Abstract
The spinal cord dorsal horn is an important action site for morphine analgesia. Wide-dynamic range (WDR) neurons in the dorsal horn are essential to spinal pain transmission and show increased excitability after repetitive noxious drive (windup). In light of differences in mu-opioid receptor distribution and neurophysiological properties of WDR neurons between deep and superficial dorsal horn, we recorded extracellular single-unit activity of WDR neurons from deep (350–700 μm) and superficial (<350 μm) dorsal horn in C57BL/6 mice and compared their responses to spinal superfusion of morphine (0.5 mM, 30 μl) and naloxone (1 mM, 30 μl). The windup level to repetitive electrical stimulation of 1.0 Hz (16 pulses, suprathreshold for C-fiber activation, 2.0 ms) was significantly decreased by morphine in deep (n=8), but not superficial (n=11), WDR neurons. However, the steady C-component response to graded intra-cutaneous electrical stimuli (0.01–5.0 mA, 2 ms) was significantly depressed by morphine only in superficial neurons. In separate experiments, spinal administration of naloxone facilitated the development of windup to 0.2 Hz stimulation in deep (n=10), but not superficial (n=8), WDR neurons. Accordingly, morphine and naloxone modulation of neuronal activity may be related to a specific effect on neuronal sensitization/plasticity in deep WDR neurons, whereas morphine inhibition may depress acute noxious inputs to superficial WDR neurons. Our study suggests that mu-opioidergic modulation may be different in deep and superficial WDR neurons.
Keywords: mu-opioid receptor, windup, wide-dynamic range neurons, mice
1. Introduction
Sensory information from primary afferents is processed by complex neuronal circuits in the spinal cord dorsal horn [29,30]. The wide-dynamic range (WDR) subtype of dorsal horn neuron represents an important component in the network of spinal pain transmission [13]. Importantly, they also function as an essential cellular mediator of a state of central neuronal hyperexcitability induced by repetitive noxious drive, which may underlie persistent pain and hyperalgesia [11,19]. Repetitive electrical stimulation of C-fibers (>0.3 Hz) progressively increases the response of WDR neurons to subsequent C-fiber input. This process, known as windup, is an electrophysiological phenomenon that results from slow temporal summation of C-fiber–mediated responses of dorsal horn neurons and represents a short form of activity-dependent neuronal plasticity [15,19]. Central processes of nociceptive and thermoreceptive Aδ- and C-afferent sensory neurons mainly terminate in the superficial dorsal horn [30]. The projection patterns of different primary afferents (e.g., C-fibers and A-fibers) and intrinsic neuronal components (e.g., inhibitory and excitatory interneurons) differ between superficial (laminae I–III) and deep (laminae III–V) dorsal horn [1,2,30]. Increasing evidence suggests that deep dorsal horn neurons, including the WDR subtype, possess neurophysiological properties that are different from those of superficial neurons [2,10,21,23]. In particular, deep WDR neurons tend to show a greater neuronal excitability in response to repetitive noxious stimuli, such as windup, than do superficial ones [15,25]. Yet, most studies that have tested the effects of drugs and roles of WDR neurons in pain have focused on deep WDR neurons.
Mu-opioids, such as morphine, remain the mainstay of analgesics used for the clinical treatment of severe and chronic pain states. The dorsal horn is an important site for morphine analgesia. Morphine depresses peripheral noxious inputs and inhibits secondary dorsal horn neurons by activating pre- and postsynaptic mu-opioid receptors (MORs) [18]. MORs are expressed mostly in small-diameter afferent sensory neurons, and more than 70% of all MORs in the spinal cord are located on C-fibers that terminate in the superficial dorsal horn [3,18]. Because both MOR distribution and the neurophysiologic properties of WDR neurons differ between deep and superficial dorsal horn, we hypothesized that the effect of morphine on acute neuronal response and plasticity may also differ between the two subpopulations. Accordingly, we compared the effects of spinal superfusion of morphine (0.5 mM) on the steady C-component response to graded electrical stimulation and on the windup to repetitive noxious inputs between deep and superficial WDR neurons in mice. Dorsal horn neuronal plasticity is also modulated by endogenous opioids [11,16,24]. Previously, we showed that spinal application of naloxone enhanced windup in WDR neurons [11]. However, because a high dose of naloxone (10 mM, 30 μl) was used in the previous work and potential nonselective drug actions may be a concern, we further examined whether naloxone at a dose 10-fold lower (1 mM, 30 μl) can also facilitates windup.
2. Materials and Methods
All procedures were reviewed and approved by the Johns Hopkins University Animal Care and Use Committee as consistent with the National Institutes of Health Guide for the Use of Experimental Animals to ensure minimal animal use and discomfort.
2.1. Animals and surgery
The detailed procedure has been described in our previous studies [11,12]. In brief, male C57BL/6 mice, aged 12–24 weeks (25–35 g), were anesthetized with sodium pentobarbital (70–80 mg/kg, i.p., Sigma-Aldrich, St. Louis, MO). A laminectomy was performed at vertebral levels T12–L1 to expose the lumbar spinal segments. To facilitate controlled ventilation (120–140 cycles/min, stroke volume 0.2–0.3 ml) and to eliminate muscular contractions during electrical stimulation, we used pancuronium bromide (0.15 mg/kg, i.p., Elkins-Sinn Inc., Cherry Hill, NJ) to paralyze the mice during neurophysiologic recording. Anesthesia was subsequently maintained with isoflurane (1.5%, Baxter, Deerfield, IL) throughout the experiment.
2.2. Dorsal horn neuron recording
Detailed procedure has also been described previously [11]. Tungsten microelectrodes (8 mΩ at 1 kHz; Frederick Haer & Co., Brunswick, ME) were used to make extracellular recordings of the activity of individual dorsal horn neurons with defined receptive fields in the plantar region of the hind paw. A real-time computerbased data acquisition and processing system (DAPSYS 6; Brian Turnquist, Johns Hopkins University) provided window discriminators for real-time sorting of different action potential (AP) waveforms (for details go to http://www.dapsys.net). Depth of recording site was estimated from the micro-positioner coordinate reading (model 650 D; David Kopf Instruments, Tujunga, CA), which has been shown to be comparable to that confirmed histologically [22,31]. Superficial (<350 μm) and deep (350–700 μm) neurons were identified according to their recording depths [11].
2.3. Experimental protocol
WDR neurons were characterized by using mechanical stimuli with intensities that ranged from mild to noxious texture: brushing skin with a small brush, indentation of the plantar skin with 1.4 g (diameter: 0.26 mm) and 4.0 g (diameter: 0.35 mm) force of von Frey monofilaments, and pinching the skin with a small serrated forceps. Neurons that responded with increased firing rates to increasing intensity of stimuli were classified as WDR cells [11,17]. WDR neurons show both A-fiber– and C-fiber–mediated responses to a single intra-cutaneous electrical stimulus that is suprathreshold for C-fiber activation. Based on the axon conduction velocities, the evoked response can be separated into three components: A-fiber-mediated responses (0–40 ms), C-fiber–mediated responses (40–250 ms), and with some neurons, a post-discharge (250–1000 ms) (Fig. 1A).
Fig. 1. Spinal administration of morphine induces different inhibitory effects on deep and superficial WDR neurons.
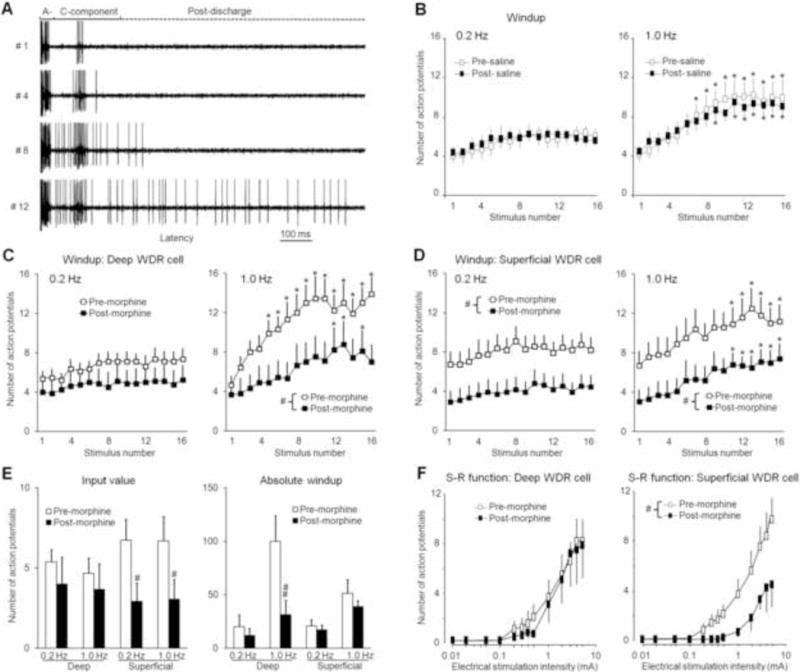
(A) Analog recordings of action potential (AP) responses of a WDR neuron to the first, fourth, eighth, and twelfth stimuli of a train of windup-inducing electrical stimuli (1.0 Hz, supra-C threshold, 2.0 ms). The WDR neuron displayed a progressive increase in the number of APs in the C-component (windup) and after-discharges. The A-component and C-component were divided by the early (0–40 ms) and late (40–250 ms) latencies to a single intracutaneous electrical stimulus. (B) The number of APs in the C-component of WDR neurons (n=8, 5 deep, 3 superficial) in response to repetitive electrical stimulation (0.2 Hz and 1.0 Hz, 16 pulses, supra-C threshold, 2.0 ms) was not changed by topical spinal application of saline. The C-component responses in (C) deep (350–700 μm, n=8) and (D) superficial (<350 μm, n=11) WDR neurons to 0.2 Hz and 1.0 Hz electrical stimuli were decreased 15–30 min after spinal superfusion of morphine (0.5 mM, 30 μl). (E) The input (number of C-component responses evoked by the first stimulus of the train) and absolute windup values before and after morphine treatment in superficial and deep WDR neurons. Absolute windup = (total number of APs evoked by the train) – (16 × input). (F) Stimulus-response (S-R) functions of the steady C-component response to graded electrical stimuli (0.01–5.0 mA, 2.0 ms) were significantly depressed in superficial, but not deep, WDR neurons 15–30 min after morphine treatment. Data are expressed as mean ± SEM. * p<0.05 versus input, # p<0.05 versus pre-drug baseline.
In pre-drug baseline tests, we established the stimulus-response functions (S-R function) of C-fiber–mediated responses to acute intra-cutaneous electrical stimuli applied in steps (0.01–5.0 mA, 2 ms) and then examined windup to a train of computercontrolled electrical stimuli (16 pulses, suprathreshold for C-fiber activation, 2.0 ms) applied at frequencies of 0.2 Hz and 1.0 Hz. Electrical stimuli were applied through a pair of fine needles inserted subcutaneously into the receptive field. At 15–30 min after baseline tests, drug solution was applied topically onto the recording spinal segment and left in place. Response profiles to the same series of test stimuli were examined from 15 to 30 min after drug application, a time period of peak drug effect. Because the drug diffusion range and elimination rate were unknown under these experimental conditions, we tested only one neuron in each animal for drug effects. Drugs examined included morphine (0.5 mM, 30 μl) and naloxone (1 mM, 30 μl). Saline (0.9%) was used as a control. The investigator who performed the experiment was blinded to the drug conditions.
2.4. Data analysis
The primary parameter for windup analysis was the number of APs in the C-component evoked by each stimulus in the train. Windup curves were then created by plotting the number of APs against the stimulation number in a train of 16 stimuli. The “absolute windup” value was calculated as (total number of APs evoked by the train) – (16 × input), where “input” is defined as the number of APs evoked by the first stimulus of the train. Because our pilot study showed that morphine changes inputs in some WDR neurons, the absolute windup, which measures the change in total number of APs during repetitive stimulation, was used for quantifying windup level. The number of APs in the C-component evoked by graded electrical stimuli was used to establish the S-R function. A two-way repeated-measures ANOVA was used to compare windup, input, and S-R function between pre-drug and post-drug conditions. The Tukey honestly significant difference post-hoc test was used. p<0.05 was considered statistically significant. Data are presented as mean ± standard error of mean (SEM).
3. Results
3.1. Superfusion with morphine exerts different modulatory effects on deep and superficial WDR neurons
Repetitive electrical stimulation at 1.0 Hz, but not 0.2 Hz, induced a progressive increase in the C-component to the subsequent stimulus in some WDR neurons (Fig. 1A). Spinal superfusion with morphine (0.5 mM, 30 μl), but not saline (Fig. 1B, n=8), depressed the windup curves to 1.0 Hz stimulation in both deep (n=8, mean depth: 443±34 μm) and superficial (n=11, mean depth: 178±20 μm) WDR neurons (Fig. 1C, D). However, further quantification analysis showed that morphine significantly decreased absolute windup (1.0 Hz), a measure of windup level that disregards the change in input, in deep, but not superficial, WDR neurons (Fig. 1E). In contrast, the same morphine treatment significantly decreased the input value only in superficial WDR neurons. Morphine also significantly depressed the S-R function of C-component to graded electrical stimuli in superficial WDR neurons (Fig. 1F).
3.2. Spinal administration of naloxone facilitates windup to 0.2 Hz simulation in deep WDR neurons
In a separate group of animals, naloxone (1.0 mM, 30 μl) facilitated windup in deep WDR neurons (n=10, mean depth: 455±28 μm) to the lower frequency stimulation of 0.2 Hz, which normally is an ineffective frequency for inducing windup (Fig. 2A–D). The effect of naloxone began a few minutes after drug application and lasted for at least for 30 min (Fig. 2B). The absolute windup to 0.2 Hz stimulation was also significantly increased from the pre-drug value after drug treatment (Fig. 2E). Although there was a trend toward increased windup (windup curve and absolute windup value) at 1.0 Hz stimulation in deep WDR neurons, these changes did not reach statistical significance. In superficial WDR neurons (n=8, mean depth: 211±17 μm), naloxone did not change windup curves significantly, but it did increase the absolute windup value at 1.0 Hz stimulation. Naloxone did not induce a significant change in input or S-R function in deep or superficial WDR neurons (Fig. 2F).
Fig. 2. Spinal administration of naloxone facilitates the development of windup in response to 0.2 Hz stimulation in deep WDR neurons.
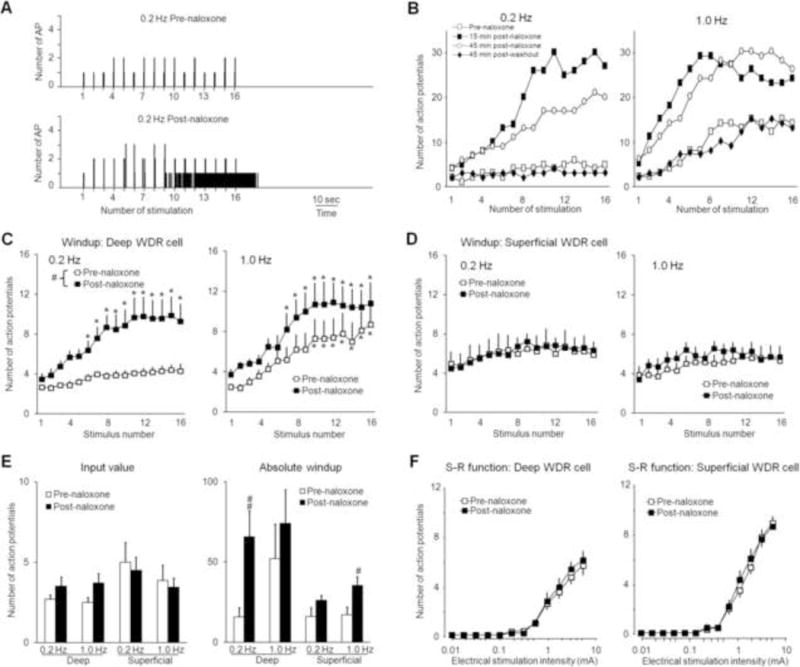
(A) Histograms show that the evoked responses of a WDR neuron to 0.2 Hz intra-cutaneous electrical stimuli (16 pulses, supra-C threshold, 2.0 ms) were markedly increased after naloxone treatment (1 mM, 30 μl). Bin sizes were 10 ms. (B) Representative graphs show the time course by which naloxone facilitates windup in a deep WDR neuron (476 μm) at 0.2 Hz and 1.0 Hz stimulation. The windup curves show the C-component responses in (C) deep (350–700 μm, n=10) and (D) superficial (<350 μm, n=8) WDR neurons to 0.2 and 1.0 Hz electrical stimuli before and 15–30 min after spinal superfusion of naloxone. (E) The input and absolute windup values before and after morphine treatment were plotted for the various frequencies in deep and superficial WDR neurons. (F) Stimulus-response (S-R) functions of C-component in deep and superficial WDR neurons to graded electrical stimuli (0.01–5.0 mA, 2 ms) did not change 15–30 min after naloxone treatment. Data are expressed as mean ± SEM. * p<0.05 versus input, # p<0.05, ## p<0.01 versus pre-drug baseline.
4. Discussion
The spinal cord dorsal horn is an important site for opioidergic control of nociceptive transmission. We show that windup, a short form of neuronal sensitization to repetitive noxious inputs, was most affected by morphine and naloxone in deep WDR neurons. However, the steady C-component response of deep WDR neurons to acute noxious inputs (e.g., S-R function) was largely unchanged. In contrast, morphine significantly depressed the steady response, but not the windup, in superficial WDR neurons. Accordingly, mu-opioidergic modulation may be different in deep and superficial WDR neurons.
How does the same dose of drug exert different actions on deep and superficial WDR neurons? Although the exact molecular mechanism remains to be determined, it may involve the following factors: 1) Different distributions of MOR in superficial and deep dorsal horn. Over 70% of the total MOR sites are on the afferent terminals that terminate mostly in the superficial dorsal horn [3,18]. Thus, the predominant site for muopioidergic modulation is pre-synaptic on these afferent terminals. A much lower concentration of MORs is found in deeper layers, and is more likely located at postsynaptic neurons. Activation of pre-synaptic MORs strongly inhibits the release of excitatory neurotransmitters from C-fiber terminals at superficial dorsal horn [20,26]. Thus, spinal topical application of morphine may preferably inhibit the steady C-component of superficial WDR neurons to graded electrical stimuli because MOR-expressing C-fibers predominantly terminate in this area. Therefore, the powerful antinociception elicited by intrathecal morphine involves a potent depression of peripheral noxious inputs in superficial dorsal horn neurons, including the WDR subtype. 2) Different neurophysiological properties of superficial and deep WDR neurons. Increasing evidence suggests that deep dorsal horn neurons possess neurophysiological properties that are different from those of superficial neurons [2,10,21,23]. Deep WDR neurons tend to show a greater neuronal excitability in response to repetitive noxious stimuli, such as windup, than do superficial ones [15,25]. Although morphine depressed the windup curves in both deep and superficial WDR neurons, it decreased the absolute windup value (indicating windup level) and the slope of the windup curve (indicating the rate of windup development) to 1.0 Hz stimulation mostly in deep WDR neurons. Windup reflects a short-term amplification mechanism in the spinal cord. Thus, activation of MORs modulates the progress of activity-dependent neuronal sensitization that involves WDR neurons, especially those located in deep dorsal horn. It is noteworthy that although morphine attenuated windup, it did not completely eliminate the eventual temporal summation of deep WDR neurons. This finding supports previous results that windup can break through the morphine inhibition, even when the steady C-component response of the cell has been significantly depressed [4,8]. 3) Network mechanism. Unlike superficial neurons, which may receive monosynaptic inputs from MOR-expressing C-fiber terminals, C-fiber inputs onto deep WDR cells may largely rely on polysynaptic transmission (i.e., relayed via interneurons). Although some deep neurons may be functionally linked to the superficial neurons, the effect of drug on deep WDR neurons can be different from that on superficial neurons, because it reflects the combined drug action on afferent terminals, superficial neurons, deep neurons bearing MORs, and interneurons in contact with them. In addition, superficial and deep dorsal horn neurons may be under different descending modulatory control from supraspinal structures. Superficial cells, especially lamina I neurons that express NK-1 receptor, are important in inducing a descending pain modulation through a spinal-supraspinal loop [28]. Accordingly, a change in superficial neuronal activity may subsequently affect the activity of deep neurons and the effect of drug on them via both network mechanism and descending pathways.
It remains unclear how windup, but not the steady C-component response, is inhibited by morphine in deep WDR neurons. It would appear that mechanism by which morphine inhibits windup in deep WDR neurons is different from that by which it inhibits the steady C-fiber inputs in superficial dorsal horn. Windup is considered to be a central phenomenon that relies primarily on temporal summation of slow cumulative depolarization of the postsynaptic neurons [15,27]. If morphine inhibition of windup involved activation of postsynaptic MORs to induce hyperpolarization in deep WDR neurons, both steady C-component and windup component would be affected. However, this was not the case. Previously, NMDA antagonists were shown to specifically affect the windup component, while not altering the steady C-component or the inputs onto the cells [7]. It is possible that interaction between MOR and NMDA may also be involved in morphine inhibition of windup in deep WDR neurons [21]. Other factors, including presynaptic mechanisms, postsynaptic receptors, membrane properties, and the network may also affect windup modulation by morphine. Others have shown that systemic administration of morphine induces significant inhibition of deep dorsal horn neuronal activity [6,9]. The discrepancy between their findings and ours may be due in part to different doses and routes of drug administration. The mild inhibition of steady response in deep WDR neurons by morphine in the current study may also be due to low MOR expression level and poor drug penetration to deep dorsal horn.
The endogenous opioidergic system is important for modulating spinal nociceptive processing. Endogenous opioids, such as endomorphine, are released into spinal cord from many sources after intense/repetitive nociceptive drive [32]. Spinal topical application of naloxone at a dose 10-fold lower than that used in our previous study did not change the S-R functions in deep or superficial WDR cells, but facilitated windup to repetitive electrical stimuli. We think that this facilitation is caused by a loss of endogenous inhibitory modulation. In general, these findings are consistent with those of our previous work with 10 mM naloxone and the work of others [11,14,23]. Accordingly, the endogenous opioidergic system may be activated by repetitive noxious inputs to counteract the development of neuronal sensitization. It is possible that naloxone at the dose tested in the current study may not represent the lowest dose required to block endogenous activation of opioid receptors, and hence even lower doses (e.g., 0.01 mM) may still be effective. The potential mechanisms underlying naloxone facilitation of windup have been described in detail in our previous study [11]. It needs to be noted that, as to most in vivo extracellular recordings, the neuron being recorded may be a small distance away from the recording site, even with using the fine-tip and high impedance micro-electrode. Thus, the depth of recording site may not precisely reflect the depth for each neuron.
In summary, our results suggest that MOR activation may differentially modulate deep and superficial WDR neuronal plasticity and excitability, supporting previously reported findings [5]. Therefore, systemic and spinal morphine analgesia may involve differential regulation of nociceptive processing in superficial and deep dorsal horn. The interplay between opioidergic inhibitory mechanisms and excitatory mechanisms may ultimately determine spinal pain transmission and neuronal sensitization to repetitive noxious inputs.
Highlights.
Neurophysiological properties differ in deep and superficial dorsal horn neurons.
Spinal morphine inhibits windup in deep wide-dynamic range (WDR) neurons.
Morphine inhibits the steady C-component response in superficial WDR neurons.
Spinal naloxone facilitates windup to 0.2 Hz simulation in deep WDR neurons.
Mu-opioidergic modulation differs in deep and superficial WDR neurons.
Acknowledgments
The authors thank Claire F. Levine, MS (scientific editor, Department of Anesthesiology/CCM, Johns Hopkins University) for editing the manuscript. This work was supported by NIH Grant NS70814 (Y.G.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The work was conducted in the Department of Anesthesiology and Critical Care Medicine, Johns Hopkins University, School of Medicine, Baltimore, MD, USA.
Reference List
- 1.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basbaum AI, Braz JM. Transgenic mouse models for the tracing of “pain” pathways. In: Kruger L, Light AR, editors. Translational Pain Research: From Mouse to Man. CRC Press; Boca Raton: 2010. [PubMed] [Google Scholar]
- 3.Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res. 1990;521:15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- 4.Chapman V, Dickenson AH. The combination of NMDA antagonism and morphine produces profound antinociception in the rat dorsal horn. Brain Res. 1992;573:321–323. doi: 10.1016/0006-8993(92)90780-d. [DOI] [PubMed] [Google Scholar]
- 5.Chen YP, Chen SR, Pan HL. Effect of morphine on deep dorsal horn projection neurons depends on spinal GABAergic and glycinergic tone: implications for reduced opioid effect in neuropathic pain. J Pharmacol Exp Ther. 2005;315:696–703. doi: 10.1124/jpet.105.091314. [DOI] [PubMed] [Google Scholar]
- 6.Chen YP, Chen SR, Pan HL. Systemic morphine inhibits dorsal horn projection neurons through spinal cholinergic system independent of descending pathways. J Pharmacol Exp Ther. 2005;314:611–617. doi: 10.1124/jpet.105.085563. [DOI] [PubMed] [Google Scholar]
- 7.Davies SN, Lodge D. Evidence for involvement of N-methylaspartate receptors in ‘wind-up’ of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987;424:402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- 8.Dickenson AH, Sullivan AF. Electrophysiological studies on the effects of intrathecal morphine on nociceptive neurones in the rat dorsal horn. Pain. 1986;24:211–222. doi: 10.1016/0304-3959(86)90044-8. [DOI] [PubMed] [Google Scholar]
- 9.Duale C, Raboisson P, Molat JL, Dallel R. Systemic morphine reduces the wind-up of trigeminal nociceptive neurons. Neuroreport. 2001;12:2091–2096. doi: 10.1097/00001756-200107200-00010. [DOI] [PubMed] [Google Scholar]
- 10.Eckert WA, III, Julius D, Basbaum AI. Differential contribution of TRPV1 to thermal responses and tissue injury-induced sensitization of dorsal horn neurons in laminae I and V in the mouse. Pain. 2006;126:184–197. doi: 10.1016/j.pain.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Guan Y, Borzan J, Meyer RA, Raja SN. Windup in dorsal horn neurons is modulated by endogenous spinal mu-opioid mechanisms. J Neurosci. 2006;26:4298–4307. doi: 10.1523/JNEUROSCI.0960-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan Y, Liu Q, Tang Z, Raja SN, Anderson DJ, Dong X. Mas-related Gprotein-coupled receptors inhibit pathological pain in mice. Proc Natl Acad Sci U S A. 2010;107:15933–15938. doi: 10.1073/pnas.1011221107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guan Y, Wacnik PW, Yang F, Carteret AF, Chung CY, Meyer RA, Raja SN. Spinal cord stimulation-induced analgesia: electrical stimulation of dorsal column and dorsal roots attenuates dorsal horn neuronal excitability in neuropathic rats. Anesthesiology. 2010;113:1392–1405. doi: 10.1097/ALN.0b013e3181fcd95c. [DOI] [PubMed] [Google Scholar]
- 14.Hartell NA, Headley PM. The effect of naloxone on spinal reflexes to electrical and mechanical stimuli in the anaesthetized, spinalized rat. J Physiol. 1991;442:513–526. doi: 10.1113/jphysiol.1991.sp018806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 16.Holden JE, Jeong Y, Forrest JM. The endogenous opioid system and clinical pain management, AACN. Clin Issues. 2005;16:291–301. doi: 10.1097/00044067-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Homma E, Collins JG, Kitahata LM, Matsumoto M, Kawahara M. Suppression of noxiously evoked WDR dorsal horn neuronal activity by spinally administered morphine. Anesthesiology. 1983;58:232–236. doi: 10.1097/00000542-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Kline RH, Wiley RG. Spinal mu-opioid receptor-expressing dorsal horn neurons: role in nociception and morphine antinociception. J Neurosci. 2008;28:904–913. doi: 10.1523/JNEUROSCI.4452-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Simone DA, Larson AA. Windup leads to characteristics of central sensitization. Pain. 1999;79:75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- 20.Lopez Soto EJ, Raingo J. A118G Mu Opioid Receptor polymorphism increases inhibitory effects on CaV2.2 channels. Neurosci Lett. 2012;523:190–194. doi: 10.1016/j.neulet.2012.06.074. [DOI] [PubMed] [Google Scholar]
- 21.Luccarini P, Sessle BJ, Woda A. Superficial and deep convergent nociceptive neurons are differentially affected by N-methyl-D-aspartate applied on the brainstem surface of the rat medullary dorsal horn. Neuroscience. 2001;107:311–316. doi: 10.1016/s0306-4522(01)00360-8. [DOI] [PubMed] [Google Scholar]
- 22.Martin WJ, Malmberg AB, Basbaum AI. PKCgamma contributes to a subset of the NMDA-dependent spinal circuits that underlie injury-induced persistent pain. J Neurosci. 2001;21:5321–5327. doi: 10.1523/JNEUROSCI.21-14-05321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mokha SS. Differential influence of naloxone on the responses of nociceptive neurons in the superficial versus the deeper dorsal horn of the medulla in the rat. Pain. 1992;49:405–413. doi: 10.1016/0304-3959(92)90248-A. [DOI] [PubMed] [Google Scholar]
- 24.Nadal X, La PC, Andreea BS, Maldonado R. Involvement of the opioid and cannabinoid systems in pain control: New insights from knockout studies. Eur J Pharmacol. 2013 doi: 10.1016/j.ejphar.2013.01.077. [DOI] [PubMed] [Google Scholar]
- 25.Schouenborg J, Sjolund BH. Activity evoked by A- and C-afferent fibers in rat dorsal horn neurons and its relation to a flexion reflex. J Neurophysiol. 1983;50:1108–1121. doi: 10.1152/jn.1983.50.5.1108. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder JE, McCleskey EW. Inhibition of Ca2+ currents by a mu-opioid in a defined subset of rat sensory neurons. J Neurosci. 1993;13:867–873. doi: 10.1523/JNEUROSCI.13-02-00867.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staud R, Robinson ME, Price DD. Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain. 2007;8:893–901. doi: 10.1016/j.jpain.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways. Nat Neurosci. 2002;5:1319–1326. doi: 10.1038/nn966. [DOI] [PubMed] [Google Scholar]
- 29.Takazawa T, MacDermott AB. Synaptic pathways and inhibitory gates in the spinal cord dorsal horn. Ann N Y Acad Sci. 2010;1198:153–158. doi: 10.1111/j.1749-6632.2010.05501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weng HR, Mansikka H, Winchurch R, Raja SN, Dougherty PM. Sensory processing in the deep spinal dorsal horn of neurokinin-1 receptor knockout mice. Anesthesiology. 2001;94:1105–1112. doi: 10.1097/00000542-200106000-00027. [DOI] [PubMed] [Google Scholar]
- 32.Zadina JE, Martin-Schild S, Gerall AA, Kastin AJ, Hackler L, Ge LJ, Zhang X. Endomorphins: novel endogenous mu-opiate receptor agonists in regions of high mu-opiate receptor density. Ann N Y Acad Sci. 1999;897:136–144. doi: 10.1111/j.1749-6632.1999.tb07885.x. [DOI] [PubMed] [Google Scholar]


