Abstract
Background
The dynorphin (DYN)/κ-opioid receptor (KOR) system undergoes neuroadaptations following chronic alcohol exposure that promote excessive operant self-administration and negative affective-like states; however, the exact mechanisms are unknown. The present studies tested the hypothesis that an upregulated DYN/KOR system mediates excessive alcohol self-administration that occurs during withdrawal in alcohol-dependent rats by assessing DYN A peptide expression and KOR function, in combination with site-specific pharmacological manipulations.
Methods
Male Wistar rats were trained to self-administer alcohol using operant behavioral strategies and subjected to intermittent alcohol vapor- or air-exposure. Changes in self-administration were assessed by pharmacological challenges during acute withdrawal. In addition, 22-kHz ultrasonic vocalizations were utilized to measure negative affective-like states. Immunohistochemical techniques assessed DYN A peptide expression and [35S]GTPγS coupling assays were performed to assess KOR function.
Results
Alcohol-dependent rats displayed increased alcohol self-administration, negative affective-like behavior, DYN A-like immunoreactivity and KOR signaling in the amygdala compared to non-dependent controls. Site-specific infusions of a KOR antagonist selectively attenuated self-administration in dependent rats whereas, a MOR/DOR antagonist cocktail selectively reduced self-administration in non-dependent rats. A MOR antagonist/partial KOR agonist attenuated self-administration in both cohorts.
Conclusion
Increased DYN A and increased KOR signaling could set the stage for a `one-two punch' during withdrawal that drives excessive alcohol consumption in alcohol-dependence. Importantly, intra-CeA pharmacological challenges functionally confirmed a DYN/KOR system involvement in the escalated alcohol self-administration. Together, the DYN/KOR system is heavily dysregulated in alcohol dependence and contributes to the excessive alcohol consumption during withdrawal.
Keywords: alcohol, amygdala, dependence, dynorphin, kappa opioid receptor, self-administration, withdrawal
Introduction
Alcohol use disorders pose a substantial risk to society with alcohol-related issues identified as the 3rd leading cause of preventable death costing US society ≤ $224 billion per year(1;2). Alcohol dependence is characterized by physiological withdrawal and the emergence of severe negative affect during withdrawal which substantially contributes to the chronic relapsing disorder. Relapse occurs in 50–90% of abstinence attempts and remains a major hurdle to successful treatment of substance abuse disorders(3). Recent data indicates that negative emotional/affective states during withdrawal drive organisms to excessively seek and use alcohol to alleviate those symptoms(4;5;6). Negative affective symptoms (e.g., depression and anxiety, 22% and 18% co-morbid with alcohol dependence, respectively)(7) are a considerable obstacle to the treatment of alcohol dependence as there are currently no FDA-approved medications indicated for the treatment of the negative affective component of alcohol withdrawal(8).
Acute alcohol stimulates β-endorphin (END), enkephalin (ENK), and dynorphins (DYN) (9) release. END and ENK, endogenous ligands for the μ- (MOR) and δ-opioid receptor (DOR), respectively, underlie the euphoric/rewarding effects of alcohol consumption(10). Conversely, DYNs are the endogenous ligands for the κ-opioid receptor (KOR) (11) and produce dysphoria in humans, aversive behavior in animals and have been implicated in alcohol abuse and dependence(12), as well as the reinforcing and rewarding properties of other drugs of abuse such as nicotine and cocaine(13;14). The dysphoric/anhedonic properties of an overactive DYN/KOR system could contribute to the excessive alcohol seeking and consumption that is observed in alcohol-dependent animals(15). Indeed, evidence shows that KOR antagonists selectively reduce dependence-induced escalation of alcohol consumption without effecting non-dependent responding (10;15;16;17;18). These selective effects implicate the recruitment of the DYN/KOR system during the transition to dependence, laying the foundation for alcohol to have negative reinforcing effects(6;19). Such preclinical evidence corresponds with human genetic associations between DYN / KOR genes and alcohol dependence(20;21).
The DYN/KOR system is expressed throughout the brain however, a dense population of KORs exists in the central nucleus of the amygdala (CeA)(22). As a component of the extended amygdala (comprised of the CeA, AcbSh and bed nucleus of the stria terminalis (BNST)(23), altered CeA function can influence emotional/affective behavior, conditional learning, motivation and decision making. Neuroadaptations within the CeA are implicated in the pathophysiology of various neuropsychiatric disorders(24) and maladaptive behaviors (craving, relapse) induced by chronic alcohol consumption(25). Recent evidence suggesting that dynamic changes are occurring in the DYN/KOR system has found upregulated KOR mRNA in the amygdala following chronic alcohol exposure(26) however, the functional importance of an upregulated DYN system has not been established. To test the hypothesis that an upregulated DYN/KOR system contributes to excessive alcohol self-administration in alcohol-dependent animals, we evaluated neuroadaptations of this system in the amygdala by assessing DYN A peptide expression and KOR function, in combination with site-specific pharmacological manipulations.
METHODS
Animals
Eighty-four male Wistar rats approximately 70 days old were housed in an environmentally controlled vivarium with food and water ad libitum. All work adhered to National Research Council's Guide for the Care and Use of Laboratory Animals(27) and followed Institutional Animal Care and Use Committee guidelines.
Operant Alcohol Self-Administration
Animals were trained to self-administer a 10% alcohol (w/v) solution using a sweetener-fade method. Standard operant chambers (Med Associates, St. Albans, VT) were utilized, allowing the animals to press a single lever and receive 0.1ml of solution. Stability of responding (<10% deviation over 3 sessions) was required prior to intracranial surgeries.
Surgical Procedures
Rats were anesthetized and bilaterally implanted with guide cannulae targeting the CeA according to stereotaxic coordinates (AP −2.3, ML ±4.2, DV −6.3, from bregma (28)). Animals received post-operative care for five days.
Intermittent Alcohol-Vapor Exposure
Following recovery, animals were exposed to alcohol-vapor or air according to an intermittent schedule (14 h on, 10 h off) shown to produce alcohol dependence-like phenotypes(29). Blood alcohol concentrations (BACs) were analyzed from samples collected prior to daily vapor termination and assessed for alcohol content using the Analox AM1 (Analox Instruments Ltd., Lunenberg, MA). Target BACs of 175–225mg% were maintained throughout the experiments and confirmed prior to testing or brain extractions.
Intracranial Infusions
Following dependence-induction, both cohorts self-administered 10% alcohol (w/v) for 30 min twice per week during acute withdrawal (6–8h after vapor termination) until stability was achieved (<10% deviation over 3 days). Next, animals received sham infusions (insertion of internal cannulae into each guide; 28GA, Plastics One, Roanoke, VA). Following stability, animals were infused with 0.5μl/side artificial cerebrospinal fluid (aCSF) until stability was achieved. Sham and aCSF (<10% deviation over 2 days) stability was required prior to neuropharmacological challenges and ensured that any deviations in self-administration were caused by the pharmacological effects of the ligands.
Pharmacology
Nor-binaltorphimine (nor-BNI; 8μg) was bilaterally infused into the CeA. A single dose of nor-BNI was utilized because nor-BNI has previously been shown to have an extended duration of action(16) that prevents the use of a multiple dosing regimen. Intra-CeA CTOP/naltrindole cocktail (equal parts; 0/0, 125/125, 250/250, 500/500ng) or nalmefene (0, 0.125, 1.25, 12.5μg) occurred according to a within-subject Latin-square design counterbalanced for drug dose to prevent any order effects. All infusions were 0.5μl/side over 2 min. The vehicle dose in the Latin-square consisted of aCSF and controlled for carry-over effects.
22-kHz Ultrasonic Vocalizations (USVs)
22-kHz USVs were elicited by a 60 psi air-puff to the nape of the neck. Two trials consisting of 15 air puffs at 15 second intervals ended 1 min after the 15th air puff or after 10 min of vocalizations. USVs were recorded using a P48 Electret Ultrasound Microphone (Avisoft Bioacoustics, Germany), E-MU Systems Audio/MIDI Interface (Scotts Valley, CA), and Avisoft Bioacoustics software (Berlin, Germany) and counted with Avisoft Bioacoustics software. Only second trial results were used for analysis.
Histology
Animals were euthanized and infused with 0.5μl/side of 0.6% cresyl violet over 4 min, their brains were removed and stored in 4% formaldehyde at 4°C until histological examination using a cryostat 1850 (Leica, Bannockburn, IL). Sections were mounted on slides, and CeA placement confirmed (only those with accurate placement were included in the analysis).
Immunohistochemistry Preparation
Animals were anesthetized 6–8 h into withdrawal using pentobarbital (200mg/kg of animal; St. Louis, MO) and perfused with 0.9% saline, 4% paraformaldehyde and 4% paraformaldehyde in 30% sucrose (150ml each; pH 7.4). Brains were stored overnight in 4% paraformaldehyde and 30% sucrose in 0.1M phosphate buffer saline (PBS; pH 7.4). After 24 hours, the brains were moved to a solution of 30% sucrose in 0.1M PBS (pH 7.4) and stored until the brains sank in the vial. 32 sections (40 microns thick) were taken from the CeA, starting at bregma −1.3 and stored in cryopreservative (150g sucrose, 5g polyvinyl pyrolidone, 150ml ethylene glycol and 250mL 0.1M phosphate buffer (PB); pH 7.4) until staining.
Immunohistochemistry
An immunoperoxidase procedure was utilized to assess DYN A content in the CeA. On day 1, sections were washed 4× in phosphate buffer with Triton X (PBTX; 5 min each) on a shaker table and incubated in 1% H2O in PBTX (10 min). Sections were washed 4× in PBTX (5 min per wash). Next sections were incubated in 1% blocking serum in PBTX (1 h). Sections were transferred into primary antibody solution (20 h, 1:1000; anti-DYN 1-17; antigen: DYN A 1-17 (Tyr-Gly-Gly-Phe-Leu-Arg-Arg-Ile-Arg-Pro-Lys-Leu-Lys-Trp-Asp-Asn-Gln); Peninsula Laboratories, San Carlos, CA). On day 2, sections were washed 4× in PBTX (5 min) and then incubated in the secondary antibody (biotinylated anti-rabbit, 1 h, 1:500; Jackson ImmunoResearch, West Grove, PA). Next, sections were washed 4× in PBTX (5 min) and incubated in vector ABC (1 h, 1:1:500; Vector Laboratories, St. Louis, Burlingame, CA). Sections were washed 4× in 0.1M PB (5 min) and transferred to a diaminobenzadine (DAB) solution (20mg DAB, 100ml PB and 34μl H2O2 for 10 min. Sections were washed 4× in 0.1M PB, mounted on slides and allowed to dry. Slides were rinsed in deionized H2O (30 sec), 30% and 50% (3 min each), 70% and 95% (5 min each) and then 100% alcohol (twice, 5 min each). Slides were rinsed in Citrosolve (twice, 20 min each) and coverslipped using DPX mountant and allowed to dry.
To ensure the DYN A-like immunoreactivity observed in dependent animals was not due to non-specific staining, a negative control condition was processed by omitting the primary antibody and measuring DYN A immunoreactivity. No DYN A-like immunoreactivity was observed, consistent with previous studies using anti-DYN 1–17 to conduct pre-absorption controls that have demonstrated no cross-reactivity with primary endogenous opioid peptides (e.g., Met/Leu ENK in the rat) (30).
Quantification of DYN A-like Immunoreactivity
Three planes of CeA sections (corresponding to bregma −2.04, −2.15, and −2.26) from each of the six animals were photographed with Infinity Analyze (Lumenera, Ottawa, Ontario CA) at 200× magnification under identical lighting/contrast conditions without photo enhancements. Using ImageJ, the well documented open-source imaging and analysis software provided by the National Institutes of Health (Bethesda, MD; http://rsb.info.nih.gov/ij/index.html), three planes (AP −2.04, −2.16, and −2.28, from bregma) from each of the air- and vapor-exposed animals were assembled into an average intensity composite z-stack. The resulting composite image was thresholded (default method, B&W color, HSB color space, brightness=125) and analyzed (pixel^2 size=0–60) with particle counts and area (pixel^2) as the output.
DYN A-stimulated [35S]GTPγS Assay
The assay was conducted as described previously(31) with modifications. Amygdala tissue was microdissected and homogenized (45 strokes; on ice) in 1.5ml of membrane buffer (pH 7.4, 50.0mM Tris–HCl, 3.0mM MgCl2, and 1.0mM EGTA). The homogenate was centrifuged (21004g, 4°C for 30 min), re-suspended in 1.5ml of membrane buffer, homogenized (12–15 strokes) and centrifuged again. The pellet was re-homogenized (12–15 strokes) in 1.5ml of assay buffer (pH 7.4, 50.0mM Tris–HCl, 3.0mM MgCl2, 0.2mM EGTA, 100.0mM NaCl). Protein estimation was conducted using a BCA protein assay (Pierce). Samples were homogenized (12–15 strokes) prior to addition of the protein homogenate (3ug for DYN A and 5ug for DAMGO). DYN A (0.0–0.3uM), DADLE (0.0–9.0uM) or DAMGO (0.0–3uM), were incubated in assay buffer in triplicate (90 min; 25°C) with 10μM GDP and 0.05nM [35S] GTPγS in 1.0ml total volume. The reaction was terminated by filtration using a 48-well cell harvester (Brandel; Gaithersburg, MD) and washed 3× in phosphate buffer (pH 7.2). Bound radioactivity was quantified by liquid scintillation spectrophotometry. Vapor- and air-exposed samples were assayed in pairs to prevent bias.
Statistical Analysis
Cohorts of animals were equally added to each condition until both significance (α=0.05) and power (1-β, β=.2) were met to ensure appropriate sample sizes and to prevent the unnecessary use of animals. If assumptions of sphericity were unmet (as determined by Mauchley's Test of Sphericity), corrected degrees of freedom were obtained using the Greenhouse-Geisser correction method and reported with the statistical results.
A mixed-model two-way ANOVA was conducted to confirm dependence-like phenotypes following one month intermittent alcohol-vapor exposure for the nor-BNI and CTOP/naltrindole cohorts and independently compare the baseline and acute withdrawal alcohol self-administration sessions for the vapor- and air-exposed animals by evaluating Session (averaged final three self-administration sessions prior to dependence induction compared to vehicle-treated post-dependence alcohol consumption) and Level (vapor-exposure) as the within- and between-group variables, respectively. Post-hoc analyses included between-group comparisons of air- and vapor-treated aCSF alcohol intake and a within-subject comparison of vapor-exposed baseline and aCSF alcohol intake.
Nor-BNI data and the dose-response curve data for CTOP/naltrindole and nalmefene for air- and vapor-exposed animals was analyzed using a mixed model two-way ANOVA with Exposure as the between-groups factor and Dose as the within-subject factor. Subsequently, if a main effect of Dose or a Dose x Exposure was identified, one-way repeated measure ANOVAs, or paired-sample t-tests for nor-BNI, were individually conducted for each exposure condition. The effects of nor-BNI on the alternate reinforcer were evaluated using a paired-sample t-test to compare aCSF- and nor-BNI treated self-administration sessions. For the nalmefene and CTOP/naltrindole-treated animals, post-hoc LSD tests were conducted if a significant main effect of dose was established.
To evaluate shifts in the potency or efficacy of the pharmacological ligands attributable to alcohol dependence, alcohol consumption (g/kg) following pharmacological challenge relative to vehicle-treated alcohol consumption (i.e., % baseline) was evaluated by two-way ANOVA for CTOP/naltrindole (vapor exposure as the between-group and dose as the within-subject) and for nor-BNI using a univariate ANOVA. Immunohistochemical counts and areas were analyzed using a univariate analysis of variance with level of vapor exposure as the independent variable and counts or area as the dependent variable. Data from the GTPγS coupling assay data was analyzed using a mixed-model two-way ANOVA to compare changes in GTPγS signaling for the vapor- and air-exposed animals. The within-subject variable was DYN concentration and the between-groups variable was exposure condition.
To address the specificity of the intracranial infusions, those animals with misplaced cannula that resulted in removal from the statistical analyses were used as negative controls. The percent reduction from vehicle was calculated for each animal and compared to zero using a one-sample t-test. Four animals were removed because the injection sites were located outside the CeA, six animals were lost due to damaged/removed head stages and three animals were removed prior to the pharmacological challenges due to unstable responding. Thus, of the 84 animals that began the study, 71 successfully completed the study and were included in the data analysis.
RESULTS
Chronic Alcohol Exposure Induces Negative Affective State and Increased DYN A-like Immunoreactivity in the CeA
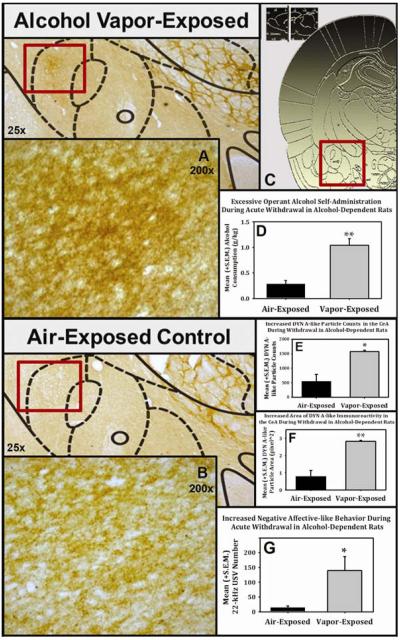
Chronic intermittent alcohol-vapor exposure induced dependence (6), escalated alcohol self-administration (Fig. 2 and 3) and significantly elevated BACs compared to non-dependent controls (Fig 2). In separate groups of animals, the expression of DYN A in the CeA was assessed by immunohistochemistry (IHC). Rats were trained to self-administer alcohol (Fig 1B) and were divided into groups matched for alcohol consumption. Following dependence induction, animals were tested for self-administration rates during acute withdrawal and demonstrated dependence-like phenotypes characterized by significantly escalated self-administration (Fig. 3D) and negative affective behavior as indexed by measurement of 22-kHz ultrasonic vocalizations (Fig. 3G); a ethologically valid behavior that easily discriminates negative affective states (32) in non-dependent vs. dependent rats. Animals continued to receive alcohol vapor- or air-exposure for an additional week and were euthanized during acute withdrawal. The brains were prepared for IHC analysis and were assessed for DYN A-like immunoreactivity in the central nucleus of the amygdala Fig. 3C). Both particle counts (Fig. 3E) and the area (Fig. 3F) of DYN A-like immunoreactivity were significantly increased (p<0.05 and p<0.01, respectively) in the capsular region of the CeA in dependent animals (Fig. 3A) compared to non-dependent controls (Fig. 3B).
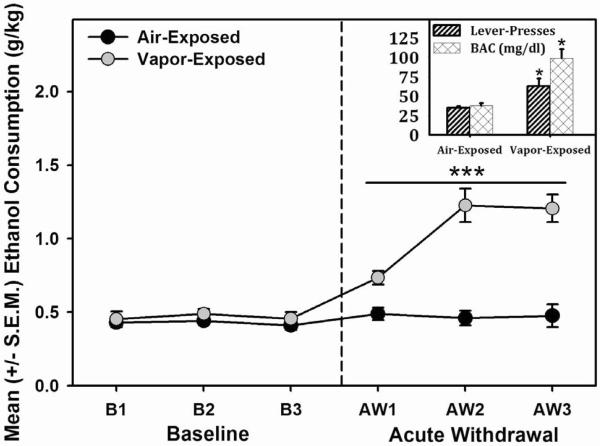
Figure 2. Escalated Alcohol Self-Administration following Intermittent Alcohol Vapor Exposure.
Mean (+/−S.E.M.) alcohol consumption for vapor-exposed rats in acute withdrawal (6–10 hrs) was increased (*** = significant interaction, F (1.538, 18.461) = 16.075, p < 0.001, power = 0.999) compared to air-exposed control animals. Inset: Lever-presses and corresponding blood alcohol concentrations (BAC) for air- and vapor-exposed animals. Both lever-pressing and BACs were significantly increased in vapor-exposed animals (* = p < 0.05 when compared to air-exposed animals).
Figure 3. Excessive Alcohol Self-Administration, Negative Affect and DYN A-Like Immunoreactivity in Alcohol-Dependent Animals.
Wistar rats exposed to alcohol vapor displayed significantly escalated alcohol self-administration (see Fig. 3D; F (1, 4) =12.8, p<0.023, power =0.832) and increased negative affect as assessed by measuring 22-kHz ultrasonic vocalizations (USVs; see Fig. 3G; F (1, 4) =9, p<0.04, power =0.802). DYN A-like immunoreactivity (DYNir) within the central nucleus of the amygdala (CeA; see Fig. 3C) was assessed during acute withdrawal (6–10 hrs) in the same alcohol vapor-treated (see Fig. 3A) or air-treated (see Fig. 3B) animals (stereotaxic schematics adapted from Paxinos and Watson, 2007). Using ImageJ (National Institutes of Health, Bethesda, MD), three planes (AP −2.04, −2.16, and −2.28, from bregma) from each of the air- and vapor-exposed animals were assembled into an average intensity composite z-stack (see Fig. 3A and 3B). The resulting single composite image was thresholded (default method, B&W color, HSB color space, brightness = 125) and analyzed (pixel^2 size = 0–60) with particle counts and area (pixel^2) as the output. Using a univariate ANOVA, a main effect of exposure condition was observed for DYNir Counts (see Fig. 3E; F (1, 4) = 17.477, p = 0.014, Power = .871) and Area (see Fig. 3F; F (1, 4) = 34.551, p = 0.005, Power = .979) in the capsular region of the CeA (* = p < 0.05 and ** = p < 0.01 when compared to air-exposed controls).
Figure 1. Experimental Timeline.
Representative timeline of the experimental procedures: 1A. Pharmacological challenges and GTPγS experiments.1B. Ultrasonic vocalization and Immunohistochemistry.
Chronic Alcohol Exposure Increases DYN A-stimulated [35S] GTPγS Coupling in the Amygdala
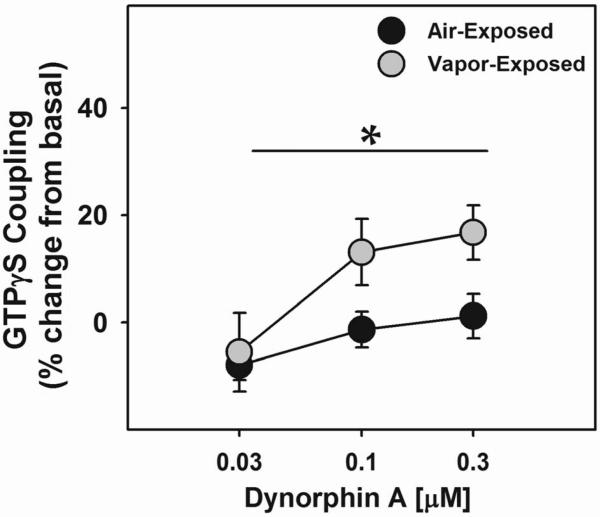
Groups were matched for alcohol consumption, subjected to three months of alcohol vapor- or air-exposure, observed for self-administration rates during withdrawal (Fig 1A), sacrificed six hours into withdrawal and the amygdalae were collected using micropunches, no pooling of animals was to achieve sufficient protein. To assess the functional state of KORs, amygdala tissue homogenates were incubated with [35S] GTPγS in the presence of different concentrations of DYN A, DAMGO (MOR agonist) or DADLE (DOR agonist). DYN A-stimulated coupling of GTPγS in amygdala tissue was shown to produce concentration-dependent increases in coupling that was blocked by nor-BNI (Fig. 4). Subsequently, DYN A, DAMGO and DADLE produced concentration dependent increases in [35S] GTPγS coupling in the amygdala of non-dependent and alcohol-dependent rats. While there were no differences between groups in DAMGO- or DADLE-stimulated GTPγS signaling (data not shown), DYN A-stimulated GTPγS signaling was significantly (p<0.05) elevated in dependent animals compared to non-dependent controls (Fig. 5).
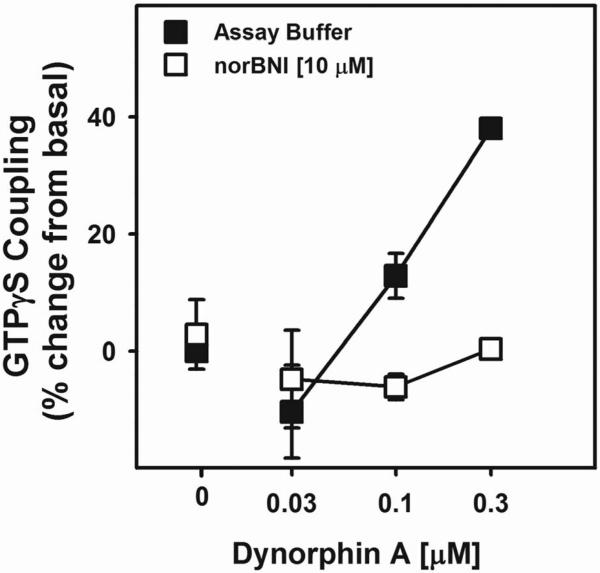
Figure 4. Dynorphin A-Stimulated GTPγS Coupling in the Amygdala of Alcohol-Naive Rats is Blocked by the KOR Antagonist Nor-Binaltorphimine.
Pretreatment with a KOR antagonist reverses DYN A-stimulated GTPγS coupling in the amygdala of alcohol-naïve rats. DYN A induced GTPγS coupling (F (2, 20) = 18.718, p = 0.000026, power = 1.0) in a concentration-dependent manner in amygdala homogenates from alcohol-naive rats which was reversed by 10 μM nor-BNI pretreatment (F (2, 20) 14.083, p = 0.000152, power = 0.995). This demonstrates that the DYN A-stimulated GTPγS coupling was a specific KOR effect.
Figure 5. Increased Dynorphin A-Stimulated GTPγS Coupling in the Amygdala of Dependent Rats during Withdrawal.
To measure the functional state of the KORs within the amygdala (AMYG) of alcohol-dependent rats, DYN A-stimulated GTPγS coupling was assessed in AMYG tissue obtained from the brains of air- or alcohol vapor-exposed rats in acute withdrawal (n=3/grp). DYN A increased [35S]GTPγS coupling in a concentration-dependent manner (F( 3, 48) = 11.713, p = 0.000007, power = 0.999). However, in alcohol-dependent rats, DYN A-stimulated [35S]GTPγS coupling was significantly increased (F (3, 48) =3.653, p = 0.01881, power = 0.827) compared to air-exposed controls. Given that the increased [35S]GTPγS coupling in the AMYG of alcohol-dependent rats was observed under conditions controlling for DYN A concentration, these data suggest that the enhanced KOR-mediated signaling occurs independent of changes in DYN A expression.
Functional Assessment of CeA KOR-Mediated Escalation of Alcohol Self-Administration during Acute Withdrawal
To functionally test the hypothesis that upregulated DYN in the CeA mediate escalated alcohol self-administration, antagonists selective for the KOR, MOR and DOR were infused intra-CeA in non-dependent and alcohol-dependent rats prior to self-administration sessions during acute withdrawal. Following stable responding to sham and artificial cerebrospinal fluid (aCSF) infusions, intra-CeA nor-BNI (8 μg) was infused prior to self-administration testing and was shown to selectively attenuate (p<0.05) escalated self-administration in dependent animals while leaving the response rates of non-dependent animals intact (Fig. 6 and Fig. 7). To assess the specificity of KOR antagonism for reducing alcohol reinforcement in dependent animals, a saccharin reinforcement control group was included. The additional rats were trained to self-administer saccharin, with the concentration of saccharin titrated until response rates were comparable to non-dependent and dependent alcohol self-administration. Following intra-CeA nor-BNI infusions, no changes in saccharin self-administration was observed (Fig S1 in Supplement).
Figure 6. Double-Dissociation between the Effects of Intra-CeA κ-opioid and μ/δ-Opioid Receptor Antagonists Exemplified by the Effects of Intra-CeA Nalmefene.
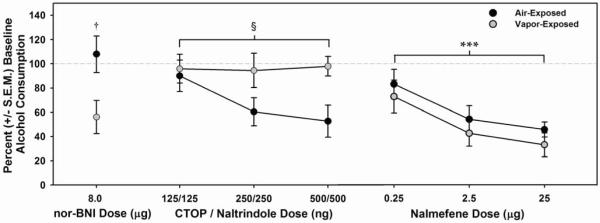
Site-specific intra-CeA infusions of the KOR antagonist nor-binaltorphimine (nor-BNI) selectively reduced (F (1, 13) = 6.833, † = p < 0.05) alcohol consumption (g/kg) in alcohol-dependent rats (while leaving non-dependent alcohol consumption unaffected), whereas infusions of the MOR / DOR antagonist cocktail (CTOP / naltrindole) selectively reduced (F (1, 6) = 7.658, § = p < 0.05) alcohol consumption (g/kg) in non-dependent rats (while leaving alcohol-dependent alcohol consumption unaffected). Intra-CeA nalmefene infusions dose-dependently decreased alcohol consumption (g/kg) in both alcohol-dependent and non-dependent rats (mixed-model two-way ANOVA, main effect of Dose, F (3, 33) = 16.853, *** = p ≤ 0.001, power = 1.0).
Figure 7. Pharmacological Challenges with Intra-CeA Nor-BNI, CTOP/Naltrindole or Nalmefene on Alcohol Self-Administration in Alcohol-Dependent and Non-Dependent Rats.
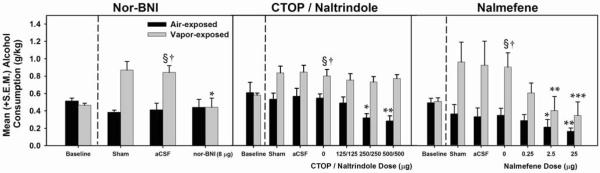
Intra-CeA infusions of nor-BNI significantly attenuated (F (1, 13) = 7.967, p = 0.014, power = 0.858) alcohol consumption (g/kg) in alcohol-dependent rats while leaving non-dependent alcohol consumption (g/kg) intact (†p = 0.003 when compared to the corresponding air-exposed group, § = p< 0.05 when compared to the baseline of the same exposure condition and * p = 0.025 when compared to the vehicle dose of the same exposure condition). CTOP / naltrindole significantly decreased (F (3, 9) = 12.319, p 0.002, power = 0.989) alcohol consumption (g/kg) in non-dependent rats, but did not affect alcohol consumption (g/kg) in alcohol-dependent rats († = p<0.05 when compared to the corresponding air-exposed group, § = p< 0.05 when compared to the baseline of the same exposure condition, * = p< 0.05 and ** = p< 0.01 when compared to the vehicle dose of the same exposure condition). Nalmefene dose-dependently decreased alcohol consumption (g/kg) in both alcohol-dependent (F (3,18) = 11.098, p = 0.000235, power = 0.996) and non-dependent rats (F (3, 15) = 5.661, p = 0.008, power = 0.980; † = p<0.05 when compared to the corresponding air-exposed group, § = p< 0.05 when compared to the baseline of the same exposure condition, * = p< 0.05, ** = p< 0.01, and ***= p<0.001 when compared to the vehicle dose of the same exposure condition).
In addition to site-specific KOR antagonism, the effect of an intra-CeA MOR/DOR antagonist cocktail (CTOP/naltrindole) was assessed on alcohol self-administration in non-dependent and alcohol-dependent rats. Non-dependent rats showed dose-dependent reductions in self-administration following infusion of the CTOP/naltrindole cocktail (p<0.05), whereas those that were alcohol-dependent were unresponsive (Fig. 6 and Fig. 7). To assess a clinically-relevant opioidergic ligand with a mechanism of action relevant to the present experiments, nalmefene (recently approved by EMA for treatment of alcohol dependence) was evaluated under identical conditions as previously described. Intra-CeA infusions of nalmefene dose-dependently attenuated (p<0.05) self-administration in non-dependent and dependent rats during acute withdrawal (Fig. 6 and Fig. 7).
DISCUSSION
To test the hypothesis that the DYN/KOR is dysregulated in dependence and contributes to escalated alcohol self-administration, dependence was induced using chronic intermittent alcohol-vapor exposure and dependent animals exhibited escalated self-administration during withdrawal that yielded significantly increased BACs compared to controls.
Negative affective states accompanying withdrawal are proposed to drive excessive alcohol seeking and consumption(33). Withdrawal-induced negative affect was confirmed by significant increases in the number of 22-kHz USVs emitted in animals that displayed escalated self-administration(29). In the same animals, increased DYN A-like immunoreactivity was observed in the capsular region of the CeA in alcohol-dependent rats compared to controls. This is the initial demonstration that DYN A is significantly upregulated in the capsular region of the CeA, a major output region that is implicated as a substrate of negative emotional states accompanying alcohol withdrawal, although it is unknown whether the increased production of DYN A results in a parallel increase in secretion. However, given that nor-BNI selectively reduced alcohol consumption in dependent animals, the inference that increased DYN A secretion drives escalated alcohol consumption is supported. The capsular region of the CeA, also termed the `nociceptive' amygdala, has extensive connectivity with midbrain and brainstem nuclei, such as the periaqueductal gray (PAG). Reciprocal projections between the CeA and PAG lay the foundation for a `FEAR' circuit designed to deter organisms from evolutionarily disadvantageous situations(34). Ascending projections from the CeA innervate nuclei involved in signaling biologically relevant information to promote survival(35), such as the ventral striatum. The dense ascending projection to the BNST integrates emotional and motivational information and is recruited in dependence to promote excessive alcohol seeking and consumption(5).
Given that acute alcohol exposure has been shown to modulate opioidergic systems in a manner that is reinforcing in animals and euphorogenic in humans(36), the Opponent-Process Theory of Motivation (37) (OPT) was used as an organizational framework. The OPT predicts that hedonic alterations will be met by compensatory responses to maintain homeostasis. It posits that initial positive changes in mood produced by alcohol will decrease following repeated exposure, while the compensatory response becomes exacerbated. Studies have examined alterations in opioid receptors following alcohol consumption; however, little is known about G-protein signaling following alcohol exposure. Evidence suggests that alcohol exposure can alter the functional state of MORs and DORs(38); however, the effect on KOR functional state is still unknown. Therefore, we evaluated the effect of chronic alcohol exposure on KOR-mediated G-protein coupling in the amygdala with an agonist-stimulated [35S] GTPγS coupling assay utilizing DYN A 1-17 (which is not known to discriminate between the k1 and putative k2 KOR subtypes) as the agonist. DYN A-stimulated coupling was compared between air- and vapor-exposed amygdalae and DYN A induced [35S] GTPγS coupling in a concentration-dependent manner in both groups. Importantly, DYN A-stimulated [35S] GTPγS signaling was significantly elevated in the alcohol-dependent group compared to controls. This is the original demonstration that alcohol dependence results in amplified KOR G-protein signaling following chronic alcohol exposure. In combination with the increased DYN A in the CeA of dependent animals that was observed, these data suggest that the DYN/KOR system is heavily dysregulated in alcohol dependence. These findings are consistent with evidence showing that KOR mRNA are upregulated in the CeA following repeated alcohol administration(26). Conversely, DAMGO- and DADLE- stimulated G-protein coupling were not different in air- and vapor-exposed amygdala tissue, an observation that is consistent with clinical evidence revealing considerable DYN/KOR system dysregulation without MOR/DOR effects in the brains of deceased human `alcoholics' compared to age-matched controls(39).
Chronic exposure to drugs of abuse, including alcohol, induce long-term changes in affective states that could result from neuroadaptations within this region of rich connectivity(40). KORs pre-synaptically modulate several neurotransmitter systems in the extended amygdala that include dopaminergic inputs to the amygdala and accumbens(41;42) GABAergic inputs to the accumbens, amygdala and BNST(43), serotonergic (5-HT) inputs to the accumbens(44), and glutamatergic inputs to the accumbens(45). KORs are positioned to modulate multiple neurotransmitter systems within motivational and emotional circuitry that have been implicated in the etiology of numerous neuropsychiatric disorders(46;47). Therefore, an upregulated DYN/KOR system could contribute to escalated alcohol self-administration and the emergence of negative affective states in dependent organisms during withdrawal.
To test the hypothesis that upregulated DYN A levels and increased KOR signaling directly contribute to escalated self-administration, intra-CeA infusions of KOR-, MOR- and DOR-selective antagonists were infused prior to acute withdrawal self-administration sessions. Following acute infusion of nor-BNI, the escalated self-administration that typifies dependence was reduced to non-dependent levels, whereas non-dependent self-administration was unaffected. It is important to note that the transient MOR affinity of nor-BNI that has been observed in mice(48) was not replicated in rats(49). Moreover, the non-dependent alcohol consumption was unaffected following nor-BNI treatment(50;16) which is not predicted if nor-BNI has MOR antagonist properties. Conversely, infusions of the CTOP/naltrindole cocktail dose-dependently decreased self-administration in non-dependent rats, whereas the dependent animals showed no change. Given that intra-CeA KOR antagonism selectively reduced self-administration in dependent animals and MOR/DOR antagonism selectively reduced self-administration in non-dependent animals, a double dissociation was identified. Considering that we did not observe functional alterations in the amygdala MOR/DOR signaling of dependent animals, downregulation of the endogenous peptides for the MOR and/or DOR in dependence could explain why the dependent animals were non-responsive. Critical to this interpretation are data showing that G-protein receptor antagonists can have negative efficacy if G-protein receptors have constitutive activity(51). Previous data has shown that both CTOP and naltrindole are neutral antagonists (i.e., produce no effects on receptor signaling) (52;53) and therefore, the results in dependent animals cannot be attributed to MOR and/or DOR-related signaling changes.
Antagonism of MOR/DORs by nalmefene presumably produced the attenuation of non-dependent self-administration (consistent with CTOP/naltrindole results), whereas the partial KOR agonist mechanism (putatively acting as a functional antagonist under conditions of heighted DYN A levels such as those confirmed by the GTPγS coupling assay) could explain the reductions in self-administration in dependent animals (observed results). Together, this double dissociation supports the predictions of the OPT by suggesting that in dependent animals, the endogenous ligands for the MOR/DOR are downregulated (reduced positive hedonic state) and the DYN/KOR-regulated component of the system is upregulated (exacerbated negative hedonic state). This assertion is supported by evidence showing that the MOR– and DOR–regulated components of the opioid system are reduced in response to chronic alcohol(38;54) and that alcohol-dependent animals have increased prodynorphin mRNA and DYN levels in the Acb(55;56) and increased KOR mRNA expression in the CeA(26).
In conclusion, a dysregulated amygdalar DYN/KOR system was identified and shown to contribute to escalated alcohol self-administration in alcohol-dependent rats. Increased DYN A, in combination with increased KOR signaling, points to the creation of a state analogous to a `one-two punch' within the amygdala of those alcohol-dependent that has considerable relevance to the treatment of other neuropsychiatric disorders. Furthermore, the pharmacological challenges, which occurred once the DYN/KOR system was already upregulated, functionally confirm intra-CeA DYN/KOR system involvement in escalated alcohol self-administration. These data not only apply to the treatment of alcohol dependence, but other neuropsychiatric conditions in which DYN dysregulation has been implicated, such as depression and anxiety(57).
Supplementary Material
ACKNOWLEDGMENTS
Support for this research was provided in part by R01AA020394-01 from the National Institute on Alcohol Abuse and Alcoholism, H. Lundbeck A/S, RGA 11-014 from the Hope for Depression Research Foundation, grants from the WSU Alcohol and Drug Abuse Research Program awarded to BMW and DJR according to the State of Washington Initiative Measure No. 171 and WSU Department of Psychology research grants awarded to DJR. The authors would like to thank Kathryn A. Nealey for her technical assistance with the neuropharmacological aspects of the experiments. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism, the National Institutes of Health or the State of Washington.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURE All authors report no biomedical financial interests or potential conflicts of interest.
Reference List
- 1.Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD. Economic costs of excessive alcohol consumption in the U.S., 2006. Am J Prev Med. 2011;41:516–524. doi: 10.1016/j.amepre.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 2.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend. 2004;74:223–234. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Charney DA, Zikos E, Gill KJ. Early recovery from alcohol dependence: factors that promote or impede abstinence. J Subst Abuse Treat. 2010;38:42–50. doi: 10.1016/j.jsat.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- 5.Koob GF. Dynamics of neuronal circuits in addiction: reward, antireward, and emotional memory. Pharmacopsychiatry. 2009;42(Suppl 1):S32–S41. doi: 10.1055/s-0029-1216356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walker BM. Conceptualizing withdrawal-induced escalation of alcohol self-administration as a learned, plasticity-dependent process. Alcohol. 2012;46:339–348. doi: 10.1016/j.alcohol.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanchez-Pena JF, Alvarez-Cotoli P, Rodriguez-Solano JJ. Psychiatric disorders associated with alcoholism: 2 year follow-up of treatment. Actas Esp Psiquiatr. 2012;40:129–135. [PubMed] [Google Scholar]
- 8.Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lam MP, Marinelli PW, Bai L, Gianoulakis C. Effects of acute ethanol on opioid peptide release in the central amygdala: an in vivo microdialysis study. Psychopharmacology (Berl) 2008;201:261–271. doi: 10.1007/s00213-008-1267-8. [DOI] [PubMed] [Google Scholar]
- 10.Nealey KA, Smith AW, Davis SM, Smith DG, Walker BM. kappa-opioid receptors are implicated in the increased potency of intra-accumbens nalmefene in ethanol-dependent rats. Neuropharmacology. 2011;61:35–42. doi: 10.1016/j.neuropharm.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215:413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 12.Walker BM, Valdez GR, McLaughlin JP, Bakalkin G. Targeting Dynorphin/Kappa Opioid Receptor Systems to Treat Alcohol Abuse and Dependence. Alcohol in press. 2012 doi: 10.1016/j.alcohol.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wee S, Orio L, Ghirmai S, Cashman JR, Koob GF. Inhibition of kappa opioid receptors attenuated increased cocaine intake in rats with extended access to cocaine. Psychopharmacology (Berl) 2009;205:565–575. doi: 10.1007/s00213-009-1563-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JS, Schindler AG, Martinelli E, Gustin RM, Bruchas MR, Chavkin C. Stress-induced activation of the dynorphin/kappa-opioid receptor system in the amygdala potentiates nicotine conditioned place preference. J Neurosci. 2012;32:1488–1495. doi: 10.1523/JNEUROSCI.2980-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker BM, Koob GF. Pharmacological Evidence for a Motivational Role of kappa-Opioid Systems in Ethanol Dependence. Neuropsychopharmacology. 2008;33:643–652. doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker BM, Zorrilla EP, Koob GF. Systemic kappa-opioid receptor antagonism by nor-binaltorphimine reduces dependence-induced excessive alcohol self-administration in rats. Addict Biol. 2011;16:116–119. doi: 10.1111/j.1369-1600.2010.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deehan GA, Jr., McKinzie DL, Carroll FI, McBride WJ, Rodd ZA. The long-lasting effects of JDTic, a kappa opioid receptor antagonist, on the expression of ethanol-seeking behavior and the relapse drinking of female alcohol-preferring (P) rats. Pharmacol Biochem Behav. 2012;101:581–587. doi: 10.1016/j.pbb.2012.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schank JR, Goldstein AL, Rowe KE, King CE, Marusich JA, Wiley JL, et al. The kappa opioid receptor antagonist JDTic attenuates alcohol seeking and withdrawal anxiety. Addict Biol. 2012;17:634–647. doi: 10.1111/j.1369-1600.2012.00455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker BM, Valdez GR, McLaughlin JP, Bakalkin G. Targeting dynorphin/kappa opioid receptor systems to treat alcohol abuse and dependence. Alcohol. 2012;46:359–370. doi: 10.1016/j.alcohol.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xuei X, Dick D, Flury-Wetherill L, Tian HJ, Agrawal A, Bierut L, et al. Association of the kappa-opioid system with alcohol dependence. Mol Psychiatry. 2006;11:1016–1024. doi: 10.1038/sj.mp.4001882. [DOI] [PubMed] [Google Scholar]
- 21.Edenberg HJ, Wang J, Tian H, Pochareddy S, Xuei X, Wetherill L, et al. A regulatory variation in OPRK1, the gene encoding the kappa-opioid receptor, is associated with alcohol dependence. Hum Mol Genet. 2008;17:1783–1789. doi: 10.1093/hmg/ddn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 23.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 24.Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 25.Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 26.D'Addario C, Caputi FF, Rimondini R, Gandolfi O, Del BE, Candeletti S, et al. Different alcohol exposures induce selective alterations on the expression of dynorphin and nociceptin systems related genes in rat brainDADDARIO2011. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00326.x. [DOI] [PubMed] [Google Scholar]
- 27.National Research Council . Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, D.C.: 1996. [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed ed. Elsevier, Academic Press; San Diego: 2007. [Google Scholar]
- 29.Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE, et al. The Effect of Intermittent Alcohol Vapor or Pulsatile Heroin on Somatic and Negative Affective Indices during Spontaneous Withdrawal in Wistar Rats. Psychopharmacology (Berl) in press. 2012 doi: 10.1007/s00213-012-2691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marvizon JC, Chen W, Murphy N. Enkephalins, dynorphins, and beta-endorphin in the rat dorsal horn: an immunofluorescence colocalization study. J Comp Neurol. 2009;517:51–68. doi: 10.1002/cne.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia YF, He L, Whistler JL, Hjelmstad GO. Acute amphetamine exposure selectively desensitizes kappa-opioid receptors in the nucleus accumbens. Neuropsychopharmacology. 2008;33:892–900. doi: 10.1038/sj.npp.1301463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brudzynski SM. Pharmacological and behavioral characteristics of 22 kHz alarm calls in rats. Neurosci Biobehav Rev. 2001;25:611–617. doi: 10.1016/s0149-7634(01)00058-6. [DOI] [PubMed] [Google Scholar]
- 33.Williams AM, Reis DJ, Powell AS, Neira LJ, Nealey KA, Ziegler CE, et al. The effect of intermittent alcohol vapor or pulsatile heroin on somatic and negative affective indices during spontaneous withdrawal in Wistar rats. Psychopharmacology (Berl) 2012 doi: 10.1007/s00213-012-2691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright JS, Panksepp J. Toward affective circuit-based preclinical models of depression: sensitizing dorsal PAG arousal leads to sustained suppression of positive affect in rats. Neurosci Biobehav Rev. 2011;35:1902–1915. doi: 10.1016/j.neubiorev.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Berthoud HR. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 36.Walker BM, Ettenberg A. Intracerebroventricular ethanol-induced conditioned place preferences are prevented by fluphenazine infusions into the nucleus accumbens of rats. Behav Neurosci. 2007;121:401–410. doi: 10.1037/0735-7044.121.2.401. [DOI] [PubMed] [Google Scholar]
- 37.Solomon RL, Corbit JD. An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- 38.Saland LC, Abeyta A, Frausto S, Raymond-Stintz M, Hastings CM, Carta M, et al. Chronic ethanol consumption reduces delta-and mu-opioid receptor-stimulated G-protein coupling in rat brain. Alcohol Clin Exp Res. 2004;28:98–104. doi: 10.1097/01.ALC.0000108658.00243.BF. [DOI] [PubMed] [Google Scholar]
- 39.Bazov I, Kononenko O, Watanabe H, Kuntic V, Sarkisyan D, Taqi MM, et al. The endogenous opioid system in human alcoholics: molecular adaptations in brain areas involved in cognitive control of addiction. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00366.x. [DOI] [PubMed] [Google Scholar]
- 40.Koob GF, Le Moal M. Review. Neurobiological mechanisms for opponent motivational processes in addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3113–3123. doi: 10.1098/rstb.2008.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grilli M, Neri E, Zappettini S, Massa F, Bisio A, Romussi G, et al. Salvinorin A exerts opposite presynaptic controls on neurotransmitter exocytosis from mouse brain nerve terminals. Neuropharmacology. 2009;57:523–530. doi: 10.1016/j.neuropharm.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 42.Werling LL, Frattali A, Portoghese PS, Takemori AE, Cox BM. Kappa receptor regulation of dopamine release from striatum and cortex of rats and guinea pigs. J Pharmacol Exp Ther. 1988;246:282–286. [PubMed] [Google Scholar]
- 43.Hjelmstad GO, Fields HL. Kappa opioid receptor activation in the nucleus accumbens inhibits glutamate and GABA release through different mechanisms. J Neurophysiol. 2003;89:2389–2395. doi: 10.1152/jn.01115.2002. [DOI] [PubMed] [Google Scholar]
- 44.Land BB, Bruchas MR, Schattauer S, Giardino WJ, Aita M, Messinger D, et al. Activation of the kappa opioid receptor in the dorsal raphe nucleus mediates the aversive effects of stress and reinstates drug seeking. Proc Natl Acad Sci U S A. 2009;106:19168–19173. doi: 10.1073/pnas.0910705106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hjelmstad GO, Fields HL. Kappa opioid receptor inhibition of glutamatergic transmission in the nucleus accumbens shell. J Neurophysiol. 2001;85:1153–1158. doi: 10.1152/jn.2001.85.3.1153. [DOI] [PubMed] [Google Scholar]
- 46.Schwarzer C. 30 years of dynorphins--new insights on their functions in neuropsychiatric diseases. Pharmacol Ther. 2009;123:353–370. doi: 10.1016/j.pharmthera.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sirohi S, Bakalkin G, Walker BM. Alcohol induced plasticity in the dynorphin/kappa opioid receptor system. Frontiers in molecular neuroscience. 2012;5:95. doi: 10.3389/fnmol.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broadbear JH, Negus SS, Butelman ER, de Costa BR, Woods JH. Differential effects of systemically administered nor-binaltorphimine (nor-BNI) on kappa-opioid agonists in the mouse writhing assay. Psychopharmacology (Berl) 1994;115:311–319. doi: 10.1007/BF02245071. [DOI] [PubMed] [Google Scholar]
- 49.Picker MJ, Mathewson C, Allen RM. Opioids and rate of positively reinforced behavior: III. Antagonism by the long-lasting kappa antagonist norbinaltorphimine. Behav Pharmacol. 1996;7:495–504. [PubMed] [Google Scholar]
- 50.Walker BM, Koob GF. Pharmacological evidence for a motivational role of kappa-opioid systems in ethanol dependence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33 doi: 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sirohi S, Dighe SV, Madia PA, Yoburn BC. The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. J Pharmacol Exp Ther. 2009;330:513–519. doi: 10.1124/jpet.109.152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Z, Bilsky EJ, Porreca F, Sadee W. Constitutive mu opioid receptor activation as a regulatory mechanism underlying narcotic tolerance and dependence. Life Sci. 1994;54:L339–L350. doi: 10.1016/0024-3205(94)90022-1. [DOI] [PubMed] [Google Scholar]
- 53.Neilan CL, Akil H, Woods JH, Traynor JR. Constitutive activity of the delta-opioid receptor expressed in C6 glioma cells: identification of non-peptide delta-inverse agonists. Br J Pharmacol. 1999;128:556–562. doi: 10.1038/sj.bjp.0702816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turchan J, Przewlocka B, Toth G, Lason W, Borsodi A, Przewlocki R. The effect of repeated administration of morphine, cocaine and ethanol on mu and delta opioid receptor density in the nucleus accumbens and striatum of the rat. Neuroscience. 1999;91:971–977. doi: 10.1016/s0306-4522(98)00637-x. [DOI] [PubMed] [Google Scholar]
- 55.Przewlocka B, Turchan J, Lason W, Przewlocki R. Ethanol withdrawal enhances the prodynorphin system activity in the rat nucleus accumbens. Neurosci Lett. 1997;238:13–16. doi: 10.1016/s0304-3940(97)00829-x. [DOI] [PubMed] [Google Scholar]
- 56.Lindholm S, Ploj K, Franck J, Nylander I. Repeated ethanol administration induces short- and long-term changes in enkephalin and dynorphin tissue concentrations in rat brain. Alcohol. 2000;22:165–171. doi: 10.1016/s0741-8329(00)00118-x. [DOI] [PubMed] [Google Scholar]
- 57.Knoll AT, Carlezon WA. Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.