HbA1c were drawn from the brachial antecubital location for later analysis. The eye with the best visual acuity was dilated with one or two drops of tropicamide (1%) and if needed, phenylephrine (2.5%) was added to obtain optimal dilation.
Experimental Protocol
After a rest period of 20 minutes to allow stabilization of baseline parameters, the subject’s retinal vessels were imaged continuously for a total of 350 seconds. This protocol consisted of a 50 second baseline period, followed by three cycles consisting of a flickering light period (light flashes at a 12.5 Hz frequency for 20 seconds) followed by a 80 second rest period (Mandecka et al. 2007; Nguyen et al. 2009; Lott et al. 2012). Blood pressure (BP) and heart rate (HR) were measured continuously during the studies.
Measurements
Retinal Vessel Diameters
The DVA (Imedos Inc., Germany) uses a modified fundus camera (Zeiss FF450, Zeiss Jena, Germany) and a video recording unit. The system visualizes retinal diameters in real time and vessel calibers can be analyzed off line (Garhofer et al. 2010). To obtain images, the subject’s fixation in the fundus camera was adjusted so that the optic nerve head was in the center of the fundus monitor. The fundus cameras’ focus and green background light were adjusted to provide crisp images on the fundus monitor. The region of interest (approximately 1.5 mm in length) was marked over a superior or inferior temporal retinal arteriole and venular between one to two optic disc diameters from the optic nerve disc. Selection criteria for the chosen segments included main vessels (segments > 80 μm), a clear contrast to fundus background, no crossing or bifurcations and avoidance of nearby vessels within one vessel diameter of the chosen segment. This region of interest was scanned at a frequency of 25 times per second and measurements were reported in arbitrary units (AU). Eye-tracking technology in the DVA compensated for small eye movements. Throughout the testing, the subject was verbally encouraged to maintain fixation and to blink frequently. All images were stored on a VHS videotape recorder for off line measurements. One observer analyzed all measurements in a standardized fashion using the DVA software and corrected for any artifacts in the tracings due to spontaneous erroneous measurements.
Hemodynamics (HR and BP)
HR, derived from the electrocardiogram, and BP, were measured continuously by use of a Finameter device (model 2300, Ohmeda, Boulder, CO, confirmed by an automated sphygmomanometer, Dinamap, Critikon, Tampa, Fl), and collected online at 200 Hz.
Plasma bloods
Plasma hs-CRP levels were assayed using radioimmunoassays (Diagnostic Products Corp., Los Angeles, CA). Plasma glucose levels were assayed using a colorimetric method (Ortho Clinical Diagnostics, Auckland, New Zealand) and insulin levels were assayed using a chemiluminescent immunoassay (Siemens Healthcare Diagnostics Inc., Tarrytown, NY).
Data Analysis
For flickering light trials, the resting baseline was averaged from the last 15 seconds prior to each flicker stimulation. Peak vasodilation period was an average of the highest diameters achieved during flicker and within approximately the first 3 seconds after the stimulus ended to capture maximal vasodilation. Percent change in vessel diameter was calculated comparing baseline diameter with the peak diameter using the following equation: percent change in diameter = ((diameterpeak-diameterbaseline)/diameterbaseline) × 100). In addition, we measured maximal vasoconstriction after peak vasodilation (i.e. lowest diameter period was calculated by averaging the three consecutive lowest diameters after peak dilation). Lastly, we calculated the range of diameter change, known as relative amplitude (percent change in relative amplitude = percent change in peak vasodilation + |percent change in vasoconstriction| (Lott et al. 2012). Vessels chosen for analysis were localized in the superior or inferior temporal quadrants. Quantitative insulin sensitivity check index (QUICKI) was calculated to assess insulin sensitivity (IR = 1/[(log insulin) + (log glucose)] (Muniyappa et al. 2008) in all subjects.
Statistical Analysis
From previous published retinal studies on healthy and diabetic populations (Garhofer et al. 2004; Mandecka et al. 2007), power was calculated for a two group comparison (control vs. prediabetic or diabetic groups) for the main outcome measure, a change in retinal diameter. Using a one-way analysis of variance for group comparisons, 18 subjects per group were calculated to yield a power of 98% in order to detect a difference in retinal diameters. Analyses of covariance were used to examine the effects of potential confounding variables (i.e. age, mean arterial blood pressure (MAP), and BMI) (Mandecka et al. 2007; Kneser et al. 2009). Post hoc testing was done with Bonferroni. Repeated measures were done for examining changes in vital signs during flicker between groups. With the groups’ data merged, the correlation between two continuous variables (i.e. changes in diameter to flickering light stimuli and hs-CRP) was assessed using Pearson’s correlation coefficients. Statistical significance was accepted at P<0.05, 95% confidence interval. All data were reported as mean ± SD.
Results
Subject Characteristics
Groups were comparable in weight and BMI (Table 1) but those with prediabetes were slightly older than the controls. The majority of the subjects were Caucasian and women. Individuals had been diagnosed with prediabetes for an average of one year or type 2 diabetes for six years. Several prediabetic individuals were using statin (23%) and antihypertensive therapy (32%) but none used diabetic medications. Most of the subjects with diabetes were treated with oral diabetic medications (84%) and several were on combination therapy with long and/or short acting insulin (28%)(Table 1). Most of the diabetic subjects were using single or multiple anti-hypertensive (80%) and statin (68%) therapy. All medications except beta blockers were held the morning of the study. Diabetic subjects had higher fasting glucose and HbA1c levels and lower insulin sensitivity compared to controls and prediabetic subjects. Diabetic compared to prediabetic subjects had lower total cholesterols and LDL levels. Type 2 diabetic subjects had higher resting HR compared to controls and prediabetic subjects. Lastly, type 2 diabetic subjects had slightly higher resting systolic and diastolic BPs.
Flicker Light-Induced Vasodilation
Resting retinal artery and vein diameters were similar at baseline between all groups (artery: 116 ± 9 AU, 123 ± 20 AU, 118 ± 17 AU and vein: 139 ± 22 AU, 149 ± 19 AU, 148 ± 24 AU in controls, prediabetic and type 2 diabetic subjects, respectively; differences between groups p > 0.05). Flickering light evoked vasodilation in all groups; however peak vein dilator responses were attenuated in the prediabetic and diabetic subjects compared to controls (Figure 1A). Despite no difference in maximal vein vasoconstriction after peak dilation, prediabetic and diabetic subjects compared to controls also had attenuated relative amplitude changes in venular diameter (Figures 1B and C). Similar findings were observed for retinal arterioles (Figures 2A, B, and C). Controlling for the covariates (i.e. age, MAP and BMI) did not alter the findings. There were no significant gender differences in arteriole or venular vasodilator or vasoconstrictor responses to the flickering light stimulus across all groups. Flickering light stimuli did not significantly change resting HR or BP in any of the groups (data not shown).
Figures 1. Prediabetes and type 2 diabetes reduces retinal vein vasodilation to flicker stimulus.
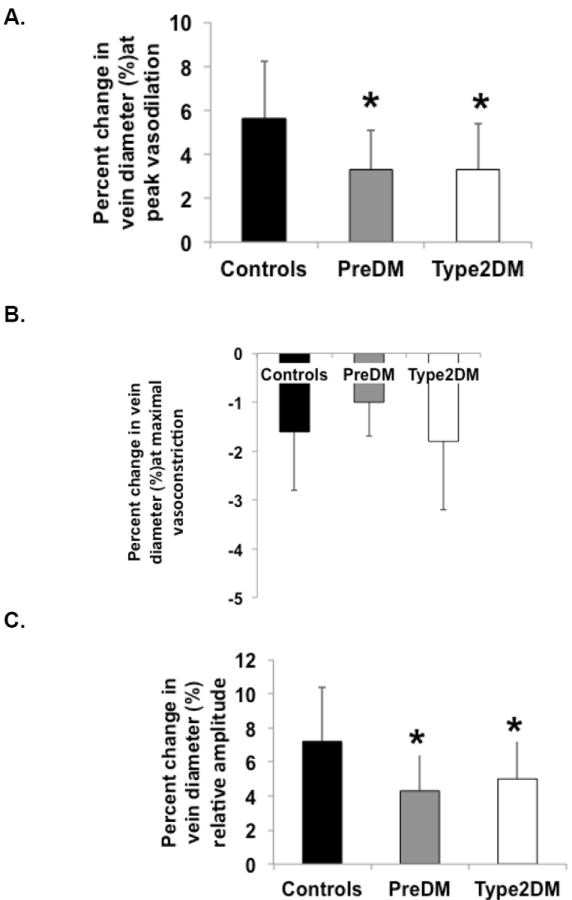
Prediabetic and diabetic subjects compared to healthy controls had attenuated retinal vein vasodilation responses to flicker-induced stimuli (p = .001; Figure 1A). There was no significant difference in vein maximal vasoconstriction after peak vasodilation comparing the different groups (p = .07; Figure 1B); however, the percent change in relative amplitude in dilation showed attenuated responses in prediabetic and diabetic subjects, compared to controls for the vein (p = .003; Figure 1C). There was no difference between groups on time to maximal vein vasoconstrictor responses (data not shown; % = percent, * = significantly different from controls; p < 0.05; prediabetics = PreDM; type 2 diabetics = Type2DM).
Figures 2. Type 2 diabetes reduces retinal artery vasodilation to flicker stimulus.

Type 2 diabetic subjects compared to controls had attenuated retinal peak artery vasodilator response to the flickering light stimulus (p = .015; Figure 2A) with a greater maximal artery vasoconstrictor responses following the peak vasodilation compared to prediabetic subjects (p = .034; Figure 2B). Prediabetic and type 2 diabetic individuals had a trend toward reduced retinal artery amplitude compared to controls (P = .084; Figure 2C). No difference between groups in time to maximal vasoconstrictor responses was observed (data not shown); * = significantly different from controls, p < 0.05; # = significantly different from prediabetic group, p < 0.05; prediabetics = PreDM; type 2 diabetics = Type2DM).
Glucose and Insulin and Blood Pressure Effects on Vasodilation
When the control, prediabetic and diabetic groups were combined, fasting insulin and HbA1c levels were associated with larger resting retinal vein diameters (r = .25, p = .043 and r = .25, p = .046). There was a trend towards an inverse relationship between fasting insulin and venous peak flicker dilation (r = -.23, p = .062) and relative amplitude change in venular diameter (r = -.21, p = .088) and HbA1c and arterial peak flicker dilation (r = -.22, p = .082). Fasting insulin was also significantly correlated to resting heart rate and mean arterial pressure (r = .37 p = .002; r = .25, p = .040, respectively). Insulin sensitivity showed an inverse relationship to BMI (r = -.43, p = .0001), HR (r = -.36, p = .003), MAP (r = -.43, p = .0001), systolic blood pressure (r = -.40, p = .001), and diastolic blood pressure (r = -.37, p = .003). Lastly, systolic blood pressure was correlated to resting vein diameters (r = .25, p = .069) and inversely correlated to venous diameter peak flicker dilation (r = -.30, p = .014) and relative amplitude change in venular diameter (r = -.32, p = .008, respectively). No correlations in retinal reactivity and the subjects’ lipid profiles were detected.
Hs-CRP Levels and Vasodilation
There was no significant difference in plasma hs-CRP levels between groups (Figure 3). When the control, prediabetic and diabetic groups were combined, higher hs-CRP levels were associated with smaller resting retinal artery diameters (r = -.30, p = .022). We observed no significant relationship between hs-CRP levels and the magnitude of the artery or vein dilator responses to flicker (i.e. peak vasodilation or relative amplitude)(Figure 4). However, hs-CRP was significantly associated with BMI (r = 0.37, p = .004).
Figure 3. Hs-CRP levels similar across groups.

There was no significant difference in hs-CRP levels between healthy controls, prediabetic and type 2 diabetic subjects (p = .71).
Figure 4. No correlation between retinal dilation to flicker response and hs-CRP.
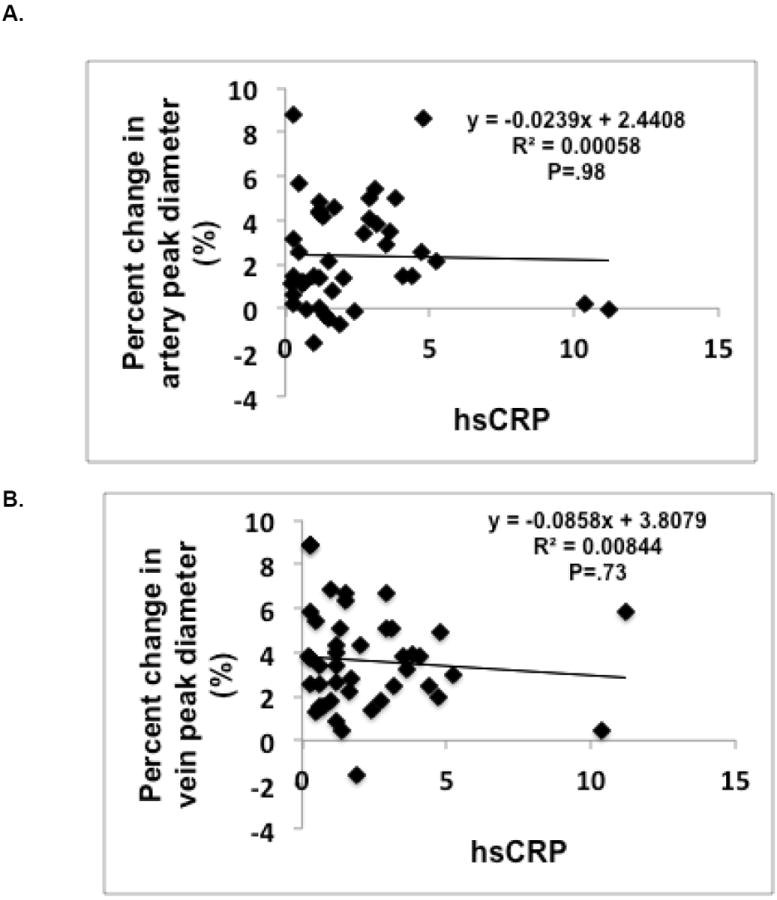
When all groups were combined, there were no significant correlations between hs-CRP and percent change in peak arterial or venular diameter to flicker dilation (p = .98 and p = .73, respectively).
Discussion
In this study, we examined 1) retinal vascular dilation responses to flicker in prediabetic and type 2 diabetic individuals and 2) the relationship between retinal vasodilator responses and an inflammation biomarker (hs-CRP). We found similar attenuated vasodilator responses in individuals with prediabetes and diabetes compared to healthy controls. There was no relationship between flicker induced vasodilation and hs-CRP levels. These data suggest that 1) prediabetic subjects already have an impaired endothelial function early in the disease which is equivalent in magnitude to those found in diabetic subjects and 2) mechanisms other than inflammatory hs-CRP responses may be involved in this early impairment.
Prediabetes, an early stage in the hyperglycemic continuum, is associated with a greater risk of later developing type 2 diabetes (Haffner 2003; Ford et al. 2010; Milman & Crandall 2011) endothelial dysfunction and cardiovascular risk (Ford et al. 2010). In prediabetes and diabetes both elevated glucose and insulin levels can lead to vasodilation of resting blood vessels potentially through an increase in vasodilators such as nitric oxide (Muniyappa & Quon 2007). Our data supports prior retinal studies (Kifley et al. 2008; Sun et al. 2009) in which higher HbA1c and insulin levels were associated with larger retinal venular diameters.
Previous studies examining retinal vessel responses to flickering light stimuli in normal subjects demonstrated robust increases in retinal diameter and blood flow responses (Michelson et al. 2002; Dorner et al. 2003; Nagel & Vilser 2004; Lott et al. 2012), whereas diabetic individuals had an attenuated retinal dilator responses (Garhofer et al. 2004; Mandecka et al. 2007; Bek et al. 2008; Nguyen et al. 2009; Pemp et al. 2009; Lott et al. 2012). In addition, retinal attenuation further increased with the progression of diabetic retinopathy (Mandecka et al. 2007; Tilma & Bek 2012). Retinal vascular dysfunction is proposed to contribute to the pathogenesis of diabetic retinopathy (Schmetterer & Wolzt 1999) but the exact microvascular changes that precede diabetes are not clearly understood (Caballero 2005).
In our study we confirmed an attenuated retinal vein vasodilator response in type 2 diabetics and showed that similar changes are already present in prediabetic individuals (diabetic individuals 3.3% ± 1.8% and prediabetic individuals 3.3% ± 2.1%) compared to healthy controls (5.6% ± 2.6%). Similar findings were found in the retinal arterioles in response to the flickering light stimuli. Since increased age, high blood pressure, and obesity are associated with attenuated flickering light responses (Mandecka et al. 2007; Kneser et al. 2009; Kotliar et al. 2011). we adjusted our analysis for age, blood pressure, and BMI as covariates but the findings did not change with these adjustments. Differences in flicker magnitude of our subject groups compared to prior studies may reflect the use of different flicker light protocols (duration and Hz) as well as the recent change in classification of prediabetes and diabetes (2010). Similar to our results, previous retinal studies have shown that the flicker response was not associated with HbA1c, in type 1 and type 2 diabetic individuals (Garhofer et al. 2004; Mandecka et al. 2007). We also demonstrated that lower insulin sensitivity was associated with an attenuated venous vasodilator response. This is in agreement with previous studies which showed that a lower QUICKI index was associated with type 2 diabetes (Katz et al. 2000) and prediabetes (Festa et al. 2003).
Since it appears that prediabetic subjects may have an increased cardiovascular risk and may develop retinopathy, we now see that individuals with prediabetes also have altered retinal microvascular function. Thus, monitoring retinal reactivity may be an early marker of microvascular disease or endothelial dysfunction that clinicians can follow non-invasively.
Reduced retinal vasodilation in response to flickering light in prediabetes and diabetes may indicate several underlying pathological processes. These include impaired autoregulation and endothelial dysfunction. Vascular abnormalities may cause retinal damage such as pericycte loss which may change the release of local metabolites. Animal and human studies suggest that part of the flickering light vasodilation can be explained by an increase in the production of nitric oxide (Kondo et al. 1997; Dorner et al. 2003). The attenuated flicker response in diabetes has been suggested to be partly due to reduced nitric oxide (Schmetterer et al. 1997). In a recent study, it was shown that retinal vessels in persons with type 1 diabetes have similar responses to exogenous NO as healthy controls (Pemp et al. 2009), implying that the diabetic retinal endothelium is not less sensitive to NO. Thus, other factors may play a role in the altered vasoreactivity observed in prediabetes and diabetes. The attenuated responses in those with prediabetes and diabetes could also be due to the arteries already being in a dilated state to meet metabolic demand. However, since we found no significant difference in resting arteriolar and venular diameters between groups, we do not think that these vessels were predilated in the diabetic or prediabetic subjects. Lastly, impaired vasomotor responses observed in those with prediabetes and diabetes could result from impaired signaling between the neurosensory retina and the vessels. These impaired neurosensory coupling mechanisms may include glial cell or retinal barrier dysfunction and altered vascular endothelium growth factor signaling pathways (Pournaras et al. 2008; Antonetti et al. 2012).
Hs-CRP, a general index of inflammation has been primarily used in large epidemiological studies. Hs-CRP is produced by the liver and rises during inflammatory processes, at least in part due to increased interleukin-6 produced by macrophages and adipocytes. Elevations of hs-CRP have been associated with increased risk of developing diabetes (Doi et al. 2005; Dehghan et al. 2007; Hu et al. 2009) and heart disease (Wilson et al. 2008; Buckley et al. 2009) and risk of a having a myocardial infarction (Ridker 2004). Elevated hs-CRP has also been associated with elevated fasting glucose (Wu et al. 2002; Aronson et al. 2004; Nakanishi et al. 2005) and HbA1c levels (Wu et al. 2002). However, there are mixed findings of elevated hs-CRP and prediabetes (Doi et al. 2005; Sabanayagam & Shankar 2011). In our study, there were no significant differences between groups on hs-CRP. Factors which may have affected these results included the use of statins (Ridker et al. 1999; Tan et al. 2002) and good glycemic control (King et al. 2003) of our diabetic individuals and/or obesity (Meng et al. 2007) in matching our controls to the other groups. In our study, we did see an association between higher hs-CRP levels and smaller resting artery diameters. However, in a large population study of individuals with a range of risk factors for coronary artery disease, elevated hs-CRP levels were associated with wider venules (Klein et al. 2006; Wong et al. 2006). Our study did not find a significant correlation between hs-CRP and vasodilator or constrictor responses to the flicker stimulus across the hyperglycemic continuum. Other studies measuring macrovascular function such as brachial flow mediated vasodilation have also not seen significant associations with hs-CRP (Vita et al. 2004; Kullo et al. 2007; Lippincott et al. 2008). Since hs-CRP is only one of several indices of inflammation, we can’t totally exclude the effects of inflammation on the changes that we observed in the retinal vascular beds of prediabetic and diabetic subjects. Thus, altered retinal vasoreactivity may prove to be a significantly more sensitive indicator of atherosclerosis and vascular risk than plasma hs-CRP.
Limitations of the study
First, it is possible that there were differences in ocular perfusion pressures between groups in which higher ocular perfusion pressures would lead to an attenuated dilator response. Although intraocular pressures were not directly measured at the study visit, all subjects had recent eye exams in which intraocular pressures were in the normal range (<21 mmHg). In addition, our findings did not change when we used blood pressure as a covariate. Second, DVA requires optimal pupil dilation. Some of our prediabetic (27%) and diabetic (52%) subjects required additional dilation with the use of phenylephrine, which theoretically could elevate MAP through increasing sympathetic stimulation; however, our findings were still observed when these individuals were excluded from the analysis. Thus, we do not feel that the use of phenylephrine negatively impacted our study results. Third, we were only able to measure diameters, not blood flow, so it is possible that velocity may be altered differently by the flicker stimulus; however, we could not measure retinal blood velocity. Lastly, our hs-CRP levels were based on only a single measurement. In addition, the lack of group differences in hs-CRP may have been due to our diabetic subjects current statin use (Tan et al. 2002). Since our study was powered for the percent change in retinal diameter (observed power was 81 to 95%), it may be that a larger sample size may be needed to see differences in hs-CRP between groups. This may also help to explain why our study showed only trends for inverse relationships between fasting insulin and vessel diameter responses to flicker.
In summary, we have demonstrated that retinal reactivity is impaired in both prediabetic and diabetic individuals, compared to healthy controls. The inflammatory biomarker, hs-CRP, was not associated with changes in retinal vasoreactivity. Retinal vasoreactivity measurements may therefore be a more sensitive non-invasive indicator of early stages of atherosclerosis than traditional markers of cardiovascular risk such as hs-CRP. Prospective studies may determine if this change in individuals with prediabetes is a harbinger of future cardiovascular disease or retinopathy.
Table 2.
Resting Hemodynamics.
| Subjects | Healthy controls | Prediabetes | Type 2 diabetes | P Value |
|---|---|---|---|---|
| HR (bpm) | 62 ± 7 | 61 ± 9 | 68 ± 9 | .01 |
| MAP (mmHg) | 93 ± 9 | 93 ± 8 | 98 ± 10 | .04 |
| SBP (mmHg) | 121 ± 11 | 128 ± 15 | 133 ± 15* | .03 |
| DBP (mmHg) | 79 ± 8 | 76 ± 7 | 81 ± 8# | .05 |
HR = heart rate; MAP = mean arterial pressure; SBP = systolic blood pressure; DBP = diastolic blood pressure; Mean ± SD
significantly different from controls;
significantly different from prediabetic individuals
Acknowledgments
We would like to thank Surju Patel, Cheryl Blaha and Jessica Mast for their help with the study. We would like to thank the individuals who participated in the studies, as well as the nursing staff of the General Clinical Research Center for technical assistance. Support was provided by a grant from Pennsylvania Tobacco Settlement Funds, UL1 RR033184, and UL TR000127 as well as C06 RR016499. The DVA was partially funded by the Penn State Diabetes and Obesity Institute Equipment Grant and the Pennsylvania Lions Sight Conservation and Eye Research Foundation and the Jack and Nancy Turner Professorship at Penn State supported Dr. Tom W Gardner. Dr. Gardner is currently supported by the A. Alfred Taubman Medical Research Institute at the University of Michigan and a Research to Prevent Blindness Physician-Scientist Award. Lastly, the studies were performed in Penn State’s Clinical Research Center which was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000127.
Footnotes
There are no conflicts of interest.
References
- American Diabetes Association. Standards of medical care in diabetes – 2010. Diabetes Care. 2010;33(Suppl 1):S11–S61. doi: 10.2337/dc10-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. The New England journal of medicine. 2012;366:1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- Aronson D, Bartha P, Zinder O, Kerner A, Shitman E, Markiewicz W, Brook GJ, Levy Y. Association between fasting glucose and C-reactive protein in middle-aged subjects. Diabet Med. 2004;21:39–44. doi: 10.1046/j.1464-5491.2003.01084.x. [DOI] [PubMed] [Google Scholar]
- Bek T, Hajari J, Jeppesen P. Interaction between flicker-induced vasodilatation and pressure autoregulation in early retinopathy of type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2008;246:763–769. doi: 10.1007/s00417-008-0766-y. [DOI] [PubMed] [Google Scholar]
- Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the U.S. Preventive Services Task Force. Ann Intern Med. 2009;151:483–495. doi: 10.7326/0003-4819-151-7-200910060-00009. [DOI] [PubMed] [Google Scholar]
- Caballero AE. Metabolic and vascular abnormalities in subjects at risk for type 2 diabetes: the early start of a dangerous situation. Arch Med Res. 2005;36:241–249. doi: 10.1016/j.arcmed.2005.03.013. [DOI] [PubMed] [Google Scholar]
- D’Souza A, Hussain M, Howarth FC, Woods NM, Bidasee K, Singh J. Pathogenesis and pathophysiology of accelerated atherosclerosis in the diabetic heart. Mol Cell Biochem. 2009;331:89–116. doi: 10.1007/s11010-009-0148-8. [DOI] [PubMed] [Google Scholar]
- Dehghan A, van Hoek M, Sijbrands EJ, Stijnen T, Hofman A, Witteman JC. Risk of type 2 diabetes attributable to C-reactive protein and other risk factors. Diabetes care. 2007;30:2695–2699. doi: 10.2337/dc07-0348. [DOI] [PubMed] [Google Scholar]
- Doi Y, Kiyohara Y, Kubo M, Ninomiya T, Wakugawa Y, Yonemoto K, Iwase M, Iida M. Elevated C-reactive protein is a predictor of the development of diabetes in a general Japanese population: the Hisayama Study. Diabetes care. 2005;28:2497–2500. doi: 10.2337/diacare.28.10.2497. [DOI] [PubMed] [Google Scholar]
- Dorner GT, Garhofer G, Huemer KH, Riva CE, Wolzt M, Schmetterer L. Hyperglycemia affects flicker-induced vasodilation in the retina of healthy subjects. Vision Res. 2003;43:1495–1500. doi: 10.1016/s0042-6989(03)00170-6. [DOI] [PubMed] [Google Scholar]
- Dorner GT, Garhofer G, Kiss B, Polska E, Polak K, Riva CE, Schmetterer L. Nitric oxide regulates retinal vascular tone in humans. Am J Physiol Heart Circ Physiol. 2003;285:H631–H636. doi: 10.1152/ajpheart.00111.2003. [DOI] [PubMed] [Google Scholar]
- Festa A, Hanley AJ, Tracy RP, D’Agostino R, Jr, Haffner SM. Inflammation in the prediabetic state is related to increased insulin resistance rather than decreased insulin secretion. Circulation. 2003;108:1822–1830. doi: 10.1161/01.CIR.0000091339.70120.53. [DOI] [PubMed] [Google Scholar]
- Ford ES, Zhao G, Li C. Pre-diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–1317. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- Garhofer G, Bek T, Boehm AG, Gherghel D, Grunwald J, Jeppesen P, Kergoat H, Kotliar K, Lanzl I, Lovasik JV, Nagel E, Vilser W, Orgul S, Schmetterer L. Use of the retinal vessel analyzer in ocular blood flow research. Acta Ophthalmol. 2010;88:717–722. doi: 10.1111/j.1755-3768.2009.01587.x. [DOI] [PubMed] [Google Scholar]
- Garhofer G, Zawinka C, Resch H, Kothy P, Schmetterer L, Dorner GT. Reduced response of retinal vessel diameters to flicker stimulation in patients with diabetes. Br J Ophthalmol. 2004;88:887–891. doi: 10.1136/bjo.2003.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner SM. Pre-diabetes, insulin resistance, inflammation and CVD risk. Diabetes Res Clin Pract. 2003;61(Suppl 1):S9–S18. doi: 10.1016/s0168-8227(03)00122-0. [DOI] [PubMed] [Google Scholar]
- Hu G, Jousilahti P, Tuomilehto J, Antikainen R, Sundvall J, Salomaa V. Association of serum C-reactive protein level with sex-specific type 2 diabetes risk: a prospective finnish study. J Clin Endocrinol Metab. 2009;94:2099–2105. doi: 10.1210/jc.2008-2260. [DOI] [PubMed] [Google Scholar]
- Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, Quon MJ. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- Kifley A, Wang JJ, Cugati S, Wong TY, Mitchell P. Retinal vascular caliber and the long-term risk of diabetes and impaired fasting glucose: the Blue Mountains Eye Study. Microcirculation. 2008;15:373–377. doi: 10.1080/10739680701812220. [DOI] [PubMed] [Google Scholar]
- King DE, Mainous AG, 3rd, Buchanan TA, Pearson WS. C-reactive protein and glycemic control in adults with diabetes. Diabetes care. 2003;26:1535–1539. doi: 10.2337/diacare.26.5.1535. [DOI] [PubMed] [Google Scholar]
- Klein BE, Klein R, Lee KE, Knudtson MD, Tsai MY. Markers of inflammation, vascular endothelial dysfunction, and age-related cataract. Am J Ophthalmol. 2006;141:116–122. doi: 10.1016/j.ajo.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Kneser M, Kohlmann T, Pokorny J, Tost F. Age related decline of microvascular regulation measured in healthy individuals by retinal dynamic vessel analysis. Med Sci Monit. 2009;15:CR436–CR441. [PubMed] [Google Scholar]
- Kondo M, Wang L, Bill A. The role of nitric oxide in hyperaemic response to flicker in the retina and optic nerve in cats. Acta Ophthalmol Scand. 1997;75:232–235. doi: 10.1111/j.1600-0420.1997.tb00762.x. [DOI] [PubMed] [Google Scholar]
- Kotliar KE, Lanzl IM, Schmidt-Trucksass A, Sitnikova D, Ali M, Blume K, Halle M, Hanssen H. Dynamic retinal vessel response to flicker in obesity: A methodological approach. Microvasc Res. 2011;81:123–128. doi: 10.1016/j.mvr.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Kullo IJ, Malik AR, Santos S, Ehrsam JE, Turner ST. Association of cardiovascular risk factors with microvascular and conduit artery function in hypertensive subjects. Am J Hypertens. 2007;20:735–742. doi: 10.1016/j.amjhyper.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Lippincott MF, Carlow A, Desai A, Blum A, Rodrigo M, Patibandla S, Zalos G, Smith K, Schenke WH, Csako G, Waclawiw MA, Cannon RO., 3rd Relation of endothelial function to cardiovascular risk in women with sedentary occupations and without known cardiovascular disease. Am J Cardiol. 2008;102:348–352. doi: 10.1016/j.amjcard.2008.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott ME, Slocomb JE, Shivkumar V, Smith B, Gabbay RA, Quillen D, Gardner TW, Bettermann K. Comparison of retinal vasodilator and constrictor responses in type 2 diabetes. Acta Ophthalmol. 2012;90:e434–e441. doi: 10.1111/j.1755-3768.2012.02445.x. [DOI] [PubMed] [Google Scholar]
- Mandecka A, Dawczynski J, Blum M, Muller N, Kloos C, Wolf G, Vilser W, Hoyer H, Muller UA. Influence of flickering light on the retinal vessels in diabetic patients. Diabetes care. 2007;30:3048–3052. doi: 10.2337/dc07-0927. [DOI] [PubMed] [Google Scholar]
- Mazzone T, Chait A, Plutzky J. Cardiovascular disease risk in type 2 diabetes mellitus: insights from mechanistic studies. Lancet. 2008;371:1800–1809. doi: 10.1016/S0140-6736(08)60768-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng YX, Ford ES, Li C, Quarshie A, Al-Mahmoud AM, Giles W, Gibbons GH, Strayhorn G. Association of C-reactive protein with surrogate measures of insulin resistance among nondiabetic US from National Health and Nutrition Examination Survey 1999-2002. Clin Chem. 2007;53:2152–2159. doi: 10.1373/clinchem.2007.088930. [DOI] [PubMed] [Google Scholar]
- Michelson G, Patzelt A, Harazny J. Flickering light increases retinal blood flow. Retina. 2002;22:336–343. doi: 10.1097/00006982-200206000-00013. [DOI] [PubMed] [Google Scholar]
- Milman S, Crandall JP. Mechanisms of vascular complications in prediabetes. Med Clin North Am. 2011;95:309–325. vii. doi: 10.1016/j.mcna.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- Muniyappa R, Quon MJ. Insulin action and insulin resistance in vascular endothelium. Curr Opin Clin Nutr Metab Care. 2007;10:523–530. doi: 10.1097/MCO.0b013e32819f8ecd. [DOI] [PubMed] [Google Scholar]
- Nagel E, Vilser W. Flicker observation light induces diameter response in retinal arterioles: a clinical methodological study. Br J Ophthalmol. 2004;88:54–56. doi: 10.1136/bjo.88.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi N, Shiraishi T, Wada M. Association between C-reactive protein and insulin resistance in a Japanese population: the Minoh Study. Intern Med. 2005;44:542–547. doi: 10.2169/internalmedicine.44.542. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Kawasaki R, Wang JJ, Kreis AJ, Shaw J, Vilser W, Wong TY. Flicker light-induced retinal vasodilation in diabetes and diabetic retinopathy. Diabetes care. 2009;32:2075–2080. doi: 10.2337/dc09-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemp B, Garhofer G, Weigert G, Karl K, Resch H, Wolzt M, Schmetterer L. Reduced retinal vessel response to flicker stimulation but not to exogenous nitric oxide in type 1 diabetes. Invest Ophthalmol Vis Sci. 2009;50:4029–4032. doi: 10.1167/iovs.08-3260. [DOI] [PubMed] [Google Scholar]
- Pemp B, Weigert G, Karl K, Petzl U, Wolzt M, Schmetterer L, Garhofer G. Correlation of flicker-induced and flow-mediated vasodilatation in patients with endothelial dysfunction and healthy volunteers. Diabetes care. 2009;32:1536–1541. doi: 10.2337/dc08-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27:284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Ridker PM. High-sensitivity C-reactive protein, inflammation, and cardiovascular risk: from concept to clinical practice to clinical benefit. Am Heart J. 2004;148:S19–S26. doi: 10.1016/j.ahj.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Pfeffer MA, Sacks F, Braunwald E. Long-term effects of pravastatin on plasma concentration of C-reactive protein. The Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1999;100:230–235. doi: 10.1161/01.cir.100.3.230. [DOI] [PubMed] [Google Scholar]
- Sabanayagam C, Shankar A. Association between plasma homocysteine and microalbuminuria in persons without hypertension, diabetes mellitus, and cardiovascular disease. Clin Exp Nephrol. 2011;15:92–99. doi: 10.1007/s10157-010-0361-5. [DOI] [PubMed] [Google Scholar]
- Schmetterer L, Findl O, Fasching P, Ferber W, Strenn K, Breiteneder H, Adam H, Eichler HG, Wolzt M. Nitric oxide and ocular blood flow in patients with IDDM. Diabetes. 1997;46:653–658. doi: 10.2337/diab.46.4.653. [DOI] [PubMed] [Google Scholar]
- Schmetterer L, Wolzt M. Ocular blood flow and associated functional deviations in diabetic retinopathy. Diabetologia. 1999;42:387–405. doi: 10.1007/s001250051171. [DOI] [PubMed] [Google Scholar]
- Sun C, Wang JJ, Mackey DA, Wong TY. Retinal vascular caliber: systemic, environmental, and genetic associations. Surv Ophthalmol. 2009;54:74–95. doi: 10.1016/j.survophthal.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Tan KC, Chow WS, Tam SC, Ai VH, Lam CH, Lam KS. Atorvastatin lowers C-reactive protein and improves endothelium-dependent vasodilation in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2002;87:563–568. doi: 10.1210/jcem.87.2.8249. [DOI] [PubMed] [Google Scholar]
- Terai N, Spoerl E, Pillunat LE, Stodtmeister R. The effect of caffeine on retinal vessel diameter in young healthy subjects. Acta Ophthalmol. 2012;90:e524–e528. doi: 10.1111/j.1755-3768.2012.02486.x. [DOI] [PubMed] [Google Scholar]
- Thomsen SB, Rathcke CN, Zerahn B, Vestergaard H. Increased levels of the calcification marker matrix Gla Protein and the inflammatory markers YKL-40 and CRP in patients with type 2 diabetes and ischemic heart disease. Cardiovasc Diabetol. 2010;9:86. doi: 10.1186/1475-2840-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilma KK, Bek T. Topical treatment for 1 week with latanoprost but not diclofenac reduces the diameter of dilated retinal arterioles in patients with type 1 diabetes mellitus and mild retinopathy. Acta Ophthalmol. 2012;90:750–755. doi: 10.1111/j.1755-3768.2011.02185.x. [DOI] [PubMed] [Google Scholar]
- Toda N, Imamura T, Okamura T. Alteration of nitric oxide-mediated blood flow regulation in diabetes mellitus. Pharmacol Ther. 2010;127:189–209. doi: 10.1016/j.pharmthera.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Vita JA, Keaney JF, Jr, Larson MG, Keyes MJ, Massaro JM, Lipinska I, Lehman BT, Fan S, Osypiuk E, Wilson PW, Vasan RS, Mitchell GF, Benjamin EJ. Brachial artery vasodilator function and systemic inflammation in the Framingham Offspring Study. Circulation. 2004;110:3604–3609. doi: 10.1161/01.CIR.0000148821.97162.5E. [DOI] [PubMed] [Google Scholar]
- Wilson PW, Pencina M, Jacques P, Selhub J, D’Agostino R, Sr, O’Donnell CJ. C-reactive protein and reclassification of cardiovascular risk in the Framingham Heart Study. Circ Cardiovasc Qual Outcomes. 2008;1:92–97. doi: 10.1161/CIRCOUTCOMES.108.831198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Islam FM, Klein R, Klein BE, Cotch MF, Castro C, Sharrett AR, Shahar E. Retinal vascular caliber, cardiovascular risk factors, and inflammation: the multi-ethnic study of atherosclerosis (MESA) Invest Ophthalmol Vis Sci. 2006;47:2341–2350. doi: 10.1167/iovs.05-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Dorn JP, Donahue RP, Sempos CT, Trevisan M. Associations of serum C-reactive protein with fasting insulin, glucose, and glycosylated hemoglobin: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2002;155:65–71. doi: 10.1093/aje/155.1.65. [DOI] [PubMed] [Google Scholar]


