Abstract
Biomaterials are being used for the healthcare applications from ancient times. But subsequent evolution has made them more versatile and has increased their utility. Biomaterials have revolutionized the areas like bioengineering and tissue engineering for the development of novel strategies to combat life threatening diseases. Together with biomaterials, stem cell technology is also being used to improve the existing healthcare facilities. These concepts and technologies are being used for the treatment of different diseases like cardiac failure, fractures, deep skin injuries, etc. Introduction of nanomaterials on the other hand is becoming a big hope for a better and an affordable healthcare. Technological advancements are underway for the development of continuous monitoring and regulating glucose levels by the implantation of sensor chips. Lab-on-a-chip technology is expected to modernize the diagnostics and make it more easy and regulated. Other area which can improve the tomorrow’s healthcare is drug delivery. Micro-needles have the potential to overcome the limitations of conventional needles and are being studied for the delivery of drugs at different location in human body. There is a huge advancement in the area of scaffold fabrication which has improved the potentiality of tissue engineering. Most emerging scaffolds for tissue engineering are hydrogels and cryogels. Dynamic hydrogels have huge application in tissue engineering and drug delivery. Furthermore, cryogels being supermacroporous allow the attachment and proliferation of most of the mammalian cell types and have shown application in tissue engineering and bioseparation. With further developments we expect these technologies to hit the market in near future which can immensely improve the healthcare facilities.
Keywords: biomaterials, nanomaterials, diagnostics, healthcare, three-dimensional matrices
Introduction
Biomaterial in medical terminology is “any natural or synthetic material (which includes polymer or metal) that is intended for introduction into living tissue especially as part of a medical device or implant” (for example artificial heart valve or joint). Biomaterials from healthcare perspective can be defined as “materials those possess some novel properties that makes them appropriate to come in immediate contact with the living tissue without eliciting any adverse immune rejection reactions.” Definition of biomaterials when intended for implant device purpose in accordance to Food, Drug, and Cosmetic Act (1976) is “an instrument, apparatus, machine, implant or other similar or related article, including any component, part or accessory which is intended for use in diagnosis of disease or in cure or prevention in man or other animals, and which does not achieve any of its principal intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of any of its principal intended purposes. These devices or any type of biomaterial is used to physically replace any hard or soft tissue which has undergone any accidental damage or destruction through some pathological processes. In addition to use of biomaterials as implant devices they have also shown applicability in other healthcare related areas like disposable medical devices, diagnostic kits, polymeric therapeutics, etc. So in general biomaterials are the devices those are used to improve the general healthcare of society and are fabricated by the process that employs or mimics biological phenomenon. Over the time biomaterials have played an imperative role in the area of bioengineering or biomedical engineering to improve the overall healthcare of society. Area of bioengineering encompasses two closely related areas of interest i.e., (1) it applies the principles of engineering science to understand how living organisms function and (2) it applies engineering technologies to design and develop new devices like diagnostic or therapeutic instruments or formulation of novel biomaterials for medical applications, design of artificial tissues or organs and development of new delivery systems. Overall bioengineering focuses on the uses of biomaterials or similar types of materials or principles to improve the healthcare services. With the advent of bioengineering concepts there has been a technological evolution in two key aspects of healthcare i.e. in diagnostic imaging and implanted therapeutic devices. Diagnostic imaging technologies have developed as novel methods to non-invasively view the abnormalities in living system or during the developmental state (in womb). Additional improvement with these technologies have revolutionized the healthcare and made the early treatment of diseases possible. Such imaging techniques like computed axial tomography (CAT), magnetic resonance imaging (MRI) or diagnostic ultrasound has substantially reduced the patient morbidity, so improving the healthcare.1 Implantable medical devices e.g. cardiac rhythm management stimulator, prosthetic heart valves, vascular stents, orthopaedic implants, implanted drug pumps have substantially enhanced the average life expectancy. More developments are expected in coming years towards the development of treatment strategies for life threatening diseases.
Emergence of Biomaterials in Healthcare
Biomaterials are being used in healthcare area from a long period of time. However, visible progress was made in the area of biomaterials since 1940s and substantial development has been observed in therapeutic medical technologies and implant devices over the past 25 years.2,3 Over the years there is a transition from the use of metals to natural tissues or their derivatives and mechanical valves are being replaced by prosthetic valves made from bovine tissue or harvested porcine valves. The major limitation with the use of these tissues is that they fail after 5–7 years of implantation. But research has shown that chemical and mechanical modification of these valves can improve their performance, so these implants are also being tried with younger patients. Also healthcare technology which is revolutionized by bioengineering concepts from past 25 years is pacemaker technology for arrhythmia management.4 Developments with sensor technology and software algorithms have now enabled the pacemakers to automatically respond to varied levels of patient’s physical activity so that they can alter the stimulation rate accordingly. In 2001, the first cardiac resynchronization therapy (CRT) devices were commercially available.5,6 CRT has proved to be a life saver for the patients with chronic heart failure. Advances in implant technologies have provided treatment to some life threatening disease like neurological abnormalities. As the blood brain barrier does not allow medication to enter the central nervous system (CNS), fully implanted programmable pumps are used to deliver precise doses of drugs like morphine to reduce severe pain. These implantable pumps are also being used for the treatment of non-malignant and cancer pain.7 One of the most remarkable applications of biomedical engineering in the area of healthcare over last decade is deep brain stimulation for the treatment of degenerative diseases like Parkinson. Techniques involve the insertion of thin electrode to a target site in brain whose other end is connected to a high frequency stimulator.8 Due to stimulations, symptoms like uncontrolled tremor, rigidity, slowness etc., disappear which improves the patient’s life quality. One of the recent advancement in degenerative disk disease is the use of metal spinal cages employing bone in-growth inducing strategies. In this approach collagen plugs containing recombinant bone morphogenetic protein (rBMP) are inserted into metal cages which encourages bone in-growth for spinal disk repair.9 In addition to above mentioned technological advancements, polymers (both natural and synthetic) have shown potential applications in healthcare. Polymethylmethacrylate has emerged as an efficient material for the fabrication of contact lenses. Biocompatable polymers like polytetrafluoroethylene (PTFE) is widely being used in the engineering of vascular system and soft tissue reconstruction. Other types of polymers those are being used for medical applications are polypropylene, polycarbonates, polyurethanes, etc. Biomaterials for the delivery of drugs or bioactive molecules emerged in 1970s and this area is showing newer developments with time. Four basic models of drug delivery are; oral, transdermal, pulmonary, site-specific delivery. Oral delivery mode is conventionally used for many pharmaceuticals. Transdermal mode delivers drug through skin by the process of diffusion but is limited for the delivery of small molecules like nicotine, etc. Pulmonary delivery systems include aerosol based systems like nasal sprays. Out of these delivery modes polymeric implants are the focus of research and are expected to further develop in coming years which can boost up the healthcare. Few products based on these concepts are already available in market e.g., NorPlant for fertility regulation and Lupron Depot for treatment of prostate cancers. Further developments are expected in this area which can improve their market and consumer applications.10
Types of Biomaterials
From healthcare perspective, biomaterials can be divided in following categories: (1) Synthetic (metals, polymers, ceramics, and composites); (2) Naturally derived (animal and plant derived); (3) Semi-synthetic or hybrid materials. All these types of biomaterials are being used in healthcare from a long time period, but ensuing developments have enhanced their utility in healthcare. Metals are the class of materials those are widely used for load bearing applications. Some of the examples include wires and screws to fracture fixation plates and artificial joints.11 During hip replacement femoral components are usually manufactured from Co-Cr-Mo or Co-Ni-Mo alloys or titanium alloys. Polymers as implants or biomedical devices are used as facial prostheses, tracheal tubes, kidney and liver parts, heart components, etc. Ultrahigh molecular weight polyethylene (UHMWPE) has shown application in knee, hip and shoulder joints. Table 1, lists the polymers those are being used in the healthcare.12
Table1. Commonly used polymers for biomedical applications.
| S. no. | Polymer | Application | |
|---|---|---|---|
| 1. |
Ultrahigh-molecular-weight polyethylene |
Knee, Hip, Shoulder Joints, Dental bridges |
|
| 2. |
Silicone rubber |
Finger Joints |
|
| 3. |
Cells/PTFE, Cells/PET, PET/collagen |
Vascular grafts |
|
| 4. |
Bioglass/PU, Bioglass/PS,CF/PS |
Spine cages, plates, rods, screws, discs |
|
| 5. |
PET/PU, PET/collagen |
Abdominal wall prosthesis |
|
| 6. |
PET/PHEMA, KF/PMA, KF/PE, GF/PU |
Tendons/ligaments |
|
| 7. |
PET/PU, PTFE/PU, CF/PTFE |
Cartilage replacement |
|
| 8. |
Polydimethyl siloxane, polyurethane, PVC |
Facial Prostheses |
|
| 9. | Polymethylmethacrylate | Bone Cement | |
Abbreviations: PVC, polyvinyl chloride; PTFE, polytetrafluoroethylene, PET, polyethylene terephthalate; PS, polysulfones; PU, polyurethane; CF, carbon fibers; PHEMA, Poly(2-hydroxyethyl methacrylate); KF, kevlar fibers; PMA, polymethacrylate; PE, polyethylene, GF-glass fiber. Reproduced with permission from reference 12.
Ceramics have revealed application as dental implants or filling materials. As ceramics have poor fracture toughness they have limited applications as load bearing materials. Composite materials are extensively used for prosthetic limbs, due to the combination of low density and high strength. Few types of composite materials like bisphenol A-glycidyl-quartz/silica filler and polymethyl methacrylate-glass filler are widely being used for dental restorations.13 Naturally derived polymers like collagen, gelatin, alginate, hyaluronic acid, etc are widely used in the areas of healthcare for the fabrication of three-dimensional (3-D) scaffolds to support cell growth and proliferation. Such 3-D cell seeded scaffolds mimic the native host tissue so has significant applicability in the area of regenerative medicine.14 As naturally derived biomaterials have limited mechanical strength it restricts their applications at load bearing regions. So such materials are being modified chemically to improve their mechanical properties. Examples include collagen chains modified with lysine and hydroxyl-lysine,15 PEGylated fibrinogen (PF),16 etc. Hybrid biomaterials are showing a substantial evolution for application in healthcare sector.
Third Generation Biomaterial and Their Applications in Healthcare
First generation of biomaterials evolved during 1960s and 1970s for their application as medical implants. Basic goal during the fabrication of these biomaterials was to maintain a balance between physical and mechanical properties together with minimal toxicity to host tissue.17 Ideal properties of the first generation biomaterials sought by surgeons were (1) appropriate mechanical properties; (2) resistance to corrosion in aqueous environment; and (3) should not elicit toxicity or carcinogenicity in living tissue. But second generation biomaterials were developed to be bioactive. A substantial progress was observed in the application of these materials for orthopedic and dental usage. Examples include bioactive glasses, ceramics, glass-ceramics and composites. Other types of materials those were developed as second generation biomaterials were resorbable. Examples include resorbable fracture fixation plates and screws used in orthopedic surgeries.18 Further developments with the biomaterial technology are now translating into the expansion of third generation biomaterials those can stimulate specific cellular response.19 Examples include bioactive glass (3rd generation) and porous foams those are designed in a manner that they activate genes those can stimulate regeneration of living tissues. Efforts are also being made to develop scaffolding materials those possess nanoscale features in order to mimic the native extracellular matrix of the host. Currently major focus of the researchers is the development of artificial tissues (as biomaterials) those have architectural features same as the natural counterpart. Development and use of biomaterials is expected to augment in coming years. New prognostic methods are being developed and are becoming available to assist the progress of innovative approaches for an affordable healthcare.
Application of Bioengineering to Healthcare
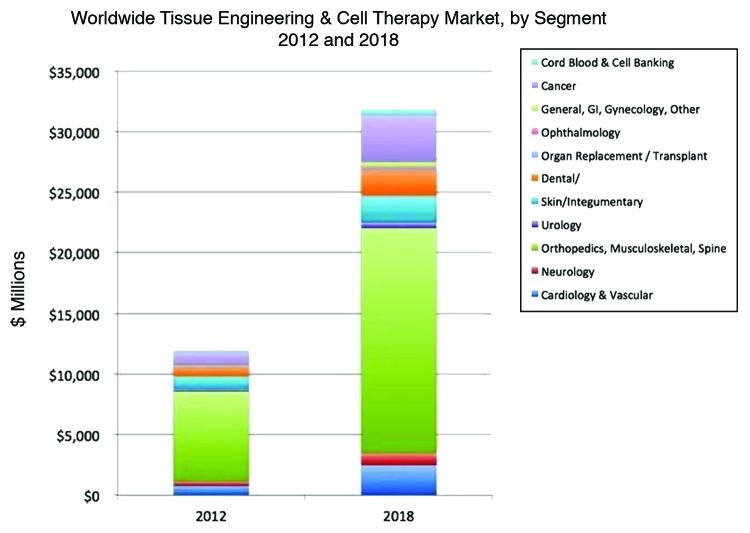
Healthcare is developing fast and a paradigm shift is being seen from replacement to regeneration using the concepts of tissue engineering and regenerative medicine. Tissue engineering works using three approaches for the repair of damaged tissue; (1) only cells (stem cells, autologous cells, etc.); (2) 3-D scaffold with or without cells which mimics native tissue. Tissue engineering promises a spectacular medical care for thousands of patients annually at reduced medical costs. Limitation with the conventional healthcare methods e.g., organ transplantation will be taken care by the emerging newer technologies or tissue engineering and regenerative medicine. Engineered organs could evade the problems associated with organ transplantation, so can prove to be a boom to tomorrow’s healthcare. Looking at the tissue engineering market in Figure 1, few tissue engineering areas have an excellent market potential for future. So in this review we have tried to focus on some of these areas.
Figure 1. Worldwide tissue engineering and cell therapy market for year 2012 and 2018. (Reproduced with permission from- Source: “Tissue Engineering, Cell Therapy and Transplantation: Products, Technologies & Market Opportunities, Worldwide, 2009-2018″, Report #S520).
Myocardial tissue engineering
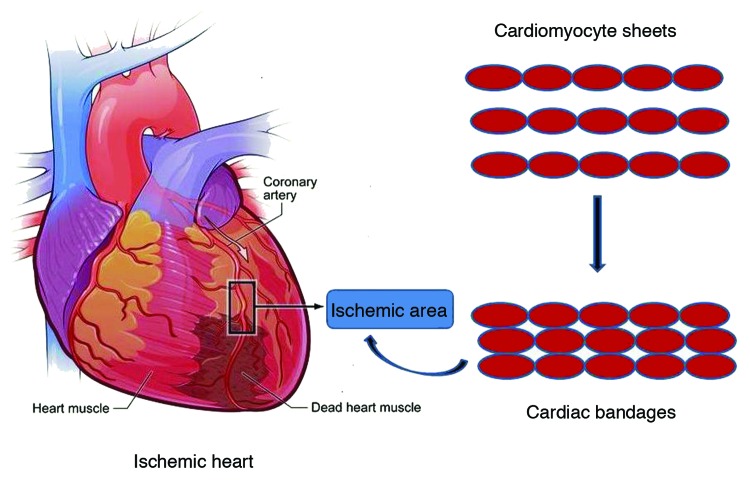
Recently cell therapy and tissue engineering are evolving as potential therapies for cardiac repair. Research activities have attempted to regenerate the ailing heart with epicardial implantation of bioengineered tissue patch pre-seeded with bone marrow cells or BM-derived mesenchymal stem cells.20 Both natural and synthetic polymers like collagen, fibrin, PGA, PLGA etc are being used for these applications. Animal derived tissues like bovine pericardia in combination with engineered cell sheet to create a sandwich like cardiac patch is also being used to regenerate ischemic heart in rat model.21 A typical approach for the fabrication of cardiac patches is depicted in Figure 2.
Figure 2. Schematic representation of use of cardiac bandages for treatment of ischemic heart (Reproduced with permission from ref. 21).
Recently in situ cardiac tissue engineering using injectable biomaterials is being used for the repair of infracted myocardium.22 This approach has certain advantages like it is less invasive and promotes repair by improving donor cell retention and neoangiogenesis.23 Alternative cell source can be adipose-tissue derived stem cells those can spontaneously differentiate into functional cardiomyocytes.24 Other potential cell source is cardiac stem cells (CSC) which has opened new avenues in the repair of cardiac injuries. CSC’s have been isolated by various research groups from human heart biopsies or murine heart using explants culture techniques and are named as cardiospheres (CSs). These CSs have differentiation potential to form bands of myocardium with varied degree of organization in vivo. Myocardial tissue engineering using cardiac stem cells is still in its infancy but a scaffold free tissue engineering approach using CSC’s was introduced by Bartosh et al.25 These cell sheets have shown potential to restore the functions of infracted myocardium in rat model. In addition to the above mentioned upcoming approaches to repair the ischemic heart gene therapy and nanotechnology hold great promises for future. From a clinical perspective a minimally invasive technique will be preferred. But bigger goals can only be achieved by employing a combination of stem cells with the appropriate biomaterials due to their associated benefits.
Orthopedic and musculoskeletal medicines
Metals (titanium alloys, stainless steel, etc.), ceramics (aluminum oxide, calcium phosphate, etc.), polymers (silicon, polylactide, etc.) and composites (ceramic-reinforced polymers) are widely being used for orthopedic applications.26 But with the advent of third generation biomaterials for bone tissue engineering, substantial progress has been observed in this area. This new class of biomaterial has an osteoinductive capacity. Osteoinductive property can be introduced into the scaffolds by methods like surface modification, incorporation of growth factors e.g., TGFβ (transforming growth factor β), BMP (bone morphogenetic protein) and VEGF (vascular endothelial growth factor), seeding bone marrow stem cells. Recent research work has shown that BMP-2 and VEGF co- loaded scaffolds enhanced vascularisation together with formation of a new bone.27 Recently gene therapy is being explored to modulate the osteoinductive properties of growth and transcriptional factors. Collagen sponge seeded with BMP-9 gene transfected MSC’s when implanted in mice have shown promising bone gap bridging results.28 Other factor which is important in bone tissue engineering and is being modulated is resorption of the scaffolds. One approach can be the use of amorphous calcium phosphate (ACP) which degrades faster and creates a calcium rich environment for faster apatite deposition. Sustained delivery of biomolecules from a porous scaffold is an emerging concept and can have wide implications for tomorrow’s healthcare. Micropores in the scaffold can be optimized in such a way that capillary action of the pores will avoid the burst release of the biomolecules. So release will occur in a time-dependent manner which will be dependent on the degradation rate of the scaffold. Combination of biomolecules can be tried those can enhance tissue integration and new bone formation.29 Emerging materials those are considered to be the future generation orthopedic biomaterials are called as nanophase biomaterials where grain size is in nanometer range. These materials have shown good osteoclast adhesion, bone remodelling, enhanced osteoblast proliferation and new bone formation.30 These materials have surface and mechanical properties similar to bone so have potential for bone tissue engineering.
Current gold standards for repair of musculoskeletal injuries are intense surgical processes which either repairs or replaces the damaged tissue. Recent approach for cartilage repair is the expansion of cells in vitro and their subsequent implantation at the defect site. This approach has been commercialized by companies like, Genzyme which provides the services to expand the autologous cells in vitro. But use of autologous cells has associated limitations which put three dimensional scaffolds (seeded with cells) to the forefront for the production of an engineered tissue. The cell source in this approach can be primary cells, bone marrow derived mesenchymal stem cells, cord derived mesenchymal stem cells, embryonic stem cells, etc.31 Scaffolds fabricated from natural (gelatin, chitosan, agarose, hyaluronic acid, etc.) and synthetic (PLA, PLGA, etc.) polymers have been used for musculoskeletal tissue engineering. More recent approach is the use of nonmaterials in which structural dimensions are less than 100 nm.32 Introduction of nanomaterials for musculoskeletal tissue repair has two advantages. (1) It is a biomimetic approach which mimics the nano-dimensional architecture of the native tissue. So these materials generate a micro-environment which signals the infiltrating cells to differentiate and form a neo-tissue.33 (2) Mechanical properties of the nano-composite materials can be tailored to match the native tissue. Successful development of nanobiomaterials in future may lead to next generation musculoskeletal substitute materials those will be applicable to biomedical device industry and can improve general healthcare.34
Strategies for skin tissue regeneration
Current gold standard for the repair of full thickness skin injuries is autologous skin grafting. But lately skin substitutes are being utilized to recreate the aesthetics of damaged skin tissue. These skin substitutes have cells seeded on a biocompatible and biodegradable 3-D scaffolds. One of the most used bioengineered products is Dermagraft (Shire Regenerative Medicine) which has a tendency to stimulate angiogenesis.35 Other commercial product is Apligraf which has been approved for the treatment of diabetic foot. In addition there are large numbers of other skin regeneration substitutes commercially available in market. Nanotechnology is an emerging technology which holds promises for future medical care and diagnostics. As already mentioned physical properties of material shows dramatic changes when it is reduced to naometric level. These nanomaterials can even be mixed with the polymeric matrix in order to improve the performance of the resultant system. Stem cell technology is emerging as a powerful technique for the treatment of wide spectrum of diseases including skin maladies. Bone marrow (BM)-derived stem cells have shown a potential to differentiate into fibroblasts of skin indicating their prospective as alternative cell source for skin tissue engineering application. Mesenchymal stem cells (MSC’s) in co-culture system have shown to differentiate into ligament fibroblasts due to synthesis of key ligament ECM component.36 Adipose derived stem cells (ADSC’s) is other potential cell source which has shown a capacity to stimulate both collagen synthesis and migration of dermal fibroblasts together with improved wrinkling and wound healing property in vivo.37 Currently available skin regeneration templates can partially repair the protective barrier functions of skin. But these templates are incapable of restoring the other important function of skin like touch, temperature sensation, excretion, perspiration, etc. Current advances in the area of biomaterials and stem cell technology may give us a hope that such products will develop in near future leading to a considerable evolution to the area of skin tissue engineering and healthcare sector.
Disposable and other medical devices
With the advances in technology we are putting a huge burden on our environment at a global scale. Large numbers of disposable medical devices fabricated from non-degradable biomaterials pose a serious environmental and economic issue. Advanced technologies in the area of bioengineering and biomaterials can be applied to tailor the biodegradability of a material in order to render them non-toxic to our environment. Biodegradable polymers like PLA, poly(glycolide), poly[D,L-(lactic-co-glycolide) have emerged as promising materials for use as disposable medical devices and are environmental friendly.38 These and other biodegradable polymers are being widely used for the fabrication of disposable medical devices which will improve the healthcare in future and will be safe to our environment too. As biodegrade polymers degrade by simple hydrolysis of ester bonds and hydrolytic products are non-toxic to mammalian tissue, they also have applicability as medical devices. Synthetic degradable polyesters have been used as suture materials, bone fixation devices, etc. Furthermore absorbable systems have many advantages when compared with metallic implants e.g., second surgery is not required for their removal from the patient’s body.39 Further advances in polymer technology and biomaterials can lead to the development of more promising biodegradable medical devices which can improve the healthcare substantially.
Drug eluting stents
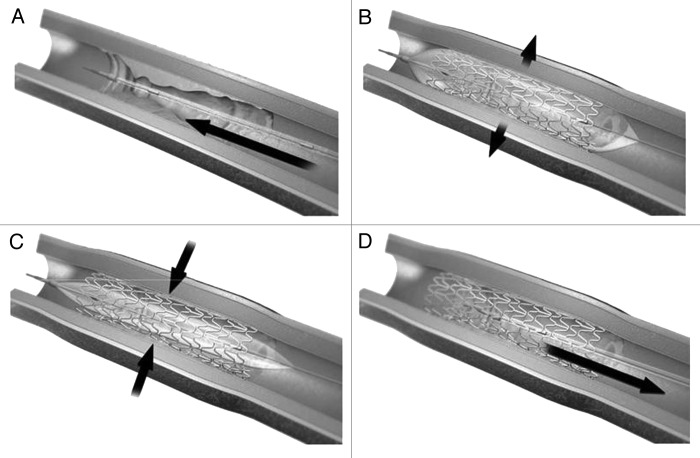
One of the latest techniques to treat atherosclerotic lesions is the implantation of stents. But bare metal stents have limited clinical use as many patients experience restenosis (narrowing of blood vessels). Lately drug eluting stents have emerged as potential biomaterials those can deliver drugs in a controlled fashion to reduce the cell proliferation ensuing implantation of metallic stent. Desirable drug or drugs can be incorporated into stents by number of ways like embedded and released from within polymeric material, surrounded and released through a carrier.40 Due to the limitations with the first and second generation drug eluting stents efforts are being made into the development of newer stents those can reduce the patient complications. This can be achieved by developing a stent which is biocompatible. This can be achieved by coating the stent surface with endothelial cell/endothelial progenitor cells, CD34 antibodies (known to attract endothelial cells), corticosteroids.41,42 Biodegradable stents have good future potential, they can either be completely biodegradable or are coated with a biodegradable polymer on a conventional metallic stent. In either case goal is to avoid the generation of an immune response. Examples of polymers used for this purpose includes PLLA, PDLLA, PLGA, etc. Major examples of biodegradable drug eluting stents those have been approved by Conformite Europeenee (CE) include BioMatrix, BioMatrix ІІ, Infinnium, etc. More recent type of drug eluting stents have microfabricated reservoirs for drugs. Reservoirs in such stents are created using laser machine techniques, wherein laser beam removes 0.1–0.5µm polymer or metal creating microchannels those can act as drug reservoirs.43 Promising class of stents which has potential for future are gene eluting stents. Nucleic acid based therapeutics, usually DNA or RNA molecules can be delivered locally to block the specific regulatory gene or genes those are responsible for pathophysiology of restenosis.44 Other alternative method is the delivery of siRNA to inhibit the corresponding mRNA from producing proteins those have a key role to play in the pathophysiology in restenosis. Gene eluting stents are developing as future drug eluting stents and have huge potential in healthcare. Figure 3 shows a typical drug eluting stent.45
Figure 3. Drug eluting stents (A) Stent is mounted on a catheter and inserted at the diseased area. (B) Balloon is inflated which expanded the stent. (C) Balloon is then deflated leaving the drug eluting stent as a scaffold. (D) Catheter with deflated balloon is removed leaving the stent at the diseased site which then releases the medication. Reproduced with permission from reference 45.
Implantable biosensors for healthcare
Biosensors work on the concept of bio-Micro Electron Mechanical System (MEMS) and are emerging as potential non-invasive approach to detect and monitor biomarkers. Biosensors are clinically being used for the early detection of diabetes, cardiovascular diseases and cancers.46 Present technology for monitoring elevated glucose level is the use of glucose meter but this is not an efficient method to monitor the levels continuously. So many efforts are being made in the development of non-invasive continuous glucose monitoring system (CGMS). Widely investigated florescence sensor is based on plant-sugar-binding protein, concanavalin A (Con A).47 Alternatively other types of CGMS are boronic acid or diborononic acid based sensors those can be implanted under the skin and fluorescence could be detected through the skin. For the early diagnosis of cardiovascular diseases cholesterol biosensors are used for the estimation of free and total cholesterol.48 But technological advances in microelectronics are likely to speed up the development of more sensitive biosensors. Biosensor technology has huge applicability for the early detection of cancer which can increase patient survival and quality of life. Affymetrix a commercially available biosensors is widely used for early detection of cancer. But technological advances are expected to make this technology more efficient which can augment healthcare facilities.
Polymer therapeutics
Complex multicomponent polymer based drugs or delivery systems like polymer-drug and polymer-protein conjugates, etc are termed as polymer therapeutics. Large number of natural, synthetic or semi-synthetics polymers have been proposed as therapeutics. Table 2 lists the polymeric therapeutics those are in clinical trials and are expected to be the future drugs for the treatment of life threatening diseases.
Table 2. List of polymeric therapeutics and conjugates those are currently under clinical development.
| S. no. | Product Name | Description | Clinical use |
|---|---|---|---|
| 1. |
VivaGel |
Lysine-based dendrimer |
Microbiocide |
| 2. |
CT-2103; Xyotax |
Poly glutamic acid |
Cancer |
| 3. |
Opaxio |
(PGA)-paclitaxel |
Cancer |
| 4. |
Prolindac |
(HPMA-copolymer-DACH platinate) |
Cancer |
| 5. |
NKTR-102 |
(PEG-irinotecan conjugate) |
Cancer-metastatic breast |
| 6. |
PEG-SN38 |
(Multiarm PEG-camptothecan derivative) |
Cancer |
| 7. |
NKTR-118 |
(PEG-naloxone) |
Opioid-induced constipation |
| 8. | XMT-1001 | (poly(1-hydroxymethylethylene hydroxymethylformal) -camptothecin conjugate) | Cancer |
PGA, Poly glutamic acid; HPMA, N-(2-Hydroxypropyl)methacrylamide; DACH, cytotoxic diaminocyclohexane. Modified from reference 51
Polymer based probes have shown application in diagnostics and are widely being used as imaging agents. Dendrimers have been widely studied for MRI imaging agents due to their capacity to carry high load of imaging probes.49 An important and rapidly growing area is the use of polymeric probes for positron emission tomography (PET) which has revolutionized the imaging technology.50 Polymeric therapeutics are currently being studied for the treatment of chronic and debilitating diseases. Emerging examples are Cimzia for rheumatoid arthritis, Macugen for age related macular degeneration, and Puricase for gout. Other promising application is the use of multivalent dendritic polyglycerolsulfate (dPGS) as anti-inflammatory agent by inhibiting leukocyte trafficking. Polymeric therapeutics are also being explored to make the diagnostic techniques more sensitive which can help in the early detection of disease.51
Lab-on-a-chip (LoC)
LoC is a device which integrates one or several lab functions on a single chip. These chips fall under the sub-set of mirco-electron mechanical system (MEMS) and are also termed as micro “total analysis systems” (µTAS). During last decade there has been extensive research on the fabrication of LoC’s using polymers like polydimethylsiloxane (PDMS), polycarbonate (PC), polymethylmethacrylate (PMMA), polycarbonate (PC), etc. These materials are cheaper than conventional ones and are suitable for the fabrication of disposable devices.52 Use of Loc’s for genomic analysis has rapidly evolved after the completion of human genome project. LoC based assays for nucleic acid analysis has a huge gamut of applications in molecular diagnosis and in the identification of human pathogens. LoC’s for the detection of mutation has been developed which can help in the identification of genetic disorders. LoC based immunoassays is other emerging area which can make the diagnosis fast. Microfluidic cartridge based LoC concept has been developed which can carry out blood group determination in 2min utilizing agglutination assay.53 As many research groups are focusing on the development of these chips we expect a large number of commercially available, disposable microfluidic devices in near future. Furthermore, in future we expect widespread usage of cheap and easy use lab-on-chips connected to complex diagnostic software for data analysis which will be connected to medical database in reference medical centers at different locations. This whole procedure can reduce the cost, and enhance the efficiency of diagnosis both at large and remote medical centers/hospitals. So this technique has huge promise for future healthcare.52
Emerging Applications of Bioengineering to Diagnostics and Healthcare
Drug delivery by microneedles (MN’s)
Limitations with conventional hypodermic needles for the drug or vaccines have put microneedles in spotlight. MN’s are fabricated using the concepts of microelectronics from silicon, metals, polymers, glass and ceramics. Different types of MN are used for different applications. For example solid MN’s are used for skin pre-treatment so that drug formulation when applied to skin can penetrate via slow diffusion. In contrast coated MN’s are coated with water soluble formulation or drug for its delivery. Coated MN’s aid in the delivery of drug to skin after which MN is removed. Other type of MN’s are classified as dissolving MN’s those degrade or dissolve in skin while they release the drug payload. Hollow MN’s are used for the infusion of liquid formulations or drugs into skin.54 By by-passing stratum corneum, MN’s make the delivery efficient across the skin. They have been employed for the delivery of low molecular weight drugs (lidocaine), biotherapeutics (insulin), vaccines (BCG tried in pigs). Figure 4 is the schematic representation of different types of MN’s.
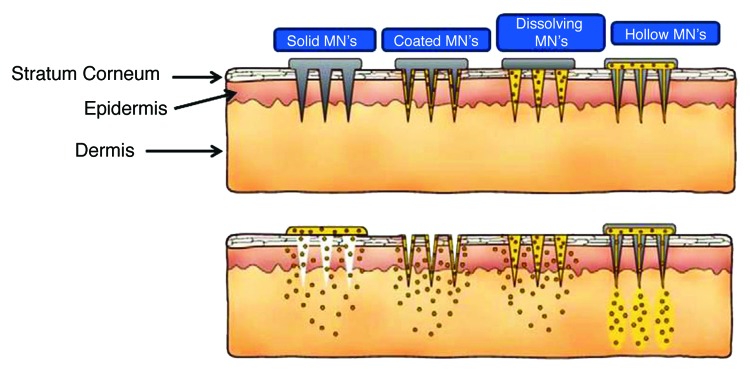
Figure 4. Schematic representation of different types of microneedles (MN’s) used for the drug delivery (Reproduced with permission from ref. 54).
Prominent MN products available include Dermaroller, Microhyala, Soluvia, etc. Microhyala are hyaluronic acid containing needles those are used to treat skin wrinkles so have huge cosmetic demand. In addition to the delivery of drugs to skin, MN’s are being studied for ocular delivery. Implantable MN’s fabricated using biodegradable polymers and methotrexate are being studied for vitreoretinal lymphoma.55 Solid MN’s have been developed for the delivery of drug in blood vessel walls for the treatment of restenosis.56 Efforts are being made for the fabrication of coated metal MN’s for the delivery of parathyroid hormone (PTH) which can benefit osteoporosis patients in future.57 Area of MN’s has matured enough that many MN systems are available for academic and clinical use. But there are few such systems those are progressing to clinical trials in humans. In future we expect this area to grow more and translate into clinics and for medical practitioners.54
Gene therapy for healthcare
Combination of gene delivery uses the concepts of tissue engineering in order to repair the ailing tissue or organ.58 This is done by the incorporation of genes into the polymeric three-dimensional matrices followed by their implantation or injection which can later promote tissue healing or regeneration. Recently researchers have tried to transfect cells present in local environment of damaged tissue using both viral and non-viral delivery modes to induce the formation of a new tissue. But due to the associated limitations with the viral transfer like patient safety, efforts are being made toward the development of non-viral modes.59 In this approach plasmid DNA is incorporated within biodegradable polymers which promote sustained delivery to promote expression over a desired period of time. Type І collagen was the first polymer used to deliver plasmid DNA encoding for BMP’s and human parathyroid hormone (PTH). Approach is also used for the repair of surgical incisions by the incorporating DNA into suture materials. Other biodegradable synthetic polymer employed for delivery of DNA is PLGA. PLGA matrices were studied for the delivery of platelet derived growth factor (PDGF) which showed effects on the formation of granulation tissue and vascularisation. Alginate being a biodegradable polymer has a huge potential in this area as it can be used to promote expression of the incorporated DNA in a specific time. Other potential polymeric systems are poly-vinyl and N-methyl-2-pyrrolidoma, PVA etc. Polymers like poly [D,L-(lactide-co-glycoide) can interact with DNA to form coated particles which protects DNA from nuclease attack and promote its delivery to cells.60 First gene therapy product which is approved by European Commission can be of great help to patients suffering with familial hyperchylomicronemia. This will be marketed under the trade name of Glybera by an Amsterdam based company called unique (http://blogs.nature.com/news/2012/11/gene-therapy-hits-european-market.html). Final approval of Glybera marks a major step toward making gene therapy available for large number of other diseases those need an immediate cure. So we expect more types of such gene therapy products in market in near future to improve the current healthcare facilities.
Bioseparation
Polymeric hydrogels are widely used for the process of bioseparation which can have diagnostic potential. Bioresponsive hydrogels fabricated by the process of biomolecular imprinting have been used for the detection of tumor markers like α-fetoprotein, α fetal protein (AFP). This was done by conjugating lectin Con A and polyclonal anti-AFP antibodies into the gels which introduces the recognition sites for specific biomarker binding site. These gels being bioresponsive shrink in response to AFP molecules which enables accurate and visible detection of biomarker molecules. Furthermore, polymeric micro/nano-spheres and micro/nano-fibers have gained popularity as potential substrates for the immobilization of biomolecules which can have applications in diagnostics and bioseparation. Due to their large surface area and small size these materials are more suitable for the immobilization of more compact biomolecules which reduces the device size and enhances the process sensitivity.38 We expect a colossal progress to be made in this area in near future which can make the diagnostics more easy and sensitive.
Hydrogels and Cryogels as Promising Scaffolds for Healthcare Applications
With the advances in material science and bioengineering new scaffold technologies are emerging. These 3-D scaffolds are being studied as potential materials for tissue regeneration, biosepartion, drug delivery, etc. So these are future materials those can revolutionize the healthcare by regenerating an ailing tissue or organ.
Hydrogels
Hydrogels are the broad class of crosslinked polymeric networks those absorb large amount of water or any other biological fluid without showing any alternations in their 3-D architecture. Retention of water by hydrogels makes them appropriate for various biomedical applications as the whole system tends to mimic the extracellular matrix environment of soft tissues. Hydrogels are being used in the area of healthcare from ages but with time hydrogels have evolved from static implants and devices to more dynamic responsive scaffolds and drug delivery vehicles. These dynamic hydrogel devices or implants have huge potential for future healthcare.61 Even though static, inert contact lenses have been successfully used for the correction of vision but developments with this technology have led to the emergence of dynamic, bioresponsive designs those are being studied for ophthalmic drug delivery, measuring or regulating ophthalmic pressure and altered glucose levels.62,63 Hydrogels composed of natural polymers like hyaluronic acid and collagen are being widely explored for tissue engineering applications. Due to the associated limitations with the natural polymers, strategies are being developed for the biofunctionalization of synthetic polymers to make them suitable for biomedical applications.64 To induce the dynamic response in hydrogel materials, different mechanisms are being employed, these user-controlled mechanisms such as temperature or electromagnetic fields have been applied to clinical applications. Hydrogels fabricated using responsive synthetic polymers like poly (N-isopropylacrylamide) (PNIPAAm) demonstrate lower critical solution temperature (LCST) behavior near physiological temperature which makes them potential materials for healthcare applications.65 Okano et al. exploited this behavior of PNIPPAm for the fabrication of 3-D stacks of functional cardiac tissue which showed improvements in systolic functions and neovascularisation when implanted into rat.66 With further improvements this polymer can have huge clinical demand for ischemic heart in future. Responsive hydrogels are also being studied for the development of on demand drug delivery technologies with remote triggers that will enable the patients with chronic diseases to control the frequency and dosage of drug. In this direction iron oxide nanoparticles have been incorporated into PEG methacrylate and PNIPAAm hyrogels. Due to alterations in the magnetic field local temperature increases which results in the deswelling of the gel to release the drug, this approach can be a potential future strategy for remote drug delivery.67 This delivery mode has also been studied for the release of anti-cancer drugs like Paclitaxel. Dynamic hydrogels are currently being tested in animal models and have huge potential to translate into clinical applications in near future. In extension to the above discussed technologies, materials are being designed to respond to endogenous signals from cells or tissues. One prominent approach is to control the hydrogel behavior using proteolysis phenomenon. These proteolytically degradable hydrogels have been studied for cell-medicated delivery of growth factors and therapeutics like VEGF, chemotherapeutic agents, etc. Other physiological stimulus is pH which has been studied for the delivery of insulin. Peppas et al. developed hydrogel complexes of poly (itaconic acid) (PIA) and PEG, hydrogen bonding between carboxylic acid group in PIA and oxygen in PEG leads to the formation of a compact network at acidic pH which protects insulin and releases it at a more neutral pH in intestines which retains the bioactivity and enhances absorption.68,69 Dynamic pH responsive hydrogels are being fabricated those can serve as sensors for the detection of pathological condition during the early stages of disease. For example high partial pressure of CO2 in stomach can be an indicative gastrointestinal ischemia and this elevated level can alter the pH which can be detected by the sensor system. This sensor has an immense potential to be translated into future diagnostics where it will be used as a tool by incorporating the sensor system into a catheter. Hydrogel sensors are the potential materials to monitor, and intervene in biological crisis situation. These sensors are currently studied for the cardiac patients. During the progression of heart disease, valve interstitial cells (VIC’s) respond to chemical and physical signals and differentiate from dormant fibroblast phenotype to active myofibroblasts. Calcification is a major problem during the progression to valve disease which influences the mechanical properties of microenvironment and surrounding extracellular matrix. In these types of pathological conditions a sensor which can read the changes related to matrix mechanical properties will be of great use.70 It will provide new insights into the pathologies of other diseases and will help in developing new strategies for their treatment.
Cryogels
Cryogels are the emerging matrices for biomedical applications and can be the tools for tomorrow’s healthcare. These supermacroporous gel matrices are synthesized at sub-zero temperature (-12°C) which results in the formation of interconnected porous network allowing the cell migration and proliferation. Over a decade’s time our group has been working on the fabrication of these gel matrices for different biomedical applications like tissue engineering, cell separation, bioartificial liver, etc. These matrices can be synthesized using both natural and synthetic polymers employing different types of crosslinkers at an optimized concentrations. Ideal properties of these matrices like large pore size (10–100 µm) together with good mechanical properties make them appropriate for large number of tissue engineering applications. An additional benefit with these gel matrices is that they can be fabricated in different formats like sheets, monoliths, discs, beads, etc71 as shown in Figure 5A. Flexibility with respect to design makes them apposite for varied number of biomedical applications. These matrices have excellent swelling capacity so can take up large volumes of solvent. This property augments the applicability of these matrices for biomedical applications. Further diversity with respect to applicability can be included by modifying the gel matrices which involves coupling of various ligands to their surface, grafting of polymer chains to the surface or fabrication of interpenetrating networks of two or more polymers. Cryogel matrices can also be modified by the surface modification phenomenon which improves their interactions with cells. Full interpenetrated network of polyacrylonitrile with gelatin was prepared for biomedical applications. These matrices showed initial cell attachment and other appropriate properties so can be utilized for tissue engineering applications.72 As these matrices possess interconnected networks they have been used as chromatography columns for the biosepartaion. Our group has established a protocol for the separation of stem cells from umbilical cord blood. For this application cryogel columns of polyacrylamide and polydimethylacrylamide were fabricated and functionalized to immobilize protein A ligand. Target cells labeled with specific antibody when allowed to pass through the column attached to the matrix through affinity to protein A. This procedure can be used as a generic type-specific cell separation approach which has potential in diagnostics and cell therapeutics.73 Cryogel matrices have been studied extensively for different tissue engineering applications. As these matrices possess large and interconnected network they allow the proliferation and migration of most of the mammalian cells. Cryogel matrices fabricated using the combination of natural polymers like alginate, agarose, gelatin, chitosan, etc., have shown encouraging results for their utility as matrices for cartilage tissue engineering. These matrices allow the growth and proliferation of goat chondrocytes together with the formation of neo-cartilage on them.74,75 These matrices have also shown a significant healing of cartilage defects when implanted in New Zealand white rabbits indicating their potentiality for treating the subchondrial cartilage defects (Unpublished results). Results with these matrices indicate that synthesized cryogel matrices hold great promise for the patients suffering from accidental injuries of cartilage or other cartilage degenerative diseases. These matrices have also been studied for their application in bone tissue engineering. For bone tissue engineering we have fabricated novel organic/inorganic composites using polyvinyl alcohol-tetraethylorthosilicate-alginate-calcium oxide (PTAC). These scaffolds supported the growth and proliferation of human osteosarcoma cell lines (MG 63) indicating their potential for the regeneration of bony tissue.76 These matrices have shown healing of cranial defects when implanted in the skull of Wistar rat (Unpublished results). So these composite cryogels have great prospective in future for the repair of bone defects. Other tissue engineering applications that are being explored include neural tissue engineering, cardiac tissue engineering, skin tissue engineering.77,78 Applicability of cryogels in tissue engineering is facilitated by their interconnected porous network as shown in Figure 5B.
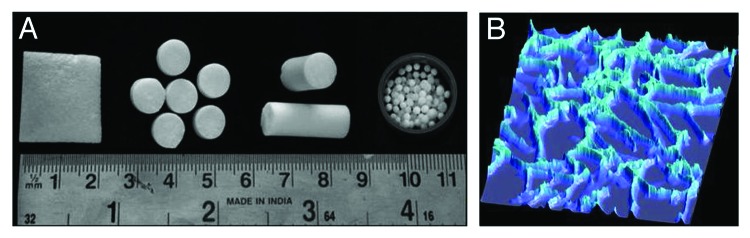
Figure 5. Digital images of cryogels showing their flexibility to be fabricated in different formats (A) (Reproduced with permission from ref. 71). Fluorescent microscopic image of cryogel section showing interconnected porous network (B).
In addition to tissue regeneration our group is involved in the fabrication of bioartificial liver devices using cryogels for the patients with acute liver failure. These devices can bridge the gap till an appropriate transplant becomes available for the patients. Cryogel matrices have also been employed for the production of therapeutics (monoclonal antibodies) using a continuous culture system.79 Research is also being performed by other groups for the fabrication of injectable cryogels80 for clinical applicatiosn like gene therapy, tissue engineering, dermal fillers, drug delivery, etc. Looking at the current research scenario cryogels are expected to evolve fast so that they can be used in clinics and diagnostics in near future.
Concluding Remarks
Current healthcare and diagnostics has many constraints like it is expensive, has limited accuracy or there is no strategy to treat some of the diseases (e.g., cancer). So there is a great demand to improve the current healthcare facilities. Research is being performed to improve the existing methods and for the development of new approaches. Bioengineering is one of the most viable option which has a potential to improve the existing healthcare scenario. It uses biomaterials and tissue engineering concepts for the repair of damaged tissue. Major goal here is to repair or regenerate the tissue or organ than to remove it. Bioengineering has also shown a good progress in diagnostics and newer methods are being developed which can make the detection easy and accurate. With the current progress in biomaterials we expect a future healthcare which will be available at an affordable price and with better services.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Authors would like to acknowledge Department of Biotechnology (DBT), Ministry of Science and Technology, Government of India for financial support. Authors would also like to acknowledge all the authors whose works are cited in this review article.
Footnotes
Previously published online: www.landesbioscience.com/journals/biomatter/article/24717
References
- 1.Paul C, Robert MN. Bioengineering: 25 years of progress—but still only a beginning. Technol Soc. 2004;26:415–31. doi: 10.1016/j.techsoc.2004.01.008. [DOI] [Google Scholar]
- 2.Chatterjee S, Gardner TJ. Factors determining selection of valve prosthesis--tissue or mechanical: current status. Adv Cardiol. 2002;39:189–94. doi: 10.1159/000058927. [DOI] [PubMed] [Google Scholar]
- 3.Rahimtoola SH. Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol. 2003;41:893–904. doi: 10.1016/S0735-1097(02)02965-0. [DOI] [PubMed] [Google Scholar]
- 4.Citron P, Smyth NP, Kleinert M, Kahn AR. Clinical experience with a new transvenous atrial lead. Chest. 1978;73:193–7. doi: 10.1378/chest.73.2.193. [DOI] [PubMed] [Google Scholar]
- 5.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, et al. Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–94. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 6.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, et al. MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–53. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 7.Rawicki B. Treatment of cerebral origin spasticity with continuous intrathecal baclofen delivered via an implantable pump: long-term follow-up review of 18 patients. J Neurosurg. 1999;91:733–6. doi: 10.3171/jns.1999.91.5.0733. [DOI] [PubMed] [Google Scholar]
- 8.Benabid AL, Koudsie A, Benazzouz A, Piallat B, Krack P, Limousin-Dowsey P, et al. Deep brain stimulation for Parkinson’s disease. Adv Neurol. 2001;86:405–12. [PubMed] [Google Scholar]
- 9.Boden SD, Kang J, Sandhu H, Heller JG. Use of recombinant human bone morphogenetic protein-2 to achieve posterolateral lumbar spine fusion in humans: a prospective, randomized clinical pilot trial: 2002 Volvo Award in clinical studies. Spine (Phila Pa 1976) 2002;27:2662–73. doi: 10.1097/00007632-200212010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Ouellette J. Biomaterials facilitate medical breakthroughs. Ind Physicist 2001; 18–21. [Google Scholar]
- 11.Davis JR. Overview of biomaterials and their use in medical devices. In: Davis JR, ed. Handbook of materials for medical devices. Illustrated edition, Ohio: ASM International, 2003: 1-11. [Google Scholar]
- 12.Ramakrishna S, Mayer J, Wintermantel E, Leong KW. Biomedical applications of polymer-composite materials: a review. Compos Sci Technol. 2001;61:1189–224. doi: 10.1016/S0266-3538(00)00241-4. [DOI] [Google Scholar]
- 13.Williams D. An Introduction to Medical and Dental Materials, Concise Encyclopedia of Medical & Dental Materials, D. Williams, Ed., Pergamon Press and The MIT Press, 1990, xvii–xx. [Google Scholar]
- 14.Rice JJ, Martino MM, De Laporte L, Tortelli F, Briquez PS, Hubbell JA. Engineering the regenerative microenvironment with biomaterials. Adv Healthc Mater. 2013;2:57–71. doi: 10.1002/adhm.201200197. [DOI] [PubMed] [Google Scholar]
- 15.Sosnik A, Sefton MV. Semi-synthetic collagen/poloxamine matrices for tissue engineering. Biomaterials. 2005;26:7425–35. doi: 10.1016/j.biomaterials.2005.05.086. [DOI] [PubMed] [Google Scholar]
- 16.Ben-David D, Srouji S, Shapira-Schweitzer K, Kossover O, Ivanir E, Kuhn G, et al. Low dose BMP-2 treatment for bone repair using a PEGylated fibrinogen hydrogel matrix. Biomaterials. 2013;34:2902–10. doi: 10.1016/j.biomaterials.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 17.Hench LL. Biomaterials. Science. 1980;208:826–31. doi: 10.1126/science.6246576. [DOI] [PubMed] [Google Scholar]
- 18.Hench LL, Thompson I. Twenty-first century challenges for biomaterials. J R Soc Interface. 2010;7(Suppl 4):S379–91. doi: 10.1098/rsif.2010.0151.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014–7. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 20.Chen CH, Wei HJ, Lin WW, Chiu I, Hwang SM, Wang CC, et al. Porous tissue grafts sandwiched with multilayered mesenchymal stromal cell sheets induce tissue regeneration for cardiac repair. Cardiovasc Res. 2008;80:88–95. doi: 10.1093/cvr/cvn149. [DOI] [PubMed] [Google Scholar]
- 21.Martinez EC, Kofidis T. Adult stem cells for cardiac tissue engineering. J Mol Cell Cardiol. 2011;50:312–9. doi: 10.1016/j.yjmcc.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 22.Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther. 2005;105:151–63. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Planat-Bénard V, Menard C, André M, Puceat M, Perez A, Garcia-Verdugo JM, et al. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–9. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 24.Missing Reference
- 25.Bartosh TJ, Wang Z, Rosales AA, Dimitrijevich SD, Roque RS. 3D-model of adult cardiac stem cells promotes cardiac differentiation and resistance to oxidative stress. J Cell Biochem. 2008;105:612–23. doi: 10.1002/jcb.21862. [DOI] [PubMed] [Google Scholar]
- 26.Barrère F, Mahmood TA. Groot de K and Blitterswijk van CA. Advanced biomaterials for skeletal tissue regeneration: Instructive and smart functions. Mater Sci Eng Rep. 2008;59:38–71. doi: 10.1016/j.mser.2007.12.001. [DOI] [Google Scholar]
- 27.Kempen DH, Lu L, Heijink A, Hefferan TE, Creemers LB, Maran A, et al. Effect of local sequential VEGF and BMP-2 delivery on ectopic and orthotopic bone regeneration. Biomaterials. 2009;30:2816–25. doi: 10.1016/j.biomaterials.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 28.Kimelman-Bleich N, Pelled G, Zilberman Y, Kallai I, Mizrahi O, Tawackoli W, et al. Targeted gene-and-host progenitor cell therapy for nonunion bone fracture repair. Mol Ther. 2011;19:53–9. doi: 10.1038/mt.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30:546–54. doi: 10.1016/j.tibtech.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster TJ. Nanostructured materials. In: Ying Jackie Y, editor. California, USA: Academic Press; 2001, 125–67. [Google Scholar]
- 31.Roberts SJ, Howard D, Buttery LD, Shakesheff KM. Clinical applications of musculoskeletal tissue engineering. Br Med Bull. 2008;86:7–22. doi: 10.1093/bmb/ldn016. [DOI] [PubMed] [Google Scholar]
- 32.Christenson EM, Anseth KS, van den Beucken JJ, Chan CK, Ercan B, Jansen JA, et al. Nanobiomaterial applications in orthopedics. J Orthop Res. 2007;25:11–22. doi: 10.1002/jor.20305. [DOI] [PubMed] [Google Scholar]
- 33.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 34.Egli RJ, Luginbuehl R. Tissue engineering - nanomaterials in the musculoskeletal system. Swiss Med Wkly. 2012;142:w13647. doi: 10.4414/smw.2012.13647. [DOI] [PubMed] [Google Scholar]
- 35.Marston WA. Dermagraft, a bioengineered human dermal equivalent for the treatment of chronic nonhealing diabetic foot ulcer. Expert Rev Med Devices. 2004;1:21–31. doi: 10.1586/17434440.1.1.21. [DOI] [PubMed] [Google Scholar]
- 36.Jayarama Reddy V, Radhakrishnan S, Ravichandran R, Mukherjee S, Balamurugan R, Sundarrajan S, et al. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen. 2013;21:1–16. doi: 10.1111/j.1524-475X.2012.00861.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci. 2007;48:15–24. doi: 10.1016/j.jdermsci.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 38.Tian H, Tang Z, Zhuang X, Chen X, Jing X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Prog Polym Sci. 2012;37:237–80. doi: 10.1016/j.progpolymsci.2011.06.004. [DOI] [Google Scholar]
- 39.Lasprilla AJ, Martinez GA, Lunelli BH, Jardini AL, Filho RM. Poly-lactic acid synthesis for application in biomedical devices - a review. Biotechnol Adv. 2012;30:321–8. doi: 10.1016/j.biotechadv.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 40.Fattori R, Piva T. Drug-eluting stents in vascular intervention. Lancet. 2003;361:247–9. doi: 10.1016/S0140-6736(03)12275-1. [DOI] [PubMed] [Google Scholar]
- 41.Garg S, Duckers HJ, Serruys PW. Endothelial progenitor cell capture stents: will this technology find its niche in contemporary practice? Eur Heart J. 2010;31:1032–5. doi: 10.1093/eurheartj/ehp591. [DOI] [PubMed] [Google Scholar]
- 42.Luo C, Zheng Y, Diao Z, Qiu J, Wang G. Review: research progress and future prospects for promoting endothelialization on endovascular stents and preventing restenosis. J Med Biol Eng. 2011;31:307–16. doi: 10.5405/jmbe.958. [DOI] [Google Scholar]
- 43.Puranik AS, Dawson ER, Peppas NA. Recent advances in drug eluting stents. Int J Pharm. 2013;441:665–79. doi: 10.1016/j.ijpharm.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Santiago FS, Khachigian LM. Nucleic acid based strategies as potential therapeutic tools: mechanistic considerations and implications to restenosis. J Mol Med (Berl) 2001;79:695–706. doi: 10.1007/s001090100272. [DOI] [PubMed] [Google Scholar]
- 45.Burt HM, Hunter WL. Drug-eluting stents: a multidisciplinary success story. Adv Drug Deliv Rev. 2006;58:350–7. doi: 10.1016/j.addr.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 46.Gouvea C. Biosensors for health applications. In: Serra PA, ed. Biosensors for health, environment and biosecurity. ISBN:978-953-307-443-6, InTech, (DOI: 10.5772/17103) 2011: 71-86. [DOI] [Google Scholar]
- 47.Ballerstadt R, Evans C, Gowda A, McNichols R. Fiber-coupled fluorescence affinity sensor for 3-day in vivo glucose sensing. J Diabetes Sci Technol. 2007;1:384–93. doi: 10.1177/193229680700100311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arya SK, Datta M, Malhotra BD. Recent advances in cholesterol biosensor. Biosens Bioelectron. 2008;23:1083–100. doi: 10.1016/j.bios.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 49.Longmire M, Choyke PL, Kobayashi H. Dendrimer-based contrast agents for molecular imaging. Curr Top Med Chem. 2008;8:1180–6. doi: 10.2174/156802608785849021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herth MM, Barz M, Moderegger D, Allmeroth M, Jahn M, Thews O, et al. Radioactive labeling of defined HPMA-based polymeric structures using [18F]FETos for in vivo imaging by positron emission tomography. Biomacromolecules. 2009;10:1697–703. doi: 10.1021/bm8014736. [DOI] [PubMed] [Google Scholar]
- 51.Duncan R. Polymer therapeutics as nanomedicines: new perspectives. Curr Opin Biotechnol. 2011;22:492–501. doi: 10.1016/j.copbio.2011.05.507. [DOI] [PubMed] [Google Scholar]
- 52.Menegatti E, Berardi D, Messina M, Ferrante I, Giachino O, Spagnolo B, et al. Lab-on-a-chip: Emerging analytical platforms for immune-mediated diseases. Autoimmun Rev. 2012 doi: 10.1016/j.autrev.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 53.Becker H, Carstens C, Gärtner C. Erythrocyte sedimentation and agglutination assays in a multi-bifurcating microfluidic cartridge. Proc. MicroTAS 2009, Jeju, Korea, 430-2. [Google Scholar]
- 54.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012;64:1547–68. doi: 10.1016/j.addr.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Palakurthi NK, Correa ZM, Augsburger JJ, Banerjee RK. Toxicity of a biodegradable microneedle implant loaded with methotrexate as a sustained release device in normal rabbit eye: a pilot study. J Ocul Pharmacol Ther. 2011;27:151–6. doi: 10.1089/jop.2010.0037. [DOI] [PubMed] [Google Scholar]
- 56.Reed ML, Wu C, Kneller J, Watkins S, Vorp DA, Nadeem A, et al. Micromechanical devices for intravascular drug delivery. J Pharm Sci. 1998;87:1387–94. doi: 10.1021/js980085q. [DOI] [PubMed] [Google Scholar]
- 57.Leroux-Roels I, Vets E, Freese R, Seiberling M, Weber F, Salamand C, et al. Seasonal influenza vaccine delivered by intradermal microinjection: A randomised controlled safety and immunogenicity trial in adults. Vaccine. 2008;26:6614–9. doi: 10.1016/j.vaccine.2008.09.078. [DOI] [PubMed] [Google Scholar]
- 58.Rauhi AM. Biomaterials body shop: diverse approaches to diverse human needs. Chem Eng News. 2000;78:33–41. doi: 10.1021/cen-v078n026.p033. [DOI] [Google Scholar]
- 59.Marshall E. Gene therapy death prompts review of adenovirus vector. Science. 1999;286:2244–5. doi: 10.1126/science.286.5448.2244. [DOI] [PubMed] [Google Scholar]
- 60.Park S, Healy KE. Nanoparticulate DNA packaging using terpolymers of poly(lysine-g-(lactide-b-ethylene glycol)) Bioconjug Chem. 2003;14:311–9. doi: 10.1021/bc025623b. [DOI] [PubMed] [Google Scholar]
- 61.Kirschnera CM, Ansetha KS. Hydrogels in healthcare: From static to dynamic material microenvironments. Acta Mater. 2013;61:931–44. doi: 10.1016/j.actamat.2012.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leonardi M, Pitchon EM, Bertsch A, Renaud P, Mermoud A. Wireless contact lens sensor for intraocular pressure monitoring: assessment on enucleated pig eyes. Acta Ophthalmol. 2009;87:433–7. doi: 10.1111/j.1755-3768.2008.01404.x. [DOI] [PubMed] [Google Scholar]
- 63.Badugu R, Lakowicz JR, Geddes CD. A glucose-sensing contact lens: from bench top to patient. Curr Opin Biotechnol. 2005;16:100–7. doi: 10.1016/j.copbio.2004.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Patterson J, Martino MM, Hubbell JA. Biomimetic materials in tissue engineering. Mater Today. 2010;13:14–22. doi: 10.1016/S1369-7021(10)70013-4. [DOI] [Google Scholar]
- 65.Jeong B, Kim SW, Bae YH. Thermosensitive sol-gel reversible hydrogels. Adv Drug Deliv Rev. 2002;54:37–51. doi: 10.1016/S0169-409X(01)00242-3. [DOI] [PubMed] [Google Scholar]
- 66.Masumoto H, Matsuo T, Yamamizu K, Uosaki H, Narazaki G, Katayama S, et al. Pluripotent stem cell-engineered cell sheets reassembled with defined cardiovascular populations ameliorate reduction in infarct heart function through cardiomyocyte-mediated neovascularization. Stem Cells. 2012;30:1196–205. doi: 10.1002/stem.1089. [DOI] [PubMed] [Google Scholar]
- 67.Meenach SA, Anderson KW, Hilt JZ. Synthesis and characterization of thermoresponsive poly(ethylene glycol)-based hydrogels and their magnetic nanocomposites. J Polym Sci Pol Chem. 2010;48:3229–35. doi: 10.1002/pola.24087. [DOI] [Google Scholar]
- 68.Betancourt T, Pardo J, Soo K, Peppas NA. Characterization of pH-responsive hydrogels of poly(itaconic acid-g-ethylene glycol) prepared by UV-initiated free radical polymerization as biomaterials for oral delivery of bioactive agents. J Biomed Mater Res A. 2010;93:175–88. doi: 10.1002/jbm.a.32510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kloxin AM, Benton JA, Anseth KS. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials. 2010;31:1–8. doi: 10.1016/j.biomaterials.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Benton JA, Kern HB, Anseth KS. Substrate properties influence calcification in valvular interstitial cell culture. J Heart Valve Dis. 2008;17:689–99. [PMC free article] [PubMed] [Google Scholar]
- 71.Tripathi A, Kumar A. Multi-featured macroporous agarose-alginate cryogel: synthesis and characterization for bioengineering applications. Macromol Biosci. 2011;11:22–35. doi: 10.1002/mabi.201000286. [DOI] [PubMed] [Google Scholar]
- 72.Jain E, Srivastava A, Kumar A. Macroporous interpenetrating cryogel network of poly(acrylonitrile) and gelatin for biomedical applications. J Mater Sci Mater Med. 2009;20(Suppl 1):S173–9. doi: 10.1007/s10856-008-3504-4. [DOI] [PubMed] [Google Scholar]
- 73.Kumar A, Srivastava A. Cell separation using cryogel-based affinity chromatography. Nat Protoc. 2010;5:1737–47. doi: 10.1038/nprot.2010.135. [DOI] [PubMed] [Google Scholar]
- 74.Bhat S, Tripathi A, Kumar A. Supermacroprous chitosan-agarose-gelatin cryogels: in vitro characterization and in vivo assessment for cartilage tissue engineering. J R Soc Interface. 2011;8:540–54. doi: 10.1098/rsif.2010.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bhat S, Lindgren L, Kumar A. In vitro neo-cartilage formation on three-dimensional composite polymeric cryogel matrix. Macromol Biosci . doi: 10.1002/mabi.201200484. [DOI] [PubMed] [Google Scholar]
- 76.Mishra R, Kumar A. Inorganic/organic biocomposite cryogels for regeneration of bony tissues. J Biomater Sci Polym Ed. 2011;22:2107–26. doi: 10.1163/092050610X534230. [DOI] [PubMed] [Google Scholar]
- 77.Vishnoi T, Kumar A. Conducting cryogel scaffold as a potential biomaterial for cell stimulation and proliferation. J Mater Sci Mater Med. 2013;24:447–59. doi: 10.1007/s10856-012-4795-z. [DOI] [PubMed] [Google Scholar]
- 78.Bhat S, Kumar A. Cell proliferation on three-dimensional chitosan-agarose-gelatin cryogel scaffolds for tissue engineering applications. J Biosci Bioeng. 2012;114:663–70. doi: 10.1016/j.jbiosc.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 79.Jain E, Karande AA, Kumar A. Supermacroporous polymer-based cryogel bioreactor for monoclonal antibody production in continuous culture using hybridoma cells. Biotechnol Prog. 2011;27:170–80. doi: 10.1002/btpr.497. [DOI] [PubMed] [Google Scholar]
- 80.Bencherif SA, Sands RW, Bhatta D, Arany P, Verbeke CS, Edwards DA, et al. Injectable preformed scaffolds with shape-memory properties. Proc Natl Acad Sci U S A. 2012;109:19590–5. doi: 10.1073/pnas.1211516109. [DOI] [PMC free article] [PubMed] [Google Scholar]