Abstract
Piperlongumine (PPLGM) is a bioactive compound isolated from long peppers that shows selective toxicity towards a variety of cancer cell types including colon cancer. The signaling pathways that lead to cancer cell death in response to PPLGM exposure have not been previously identified. Our objective was to identify the intracellular signaling mechanisms by which PPLGM leads to enhanced colon cancer cell death. We found that PPLGM inhibited the growth of colon cancer cells in time- and concentration-dependent manners, but was not toxic toward normal colon mucosal cells at concentrations below 10 μM. Acute (0-60 minutes) and prolonged (24 hours) exposure of HT-29 cells to PPLGM resulted in phosphorylation of ERK. To investigate whether ERK signaling was involved in PPLGM-mediated cell death, we treated HT-29 cells with the MEK inhibitor U0126, prior to treating with PPLGM. We found that U0126 attenuated PPLGM-induced activation of ERK and partially protected against PPLGM-induced cell death. These results suggest that PPLGM works, at least in part, through the MEK/ERK pathway to result in colon cancer cell death. A more thorough understanding of the molecular mechanisms by which PPLGM induces colon cancer cell death will be useful in developing therapeutic strategies to treat colon cancer.
Keywords: piperlongumine, colon cancer, MEK/ERK, cell signaling, apoptosis
Introduction
Bioactive food components extracted from botanicals are effective chemopreventive and therapeutic agents for various human cancers (Greenwald et al., 2002). Specifically, extracts from Piper (pepper) plants have been used for medicinal purposes for centuries and show numerous pharmacological activities such as anti-cancer (Bezerra et al., 2005), antiinflammatory (Bang et al., 2009), anti-platelet (Fontenele et al., 2009), anti-depressant (Lee et al., 2005b), analgesic (Vedhanayaki et al., 2003), and cardioprotective effects (Wakade et al., 2008). A primary constituent of Piper longum (long pepper) is piperlongumine (PPLGM; aka piplartine), an alkaloid which is found not only in the fruits but also the roots of the plant (Park et al., 2007).
PPLGM displays potent growth-inhibitory properties in a cancer cell-selective manner in a variety of cancer cell lines including colon cancer (Raj et al., 2011). A proposed mechanism by which PPLGM induces cancer-selective cell death is through the elevation of reactive oxygen species (ROS). Relative to healthy cells, cancer cells have heightened levels of ROS and a highly active antioxidant defense system (Szatrowski and Nathan, 1991). As a result, cancer cells, but not healthy cells, are unable to recover from additional oxidative stress and die (Schumacker, 2006; Trachootham et al., 2009). PPLGM directly binds to and inhibits the antioxidant enzyme glutathione S-transferase pi 1 (GSTP1) resulting in elevated levels of ROS and subsequent cancer-selective cell death (Raj et al., 2011). However, little is known about the signaling mechanisms by which PPLGM causes cell death in cancer cells, particularly colon cancer.
The extracellular signal-regulated kinase pathway mediates signal transduction involved in cell proliferation, differentiation, and migration (Dhillon et al., 2007). Paradoxically, a growing number of studies have shown that activation of ERK by chemopreventive compounds (i.e. resveratrol and quercetin) results in anti-proliferative effects such as apoptosis, senescence, or autophagy in cancer cells (Aggarwal and Shishodia, 2006; Kim et al., 2008; She et al., 2001; Shih et al., 2002). Once activated, ERK can activate apoptotic enzymes or phosphorylate transcription factors that regulate the expression of pro-apoptotic genes (Cagnol and Chambard, 2010). Previous studies have shown that cell death in PPLGM-treated cells was accompanied by increased ROS levels, increased p53 and PUMA expression, decreased Bcl-2 expression, depolarization of the mitochondrial membrane, cleaved caspase-3, and DNA fragmentation (Kong et al., 2008; Raj et al., 2011). The association of chemopreventive compounds with enhanced ERK signaling and the fact that elevated levels of ROS can activate ERK signaling led us to hypothesize that PPLGM induces apoptosis in colon cancer cells via activation of the MEK/ERK pathway.
In the present study, we investigated the effect of PPLGM on MEK/ERK signal transduction activity in HT-29 colon cancer cells. Further, we identified the contribution of the MEK/ERK pathway in PPLGM-mediated colon cancer cell death. Colorectal cancer is a leading cause of cancer-related deaths in the US. A better understanding of the signal transduction pathways used by PPLGM to induce colon cancer cell death will provide a stronger rationale for the use of PPLGM as an adjuvant therapy for colon cancer treatment.
Materials and Methods
Materials
Piperlongumine (PPLGM) was purchased from Indofine Chemical Company (Catalog #: P-004, 97%, Hillsborough, NJ). PPLGM was dissolved in 100% DMSO at a stock concentration of 10 mM and then diluted in water for experiments. The final concentration of PPLGM was in the range of 1-40 μM with a maximum DMSO concentration of 0.2%. In all experiments, DMSO was added to control cells at a final concentration of 0.2% in culture medium. Antibodies (total and phospho-ERK, total and cleaved caspase-3, and GAPDH) and the MEK inhibitor U0126 were purchased from Cell Signaling Technologies (Danvers, MA). The anti-rabbit secondary antibody was purchased from Jackson Immuno Research (West Grove, PA).
Cell culture
HT-29 and HCT 116 cell lines were obtained from ATCC (Manassas, VA) and cultured at 37°C with 5% carbon dioxide in McCoy's 5a (Thermo Scientific; Waltham, MA) medium supplemented with 10% fetal bovine serum (Atlanta Biologicals; Lawrenceville, GA). NCM460, a normal human colon mucosal epithelial cell line was obtained from INCELL Corporation (San Antonio, TX) and grown in M3 medium (INCELL) supplemented with 10% FBS and 1% penicillin streptomycin (Thermo Fisher Scientific, Waltham, MA). Cell lines were subcultured by enzymatic digestion with 0.25% trypsin / 1 mM EDTA solution (Thermo Fisher) when they reached approximately 70% confluency. Both floating and attached cells were harvested in the case of NCM460 for subsequent analysis.
AlamarBlue® cell toxicity assay
HT-29, HCT 116, and NCM460 cells (5 ×103) were seeded into individuals wells of a 96-well plate, and 24 hours later were treated with PPLGM (0-40 μM) after which alamarBlue® (AbD Serotech; Raleigh, North Carolina) was added at a final concentration of 10% and incubated at 37°C for 4 hours. The oxidized form of the dye is converted into the reduced form by a mitochondrial enzyme in the viable cells. Absorbance was measured at 570 and 600 nm on a plate reader and the cell viability was calculated by plotting percent reduction in alamarBlue® over time for each treatment. The cells were monitored daily over a 3-day period to gauge a shift in absorbance. The data represent the average percent reduction of alamarBlue® +/- standard deviation in eight replicate wells per treatment for three independent experiments. A reduction in alamarBlue® absorbance correlates to a decrease in cell viability. The half maximal inhibitory concentrations (IC50s) were calculated by fitting the dose-response curves derived after plotting the percentage reduction of alamarBlue® against the log concentration.
Trypan blue exclusion assay
HT-29 cells in logarithmic growth phase were seeded at 8 ×103/ml into each well of a 48-well plate, and were treated with different concentrations of PPLGM (0-40 μM). Cells were collected each day after 24, 48, and 72 hours, and counted under a light microscope after trypan blue exclusion. There were six replicates for each concentration and the experiment was performed three times.
Apoptosis assay by acridine orange/ethidium bromide staining
HT-29 cells were seeded in a 6-well plate and treated with either DMSO alone or increasing concentrations of PPLGM (2.5 – 40 μM) for 24 h. Alternatively, HT-29 cells were pretreated with 10 μM U0126 for 2 h followed by 15 μM PPLGM for 24 h. The next day, the cells were washed with PBS and stained with 100 μg/ml acridine orange and 100 μg/ml ethidium bromide in PBS just prior to microscopy. The cells were viewed using a Zeiss inverted Axio Observer Z1 microscope with excitation at 480 nm and emission at 535 nm. For quantification of cell viability, the total number of viable cells was determined for eight replicate microscopic views for each of three independent experiments. A minimum of 200 cells were counted for each replicate well in each independent experiment. The average numbers of viable cells in the treatment groups were then expressed as percentages of the control.
SDS-PAGE and western blotting
Cell pellets were lysed using an SDS lysis buffer (Cell Signaling Technologies) containing protease and phosphatase inhibitors (Roche; Indianapolis, IN). Samples were briefly sonicated to dissociate cell membranes. Fifty μg of total protein isolated from PPLGM or U0126-treated HT-29 cells were separated on 10% SDS-polyacrylamide gels at 100 V for 1 h. Proteins were transferred to nitrocellulose membranes at 100 V for 75 min at 4 °C. Blots were then probed overnight at 4°C with primary antibodies. The next day, blots were rinsed with 1× TBS-tween (0.1%) and probed with anti-rabbit secondary antibody for 1 h at room temperature. The western blots were analyzed using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific; Rockford, IL) and images were captured using the MultiImage™ Light Cabinet (Alpha Innotech; San Leandro, CA). Phospho-ERK levels were normalized to total ERK or GAPDH expression. Immunoblots were performed in triplicate and the images in the figures represent one typical replicate.
Statistical analyses
Data are presented as means ± standard deviation for at least 3 independent experiments. The significance of differences between groups was determined using a student's t-test with statistical significance defined as p<0.05. Statistical analyses were performed using SigmaPlot v12.
Results
PPLGM causes concentration and time-dependent growth inhibition of HT-29 and HCT 116 cells and is less toxic toward NCM460 normal colon mucosal cells
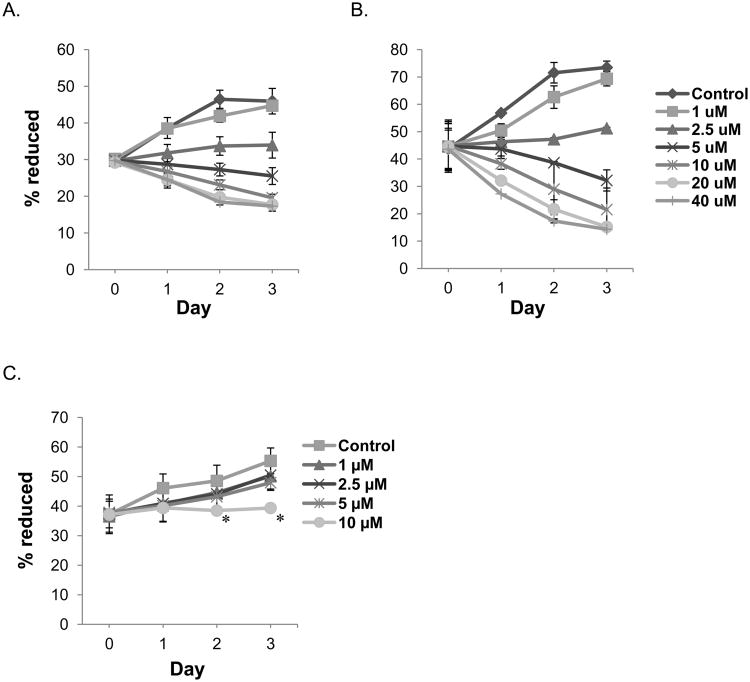
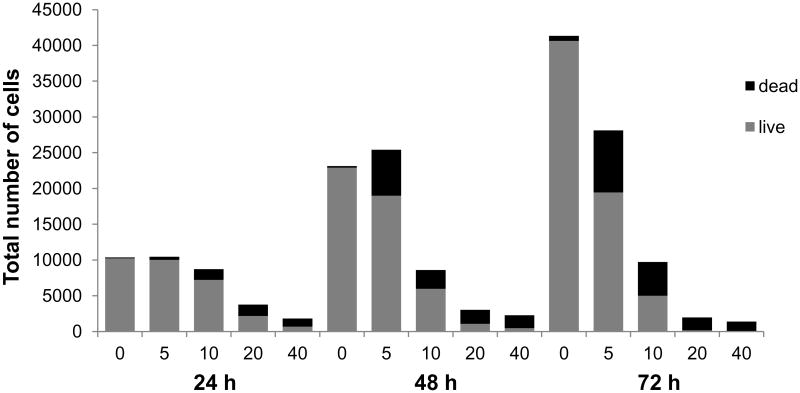
We examined the growth inhibitory effect of PPLGM on HT-29 and HCT 116 colon cancer cells and NCM460 normal colon mucosal cells using the alamarBlue® assay. Exponentially growing HT-29 and HCT 116 cells were treated with either the vehicle control (DMSO) or increasing concentrations of PPLGM (1 – 40 μM) over a course of 3 days and cell growth was assessed each day. PPLGM induced a concentration and time-dependent decrease in the viability of both HT-29 and HCT 116 cells, with an IC50 value of 10.1 μM in HT-29 and 6.4 μM in HCT 116 cells at 48 hours (Figures 1A and B), but only inhibited the growth of NCM460 normal colon mucosal cells at concentrations of 10 μM after 48 hours (Figure 1C). Moreover, total cell counts determined by trypan blue exclusion assay in HT-29 cells treated with different concentration of PPLGM (5 – 40 μM) for 24, 48 and 72 hours demonstrated that PPLGM is inhibiting the proliferation of and inducing cell death in HT-29 cells (Figure 2).
Figure 1.
Growth curve showing the concentration and time-dependent effects of PPLGM on the viability of colon cancer and normal colon mucosal cells. The growth of A) HT-29 and B) HCT 116 colon cancer cells and C) NCM460 normal colon mucosal cells was assessed by the percentage of alamarBlue® reduction in the presence of PPLGM (0-40 μM) over the course of 3 days. Data represent the average ± standard deviation of eight replicate wells for three independent experiments for HT-29, HCT 116, and NCM460 cells. PPLGM (2.5-40 μM) significantly inhibited the growth of the colon cancer cells at all time points; whereas, PPLGM (10 μM) inhibited the growth of NCM460 cells after 48 hours of treatment, where * denotes statistical significance (p<0.05).
Figure 2.
Total cell count of HT-29 cells showing the number of live and dead cells assessed by the trypan blue exclusion assay upon treatment of PPLGM (0-40 μM) for 24, 48, and 72 hours. The bar graph shows average cell counts of six replicate wells for three independent experiments.
PPLGM induces apoptosis in HT-29 colon cancer cells
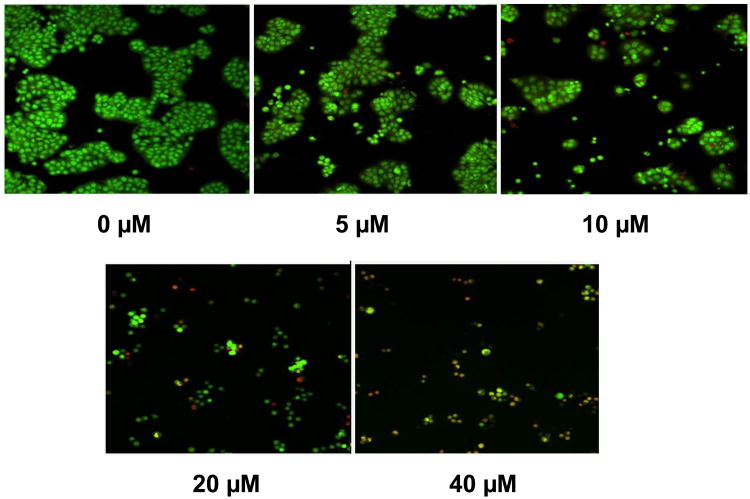
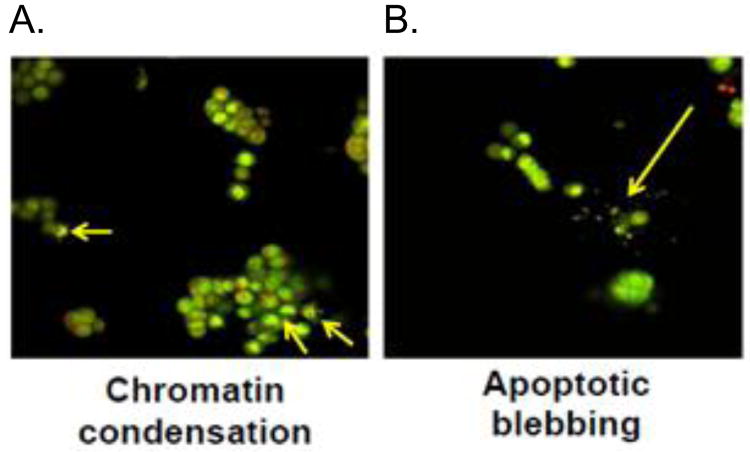
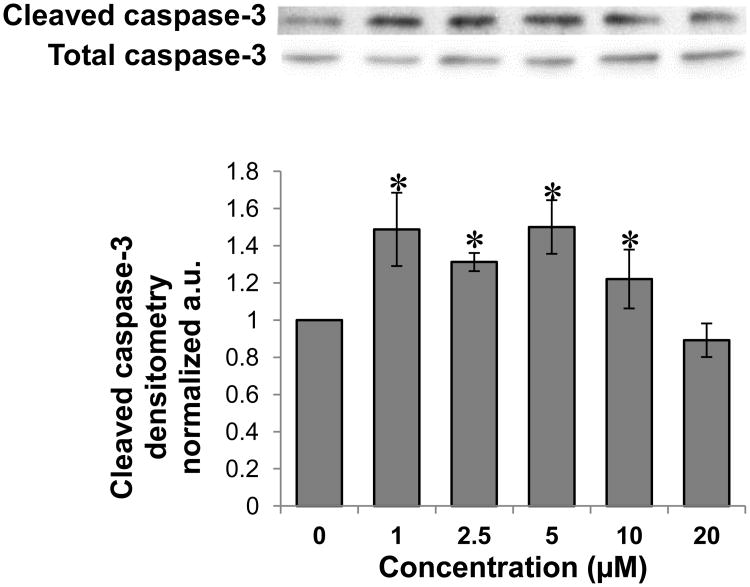
To determine whether the inhibition of colon cancer cell proliferation by PPLGM was due to the induction of apoptosis or necrosis, we assessed cell death using the acridine orange/ ethidium bromide method. HT-29 cells were treated with DMSO alone or different concentrations of PPLGM (5 - 40 μM) for 24 hours and stained using acridine orange and ethidium bromide. Figure 3 shows a decrease in the number of cells upon treatment with increasing concentrations of PPLGM. Live cells were uniformly green whereas apoptotic cells were characterized by chromatin condensation, loss of membrane integrity, and apoptotic blebbing (Figure 4). In addition, lower concentrations of PPLGM (1 - 10 μM) enhanced the expression of cleaved caspase 3 (Figure 5).
Figure 3.
Acridine orange/ethidium bromide staining of HT-29 cells treated with increasing concentrations of PPLGM. PPLGM treatment (0-40 μM) of HT-29 cells for 24 hours resulted in concentration-dependent cell death as assayed by acridine orange/ethidium bromide staining. Three independent experiments were performed, and the image shown here is representative of one of these experiments.
Figure 4.
Acridine orange/ethidium bromide staining of HT-29 cells treated with PPLGM reveals characteristics of apoptotic cell death. PPLGM treatment (10 μM for 24 hours) induced apoptosis as observed by A) chromatin condensation and B) apoptotic blebbing in HT-29 cells assayed using acridine orange/ethidium bromide. Three independent experiments were performed, and the image shown here is representative of one of these experiments.
Figure 5.
Western blot analysis and quantitative densitometry of cleaved caspase-3 expression in HT-29 cells treated with various concentrations of PPLGM. A) Treatment (24 hours) of HT-29 cells with PPLGM (0-20 μM) resulted in enhanced cleaved caspase-3 expression. B) Densitometry shows the results of PPLGM treatment on cleaved caspase-3 expression normalized to total caspase-3 in three replicate experiments ± standard deviation, where * denotes statistical significance (p<0.05).
PPLGM induces phosphorylation of ERK in time- and concentration-dependent manners
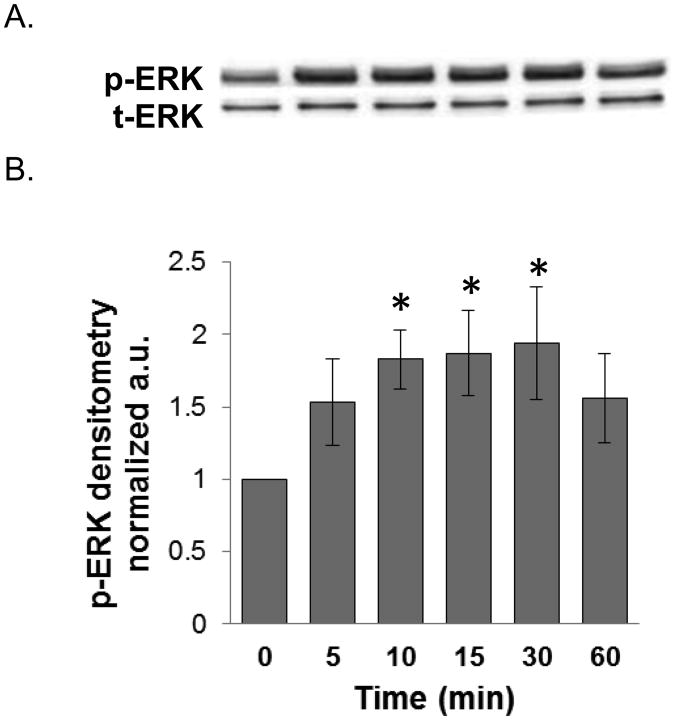
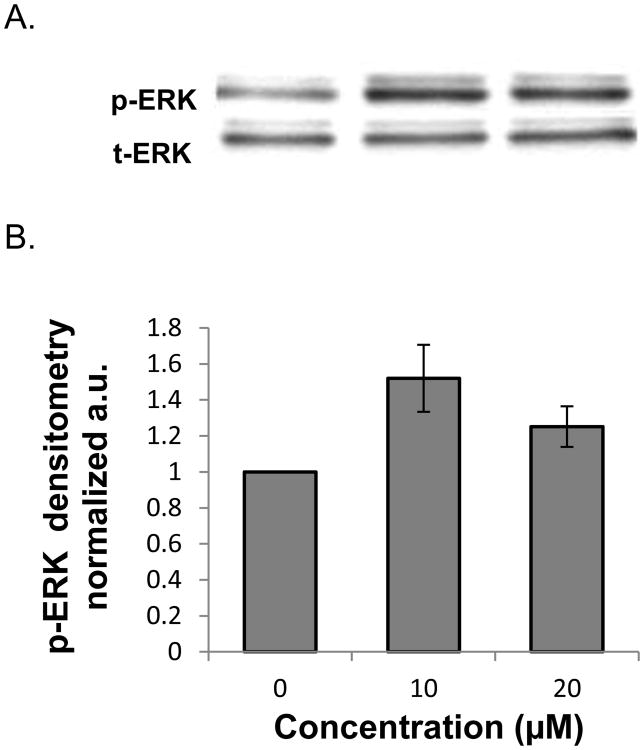
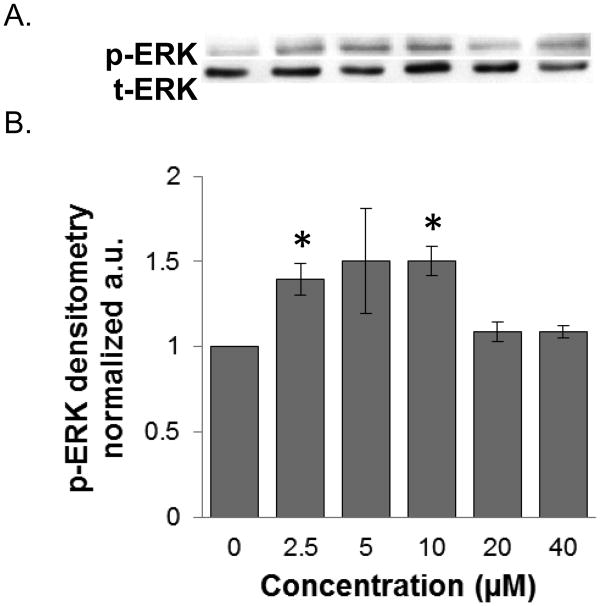
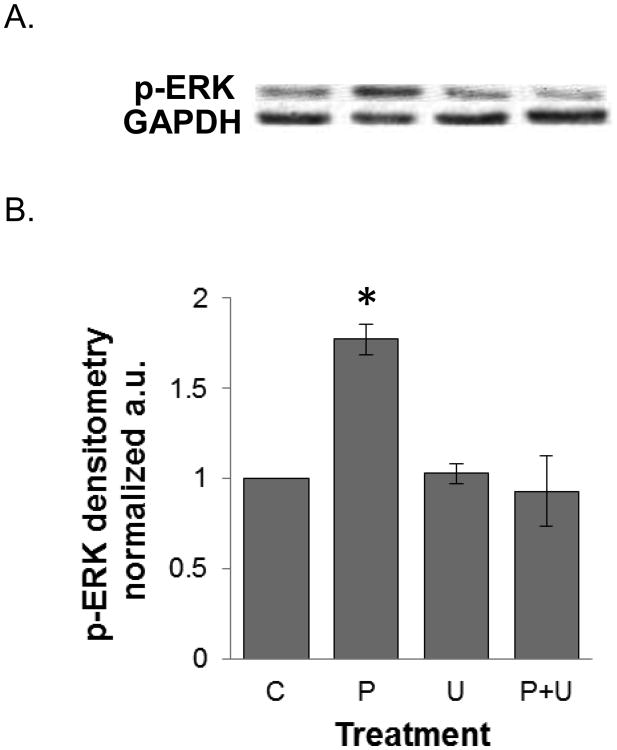
We investigated the signaling mechanisms by which PPLGM induced colon cancer cell death. We found that 10 μM PPLGM results in a rapid (5-60 min) activation of phosphorylated ERK (p-ERK) in HT-29 cells (Figure 6) that was sustained for at least 24 h (Figure 7). Maximal increases in p-ERK expression were observed with the lower concentrations (2.5 - 10 μM) of PPLGM; whereas, higher concentrations of PPLGM (20 - 40 μM) produced only slightly elevated levels of p-ERK (Figure 8). To further investigate the signaling mechanisms mediated by PPLGM, we pre-treated HT-29 cells with 10 μM of the MEK inhibitor U0126 for 1 hour followed by 10 μM PPLGM for 15 min. MEK acts upstream of ERK to lead to its activation. An increase in p-ERK protein expression was observed upon treatment of HT-29 cells with PPLGM alone, when compared to the vehicle-treated control cells. Pre-treatment with U0126 followed by PPLGM treatment abrogated this effect (Figure 9).
Figure 6.
Western blot analysis and quantitative densitometry of phosphorylated ERK expression in HT-29 cells treated with PPLGM for various durations. A) Short-term treatment (5-60 minutes) of HT-29 cells with PPLGM (10 μM) resulted in enhanced phosphorylated ERK (p-ERK) expression, but no effect on total ERK (t-ERK) expression. B) Densitometry shows the results of PPLGM treatment on p-ERK expression normalized to t-ERK in three replicate experiments ± standard deviation, where * denotes statistical significance (p<0.05).
Figure 7.
Western blot analysis and quantitative densitometry of phosphorylated ERK expression in HT-29 cells treated with varying concentrations of PPLGM. A) Treatment (24 hours) of HT-29 cells with PPLGM (10 and 20 μM) resulted in enhanced phosphorylated ERK (p-ERK) expression. B) Densitometry shows the results of PPLGM treatment on p-ERK expression normalized to t-ERK in three replicate experiments ± standard deviation.
Figure 8.
Western blot analysis and quantitative densitometry of phosphorylated ERK expression in HT-29 cells treated with varying concentrations of PPLGM. A) Treatment (10 minutes) of HT-29 cells with PPLGM (0-40 μM) resulted in enhanced phosphorylated ERK (p-ERK) expression. B) Densitometry shows the results of PPLGM treatment on p-ERK expression normalized to t-ERK in three replicate experiments ± standard deviation, where * denotes statistical significance (p<0.05).
Figure 9.
Western blot analysis and quantitative densitometry of phosphorylated ERK expression in HT-29 cells treated with PPLGM ± a MEK inhibitor. HT-29 cells were treated with the vehicle (C), 10 μM PPLGM alone for 15 minutes (P), 10 μM U0126 alone for 1 hour (U), or pre-treated with 10 μM U0216 for 1 hour followed by treatment with 10 μM PPLGM for 15 minutes (P+U). A) The western blot shows that PPLGM alone enhanced p-ERK expression while U0126 abrogated this effect. B) Densitometry shows the results of PPLGM and/or U0126 treatment on p-ERK expression normalized to GAPDH in three replicate experiments ± standard deviation, where * denotes statistical significance (p<0.05).
Inhibition of ERK signaling by U0126 attenuates cell death induced by PPLGM
Based upon our independent observations that PPLGM induces apoptosis and PPLGM activates ERK signaling in colon cancer cells, we next investigated the involvement of ERK signaling in PPLGM-induced apoptosis in HT-29 cells. Two hour treatment of HT-29 cells with the MEK inhibitor U0126 (10 μM) alone did not influence the average percentage of cell viability. Conversely, as shown in previous figures, PPLGM was toxic to HT-29 cells and reduced their viability by over 60%. The toxic effects of PPLGM were partially blocked by pre-treating cells with U0126. U0126 + PPLGM treatment resulted in a 25% increase in cell viability compared to PPLGM treatment alone (Table 1).
Table 1.
Average percent of viable HT-29 cells relative to the vehicle-treated control following treatment with 15 μM PPLGM for 24 hours and/or pre-treatment with 10 μM U0126 for 2 hours.
Letters denote statistically distinct groups and
p<0.05,
p<0.01.
Discussion
The natural compound piperlongumine (PPLGM) displays potent growth-inhibitory effects in many different types of cancers (Bezerra et al., 2006; Bezerra et al., 2007; Raj et al., 2011). However, the intracellular signaling mechanism involved in PPLGM-mediated cancer cell death has never been evaluated and, thus, was the focus of this study. Here we show that PPLGM is toxic to both HT-29 and HCT 116 colon cancer cells, and less toxic to NCM460 normal colon cells. Further, we showed that PPLGM induced apoptosis in HT-29 cells as evidenced by apoptotic blebbing, chromatin condensation, and caspase-3 cleavage. PPLGM enhanced ERK phosphorylation in time- and concentration-dependent manners. PPLGM-induced ERK signaling and cell death were reduced in cells pre-treated with a MEK inhibitor. We suggest that activation of ERK signaling plays a role in PPLGM-induced apoptosis in HT-29 cells. Further studies using RNAi to knock-down MEK or ERK are needed to confirm the results shown here.
The selective anti-cancer effects of PPLGM have been recently reported (Raj et al., 2011). PPLGM, as high as 15 μM, did not cause toxic effects in six different noncancerous cell types; whereas, in thirteen cancer cell lines, PPLGM showed an average IC50 of 7 μM (Raj et al., 2011). Similarly, our data show that PPLGM is toxic toward HT-29 and HCT 116 colon cancer cells (IC50s of 6.4 and 10.1 μM, respectively) and only slightly inhibits the growth of NCM460 normal colon mucosal cells at concentrations 10 μM and below (Figure 1). The mechanisms by which PPLGM causes cancer-selective cell death have been explored and found to be dependent upon elevation of reactive oxygen species (ROS).
ROS are essential for normal cell function where they play important roles in regulating signal transduction events, enzyme activity, and cytokine production (Valko et al., 2007a). However, deregulation of redox homeostasis can cause irreparable cell damage and is associated with cancer initiation, promotion, and therapy resistance (Behrend et al., 2003; Valko et al., 2007b; Wu, 2006). Cancer cells become more dependent on elevated ROS levels and a highly functional anti-oxidant system than healthy cells, and as a consequence, are more vulnerable to agents that impair antioxidant capacity or induce further oxidative stress levels (Pelicano et al., 2004). Recent studies have revealed that increasing reactive species above a certain threshold can induce cancer cell death (Fruehauf and Meyskens, 2007). Many natural products, including sulforaphane (SFN), curcumin (CUR), phenethyl isothiocyanate (PEITC), and PPLGM increase ROS in cancer cells leading to cell cycle arrest or cell death_(Jutooru et al., 2010; Naumann et al., 2011; Pham et al., 2004; Qanungo et al., 2005; Raj et al., 2011). The presence of elevated ROS can lead to sustained ERK activity resulting in cell death (Kong et al., 2008; Son, 2011). Indeed, we found that PPLGM treatment of colon cancer cells induced ERK signaling (Figures 6 and 8) that was sustained for at least 24 hours (Figure 7), at which point cell death was noted (Figure 2 and 3).
A cascade of signaling events initiated by Ras small GTPases can lead to ERK activation and its associated cellular responses such as enhanced proliferation. Ras proteins phosphorylate and activate downstream effectors such as B-raf which, in turn, phosphorylate MEK1/2 leading to activation of ERK1/2 (Roberts and Der, 2007). Mutations in KRAS, one form of RAS, are detected in approximately 50% of colorectal cancers (Vogelstein et al., 1988) and are thought to play an important role in disease progression and resistance to chemotherapies (Haigis et al., 2008; Lievre and Laurent-Puig, 2009). In this study, we compared the effects of PPLGM on the growth of two different colon cancer cell lines, HCT 116 and HT-29. The HCT 116 cell line contains a KRAS mutation, but has wildtype BRAF and p53, while the HT-29 colon cancer cell line contains wild-type KRAS, but has BRAF and p53 mutations (Yeh et al., 2009). We found that the HCT 116 was slightly more sensitive to PPLGM than the HT-29 cell line (Figure 1A and B). Interestingly, recent studies have revealed that small molecule inducers of oxidative stress can effectively kill KRAS mutant cancer cells (Lebedeva et al., 2005; Shaw et al., 2011). This is a promising result since it suggests that PPLGM may be an effective treatment strategy even for colon cancer patients with oncogenic RAS mutations that may not respond to current treatments. Further evaluation of the effect of PPLGM on normal and mutant KRAS colon tumors and a potential role for elevated ROS levels in resulting cancer cell death is needed.
While the ERK pathway is normally associated with enhanced cell proliferation, many studies on bioactive food components have shown that ERK activation up-regulates the expression of apoptotic genes which contribute to enhanced cell death (Blagosklonny et al., 1996; Cagnol and Chambard, 2010; Ellington et al., 2006). The ERK pathway has been shown to modulate the activity and expression of numerous protein targets including pro- and anti-apoptotic proteins (Lee et al., 2005a). Activation of ERK signaling leads to transcriptional events such as enhanced gene expression of p53 and p53 up-regulated modulator of apoptosis (PUMA) and decreased expression of the anti-apoptotic protein Bcl-2 (Liu et al., 2008; She et al., 2001). Interestingly, PPLGM enhances p53 and PUMA expression and reduces Bcl-2 expression in various cancer cells (Raj et al., 2011). It is likely that ERK signaling plays a role in the apoptotic response of cancer cells to PPLGM via these targets.
A previous study showed that lower concentrations (2.5 μg/ml) of PPLGM induced apoptosis in HL-60 human leukemia cells as assessed by caspase-3 activation (Bezerra et al., 2007). However, higher concentrations of PPLGM (10 μg/ml) resulted in an increased number of necrotic cells as determined by acridine orange and ethidium bromide staining. Our data show that lower concentrations of PPLGM increase pERK and cleaved caspase-3 levels; whereas higher concentrations do not (Figure 5 and 7). We suggest that lower concentrations of PPLGM lead to ERK activation resulting in apoptosis; whereas higher concentrations of PPLGM trigger necrosis without activating ERK.
In our study, PPLGM lead to rapid elevation of p-ERK levels in a concentration dependent manner (Figure 6 and 8) which was determined for total cell lysates. It is possible that we would have observed greater pERK expression in PPLGM-treated cells from nuclear extracts. Further, we showed that the MEK inhibitor U0126 could partially block PPLGM-induced colon cancer cell death (Table 1; U0126 resulted in a 25% increase in cell viability compared to cells treated with PPLGM alone). It is likely that ERK is not solely responsible for PPLGM-mediated cell death given that inhibition of its upstream activator MEK was unable to completely block PPLGM-induced cell death.
ERK activity is regulated by dual-specificity phosphatases (DUSPs) that dephosphorylate and inactivate MAPKs. Interestingly, DUSP9 was 12× repressed by PPLGM (Raj et al., 2011), which would enhance ERK signaling in the presence of PPLGM. Additionally, DUSPs are sensitive to ROS; therefore, elevated levels of ROS triggered by PPLGM treatment would inhibit DUSPs which would result in activation of ERK and promote cancer cell death. These findings support our results showing that the ERK pathway plays a role in PPLGM-induced cell death.
Collectively, our data support the conclusion that PPLGM induces ERK phosphorylation leading to colon cancer cell death. This study suggests a mechanism by which PPLGM can trigger colon cancer cell death and supports the need for further investigation of PPLGM as a potential cancer therapy.
Highlights.
PPLGM inhibits colon cancer cell proliferation and induces apoptosis
PPLGM activates ERK signaling
Inhibition of ERK signaling reduces PPLGM-induced colon cancer cell death
Acknowledgments
The authors would like to thank Dr. Pawel Borowicz of the Advanced Imaging and Microscopy Lab at NDSU for technical assistance with cell imaging. Further, the authors acknowledge Dr. Tao Wang and the NDSU Center for Protease Research Core Biology Facility for access to equipment used to collect data for this study. This publication, and the use of the Core Biology Facility, was made possible, in part, by National Institutes of Health (NIH) Grant Numbers 2P20 RR015566 and P30 GM103332-01 from the National Center for Research Resources and National Science Foundation (NSF) Grant Number HRD-0811239 to the NDSU Advance FORWARD program. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NSF.
Abbreviations
- ERK
Extracellular-signal-regulated kinase
- RAS
Rat sarcoma oncogene
- MAPK
Mitogen-activated protein kinase
- MEK
Mitogen-activated protein kinase kinase
- PPLGM
Piperlongumine
- ROS
Reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397–1421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Bang JS, Oh da H, Choi HM, Sur BJ, Lim SJ, Kim JY, Yang HI, Yoo MC, Hahm DH, Kim KS. Anti-inflammatory and antiarthritic effects of piperine in human interleukin 1beta-stimulated fibroblast-like synoviocytes and in rat arthritis models. Arthritis Res Ther. 2009;11:R49. doi: 10.1186/ar2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrend L, Henderson G, Zwacka RM. Reactive oxygen species in oncogenic transformation. Biochem Soc Trans. 2003;31:1441–1444. doi: 10.1042/bst0311441. [DOI] [PubMed] [Google Scholar]
- Bezerra DP, Castro FO, Alves AP, Pessoa C, Moraes MO, Silveira ER, Lima MA, Elmiro FJ, Costa-Lotufo LV. In vivo growth-inhibition of Sarcoma 180 by piplartine and piperine, two alkaloid amides from Piper. Braz J Med Biol Res. 2006;39:801–807. doi: 10.1590/s0100-879x2006000600014. [DOI] [PubMed] [Google Scholar]
- Bezerra DP, Militao GC, de Castro FO, Pessoa C, de Moraes MO, Silveira ER, Lima MA, Elmiro FJ, Costa-Lotufo LV. Piplartine induces inhibition of leukemia cell proliferation triggering both apoptosis and necrosis pathways. Toxicol In Vitro. 2007;21:1–8. doi: 10.1016/j.tiv.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Bezerra DP, Pessoa C, de Moraes MO, Silveira ER, Lima MA, Elmiro FJ, Costa-Lotufo LV. Antiproliferative effects of two amides, piperine and piplartine, from Piper species. Z Naturforsch C. 2005;60:539–543. doi: 10.1515/znc-2005-7-805. [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV, Schulte T, Nguyen P, Trepel J, Neckers LM. Taxol-induced apoptosis and phosphorylation of Bcl-2 protein involves c-Raf-1 and represents a novel c-Raf-1 signal transduction pathway. Cancer Res. 1996;56:1851–1854. [PubMed] [Google Scholar]
- Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death--apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- Ellington AA, Berhow MA, Singletary KW. Inhibition of Akt signaling and enhanced ERK1/2 activity are involved in induction of macroautophagy by triterpenoid B-group soyasaponins in colon cancer cells. Carcinogenesis. 2006;27:298–306. doi: 10.1093/carcin/bgi214. [DOI] [PubMed] [Google Scholar]
- Fontenele JB, Leal LK, Silveira ER, Felix FH, Bezerra Felipe CF, Viana GS. Antiplatelet effects of piplartine, an alkamide isolated from Piper tuberculatum: possible involvement of cyclooxygenase blockade and antioxidant activity. J Pharm Pharmacol. 2009;61:511–515. doi: 10.1211/jpp/61.04.0014. [DOI] [PubMed] [Google Scholar]
- Fruehauf JP, Meyskens FL., Jr Reactive oxygen species: a breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- Greenwald P, Milner JA, Anderson DE, McDonald SS. Micronutrients in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:217–230. doi: 10.1023/a:1021202709003. [DOI] [PubMed] [Google Scholar]
- Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A, Sebolt-Leopold J, Shannon KM, Settleman J, Giovannini M, Jacks T. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutooru I, Chadalapaka G, Lei P, Safe S. Inhibition of NFkappaB and pancreatic cancer cell and tumor growth by curcumin is dependent on specificity protein down-regulation. J Biol Chem. 2010;285:25332–25344. doi: 10.1074/jbc.M109.095240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Lee DH, Jeong JH, Guo ZS, Lee YJ. Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem Pharmacol. 2008;75:1946–1958. doi: 10.1016/j.bcp.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong EH, Kim YJ, Cho HJ, Yu SN, Kim KY, Chang JH, Ahn SC. Piplartine induces caspase-mediated apoptosis in PC-3 human prostate cancer cells. Oncol Rep. 2008;20:785–792. [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Sarkar D, Gopalkrishnan RV, Waxman S, Yacoub A, Dent P, Fisher PB. Induction of reactive oxygen species renders mutant and wild-type K-ras pancreatic carcinoma cells susceptible to Ad.mda-7-induced apoptosis. Oncogene. 2005;24:585–596. doi: 10.1038/sj.onc.1208183. [DOI] [PubMed] [Google Scholar]
- Lee ER, Kang YJ, Kim JH, Lee HT, Cho SG. Modulation of Apoptosis in HaCaT Keratinocytes via Differential Regulation of ERK Signaling Pathway by Flavonoids. Journal of Biological Chemistry. 2005a;280:31498–31507. doi: 10.1074/jbc.M505537200. [DOI] [PubMed] [Google Scholar]
- Lee SA, Hong SS, Han XH, Hwang JS, Oh GJ, Lee KS, Lee MK, Hwang BY, Ro JS. Piperine from the fruits of Piper longum with inhibitory effect on monoamine oxidase and antidepressant-like activity. Chem Pharm Bull (Tokyo) 2005b;53:832–835. doi: 10.1248/cpb.53.832. [DOI] [PubMed] [Google Scholar]
- Lievre A, Laurent-Puig P. Genetics: Predictive value of KRAS mutations in chemoresistant CRC. Nat Rev Clin Oncol. 2009;6:306–307. doi: 10.1038/nrclinonc.2009.69. [DOI] [PubMed] [Google Scholar]
- Liu J, Mao W, Ding B, Liang Cs. ERKs/p53 signal transduction pathway is involved in doxorubicin-induced apoptosis in H9c2 cells and cardiomyocytes. American Journal of Physiology - Heart and Circulatory Physiology. 2008;295:H1956–H1965. doi: 10.1152/ajpheart.00407.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann P, Fortunato F, Zentgraf H, Buchler MW, Herr I, Werner J. Autophagy and cell death signaling following dietary sulforaphane act independently of each other and require oxidative stress in pancreatic cancer. Int J Oncol. 2011;39:101–109. doi: 10.3892/ijo.2011.1025. [DOI] [PubMed] [Google Scholar]
- Park BS, Son DJ, Park YH, Kim TW, Lee SE. Antiplatelet effects of acidamides isolated from the fruits of Piper longum L. Phytomedicine. 2007;14:853–855. doi: 10.1016/j.phymed.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pham NA, Jacobberger JW, Schimmer AD, Cao P, Gronda M, Hedley DW. The dietary isothiocyanate sulforaphane targets pathways of apoptosis, cell cycle arrest, and oxidative stress in human pancreatic cancer cells and inhibits tumor growth in severe combined immunodeficient mice. Mol Cancer Ther. 2004;3:1239–1248. [PubMed] [Google Scholar]
- Qanungo S, Das M, Haldar S, Basu A. Epigallocatechin-3-gallate induces mitochondrial membrane depolarization and caspase-dependent apoptosis in pancreatic cancer cells. Carcinogenesis. 2005;26:958–967. doi: 10.1093/carcin/bgi040. [DOI] [PubMed] [Google Scholar]
- Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Shaw AT, Winslow MM, Magendantz M, Ouyang C, Dowdle J, Subramanian A, Lewis TA, Maglathin RL, Tolliday N, Jacks T. Selective killing of K-ras mutant cancer cells by small molecule inducers of oxidative stress. Proc Natl Acad Sci U S A. 2011;108:8773–8778. doi: 10.1073/pnas.1105941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- She QB, Bode AM, Ma WY, Chen NY, Dong ZG. Resveratrol-induced activation of p53 and apoptosis is mediated by extracellular-signal-regulated protein kinases and p38 kinase. Cancer Research. 2001;61:1604–1610. [PubMed] [Google Scholar]
- Shih A, Davis FB, Lin HY, Davis PJ. Resveratrol induces apoptosis in thyroid cancer cell lines via a MAPK- and p53-dependent mechanism. J Clin Endocrinol Metab. 2002;87:1223–1232. doi: 10.1210/jcem.87.3.8345. [DOI] [PubMed] [Google Scholar]
- Son Y, et al. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J Signal Transduct. 2011;2011:792639. doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007a;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry & Cell Biology. 2007b;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Vedhanayaki G, Shastri GV, Kuruvilla A. Analgesic activity of Piper longum Linn. root. Indian J Exp Biol. 2003;41:649–651. [PubMed] [Google Scholar]
- Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Wakade AS, Shah AS, Kulkarni MP, Juvekar AR. Protective effect of Piper longum L. on oxidative stress induced injury and cellular abnormality in adriamycin induced cardiotoxicity in rats. Indian J Exp Biol. 2008;46:528–533. [PubMed] [Google Scholar]
- Wu WS. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006;25:695–705. doi: 10.1007/s10555-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Yeh JJ, Routh ED, Rubinas T, Peacock J, Martin TD, Shen XJ, Sandler RS, Kim HJ, Keku TO, Der CJ. KRAS/BRAF mutation status and ERK1/2 activation as biomarkers for MEK1/2 inhibitor therapy in colorectal cancer. Mol Cancer Ther. 2009;8:834–843. doi: 10.1158/1535-7163.MCT-08-0972. [DOI] [PMC free article] [PubMed] [Google Scholar]